Italian Biodiversity: A Source of Edible Plant Extracts with Protective Effects Against Advanced Glycation End Product-Related Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Plant Material
2.3. Reagents
2.4. Evaluation of Amadori Product Inhibition
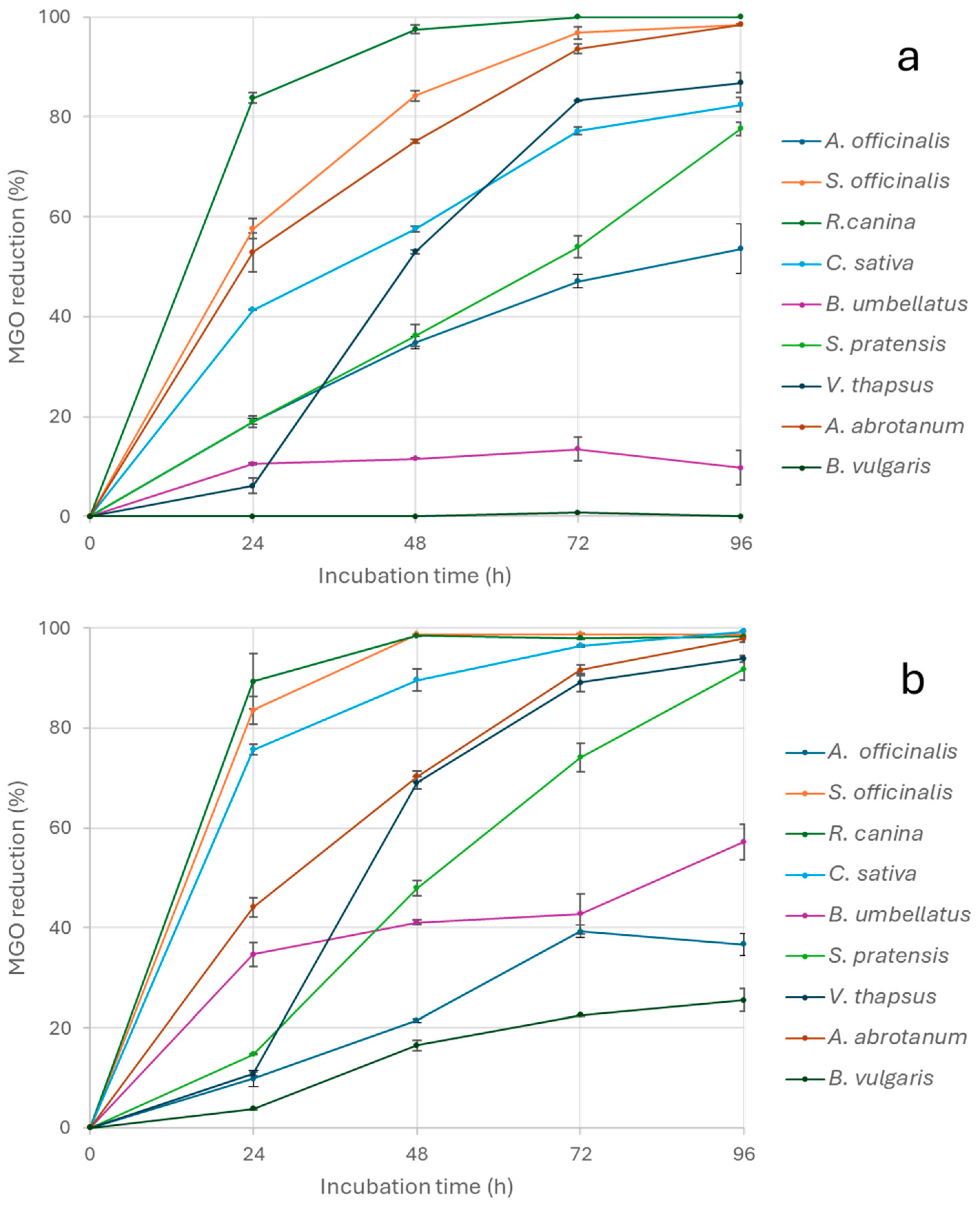
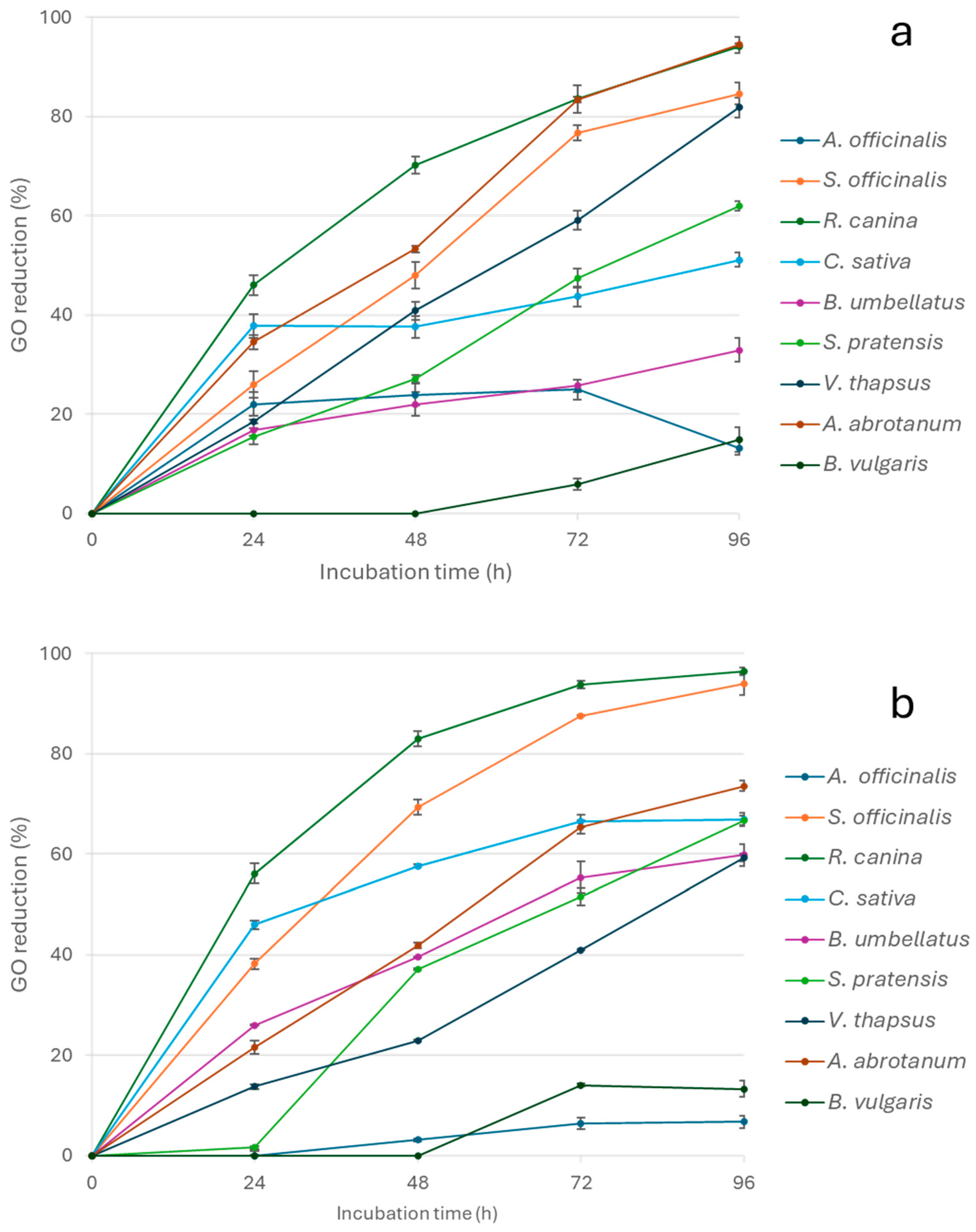
2.5. Evaluation of MGO and GO Trapping Capacity Using the RP-UHPLC-DAD Method
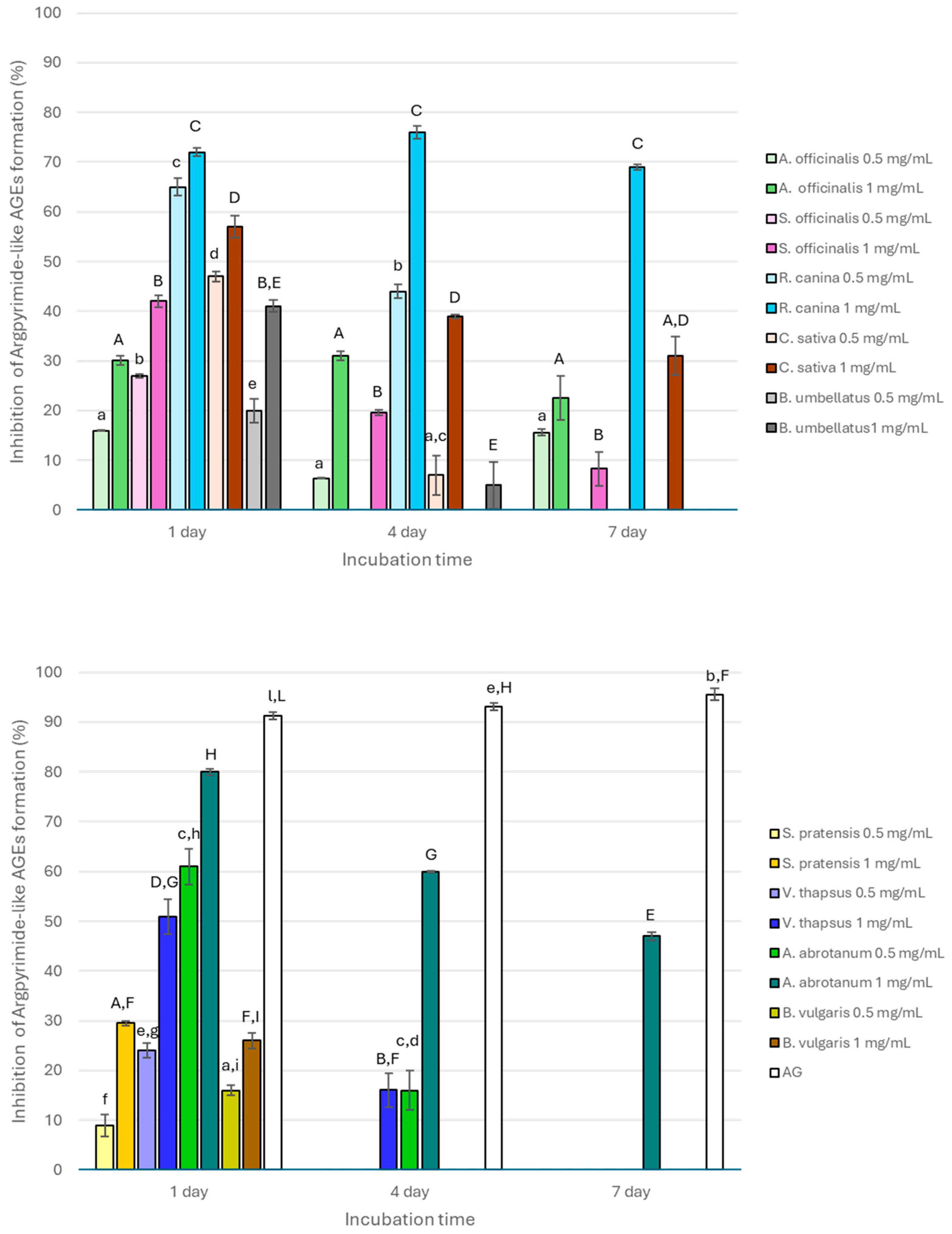
2.6. Evaluation of the Extracts’ Capacity to Inhibit AGE Formation
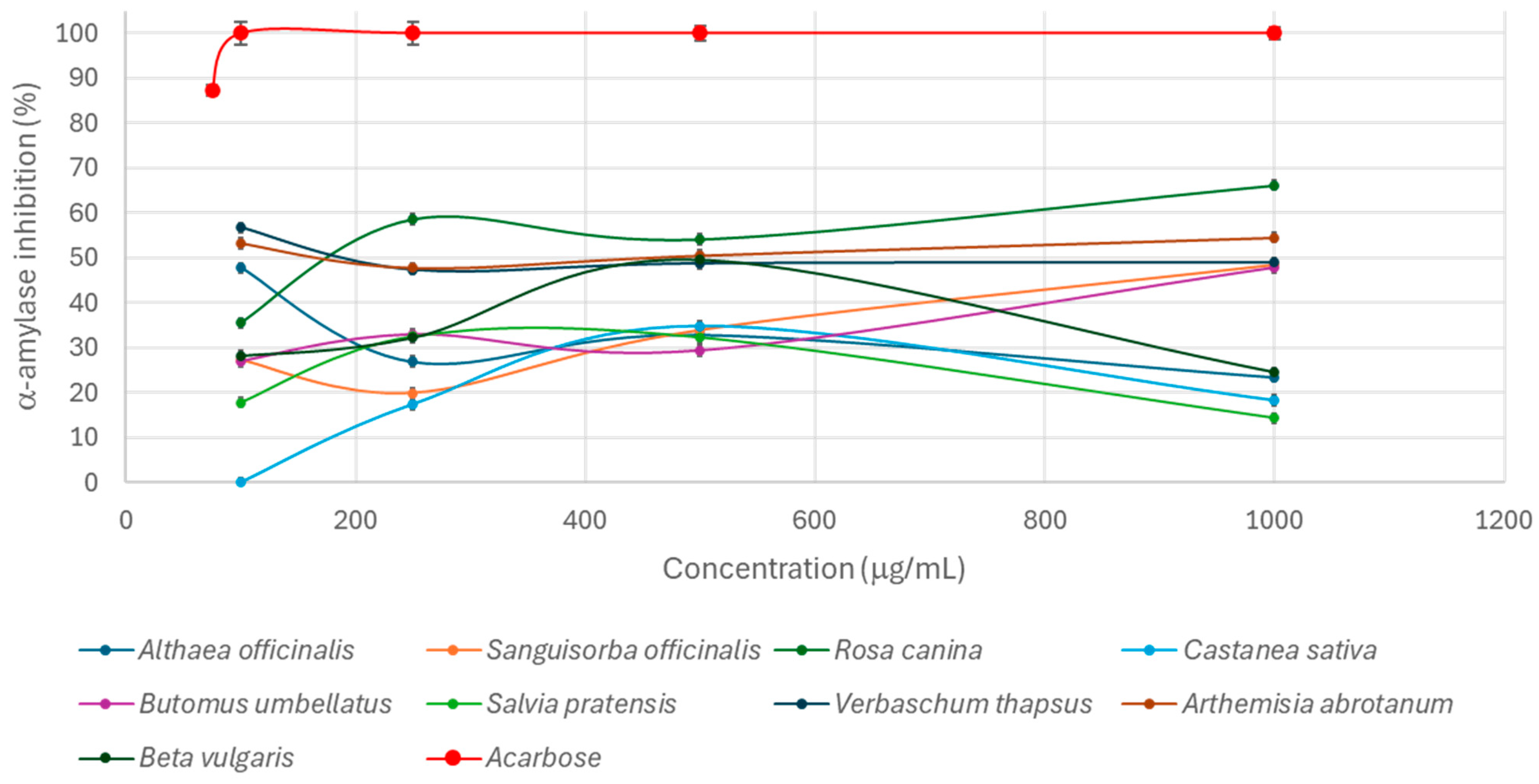
2.7. Evaluation of the Extracts’ Capacity to Inhibit α-Amylase Activity
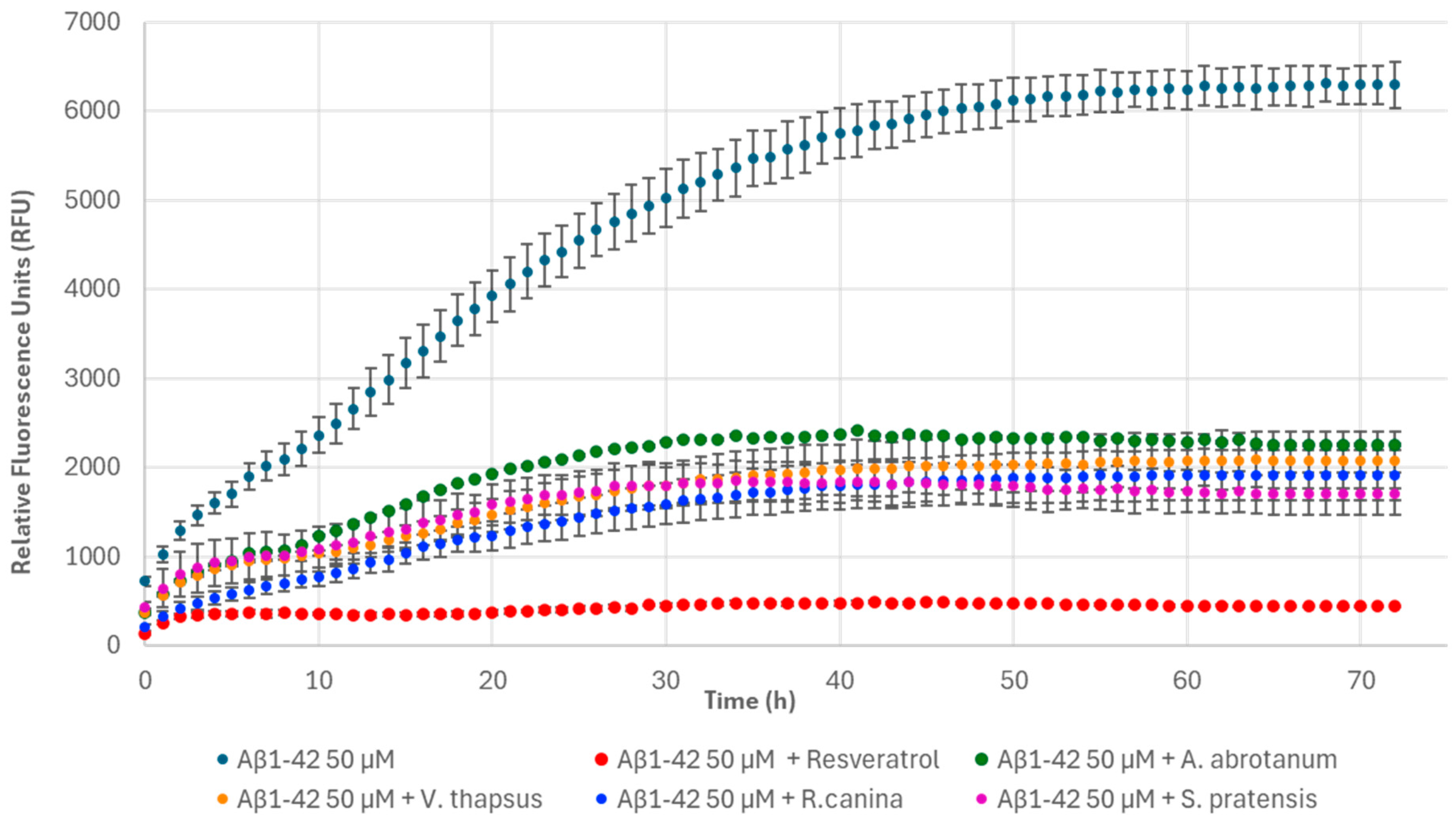
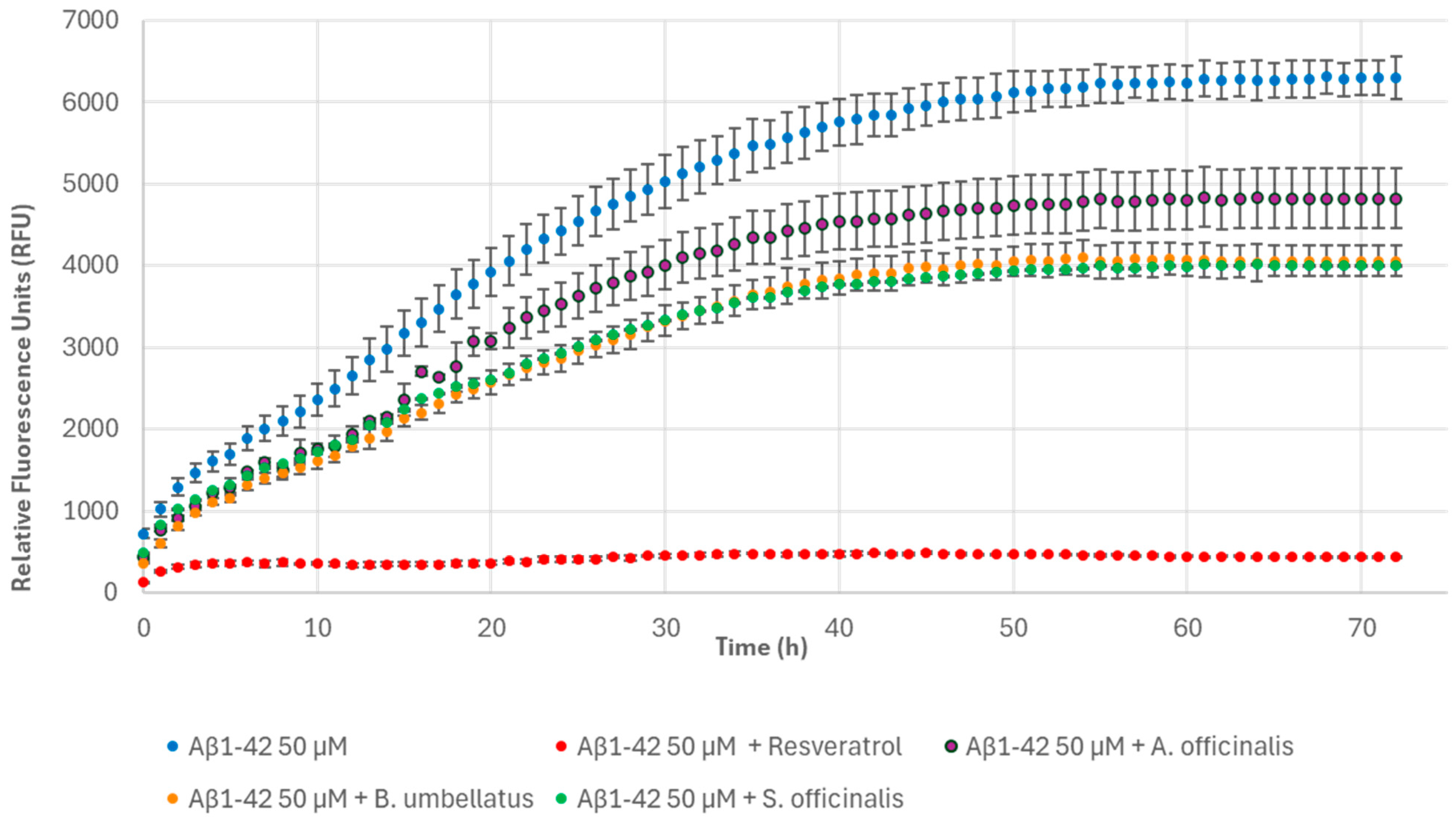
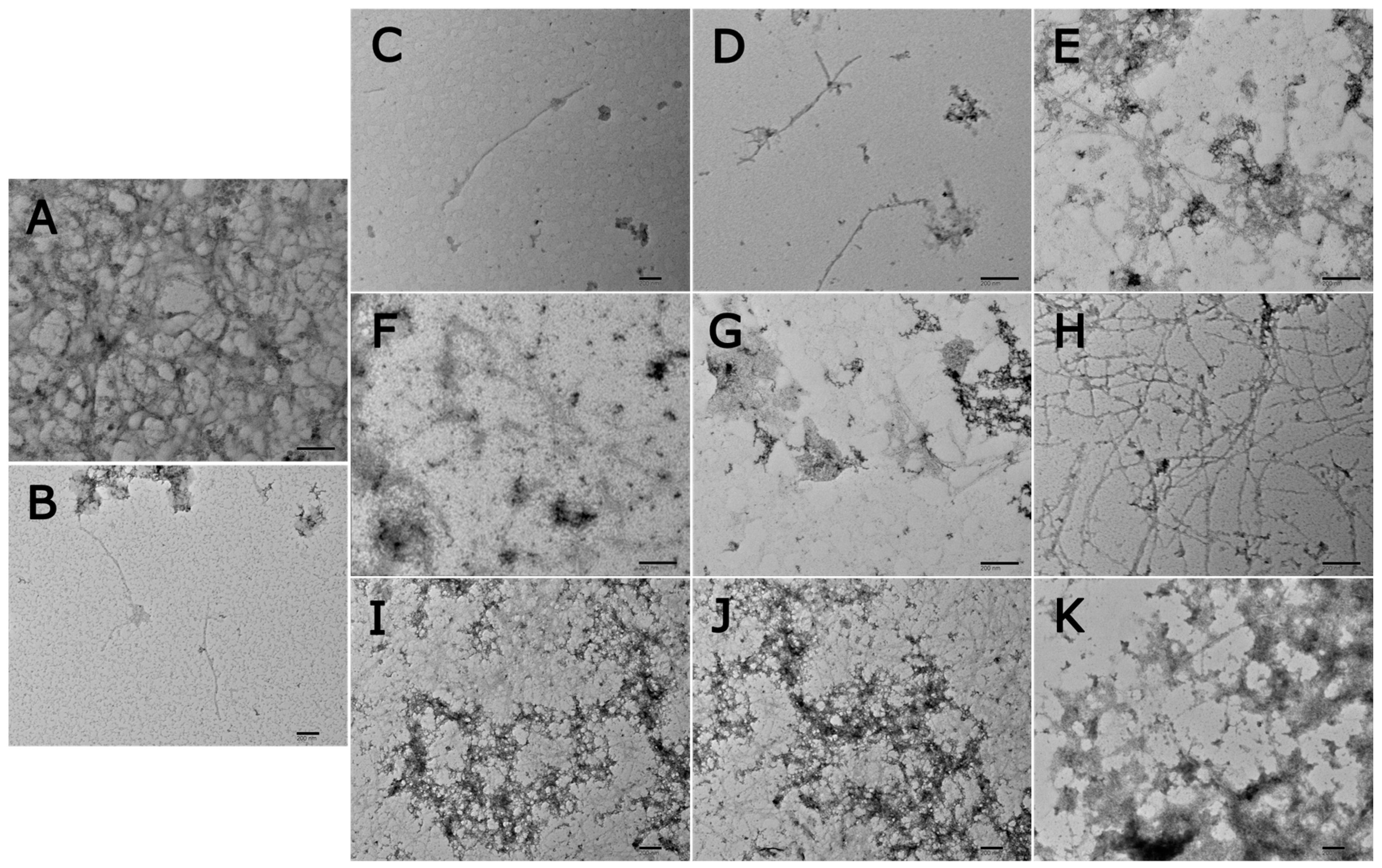
2.8. Thioflavin T Fluorescence Assay
2.9. Transmission Electron Microscopy (TEM)
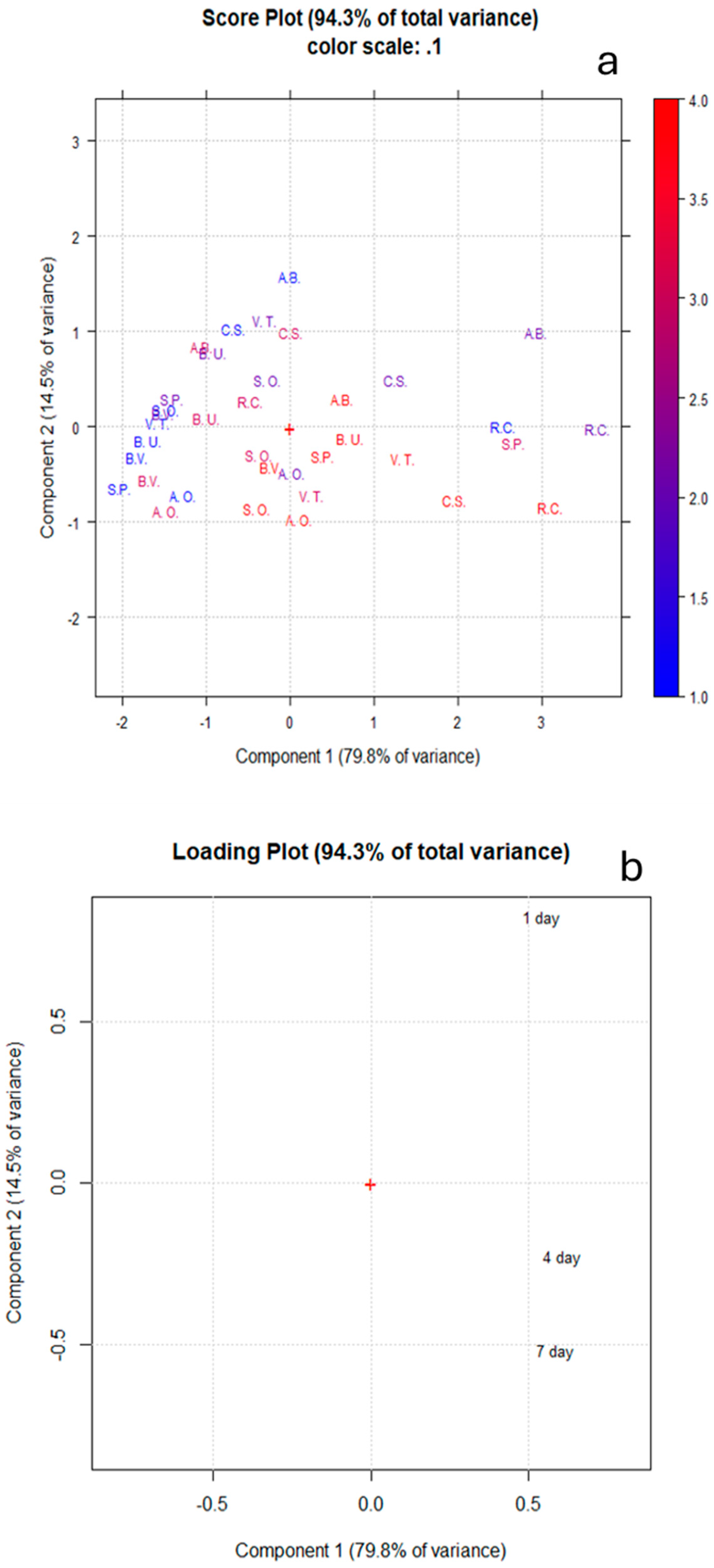
2.10. Statistical Analysis
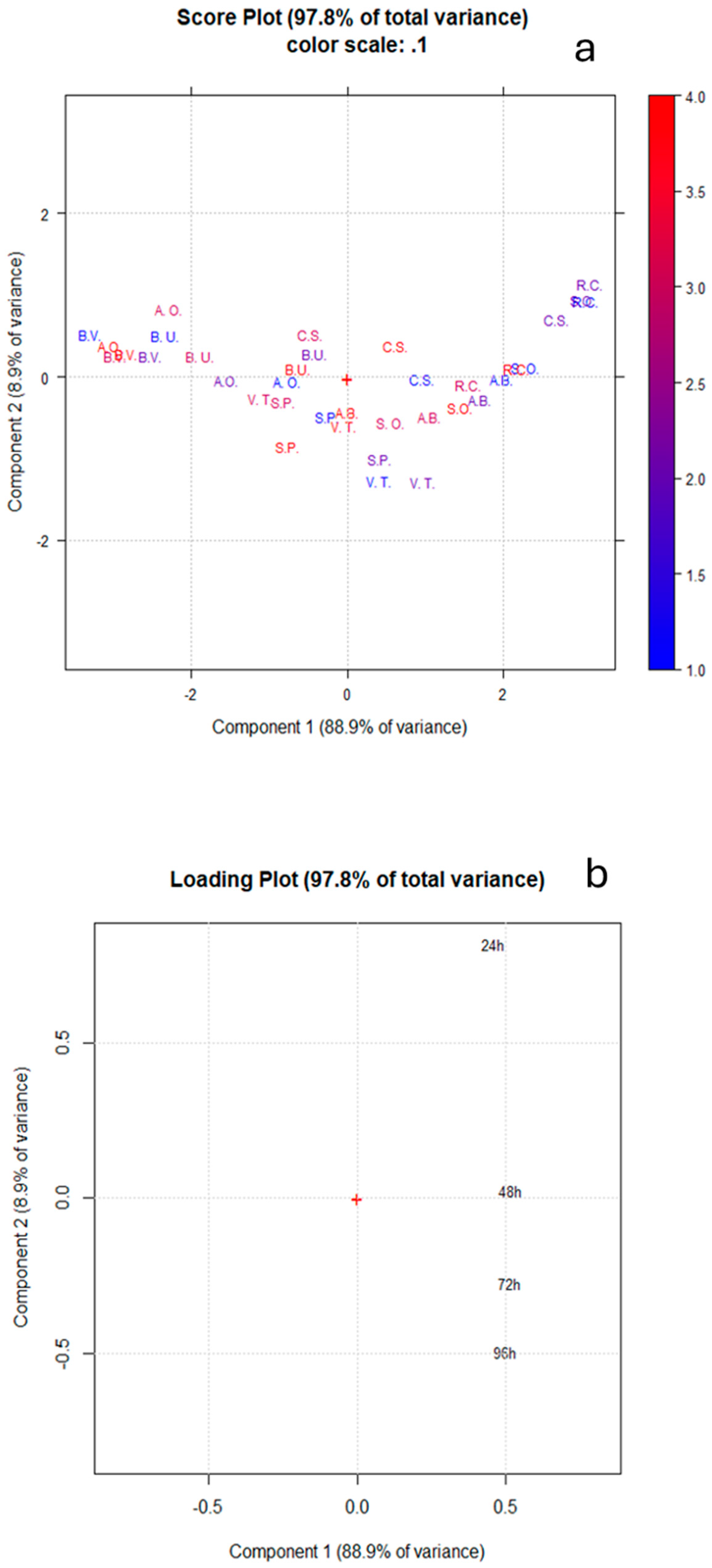
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbs, E.P.J. The evolution of One Health: A decade of progress and challenges for the future. Vet. Rec. 2014, 174, 85–91. [Google Scholar] [CrossRef]
- Linhares, Y.; Kaganski, A.; Agyare, C.; Kurnaz, I.A.; Neergheen, V.; Kolodziejczyk, B.; Kędra, M.; Wahajuddin, M.; El-Youssf, L.; Dela Cruz, T.E.; et al. Biodiversity: The overlooked source of human health. Trends Mol. Med. 2023, 29, 173–187. [Google Scholar] [CrossRef]
- Marselle, M.R.; Hartig, T.; Cox, D.T.C.; de Bell, S.; Knapp, S.; Lindley, S.; Triguero-Mas, M.; Böhning-Gaese, K.; Braubach, M.; Cook, P.A.; et al. Pathways linking biodiversity to human health: A conceptual framework. Environ. Int. 2021, 150, 106420. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bassani, B.; Baci, D.; Dallaglio, K.; Gallazzi, M.; Corradino, P.; Bruno, A.; Noonan, D.M. Nutraceuticals and “Repurposed” drugs of phytochemical origin in prevention and interception of chronic degenerative diseases and cancer. Curr. Med. Chem. 2019, 26, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Džamić, A.M.; Matejić, J.S. Plant products in the prevention of diabetes mellitus. Mini Rev. Med. Chem. 2022, 22, 1395–14198. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.K.; Batkulwar, K.B.; Kulkarni, M.J.; Sengupta, N. Glycation induces conformational changes in the amyloid-β peptide and enhances its aggregation propensity: Molecular insights. Phys. Chem. Chem. Phys. 2016, 18, 31446–31458. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. Understanding the role of protein glycation in the amyloid aggregation. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, A.; Knut, E.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia abrotanum L. (Southern Wormwood)-history, current knowledge on the chemistry, biological activity, traditional use and possible new pharmaceutical and cosmetological applications. Molecules 2021, 26, 2503. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Moretti, R.M.; Nasti, R.; Cincinelli, R.; Dallavalle, S.; Montagnani Marelli, M. Apoptosis-mediated anticancer activity in prostate cancer cells of a chestnut honey (Castanea sativa L.) quinoline-pyrrolidine gamma-lactam alkaloid. Amino Acids 2021, 53, 869–880. [Google Scholar] [CrossRef]
- Gupta, A.; Atkinson, A.N.; Pandey, A.K.; Bishayee, A. Health-promoting and disease-mitigating potential of Verbascum thapsus L. (common mullein): A review. Phytother. Res. 2022, 36, 1507–1522. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Samynathan, R.; Chandar, S.R.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A comprehensive review of beetroot (Beta vulgaris L.) bioactive components in the food and pharmaceutical industries. Crit. Rev. Food Sci. Nutr. 2024, 64, 708–739. [Google Scholar] [CrossRef]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Fang, J.C.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional uses, chemical constituents and biological activities of plants from the genus Sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Negrean, O.R.; Farcas, A.C.; Nemes, S.A.; Cic, D.-E.; Socaci, S.A. Recent advances and insights into the bioactive properties and applications of Rosa canina L. and its by-products. Heliyon 2024, 10, e30816. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Angeloni, C.; Zambonin, L.; Hrelia, S. Role of methylglyoxal in Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 238485. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, X.; Dong, L.L. Antioxidant and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Corana, F.; Papetti, A. Cretan tea (Origanum dictamnus L.) as a functional beverage: An investigation on antiglycative and carbonyl trapping activities. Food Funct. 2018, 9, 1545–1556. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Ferron, L.; Colombo, R.; Mannucci, B.; Papetti, A. A New Italian purple corn variety (Moradyn) byproduct extract: Antiglycative and hypoglycemic in vitro activities and preliminary bioaccessibility studies. Molecules 2020, 25, 1958. [Google Scholar] [CrossRef]
- Bisceglia, F.; Natalello, A.; Serafini, M.M.; Colombo, R.; Verga, L.; Lanni, C.; De Lorenzi, E. An integrated strategy to correlate aggregation state, structure and toxicity of Aß 1–42 oligomers. Talanta 2018, 188, 17–26. [Google Scholar] [CrossRef]
- Li, F.; Zhan, C.; Dong, X.; Wei, G. Molecular mechanisms of resveratrol and EGCG in the inhibi-tion of Aβ42 aggregation and disruption of Aβ42 protofibril: Similarities and differences. Phys. Chem. Chem. Phys. 2021, 23, 18843–18854. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Rad. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef]
- Sharma, S.D.; Pandey, B.N.; Mishra, K.P.; Sivakami, S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J. Biochem. Mol. Biol. Biophys. 2002, 6, 233–242. [Google Scholar]
- Klöpfer, A.; Spanneberg, R.; Glomb, M.A. Formation of arginine modifications in a model system of N α-tert-butoxycarbonyl (Boc)-arginine with methylglyoxal. J. Agric. Food Chem. 2011, 59, 394–401. [Google Scholar] [CrossRef]
- Sompong, W.; Meeprom, A.; Cheng, H.; Adisakwattana, S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage on bovine serum albumin. Molecules 2013, 18, 13886–13903. [Google Scholar] [CrossRef] [PubMed]
- Saheem, A.; Mohd, Y.K.; Zeeshan, R.; Hamda, K.; Zeba, S.; Shahnawaz, R.; Uzma, S.; Mohd, S.K.; Mohd, S.; Sultan, A.; et al. Oxidation, glycation and glycoxidation—The vicious cycle and lung cancer. Semin. Cancer Biol. 2018, 49, 29–36. [Google Scholar]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2002, 44, 129–146. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Hou, G.Y.; Wang, L.; Liu, S.; Song, F.R.; Liu, Z.Q. Inhibitory effect of eleven herbal extracts on advanced glycation end-products formation and aldose reductase activity. Chin. Chem. Lett. 2014, 25, 1039–1043. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Rashedinia, M.; Rasti Arbabi, Z.; Sabet, R.; Emami, L.; Poustforoosh, A.; Sabahi, Z. Comparison of protective effects of phenolic acids on protein glycation of BSA supported by in vitro and docking studies. Biochem. Res. Int. 2023, 2023, 9984618. [Google Scholar] [CrossRef]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Bertelli, D. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceuticals for the management of diabetes mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Fecka, I.; Bednarska, K.; Kowalczyk, A. In vitro antiglycation and methylglyoxal trapping effect of peppermint leaf (Mentha piperita L.) and its polyphenols. Molecules 2023, 28, 2865. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, L.; Lv, L.; Sang, S. Trapping methylglyoxal by myricetin and its metabolites in mice. J. Agric. Food Chem. 2020, 68, 9408–9414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. The effect of molecular structure of polyphenols on the kinetics of the trapping reactions with methylglyoxal. Food Chem. 2020, 319, 126500. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wan, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Abu Soud, R.S.; Hamdan, L.I.; Afifi, F.U. Alpha amylase inhibitory activity of some plant extracts with hypoglycemic activity. Sci. Pharm. 2004, 72, 25–33. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B.M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Córković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Qunqin, F.; Yuan, G.; Xin, Z.; Yi, S.; Bing, H.; Li, Z.; Saqib, J.; Xiaoxiong, Z. Effects of Oolong tea polyphenols, EGCG, and EGCG3′′Me on pancreatic α-amylase activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar]
- Djeridane, A.; Hamdi, A.; Bensania, W.; Cheifa, K.; Lakhdari, I.; Yousfi, M. The in vitro evaluation of antioxidative activity, α-glucosidase and α-amylase enzyme inhibitory of natural phenolic extracts. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Interaction of thioflavin T with amyloid fibrils: Fluorescence quantum yield of bound dye. J. Phys. Chem. B 2012, 116, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Woo, E.R.; Chi, E.Y.; Sharoar, M.G.; Jin, H.G.; Shin, S.Y.; Park, I.S. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-β toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry 2011, 50, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Liu, R.T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef]
- Kumar, S.; Harris, R.J.; Seal, C.J.; Okello, E.J. An aqueous extract of Withania somnifera root inhibits amyloid β fibril formation in vitro. Phytother. Res. 2012, 26, 113–117. [Google Scholar] [CrossRef]
- Stefanescu, R.; Stanciu, G.D.; Luca, A.; Paduraru, L.; Tamba, B.I. Secondary metabolites from plants possessing inhibitory properties against beta-amyloid aggregation as revealed by thioflavin-T assay and correlations with investigations on transgenic mouse models of Alzheimer’s disease. Biomolecules 2020, 10, 870. [Google Scholar] [CrossRef]
- Ngoungoure, V.L.N.; Schluesener, J.; Moundipa, P.F.; Schluesener, H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dhouafli, Z.; Cuanalo-Contreras, K.; Hayouni, E.A.; Mays, C.E.; Soto, C.; Moreno-Gonzalez, I. Inhibition of protein misfolding and aggregation by natural phenolic compounds. Cell. Mol. Life Sci. 2018, 75, 3521–3538. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, A.; Leri, M.; Bucciantini, M.; Najjaa, H.; Ben Arfa, A.; Stefani, M.; Neffati, M. Allium roseum L. extract inhibits amyloid beta aggregation and toxicity involved in Alzheimer’s disease. PLoS ONE 2020, 15, e0223815. [Google Scholar] [CrossRef] [PubMed]
- Rho, T.; Choi, M.S.; Jung, M.; Kil, H.W.; Hong, Y.D.; Yoon, K.D. Identification of fermented tea (Camellia sinensis) polyphenols and their inhibitory activities against amyloid-beta aggregation. Phytochemistry 2019, 160, 11–18. [Google Scholar] [CrossRef]
- Liu, Y.; Pukala, T.L.; Musgrave, I.F.; Williams, D.M.; Dehle, F.C.; Carver, J.A. Gallic acid is the major component of grape seed extract that inhibits amyloid fibril formation. Bioorg. Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Influence of in vitro neuronal membranes on the anti-amyloidogenic activity of gallic acid: Implication for the therapy of Alzheimer’s disease. Arch. Bioch. Biophys. 2021, 711, 109022. [Google Scholar] [CrossRef]
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory effect of quercetin and apigenin on acetylcholinesterase, amyloid-β aggregation and acetylcholinesterase-amyloid interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef]



| Species | Family | Place of Sampling/Supplier | n | Organs Collected |
|---|---|---|---|---|
| Artemisia abrotanum L. | Asteraceae | Padua Botanical Garden | 2 | Leaves and young stems |
| Salvia pratensis L. | Lamiaceae | Veneto Agricoltura nursery * | 2 | Leaves |
| Althaea officinalis L. | Malvaceae | Veneto Agricoltura nursery * | 2 | Leaves |
| Butomus umbellatus L. | Butomaceae | Padua Botanical Garden | 5 | Leaves and young stems |
| Castanea sativa Mill. | Fagaceae | Padua Botanical Garden | 1 | Leaves |
| Verbascum thapsus L. | Scrophulariaceae | Padua Botanical Garden | 2 | Leaves |
| Sanguisorba officinalis L. | Rosaceae | Padua Botanical Garden | 5 | Leaves and young stems |
| Rosa canina L. | Rosaceae | Padua Botanical Garden | 2 | Leaves |
| Beta vulgaris L. | Amaranthaceae | University of Verona | 10 | Leaves |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretto, G.; Colombo, R.; Negri, S.; Cena, H.; Vailati, L.; Papetti, A. Italian Biodiversity: A Source of Edible Plant Extracts with Protective Effects Against Advanced Glycation End Product-Related Diseases. Nutrients 2025, 17, 935. https://doi.org/10.3390/nu17060935
Moretto G, Colombo R, Negri S, Cena H, Vailati L, Papetti A. Italian Biodiversity: A Source of Edible Plant Extracts with Protective Effects Against Advanced Glycation End Product-Related Diseases. Nutrients. 2025; 17(6):935. https://doi.org/10.3390/nu17060935
Chicago/Turabian StyleMoretto, Giulia, Raffaella Colombo, Stefano Negri, Hellas Cena, Lorena Vailati, and Adele Papetti. 2025. "Italian Biodiversity: A Source of Edible Plant Extracts with Protective Effects Against Advanced Glycation End Product-Related Diseases" Nutrients 17, no. 6: 935. https://doi.org/10.3390/nu17060935
APA StyleMoretto, G., Colombo, R., Negri, S., Cena, H., Vailati, L., & Papetti, A. (2025). Italian Biodiversity: A Source of Edible Plant Extracts with Protective Effects Against Advanced Glycation End Product-Related Diseases. Nutrients, 17(6), 935. https://doi.org/10.3390/nu17060935









