Abstract
Objectives: The objective of this study was to examine the association between non-high-density lipoprotein cholesterol (non-HDL-C) to high-density lipoprotein cholesterol (HDL-C) ratio (NHHR) and chronic kidney disease (CKD) in Chinese adults with type 2 diabetes mellitus (T2DM). Methods: This study originated from a survey carried out in Zhejiang Province, located in eastern China, between March and November 2018. To explore the relationship between NHHR and CKD, a multivariable logistic regression model was employed. The dose–response relationship was assessed using restricted cubic spline (RCS) analysis, while generalized additive models (GAMs) were applied to examine the associations between NHHR and urinary albumin-to-creatinine ratio (UACR) as well as estimated glomerular filtration rate (eGFR). Subgroup analyses were performed across various demographic and clinical categories to assess the consistency of the NHHR–CKD association. The optimal NHHR cutoff for CKD diagnosis, its predictive accuracy, and its comparison with its components and HbA1c were determined through receiver operating characteristic (ROC) curve analysis. Results: The study enrolled 1756 participants, including 485 individuals with CKD and 1271 without CKD. Multivariable logistic regression revealed a significant positive association between NHHR and CKD, with each standard deviation (SD) increase in NHHR linked to a 23% higher odds of CKD (OR = 1.23, 95% CI: 1.09–1.37) after adjusting for potential confounders. When comparing quartiles, the fully adjusted ORs for Q2, Q3, and Q4 were 1.29 (0.92–1.79), 1.31 (0.94–1.83), and 1.87 (1.34–2.60), respectively, relative to Q1 (p for trend < 0.01). RCS analysis confirmed a linear dose–response relationship between NHHR and CKD in both sexes (p for nonlinearity > 0.05). GAMs indicated a significant positive correlation between NHHR and UACR (ρ = 0.109, p < 0.001) but no significant association with eGFR (ρ = −0.016, p = 0.502). Subgroup analyses demonstrated consistent associations across most subgroups, except for the 18–44 years age group, the well-controlled glycemic group, and the non-alcohol drinking group (p > 0.05). ROC curve analysis identified an optimal NHHR cutoff of 3.48 for CKD prediction, with an area under the curve (AUC) of 0.606 (95% CI: 0.577–0.635). Notably, NHHR outperformed its individual components and HbA1c in predictive performance. Conclusions: This study revealed a linear link between higher NHHR levels and increased CKD prevalence in Chinese T2DM patients. NHHR may also serve as a potential complementary biomarker for early CKD detection, though further prospective studies are needed to confirm its predictive value and clinical utility in high-risk T2DM populations.
1. Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has risen sharply globally, with China facing a particularly severe burden [1]. Recent epidemiological data reveal that 12.4% of the adult population in mainland China are living with diabetes, and the majority of these cases are classified as T2DM [2]. In addition to being a metabolic condition, T2DM, a metabolic disorder, significantly increases the risk of systemic complications, particularly cardiovascular disease and chronic kidney disease (CKD) [3]. These associated conditions are major drivers of elevated morbidity and mortality rates among individuals with T2DM [4,5].
CKD is one of the most serious complications associated with T2DM, marked by progressive albuminuria, a reduction in glomerular filtration rate, and an increased risk of cardiovascular events [6]. In China, over the past three decades, the absolute count of deaths and disability-adjusted life years associated with CKD linked to diabetes has shown a consistent upward trend [7]. Considering the chronic and typically irreversible trajectory of CKD, implementing timely detection methods for high-risk populations and adopting focused intervention approaches are critical to decelerating the advancement of the condition and enhancing long-term health results for affected individuals.
In patients with T2DM, lipid abnormalities play a significant role in the development and progression of CKD [8]. Among the lipid markers, high-density lipoprotein cholesterol (HDL-C) has protective effects on vascular and renal health due to its anti-inflammatory, antioxidant, and cholesterol reverse transport functions [9]. Low HDL-C levels are associated with an increased risk of CKD, potentially due to reduced endothelial protection and impaired renal function [10,11]. However, the relationship between HDL-C and clinical outcomes is complex and likely nonlinear, as evidenced by studies showing diminishing protective effects at higher HDL-C levels [12]. Moreover, interventions to raise HDL-C have failed to demonstrate clinical benefits [13]. Conversely, non-high-density lipoprotein cholesterol (non-HDL-C), calculated as total cholesterol (TC) minus HDL-C, represents the total atherogenic lipoprotein burden, including low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), and lipoprotein(a) [14]. Elevated non-HDL-C levels have been linked to a decline in estimated glomerular filtration rate (eGFR) and an increased risk of proteinuria, suggesting that excessive atherogenic lipids contribute to endothelial dysfunction, inflammation, and glomerular injury, thereby accelerating CKD progression in T2DM patients [15].
The non-HDL-C/HDL-C ratio (NHHR) reflecting the balance between protective and atherogenic lipoproteins, has been linked to endothelial dysfunction and inflammation, key pathways in CKD progression [16]. Currently, some evidence suggests that specific lipid ratios may better reflect lipid-related renal risk compared to traditional lipid parameters [17,18]. Similarly, NHHR has also emerged as a potential predictor of atherosclerotic cardiovascular disease and metabolic disorders [19]. However, the validity of such ratios is debated, particularly given the nonlinear effects of HDL-C and the lack of evidence supporting their superiority in guiding individual treatment decisions [20,21].
Given that T2DM involves chronic inflammation, insulin resistance (IR), and vascular dysfunction [22], lipid ratios like NHHR may offer insights into systemic metabolic risks that contribute to CKD alongside traditional renal markers like urinary albumin-to-creatinine ratio (UACR) and eGFR. While UACR and eGFR are cost-effective and directly reflect renal damage, lipid ratios could potentially identify at-risk individuals earlier by capturing dyslipidemia-related pathways, though their added value remains unproven. Our study explores NHHR’s association with CKD to determine if it complements the existing clinical tools, especially among high-risk groups, such as Chinese T2DM patients.
To address this gap in understanding, our study employs a cross-sectional dataset to examine the relationship between NHHR and CKD in Chinese adults diagnosed with T2DM. By elucidating this association, our results have the potential of contributing to a deeper understanding of the metabolic-renal interface and informing risk stratification strategies in clinical practice.
2. Materials and Methods
2.1. Study Subjects
The research subjects were drawn from a comprehensive investigation focused on complications associated with T2DM, conducted between March and November 2018 in Zhejiang Province, eastern China. The study included local residents aged 18 years or older who had been diagnosed with T2DM and recorded in the local health information platform.
A multi-stage randomized sampling strategy was employed to select participants. In stage one, two districts and two counties were randomly chosen from the province. During the second stage, four townships or subdistricts were randomly selected from each of the previously chosen districts and counties. In stage three, a random sample of 120 individuals diagnosed with T2DM was selected from each township or subdistrict, ensuring a balanced distribution across sex and age categories. This approach led to the inclusion of 1920 participants in the study. Further details regarding the study design and methodology are available in the published protocol [23].
Following the application of exclusion criteria, 164 individuals were excluded, leaving a final analytical sample of 1756 participants (Figure 1).

Figure 1.
The detailed procedure of participant selection.
2.2. Sample Size Calculation
The determination of the required number of participants was derived using the equation N = μ2 × p × (1 − p)/d2. Within this equation, the variables were specified as follows: p denoted the estimated proportion of CKD cases among Chinese individuals with T2DM, set at 0.271 [24]; μ was assigned a value of 1.96; and the relative error (d) was fixed at 0.05. Utilizing these parameters, the necessary sample size for each subgroup was calculated to be 304 individuals. Considering the division of the target population into 4 subgroups (urban and rural regions, along with sex) and anticipating a non-participation rate of 15.0%, the total number of subjects needed for the research was projected to be 1430. Consequently, the participant count in this study was deemed sufficient.
2.3. Data Collection
In this investigation, participants were required to complete a face-to-face questionnaire, undergo physical examinations (e.g., height, weight, and blood pressure), and provide fasting blood and urine samples for laboratory analyses. These analyses encompassed assessments of hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), lipid profiles, renal function markers (urea, creatinine, and serum uric acid [SUA]), urinary creatinine and urinary albumin, etc. All procedures were conducted by trained personnel from township health centers, who had substantial professional experience and had undergone specific training for this research initiative.
Standardized instruments were utilized for anthropometric measurements: height was measured using a stadiometer, weight with an electronic scale (HD-390, TANITA, Tokyo, Japan), and waist circumference with a flexible retractable tape. Blood pressure and heart rate were recorded using an automated blood pressure monitor (HBP-1300, OMRON, Kyoto, Japan).
Fasting blood samples were collected from all participants after a 10–12 h overnight fast, along with first-morning urine specimens. FPG levels were assessed at local laboratories that had met qualification standards, employing either the glucose oxidase or hexokinase method. The remaining blood and urine samples were processed on-site, where they were centrifuged, aliquoted, and stored according to standardized preservation protocols. These samples were then transported by a logistics provider designated by the national project team for centralized analysis. HbA1c levels were measured using high-performance liquid chromatography (D10, Bio-Rad, Berkeley, CA, USA). Lipid profiles, including triglycerides (TG), TC, HDL-C, and LDL-C, as well as SUA and creatinine levels in blood and urine, were determined enzymatically (Cobas C701, Roche, Basel, Switzerland). Urinary albumin concentrations were quantified using an immunoturbidimetric method (Cobas C701, Roche, Basel, Switzerland).
2.4. Definitions of Variables
2.4.1. Definitions of CKD and NHHR
CKD was diagnosed based on two primary criteria: the presence of albuminuria, defined as a UACR exceeding 30 mg/g; or impaired kidney function, characterized by an eGFR below 60 mL/min per 1.73 m2 [25,26]. The eGFR values were calculated utilizing the chronic kidney disease epidemiology collaboration equation [27]. Additionally, the NHHR was calculated by dividing the non-HDL-C concentration by the HDL-C concentration [28]. Non-HDL-C levels were obtained by subtracting HDL-C values from TC measurements.
2.4.2. Definitions of Covariates
Hypertension was characterized by systolic blood pressure readings of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or above, combined with a self-reported diagnosis of hypertension documented by medical institutions [29]. The body mass index (BMI) was calculated as weight (kg) divided by height squared (m2) and was categorized as <24 kg/m2 and ≥24 kg/m2 for the subgroup analysis [30]. Elevated glycemic markers were defined as HbA1c levels of 7.0% or more or FPG concentrations of 7.0 mmol/L or higher [31]. Educational background was categorized into three groups: secondary education or less, senior high school completion, and attainment of college education or higher. Marital status was divided into two classifications: married and other (including single, divorced, or widowed). Participants were stratified by age into young adults (18–44 years), middle-aged adults (45–59 years), and older adults (60 years and above). Residential status was classified as urban or rural based on the geographic location of participants’ homes. Routine exercise was defined as engaging in exercise for a minimum of 30 min per day, at least five days per week [32]. Smoking status was determined by current daily or occasional cigarette use, while alcohol drinking was recorded if participants had consumed alcoholic beverages within the past 30 days [33].
2.5. Statistical Analysis
Numerical variables were summarized using two distinct approaches based on their distributional properties: means accompanied by standard deviations (SD) were used for normally distributed data, while medians with interquartile ranges (IQR) were reported for data that deviated from normality. Categorical variables were expressed as counts and percentages. For normally distributed data, comparisons between groups were performed using independent t-tests for two groups and analysis of variance (ANOVA) for multiple groups. Non-normally distributed data were analyzed using the Mann–Whitney U test for two groups and the Kruskal–Wallis test for multiple groups. Categorical comparisons were conducted using the chi-square test. Participants were categorized into four groups according to NHHR quartiles: the first quartile (Q1), second quartile (Q2), third quartile (Q3), and fourth quartile (Q4). To reduce potential confounding and achieve balanced group characteristics, propensity score matching (PSM) was performed, with standardized mean differences (SMD) used to assess balance between CKD and non-CKD participants (Table S1). To investigate the association between NHHR and CKD, multivariable logistic regression models were utilized. Restricted cubic splines (RCS) were applied to examine potential non-linear relationships between NHHR and CKD, with a piecewise logistic regression model to further explore this relationship. Three regression models were constructed: Model 1 was unadjusted and included no covariates; Model 2 adjusted for age and sex; and Model 3 incorporated additional adjustments for education, marital status, residence, body mass index, serum uric acid, triglyceride, hemoglobin A1c and fasting plasma glucose, systolic blood pressure, diastolic blood pressure, smoking, alcohol drinking, routine exercise, and diabetes duration. To assess potential multicollinearity in our dataset, the generalized variance inflation factor (GVIF) was calculated for all included variables. The results confirmed no significant multicollinearity (all GVIF1/2Df < 2) (Table S2). Generalized additive models (GAMs) were employed to assess the relationships between NHHR and both UACR and eGFR. Furthermore, subgroup analyses were performed to evaluate the consistency of the NHHR–CKD association across various strata, including age, sex, BMI, hypertension status, glycemic control, smoking, and alcohol drinking status. The ROC curve was utilized to assess NHHR’s capability in differentiating CKD patients from non-CKD individuals, quantified by the area under curve (AUC). The DeLong test was applied to compare the diagnostic performance across various markers. Statistical significance was defined as a two-sided p-value of less than 0.05. To account for multiple comparisons and determine statistical significance, the Bonferroni correction was applied, setting the adjusted significance threshold at α = 0.007 for subgroup interaction tests (calculated as 0.05 divided by 7 subgroups) [34]. All analyses were conducted using R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Basic Characteristics of Participants
As summarized in Table 1, the study comprised 1756 participants, stratified by CKD status into 485 individuals with CKD and 1271 without CKD. The mean age of the overall population was 57.23 ± 10.15 years, with participants in the CKD group being significantly older than those in the non-CKD group (p < 0.001). The sex distribution was equal, with 50.11% females and 49.89% males, and no significant sex-based differences were observed between the CKD and non-CKD groups (p > 0.05). The overall mean BMI was 24.76 ± 3.43 kg/m2, with the CKD group demonstrating a significantly higher BMI compared to the non-CKD group (p < 0.001). Hypertension was markedly more prevalent in the CKD group than in the non-CKD group (p < 0.001). Further analysis of lipid profiles revealed significant disparities, including higher TG, TC, non-HDL-C levels and lower HDL-C levels in the CKD group compared to the non-CKD group (all p < 0.001). Glycemic control was notably worse in the CKD group, as evidenced by elevated HbA1c levels and FPG concentrations (both p < 0.001). Additionally, SUA levels were significantly higher in the CKD group (p < 0.001). Regarding lifestyle factors, alcohol consumption was less common in the CKD group compared to the non-CKD group, while no significant differences were observed in smoking or routine exercise between the two groups (all p > 0.05).

Table 1.
Participants’ basic characteristics by CKD status (n = 1756).
Table 2 presents the basic characteristics of participants stratified by NHHR quartiles. Significant differences were observed across quartiles for age, BMI, HDL-C, non-HDL-C, SUA levels, diabetes duration, and UACR (all p < 0.05). Additionally, the prevalence of elevated HbA1c, FPG, smoking, and CKD varied significantly (all p < 0.01), with the highest rates observed in the fourth quartile (Q4). In contrast, no significant differences were found for sex, educational attainment, residence, alcohol consumption, routine exercise, or eGFR distribution across the NHHR quartiles (all p > 0.05).

Table 2.
Participants’ basic characteristics by NHHR quartile (n = 1756).
3.2. Multivariable Regression Analysis of NHHR Quartiles in Relation to CKD
To evaluate the relationship between NHHR and CKD, multivariable logistic regression models were employed. Odds ratios (ORs) and their 95% confidence intervals (CIs) were computed for NHHR quartiles, using Q1 as the reference group (Table 3). Initially, NHHR was analyzed as a continuous variable. In the unadjusted model (Model 1), each SD increase in NHHR was associated with ORs of 1.34 (1.21–1.49) in the overall population, 1.38 (1.19–1.60) in females, and 1.32 (1.14–1.53) in males. After adjusting for age and sex, the ORs remained largely unchanged. Further adjustments for covariates such as education, marital status, residence, body mass index, serum uric acid, triglyceride, hemoglobin A1c, fasting plasma glucose, systolic and diastolic blood pressure, smoking, alcohol consumption, physical activity, and diabetes duration yielded ORs of 1.23 (1.09–1.37) in the overall population, 1.22 (1.05–1.45) in females, and 1.25 (1.03–1.47) in males.

Table 3.
Multivariable regression analysis of NHHR quartiles in relation to CKD (n = 1756).
When NHHR was assessed categorically, the unadjusted model showed ORs for CKD of 1.36 (0.99–1.87) for Q2, 1.53 (1.12–2.09) for Q3, and 2.26 (1.67–3.05) for Q4 compared to Q1 in the overall population. In females, the ORs were 1.11 (0.71–1.73), 1.16 (0.75–1.81), and 2.16 (1.42–3.27), while in males, they were 1.57 (1.00–2.49), 2.06 (1.32–3.12), and 2.48 (1.59–3.85). After adjusting for age and sex in Model 2, the ORs exhibited minimal changes. In the fully adjusted Model 3, the ORs for Q2, Q3, and Q4 were 1.29 (0.92–1.79), 1.31 (0.94–1.83), and 1.87 (1.34–2.60), respectively, in the overall population. For females, the ORs were 0.95 (0.59–1.52), 1.08 (0.68–1.72), and 1.77 (1.13–2.79), while for males, they were 1.62 (1.00–2.64), 1.86 (1.15–3.03), and 2.19 (1.34–3.60). Significant trends were observed across all models in the overall population, females, and males (all p for trend < 0.05). The multivariable regression analysis conducted on the dataset after PSM further confirmed the robustness of these results (Table S3).
3.3. RCS Analysis of the Association Between NHHR and CKD
To explore potential non-linear relationships between NHHR and CKD, RCS analysis was conducted, with the results presented in Figure 2. After adjusting for covariates, a consistent linear dose–response relationship was observed between NHHR and CKD in the overall population, as well as in both females and males (all p for nonlinearity > 0.05). This suggests that higher NHHR levels are associated with an increased likelihood of CKD. Additionally, a linear relationship between NHHR and CKD was observed in the dataset after PSM (Figure S1). Furthermore, a piecewise regression analysis was conducted to evaluate the threshold effect of NHHR on CKD. The log-likelihood ratio test yielded a p-value > 0.05, suggesting that the relationship between NHHR and CKD does not significantly deviate from a linear model (Table S4).
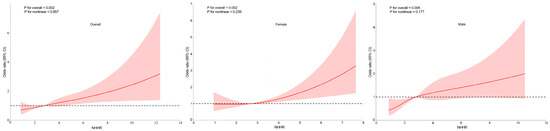
Figure 2.
Association between the non-high-density lipoprotein cholesterol (non-HDL-C) to HDL-C ratio (NHHR) and chronic kidney disease (CKD) in overall population, females and males, with 95% confidence intervals (CIs). The figure depicts the association between NHHR and CKD, considering potential nonlinear associations. X-axis: Represents the levels of NHHR. Y-axis: Represents the odds ratios for CKD, indicating the relative likelihood of CKD presence associated with different levels of NHHR. The solid lines represent the fitted curves illustrating the association between NHHR and CKD. The dotted horizontal lines denote an OR of 1, representing the absence of association. The red areas surrounding the fitted curves indicate the 95% CIs for the predicted ORs. Adjusted for age, sex, education, marital status, residence, body mass index, serum uric acid, triglyceride, hemoglobin A1c and fasting plasma glucose, systolic blood pressure, diastolic blood pressure, smoking, alcohol drinking, routine exercise, and diabetes duration.
3.4. GAM Analysis of NHHR with UACR and eGFR
To further investigate these relationships, GAMs were used to evaluate the associations of NHHR with both UACR and eGFR in the overall population, as well as in females and males separately. As shown in Figure 3A,C,E, a statistically significant positive correlation was observed between NHHR and UACR (mg/g), with Spearman correlation coefficients of ρ = 0.109 (p < 0.001) in the overall population, ρ = 0.118 (p < 0.001) in females, and ρ = 0.106 (p = 0.002) in males. The smooth-fitted curves demonstrated a modest upward trend, suggesting that higher NHHR levels may be associated with increased UACR values. In contrast, no statistically significant correlation was found between NHHR and eGFR (mL/min/1.73 m2) in the overall population (ρ = −0.016, p = 0.502; Figure 3B), females (ρ = 0.005, p = 0.850; Figure 3D), or males (ρ = −0.033, p = 0.325; Figure 3F), indicating no significant relationship between NHHR and eGFR.
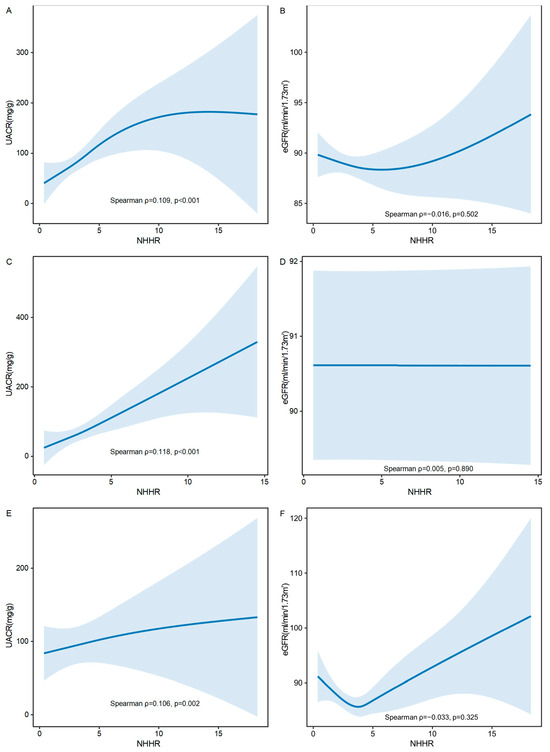
Figure 3.
Correlation of the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) with both the urinary albumin-to-creatinine ratio (UACR) and the estimated glomerular filtration rate (eGFR), with 95% confidence intervals (CIs). (A) Correlation of NHHR with UACR in overall population. (B) Correlation of NHHR with eGFR in overall population. (C) Correlation of NHHR with UACR in females. (D) Correlation of NHHR with eGFR in females. (E) Correlation of NHHR with UACR in males. (F) Correlation of NHHR with eGFR in males. X-axis: Represents the levels of NHHR. Higher values on this axis indicate higher NHHR levels. Y-axis: Represents the levels of UACR or eGFR. The solid lines represent the fitted curves illustrating the trend of the relationship between NHHR with UACR and eGFR. Blue areas: Represent the 95% CIs around the fitted curves, indicating the precision of the estimated relationships. UACR, urinary albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate.
3.5. Results of Subgroup Analyses
Subgroup analyses were conducted across a range of demographic and clinical variables, including age categories (18–44 years, 45–59 years, and ≥60 years), sex (female or male), BMI (≥24 kg/m2 or <24 kg/m2), hypertension status (no or yes), glycemic control (poor or good), smoking status (no or yes), and alcohol consumption (no or yes) (Figure 4). The findings revealed statistically significant associations (all p < 0.05) in most subgroups, except for the 18–44 years age group, the good glycemic control group, and the non-alcohol drinking group, where the associations were not significant (p > 0.05). No significant interactions were detected between NHHR and the stratified variables, suggesting that the positive relationship between NHHR and CKD was consistent across all examined subgroups (all p for interaction > 0.007).
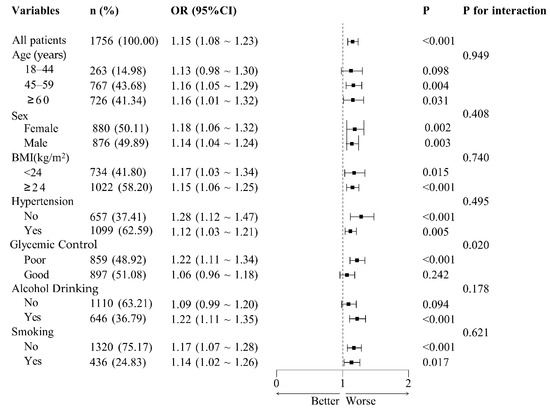
Figure 4.
Subgroup analysis of adjusted odds ratios for non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and chronic kidney disease (CKD). Adjusted odds ratios (ORs) for the association between the NHHR and CKD across subgroups. Adjustments were made for age, sex, education, marital status, residence, body mass index, serum uric acid, triglyceride, hemoglobin A1c and fasting plasma glucose, systolic blood pressure, diastolic blood pressure, smoking, alcohol drinking, routine exercise, and diabetes duration. Each subgroup analysis excluded adjustments for the variable defining the subgroup. The central dashed line represents that OR = 1. Each horizontal bar represents the confidence interval (CI) for the OR of a subgroup. OR, odds ratio; BMI, body mass index.
3.6. Roc Curve Assessment
The ability of NHHR to diagnose CKD in T2DM individuals was evaluated using ROC curve analysis, as depicted in Figure 5. The analysis produced an AUC (95% CI) of 0.606 (0.577–0.635), indicating moderate diagnostic precision. The optimal threshold for NHHR was determined to be 3.48, with sensitivity and specificity values of 60.2% and 55.2%, respectively. Additionally, NHHR was compared with its individual elements (non-HDL-C and HDL-C) and HbA1c. The AUC (95% CI) values for non-HDL-C, HDL-C, and HbA1c were 0.551 (0.521–0.582), 0.576 (0.545–0.606), and 0.578 (0.548–0.607), respectively. According to the DeLong test, NHHR demonstrated significantly better predictive performance than HbA1c, non-HDL-C, and HDL-C (all p < 0.05), while no notable differences were observed among the other markers (all p > 0.05). These findings indicate that the composite NHHR offers greater diagnostic value.
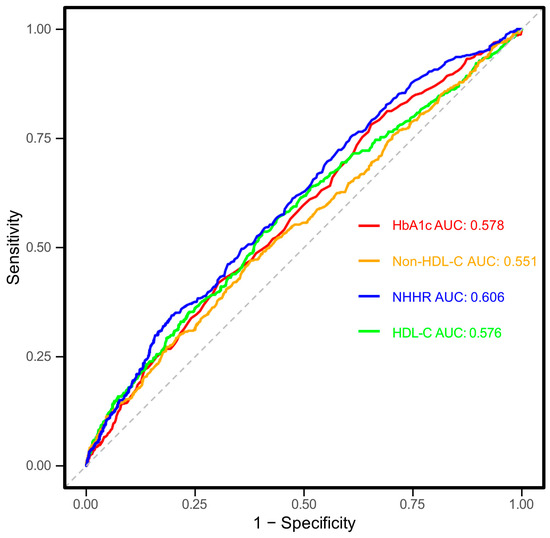
Figure 5.
Receiver operating characteristic (ROC) curves for the association between non-high-density lipoprotein cholesterol (non-HDL-C) to high-density lipoprotein cholesterol ratio (NHHR), non-HDL-C, HDL-C and hemoglobin A1c (HbA1c) with chronic kidney disease (CKD). X-axis (1-Specificity): Represents the false-positive rate, indicating the proportion of individuals without CKD who are incorrectly classified as having CKD. Y-axis (Sensitivity): Represents the true-positive rate, indicating the proportion of individuals with CKD who are correctly classified as having CKD. The solid lines represent the ROC curves for each indicator, while the dashed line indicates the reference line representing no discriminatory ability. The area under the curve (AUC) values is provided for each indicator to quantify the discriminatory ability of NHHR for CKD. Higher AUC values (closer to 1) indicate better classification performance. HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; NHHR, non-HDL-C to HDL-C ratio; AUC, area under curve.
4. Discussion
This study revealed a significant positive association between NHHR and CKD among Chinese individuals aged 18 years and older with T2DM. Higher NHHR levels and quartiles were linked to a progressively elevated prevalence of CKD, a finding reinforced by RCS and piecewise analyses, which demonstrated a linear dose–response relationship, both in females and males. After adjusting for covariates such as age, sex, education, marital status, residence, body mass index, serum uric acid, triglyceride, hemoglobin A1c and fasting plasma glucose, systolic blood pressure, diastolic blood pressure, smoking, alcohol drinking, routine exercise, and diabetes duration, the association remained significant (p < 0.05) across most subgroups. However, no significant associations were observed in the 18–44 years age group, the good glycemic control group, or the non-alcohol drinking group (p > 0.05). GAMs further confirmed a significant positive correlation between NHHR and UACR (ρ = 0.109, p < 0.001). ROC curve analysis demonstrated NHHR’s diagnostic potential, with an optimal cut-off value of 3.48 showing better predictive performance compared to its individual components and HbA1c. Sensitivity analyses conducted on the PSM-adjusted dataset further reinforced the robustness and reliability of our primary findings. These results highlight NHHR’s promise as a biomarker for assessing CKD risk in patients with T2DM.
The positive linear association between NHHR and CKD may be explained by several interrelated mechanisms, including atherogenic lipid metabolism abnormalities, oxidative stress, chronic inflammation, IR, sympathetic overactivity, lipotoxicity in renal tubules, and microvascular damage leading to fibrosis. These factors collectively contribute to renal impairment and CKD progression. Elevated NHHR reflects an imbalance between atherogenic lipoproteins (non-HDL-C) and protective lipoproteins (HDL-C), indicating a pro-atherogenic state. Elevated non-HDL-C, particularly LDL-C and VLDL-C, can penetrate the glomerular capillary wall, undergo oxidation (oxLDL), and trigger macrophage infiltration and foam cell formation, promoting glomerulosclerosis [35]. Simultaneously, reduced HDL-C impairs cholesterol efflux, weakens endothelial protection, and accelerates renal vascular dysfunction. This imbalance contributes to endothelial nitric oxide deficiency, leading to renal hypoperfusion and chronic hypoxia, further exacerbating CKD.
Oxidative stress and inflammation play a central role in NHHR-induced renal damage. oxLDL activates the NF-κB pathway, increasing pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which drive tubulointerstitial inflammation and fibrosis [36]. Meanwhile, HDL-C possesses anti-inflammatory and antioxidant properties, which are diminished in individuals with a high NHHR. This chronic inflammatory state disrupts tubuloglomerular feedback, leading to proteinuria and progressive nephron loss [37].
NHHR is also closely linked to IR, a key driver of CKD. IR contributes to glomerular hyperfiltration by impairing afferent arteriolar relaxation and increasing tubular sodium reabsorption, leading to intraglomerular hypertension [38]. Additionally, IR dysregulates lipid metabolism, promoting the overproduction of VLDL and small dense LDL-C while reducing HDL-C levels, further elevating NHHR [39]. These metabolic disturbances exacerbate oxidative stress and renal endothelial dysfunction, accelerating CKD progression. Sympathetic nervous system overactivity may further mediate the NHHR–CKD relationship. Increased sympathetic activity enhances renin–angiotensin–aldosterone system (RAAS) activation, raising glomerular pressure and worsening renal injury [40]. Lipotoxicity in renal tubular cells provides another potential link between NHHR and CKD. Elevated free fatty acids in a high-NHHR state accumulate in tubular epithelial cells, inducing mitochondrial dysfunction and apoptosis [41]. Lipid peroxidation products such as 4-hydroxynonenal contribute to tubular injury and interstitial fibrosis, exacerbating renal decline [42]. These effects may explain the observed association between NHHR and increased UACR, an early marker of renal damage. Microvascular injury and fibrosis may also underlie the NHHR–CKD association. Increased non-HDL-C promotes transforming growth factor-β1 expression, driving renal fibrosis [43]. Reduced HDL-C impairs cholesterol efflux from mesangial cells, facilitating mesangial expansion and glomerulosclerosis [44]. Persistent oxidative stress, inflammation, and IR further accelerate extracellular matrix deposition, leading to irreversible renal impairment [45].
The significant positive correlation between NHHR and UACR, but not eGFR, suggests that NHHR primarily reflects early glomerular and endothelial dysfunction rather than overt reductions in filtration capacity. UACR is a sensitive marker of glomerular endothelial injury and increased permeability, both of which are strongly influenced by lipid abnormalities [46]. A high NHHR indicates an imbalance favoring atherogenic lipoproteins (non-HDL-C) over protective HDL-C, leading to oxidative stress, inflammation, and endothelial dysfunction. oxLDL promotes podocyte injury and glomerular basement membrane thickening, increasing albumin leakage [47]. Additionally, impaired HDL function reduces cholesterol efflux from renal cells, exacerbating lipid accumulation and inflammatory cytokine release, further driving microvascular damage and albuminuria [48]. In contrast, eGFR reflects overall renal filtration capacity, which tends to decline later in CKD progression [49]. In early kidney dysfunction, compensatory hyperfiltration can mask reductions in eGFR despite ongoing glomerular damage. This compensatory response is particularly pronounced in diabetes, where intraglomerular hypertension initially preserves filtration despite endothelial injury [50]. Furthermore, eGFR estimation is influenced by muscle mass and hydration status, potentially reducing its sensitivity to NHHR-related microvascular injury [51].
The lack of a significant association between NHHR and CKD in the 18–44 years age group, the good glycemic control group, and the non-alcohol drinking group may be attributed to a combination of physiological resilience, metabolic stability, compensatory renal mechanisms, and the influence of additional protective factors in these subpopulations. Younger individuals (18–44 years) generally have greater physiological resilience and renal reserve, which may mitigate the adverse effects of dyslipidemia on kidney function [52]. Renal aging involves progressive structural and functional changes, including reduced nephron number, glomerular hypertrophy, and increased susceptibility to oxidative stress and inflammation [53]. In contrast, younger individuals have a more robust capacity for glomerular autoregulation and lipid metabolism [54], which may buffer the detrimental impact of an elevated NHHR. In individuals with good glycemic control, this may be due to lower systemic metabolic stress and reduced vascular damage. Hyperglycemia exacerbates dyslipidemia-induced renal injury by promoting advanced glycation end-products, increasing oxidative stress, and activating the RAAS [55]. In contrast, patients with well-controlled blood glucose levels experience less endothelial dysfunction, lower inflammation, and reduced lipid oxidation, which may attenuate the nephrotoxic effects of NHHR [56]. Moreover, proper glycemic control may help maintain HDL-C functionality, preserving its anti-inflammatory, antioxidant, and endothelial-protective properties, thereby weakening the NHHR–CKD association in this subgroup [57]. The lack of an association in the non-alcohol drinking group suggests a potential modifying effect of alcohol consumption on lipid metabolism and renal function. Non-alcohol drinkers may exhibit healthier lifestyle patterns that mitigate the adverse effects of dyslipidemia on renal health. In contrast, heavy alcohol consumption may contribute to lipid abnormalities, hypertension, and oxidative stress, which may exacerbate CKD risk in those with an elevated NHHR [58].
Our results align with prior research highlighting the significant association between NHHR and renal function. For instance, Pan et al. analyzed data from the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2016, involving 4177 diabetic patients, and identified a positive but non-linear relationship between NHHR and diabetic kidney disease (DKD) [59]. Similarly, Zhang et al., using NHANES data from 1999 to 2020 with 8329 diabetic participants, also reported a non-linear positive correlation between NHHR and DKD [60]. Further corroborating these findings, Huang et al. examined data from 19,458 individuals in the 1999–2018 NHANES and found that higher NHHR levels were associated with an increased likelihood of macroalbuminuria in U.S. adults [61]. Additionally, Hong et al. demonstrated that elevated NHHR levels were significantly linked to a higher risk of kidney stone development and recurrence in the U.S. adult population [62]. Du et al. further supported this by suggesting a strong connection between increased NHHR levels and a heightened risk of kidney stones among American adults [63].
This study has several notable strengths. First, it represents the first investigation into the association between NHHR and CKD in a well-characterized sample of Chinese adults with T2DM, providing critical insights into this relationship within a high-risk population. Second, the use of standardized data collection methods enhances the reliability and consistency of the findings.
However, certain limitations should be acknowledged. The study population was limited to T2DM patients from Eastern China, which may restrict the generalizability of the results to other regions or demographic groups. Although extensive adjustments were made for various confounders, the possibility of residual confounding cannot be entirely eliminated. Additionally, the analysis did not account for medication use, which could influence the observed outcomes. What is more, the reliance on eGFR calculated from serum creatinine, rather than direct measurement of creatinine clearance, may introduce systematic inaccuracies due to assumptions in the estimation formula or variability in creatinine production across individuals. This limitation is contextualized by the study’s alignment with a national diabetes complications investigation, which standardized eGFR calculation over direct creatinine measurement for consistency across sites. Finally, the cross-sectional nature of the study design precludes the establishment of causal relationships between the variables examined.
5. Conclusions
This study identifies a significant positive association between NHHR and CKD prevalence among Chinese adults with T2DM, suggesting that NHHR may serve as a complementary marker to existing clinical tools for identifying individuals at risk of early renal function deterioration in this population. However, longitudinal research is needed to confirm its predictive value for CKD onset and progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17071125/s1, Table S1: Covariates before and after PSM. Table S2: Assessment of multicollinearity among independent variables (n = 1756). Table S3: Multivariable regression analysis of NHHR quartiles in relation to CKD after PSM (n = 970). Table S4: Threshold effect analysis of NHHR on CKD (n = 970). Figure S1: Association between the non-high-density lipoprotein cholesterol (non-HDL-C) to HDL-C ratio (NHHR) and chronic kidney disease (CKD), with 95% confidence intervals (CIs).
Author Contributions
Conceptualization, X.C.; methodology, M.L.; software, X.C. and C.X.; validation, L.C. and X.C.; formal analysis, X.C. and J.Z. (Jieming Zhong); investigation, J.Z. (Jie Zhang) and R.H.; resources, R.H.; data curation, X.C. and J.Z. (Jieming Zhong); writing—original draft preparation, X.C. and J.Z. (Jieming Zhong); writing—review and editing, J.Z. (Jieming Zhong) and X.C.; visualization, X.C.; supervision, R.H.; project administration, X.C. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Noncommunicable Chronic Diseases-National Science and Technology Major Project (grant number: 2023ZD0509800, 2023ZD0509806).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (Approval No: 2018-010, 13 January 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
We would like to express our profound appreciation to the dedicated personnel at the primary healthcare facilities who played an important role in this research, for their exceptional contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HDL-C | High-density lipoprotein cholesterol |
| Non-HDL-C | Non-high-density lipoprotein cholesterol |
| NHHR | Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio |
| T2DM | Type 2 diabetes mellitus |
| CKD | Chronic kidney disease |
| RCS | Restricted cubic spline |
| GAM | Generalized additive models |
| UACR | Urinary albumin-to-creatinine ratio |
| eGFR | Estimated glomerular filtration rate |
| BMI | Body mass index |
| TC | Total cholesterol |
| TG | Triglycerides |
| SUA | Serum uric acid |
| LDL-C | Low-density lipoprotein cholesterol |
| VLDL-C | Very-low-density lipoprotein cholesterol |
| IR | Insulin resistance |
| HbA1c | Hemoglobin A1c |
| FPG | Fasting blood glucose |
| SD | Standard deviation |
| IQR | Interquartile range |
| OR | Odds ratio |
| CI | Confidence interval |
| Q1 | First quartile |
| Q2 | Second quartile |
| Q3 | Third quartile |
| Q4 | Fourth quartile |
| PSM | Propensity score matching |
| SMD | Standardized mean difference |
| ANOVA | Analysis of variance |
| RAAS | Renin-angiotensin-aldosterone system |
| NHANES | National Health and Nutrition Examination Survey |
References
- Deng, W.; Zhao, L.; Chen, C.; Ren, Z.; Jing, Y.; Qiu, J.; Liu, D. National burden and risk factors of diabetes mellitus in China from 1990 to 2021: Results from the Global Burden of Disease study 2021. J. Diabetes 2024, 16, e70012. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA-J. Am. Med. Assoc. 2021, 326, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Presslie, C.; Rutter, M.K.; McGuire, D.K. Cardiovascular and Kidney Risks in Individuals with Type 2 Diabetes: Contemporary Understanding with Greater Emphasis on Excess Adiposity. Diabetes Care 2024, 47, 531–543. [Google Scholar] [CrossRef]
- Chaudhry, K.; Karalliedde, J. Chronic kidney disease in type 2 diabetes: The size of the problem, addressing residual renal risk and what we have learned from the CREDENCE trial. Diabetes Obes. Metab. 2024, 26 (Suppl. S5), 25–34. [Google Scholar] [CrossRef]
- Sardar, M.B.; Nadeem, Z.A.; Babar, M. Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr. Probl. Cardiol. 2024, 49, 102489. [Google Scholar] [CrossRef]
- Triozzi, J.L.; Parker, G.L.; Virani, S.S.; Navaneethan, S.D. Management of type 2 diabetes in chronic kidney disease. BMJ Open Diab. Res. Care 2021, 9, e002300. [Google Scholar] [CrossRef]
- Pan, X.; Lin, X.; Huang, X.; Xu, J.; Ye, L.; Zhang, T.; Hu, S.; Jiang, H.; Ren, Y.; Shan, P.F. The Burden of Diabetes-Related Chronic Kidney Disease in China From 1990 to 2019. Front. Endocrinol. 2022, 13, 892860. [Google Scholar] [CrossRef]
- Giardini, E.; Moore, D.; Sadlier, D.; Godson, C.; Brennan, E. The dual role of lipids in chronic kidney disease: Pathogenic culprits and therapeutic allies. Atherosclerosis 2024, 398, 118615. [Google Scholar] [CrossRef]
- Chiesa, S.T.; Charakida, M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc. Drugs Ther. 2019, 33, 207–219. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Franceschini, G. Endothelial protection by high-density lipoproteins: From bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1724–1731. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar]
- Kaur, N.; Pandey, A.; Negi, H.; Shafiq, N.; Reddy, S.; Kaur, H.; Chadha, N.; Malhotra, S. Effect of HDL-raising drugs on cardiovascular outcomes: A systematic review and meta-regression. PLoS ONE 2014, 9, e94585. [Google Scholar]
- Guan, X.M.; Shi, H.P.; Xu, S.; Chen, Y.; Zhang, R.F.; Dong, Y.X.; Gao, L.J.; Wu, S.L.; Xia, Y.L. Cumulative non-high-density lipoprotein cholesterol burden and risk of atherosclerotic cardiovascular disease: A prospective community-based study. Front. Cardiovasc. Med. 2023, 10, 1105342. [Google Scholar]
- Roumeliotis, S.; Roumeliotis, A.; Georgianos, P.I.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Is Associated with eGFR Decline in Proteinuric Diabetic Kidney Disease: A Cohort Study. Oxidative Med. Cell. Longev. 2021, 2021, 2968869. [Google Scholar]
- Zhang, F.; Li, Z.; Wang, M.; Wang, Y.; Lu, C. Association of non-highdensity lipoprotein cholesterol to highdensity lipoprotein cholesterol ratio (NHHR) and subsequent hypertension and heart diseases: Findings from the CHARLS cohort. Aging Clin. Exp. Res. 2025, 37, 26. [Google Scholar]
- Han, Y.Z.; Du, B.X.; Zhu, X.Y.; Wang, Y.Z.; Zheng, H.J.; Liu, W.J. Lipid metabolism disorder in diabetic kidney disease. Front. Endocrinol. 2024, 15, 1336402. [Google Scholar]
- Lu, C.F.; Liu, W.S.; Chen, Z.H.; Hua, L.Y.; Wang, X.Q.; Huang, H.Y. Comparisons of the Relationships Between Multiple Lipid Indices and Diabetic Kidney Disease in Patients with Type 2 Diabetes: A Cross-Sectional Study. Front. Endocrinol. 2022, 13, 888599. [Google Scholar]
- Su, X.; Rao, H.; Zhao, C.; Wu, J.; Zhang, X.; Li, D. Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality among hypertension patients. Sci. Rep. 2025, 15, 6012. [Google Scholar]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Ko, Y.A.; Mehta, A.; Alkhoder, A.A.; Alras, Z.; Desai, S.R.; Patel, K.J.; Hooda, A.; et al. Association Between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations. JAMA Cardiol. 2022, 7, 672–680. [Google Scholar]
- Hashem, C.; Altin, S.E.; Guyton, J.R.; Boden, W.E. Nonlinearity of the inverse relationship between high-density lipoprotein (HDL) cholesterol and incident cardiovascular risk: Is it time to revisit the “HDL hypothesis”? J. Clin. Lipidol. 2024, in press. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.H.; Wang, L.M.; Chen, S.Y.; Liang, Y.B.; Zhang, M.; Huang, Z.J.; Chen, H.L.; Wu, J.Z.; Wu, J.; Jia, W.P. Data Resource Profile: A Protocol of China National Diabetic Chronic Complications Study. Biomed. Environ. Sci. 2022, 35, 633–640. [Google Scholar] [PubMed]
- Guo, K.; Zhang, L.; Zhao, F.; Lu, J.; Pan, P.; Yu, H.; Bao, Y.; Chen, H.; Jia, W. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J. Diabetes Complicat. 2016, 30, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Stempniewicz, N.; Vassalotti, J.A.; Cuddeback, J.K.; Ciemins, E.; Storfer-Isser, A.; Sang, Y.; Matsushita, K.; Ballew, S.H.; Chang, A.R.; Levey, A.S.; et al. Chronic Kidney Disease Testing Among Primary Care Patients with Type 2 Diabetes Across 24 U.S. Health Care Organizations. Diabetes Care 2021, 44, 2000–2009. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA-J. Am. Med. Assoc. 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.R.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Luo, D.; Chen, B.; Lai, C.; He, C.; Li, S. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and cardiometabolic multimorbidity among middle-aged and older adults in China. BMC Public Health 2025, 25, 570. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, W.; Li, Y.; Liu, L. Hypertension in China: Epidemiology and treatment initiatives. Nat. Rev. Cardiol. 2023, 20, 531–545. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, N.; Pan, X.F.; Chen, L.; Pan, A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 393–405. [Google Scholar] [CrossRef]
- Wang, X.; Tian, B.; Zhang, S.; Zhang, J.; Yang, W.; Li, J.; Wang, W.; Wang, Y.; Zhang, W. Diabetes knowledge predicts HbA1c levels of people with type 2 diabetes mellitus in rural China: A ten-month follow-up study. Sci. Rep. 2023, 13, 18248. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Y.; Bai, Z.; Xu, X.; Su, D.; Chen, J.; He, R.; Sun, J. The Association Between Family Health and Frailty with the Mediation Role of Health Literacy and Health Behavior Among Older Adults in China: Nationwide Cross-Sectional Study. JMIR Public Health Surveill. 2023, 9, e44486. [Google Scholar]
- Chen, X.; Du, X.; Lu, F.; Zhang, J.; Xu, C.; Liang, M.; Chen, L.; Zhong, J. The Association Between the Triglyceride-Glucose Index, Its Combination with the Body Roundness Index, and Chronic Kidney Disease in Patients with Type 2 Diabetes in Eastern China: A Preliminary Study. Nutrients 2025, 17, 492. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, M.; Yu, Z.; Zheng, T.; Feng, X.; Gao, A.; Zhang, H.; Gao, R. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999–2018. BMC Med. 2024, 22, 317. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Wang, W.; Muniyappa, H.; Deshmukh, A.; Hu, C.; Das, K.; Mehta, J.L. Abrogation of lectin-like oxidized LDL receptor-1 attenuates acute myocardial ischemia-induced renal dysfunction by modulating systemic and local inflammation. Kidney Int. 2012, 82, 436–444. [Google Scholar]
- Xu, Z.; Yang, S.; Cui, L. Understanding the heterogeneity and dysfunction of HDL in chronic kidney disease: Insights from recent reviews. BMC Nephrol. 2024, 25, 400. [Google Scholar]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. S2), S165–S171. [Google Scholar] [CrossRef]
- Huang, J.K.; Lee, H.C. Emerging Evidence of Pathological Roles of Very-Low-Density Lipoprotein (VLDL). Int. J. Mol. Sci. 2022, 23, 4300. [Google Scholar] [CrossRef]
- Ma, K.; Gao, W.; Xu, H.; Liang, W.; Ma, G. Role and Mechanism of the Renin-Angiotensin-Aldosterone System in the Onset and Development of Cardiorenal Syndrome. J. Renin-Angiotensin-Aldosterone Syst. 2022, 2022, 3239057. [Google Scholar]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [PubMed]
- Long, Z.; Luo, Y.; Yu, M.; Wang, X.; Zeng, L.; Yang, K. Targeting ferroptosis: A new therapeutic opportunity for kidney diseases. Front. Immunol. 2024, 15, 1435139. [Google Scholar]
- Tang, P.M.; Zhang, Y.Y.; Mak, T.S.; Tang, P.C.; Huang, X.R.; Lan, H.Y. Transforming growth factor-beta signalling in renal fibrosis: From Smads to non-coding RNAs. J. Physiol. 2018, 596, 3493–3503. [Google Scholar]
- Liu, K.; Cooper, M.E.; Chai, Z.; Liu, F. High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe? Int. J. Mol. Sci. 2025, 26, 1683. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar]
- Hwang, S.W.; Lee, T.; Uh, Y.; Lee, J.Y. Urinary albumin creatinine ratio is associated with lipid profile. Sci. Rep. 2024, 14, 14870. [Google Scholar]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar]
- Zsom, L.; Zsom, M.; Salim, S.A.; Fulop, T. Estimated Glomerular Filtration Rate in Chronic Kidney Disease: A Critical Review of Estimate-Based Predictions of Individual Outcomes in Kidney Disease. Toxins 2022, 14, 127. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Nankivell, L.; Elder, G.J.; Gruenewald, S.M. How unmeasured muscle mass affects estimated GFR and diagnostic inaccuracy. Eclinicalmedicine 2020, 29–30, 100662. [Google Scholar] [CrossRef] [PubMed]
- Bowling, C.B.; Olsen, M.K.; Berkowitz, T.; Smith, B.; Floyd, B.; Majette, N.; Miles, A.L.; Crowley, S.D.; Wang, V.; Maciejewski, M.L.; et al. Reserve and resilience in CKD: Concept introduction and baseline results from the Physical REsilience Prediction in Advanced REnal Disease (PREPARED) study. BMC Nephrol. 2022, 23, 418. [Google Scholar]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health-Part B-Crit. Rev. 2017, 20, 55–80. [Google Scholar]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C. Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease. Antioxidants 2024, 13, 455. [Google Scholar] [CrossRef]
- Denimal, D. Antioxidant and Anti-Inflammatory Functions of High-Density Lipoprotein in Type 1 and Type 2 Diabetes. Antioxidants 2023, 13, 57. [Google Scholar] [CrossRef]
- Fan, Z.; Yun, J.; Yu, S.; Yang, Q.; Song, L. Alcohol Consumption Can be a “Double-Edged Sword” for Chronic Kidney Disease Patients. Med. Sci. Monit. 2019, 25, 7059–7072. [Google Scholar]
- Pan, J.; Li, C.; Zhang, J.; Sun, Z.; Yu, X.; Wan, Q.; Ruan, Z.; Wang, W.; Li, Y. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and diabetic kidney disease in patients with diabetes in the United States: A cross-sectional study. Lipids Health Dis. 2024, 23, 317. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, D.; Zhu, T.; Geng, L.; Gan, L.; Ou, S.; Yin, D. The ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol is associated with diabetic kidney disease: A cross-sectional study. PLoS ONE 2024, 19, e311620. [Google Scholar] [CrossRef]
- Huang, D.; He, Y. Association between non-high-density lipoprotein cholesterol-to-high-density lipoprotein cholesterol ratio and macroalbuminuria: Evidence from NHANES 1999–2018. Front. Endocrinol. 2025, 16, 1503780. [Google Scholar]
- Hong, H.; He, Y.; Gong, Z.; Feng, J.; Qu, Y. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and kidney stones: A cross-sectional study. Lipids Health Dis. 2024, 23, 102. [Google Scholar]
- Du, Y.Z.; Dong, Q.X.; Hu, H.J.; Guo, B.; Li, Y.H.; Zhang, J.; Li, F.C.; Guo, J. A cross-sectional analysis of the relationship between the non-high density to high density lipoprotein cholesterol ratio (NHHR) and kidney stone risk in American adults. Lipids Health Dis. 2024, 23, 158. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).