Dietary Fatty Acids and Antinuclear Antibodies Among Adults with Arthritis in the United States: NHANES 1999–2004
Abstract
1. Introduction
2. Materials and Methods
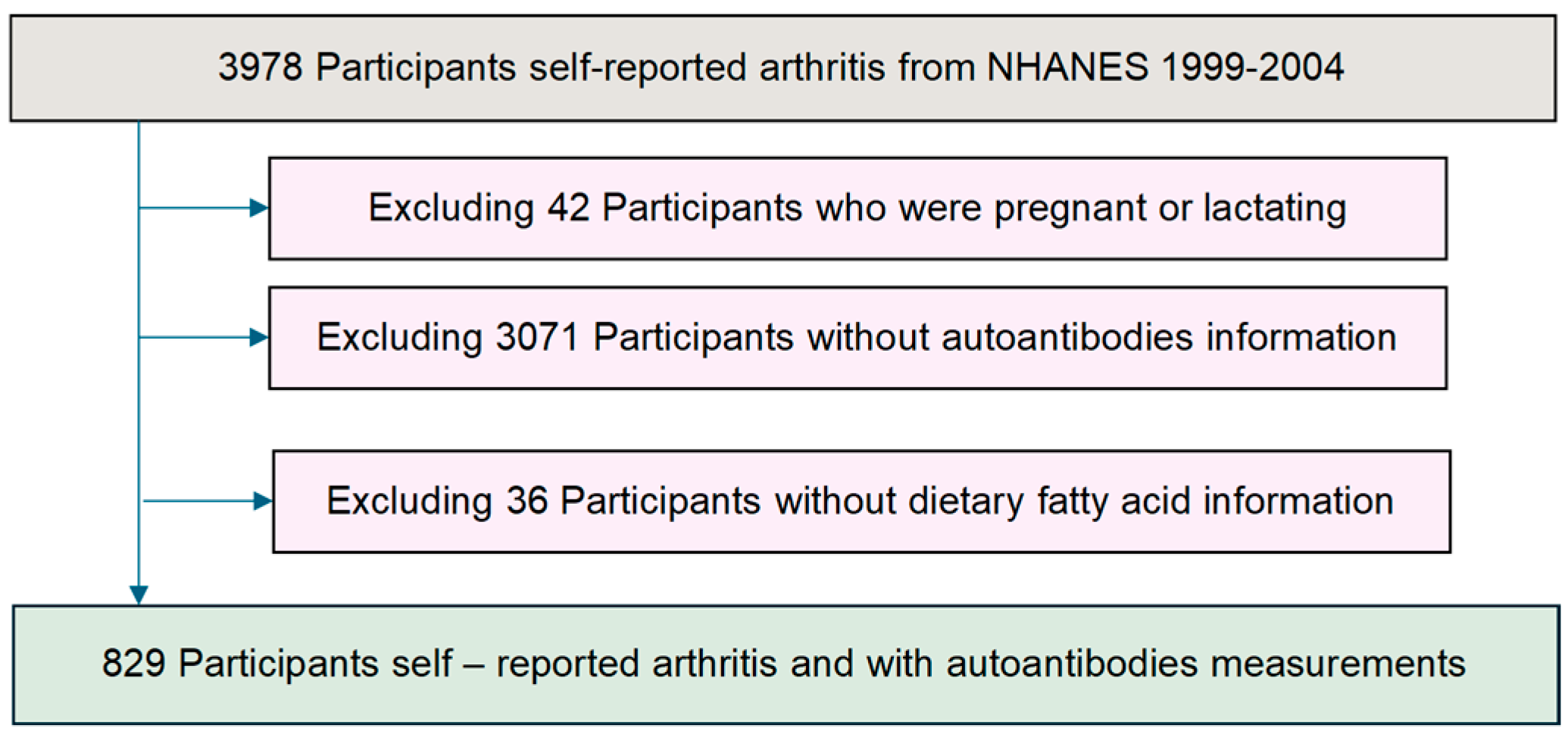
2.1. Study Population
2.2. Assessment of Dietary Fatty Acids
2.3. Assessment of ANA Test
2.4. Covariates
2.5. Statistical Analysis
2.6. Ethical Approval and Consent to Participate
3. Results
3.1. Baseline Characteristics
3.2. Associations of Dietary Fatty Acids Intake with the Probability of Being ANA Positive
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANA | antinuclear antibodies |
| CI | confidence interval |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| MUFA | monounsaturated fatty acid |
| NHANES | National Health and Nutrition Examination Survey |
| OR | odds ratio |
| PUFA | polyunsaturated fatty acid |
| SFA | saturated fatty acid |
References
- Wang, F.F.; Liu, J.; Fang, Y.Y.; Wen, J.T.; He, M.Y.; Li, X.; Han, Q. Effect of Siegesbeckiae Herba on immune-inflammation of rheumatoid arthritis: Data mining and network pharmacology. Eur. J. Integr. Med. 2023, 59, 102242. [Google Scholar] [CrossRef]
- Chen, W.J.; Sun, Z.; Xiong, X.H.; Tan, H.T.; Hu, J.H.; Liu, C.R.; Chen, C. Exploring the causal link among statin drugs and the osteoarthritis risk based on Mendelian randomization research. Front. Genet. 2024, 15, 1390387. [Google Scholar] [CrossRef] [PubMed]
- Akey, K.S.; Esakkimuthukumar, M.; Saranya, R.; Chandru, M.; Sowbarnika, S.; Sudharsan, J.; Dhanush, V.; Prabha, T.; Jubie, S. Exploring Potential Bioactive Components of Persea americana for the Treatment of Rheumatoid Arthritis through Network Pharmacology. Curr. Rheumatol. Rev. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Stándo, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef]
- Laing, B.B.; Cavadino, A.; Ellett, S.; Ferguson, L.R. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis 2021, 324, 27–37. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Yang, H.T.; Liu, C.D.; Song, C.Y.; Cheng, Y.Y.; He, C.; Zou, Z.; Zhou, D.; Wu, G.; et al. Eicosapentaenoic acid and docosahexaenoic acid suppress colonic tumorigenesis in obese mice. J. Funct. Foods 2024, 116, 106164. [Google Scholar] [CrossRef]
- Tan, W.F.; Mao, L.Z.; Yu, S.Y.; Huang, J.; Xie, Q.Y.; Hu, M.J.; Mao, L. DHA and EPA improve liver IR in HFD-induced IR mice through modulating the gut microbiotas-LPS-liver axis. J. Funct. Foods 2024, 112, 105917. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Moon, Y.S.; Cho, K.K. ω-6 and ω-3 Polyunsaturated Fatty Acids: Inflammation, Obesity and Foods of Animal Resources. Food Sci. Anim. Resour. 2024, 44, 988–1010. [Google Scholar] [CrossRef]
- Yan, D.; Ye, S.Y.; He, Y.; Wang, S.D.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty acids and lipid mediators in inflammatory bowel disease: From mechanism to treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Solares, A.; Eslamloo, K.; Hall, J.R.; Katan, T.; Emam, M.; Xue, X.; Taylor, R.G.; Balder, R.; Parrish, C.C.; Rise, M.L. Vegetable omega-3 and omega-6 fatty acids differentially modulate the antiviral and antibacterial immune responses of Atlantic salmon. Sci. Rep. 2024, 14, 10947. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Spencer, D.M.; Rovin, B.; Lipsky, P.E. Role of ANA testing in the classification of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, e124. [Google Scholar] [CrossRef] [PubMed]
- Kondeti, R.D.; Venkatesh, K.; Murthy, D.; Kameti, S.; Devi, K.S.; Chandrika, K.V. A Study of Clinical Manifestations and their Association with Antinuclear Antibodies in Various Autoimmune Connective Tissue Disorders. Indian J. Dermatol. 2023, 68, 486. [Google Scholar] [CrossRef]
- Andrade, L.E.C.; Damoiseaux, J.; Vergani, D.; Fritzler, M.J. Antinuclear antibodies (ANA) as a criterion for classification and diagnosis of systemic autoimmune diseases. J. Transl. Autoimmun. 2022, 5, 100145. [Google Scholar] [CrossRef]
- Nanda, R.; Gupta, P.; Patel, S.; Shah, S.; Mohapatra, E. Uncommon antinuclear antibody patterns as diagnostic indicators. Clin. Biochem. 2021, 90, 28–33. [Google Scholar] [CrossRef]
- Kuwana, M.; Gil-Vila, A.; Selva-O’Callaghan, A. Role of autoantibodies in the diagnosis and prognosis of interstitial lung disease in autoimmune rheumatic disorders. Ther. Adv. Musculoskel. 2021, 13, 1759720X211032457. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Wang, C.M.; Marion, T.N. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 2012, 82, 184–192. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Li, Y.F.; Tang, H.; Yang, X.T.; Ma, L.L.; Zhou, H.Q.; Zhang, G.J.; Chen, X.; Ma, L.; Gao, J.; Ji, W. Associations of ω-3, ω-6 polyunsaturated fatty acids intake and ω-6: ω-3 ratio with systemic immune and inflammatory biomarkers: NHANES 1999-2020. Front. Nutr. 2024, 11, 1410154. [Google Scholar] [CrossRef]
- Huang, S.Q.; Jiang, J.C.; Gong, H.Y. Association between dietary omega-3 fatty acid intake and all-cause mortality in patients with osteoarthritis: A population-based prospective cohort study. Sci. Rep. 2024, 14, 26516. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Xi, Y.; Qian, T.; Lu, S.; Du, M.; Shi, X.; Hou, X. Exploring the link between dietary omega-3 and omega-6 fatty acid intake and rheumatoid arthritis risk: NHANES 1999-2020 study. Clin. Exp. Rheumatol. 2024, 42, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Staruszkiewicz, M.; Pituch-Noworolska, A.; Skoczen, S. Uncommon types of autoantibodies—Detection and clinical associations. Autoimmun. Rev. 2023, 22, 103263. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Ganser, M.A.; Warren, J.S.; Basu, N.; Wang, L.; Zick, S.M.; Park, S.K. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Persp. 2015, 123, 792–798. [Google Scholar] [CrossRef]
- Gui, Z.; Li, S.Y.; Yu, H.Q.; Chang, L.; Chang, Y. Sex-specific relationship between serum 25-hydroxyvitamin D concentrations and antinuclear antibodies in US adults, NHANES 2001–2004. BMC Immunol. 2024, 25, 81. [Google Scholar] [CrossRef]
- Jiang, S.S.; Yang, W.H.; Li, Y.M.; Feng, J.Y.; Miao, J.J.; Shi, H.M.; Xue, H. Monounsaturated and polyunsaturated fatty acids concerning prediabetes and type 2 diabetes mellitus risk among participants in the National Health and Nutrition Examination Surveys (NHANES) from 2005 to March 2020. Front. Nutr. 2023, 10, 1284800. [Google Scholar] [CrossRef]
- Che, J.H.; He, N.; Kuang, X.; Zheng, C.Y.; Zhou, R.Y.; Zhan, X.D.; Liu, Z. Dietary n-3 Fatty Acids Intake and All-Cause and Cardiovascular Mortality in Patients With Prediabetes and Diabetes. J. Clin. Endocr. Metab. 2024, 109, 2847–2856. [Google Scholar] [CrossRef]
- Susai, S.R.; Mongan, D.; Healy, C.; Cannon, M.; Nelson, B.; Markulev, C.; Schäfer, M.R.; Berger, M.; Mossaheb, N.; Schlögelhofer, M.; et al. The association of plasma inflammatory markers with omega-3 fatty acids and their mediating role in psychotic symptoms and functioning: An analysis of the NEURAPRO clinical trial. Brain Behav. Immun. 2022, 99, 147–156. [Google Scholar] [CrossRef]
- Abd Alhusen, S.K.; Hasan, A.F. Evaluating the renoprotective effects of omega-3-6-9 against cisplatin-induced nephrotoxicity in mice. J. Med. Life 2023, 16, 1756–1759. [Google Scholar] [CrossRef]
- Fares, S.; Omar, M.; Laurence, A.; Abu-Baker, S.; Shaza, A.; Fadi, H.; Jonathan, M.; Georges, K.; Koushik, S.; Elie, B.S.; et al. Over-the-Counter Anti-inflammatory Supplements for Adjunctive Rheumatoid Arthritis Therapy: A Comprehensive Narrative Review. Aging Dis. 2025, 16, 408. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Philippou, E. Nutrition and its role in prevention and management of rheumatoid arthritis. Autoimmun. Rev. 2023, 22, 103333. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Li, Z.; Zeng, J.W.; Peng, Y.R.; Wang, S.; Bi, X.Y.; Zhao, Z.; Zhou, S.; Zhao, A.Z.; Mu, Y.; et al. ω-3 polyunsaturated fatty acid alleviates systemic lupus erythematosus by suppressing autoimmunity in a murine model. Int. Immunopharmacol. 2024, 126, 111299. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.L.; Li, L.Y.; Li, Q.; Switzer, K.; Liu, M.Y.; Han, S.H.; Zheng, B. Docosahexaenoic acid ameliorates autoimmune inflammation by activating GPR120 signaling pathway in dendritic cells. Int. Immunopharmacol. 2021, 97, 107698. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kang, E.Y.; Go, G.W. Recent insights into dietary w-6 fatty acid health implications using a systematic review. Food Sci. Biotechnol. 2022, 31, 1365–1376. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. [Google Scholar] [CrossRef]
- Ludovico, I.D.; Sarkar, S.; Elliott, E.; Virtanen, S.M.; Erlund, I.; Ramanadham, S.; Mirmira, R.G.; Metz, T.O.; Nakayasu, E.S. Fatty acid-mediated signaling as a target for developing type 1 diabetes therapies. Expert Opin. Ther. Targets 2023, 27, 793–806. [Google Scholar] [CrossRef]
- Yelken, H.D.; Elci, M.P.; Turker, P.F.; Demirkaya, S. Omega fatty acid ratios and neurodegeneration in a healthy environment. Prostaglandins Other Lipid Mediat. 2024, 170, 106799. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Kinnear, D.; Triggs, N.; Quintanilla, N.M.; Himes, R. Clinical, Laboratory, and Histologic Correlates of Serum Antinuclear Antibody in Hispanic Pediatric Patients With Nonalcoholic Fatty Liver Disease. Am. J. Clin. Pathol. 2022, 158, 221–227. [Google Scholar] [CrossRef]
- Gorczyca, D.; Szponar, B.; Pasciak, M.; Czajkowska, A.; Szmyrka, M. Serum levels of n-3 and n-6 polyunsaturated fatty acids in patients with systemic lupus erythematosus and their association with disease activity: A pilot study. Scand. J. Rheumatol. 2022, 51, 230–236. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef]
- Jalili, M.; Hekmatdoost, A. Dietary ω-3 fatty acids and their influence on inflammation via Toll-like receptor pathways. Nutrition 2021, 85, 111070. [Google Scholar] [CrossRef] [PubMed]
- Hellwing, C.; Schoeniger, A.; Roessler, C.; Leimert, A.; Schumann, J. Lipid raft localization of TLR2 and its co-receptors is independent of membrane lipid composition. PeerJ 2018, 6, e4212. [Google Scholar] [CrossRef] [PubMed]
- Schoeniger, A.; Fuhrmann, H.; Schumann, J. LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition. PeerJ 2016, 4, e1663. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Y.; Li, Y.; Li, X.; Wu, Y.; Yao, Q. Omega-3 polyunsaturated fatty acids play a protective role in a mouse model of Parkinson’s disease by increasing intestinal inducible Treg cells. Cell. Mol. Biol. 2024, 70, 107–112. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef]
- Montserrat-de La Paz, S.; Naranjo, M.C.; Lopez, S.; Abia, R.; Muriana, F.; Bermudez, B. Niacin and olive oil promote skewing to the M2 phenotype in bone marrow-derived macrophages of mice with metabolic syndrome. Food Funct. 2016, 7, 2233–2238. [Google Scholar] [CrossRef]
- Chung, M.Y.; Kim, B.H. Fatty acids and epigenetics in health and diseases. Food Sci. Biotechnol. 2024, 33, 3153–3166. [Google Scholar] [CrossRef]
- Aslibekyan, S.; Wiener, H.W.; Have, P.J.; Stanhope, K.L.; O’Brien, D.M.; Hopkins, S.E.; Absher, D.M.; Tiwari, H.K.; Boyer, B.B. DNA Methylation Patterns Are Associated with n-3 Fatty Acid Intake in Yup’ik People. J. Nutr. 2014, 144, 425–430. [Google Scholar] [CrossRef]
- Ahlberg, E.; Martí, M.; Govindaraj, D.; Severin, E.; Duchén, K.; Jenmalm, M.C.; Tingö, L. Immune-related microRNAs in breast milk and their relation to regulatory T cells in breastfed children. Pediatr. Allergy Immunol. 2023, 34, e13952. [Google Scholar] [CrossRef]
- Abrescia, P.; Treppiccione, L.; Rossi, M.; Bergamo, P. Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog. Lipid Res. 2020, 80, 101066. [Google Scholar] [CrossRef]
- Zhu, X.L.; Bi, Z.C.; Yang, C.; Guo, Y.H.; Yuan, J.L.; Li, L.J.; Guo, Y. Effects of different doses of omega-3 polyunsaturated fatty acids on gut microbiota and immunity. Food Nutr. Res. 2021, 65, 6263. [Google Scholar] [CrossRef]
- Fu, Y.W.; Wang, Y.D.; Gao, H.; Li, D.H.; Jiang, R.R.; Ge, L.R.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]

| Characteristics | Total | ANA Positive | ANA Negative | p-Value |
|---|---|---|---|---|
| Age, mean (SD), years | 59.8 (0.6) | 62.4 (1.5) | 59.3 (0.6) | 0.099 |
| Sex (%) | 0.018 | |||
| Female | 492 (60.9) | 88 (70.1) | 404 (59.2) | |
| Male | 337 (39.1) | 46 (29.9) | 291 (40.8) | |
| Race/Ethnicity (%) | 0.530 | |||
| Mexican American | 142 (3.6) | 19 (3.3) | 123 (3.6) | |
| Non-Hispanic White | 490 (77.1) | 76 (73.6) | 414 (77.8) | |
| Non-Hispanic Black | 147 (11.4) | 29 (14.9) | 118 (10.7) | |
| Other Hispanic or other race | 50 (7.9) | 10 (8.2) | 40 (7.9) | |
| Married status (%) | 0.178 | |||
| Married or living with a partner | 472 (63.2) | 68 (56.6) | 404 (64.4) | |
| Not married nor living with a partner | 332 (36.8) | 63 (43.4) | 269 (35.6) | |
| Poverty-to-income ratio (%) | 0.485 | |||
| <1.3 | 240 (24.2) | 34 (20.2) | 206 (24.9) | |
| 1.3–3.5 | 313 (40.2) | 54 (45.8) | 259 (39.2) | |
| ≥3.5 | 211 (35.6) | 33 (34.0) | 178 (35.9) | |
| Highest education (%) | 0.859 | |||
| Less than high school | 319 (27.2) | 49 (26.8) | 270 (27.3) | |
| High school or equivalent | 193 (27.5) | 33 (29.6) | 160 (27.1) | |
| College or above | 315 (45.3) | 52 (43.7) | 263 (45.6) | |
| Smoking status (%) | <0.001 | |||
| Current | 162 (20.8) | 17 (11.6) | 145 (22.5) | |
| Former | 302 (36.3) | 45 (30.4) | 257 (37.4) | |
| Never | 365 (42.9) | 72 (58.0) | 293 (40.1) | |
| Drinking status (%) | 0.242 | |||
| Non-drinker | 296 (34.7) | 41 (28.9) | 255 (35.7) | |
| Drinker | 505 (65.3) | 85 (71.1) | 420 (64.3) | |
| Total energy, mean (SD), kcal | 1987 (42.5) | 1874.9 (78.5) | 2008.9 (48.6) | 0.182 |
| Omega 3 PUFAs, mean (SD), g | 1.6 (0.0) | 1.4 (0.1) | 1.6 (0.1) | 0.031 |
| Decanoic (g) | 0.4 (0.0) | 0.4 (0.0) | 0.4 (0.0) | 0.620 |
| Decanoic: omega 3 PUFAs ratio | 0.3 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.501 |
| Omega 6 PUFAs (g) | 14.4 (0.4) | 12.7 (0.6) | 14.7 (0.4) | 0.014 |
| Omega 3: omega 6 PUFAs ratio | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | 0.021 |
| SFAs (g) | 25.1 (0.7) | 23.2 (1.3) | 25.5 (0.8) | 0.170 |
| MUFAs (g) | 28.5 (0.8) | 25.7 (1.3) | 29.1 (0.9) | 0.045 |
| PUFAs (g) | 16.2 (0.4) | 14.3 (0.7) | 16.5 (0.5) | 0.016 |
| Dietary Fatty Acid | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Decanoic (g) | |||
| Tertile 1 (≤0.17) | Reference | Reference | Reference |
| Tertile 2 (>0.17, ≤0.42) | 0.66 (0.36–1.21) | 0.59 (0.31–1.16) | 0.58 (0.28–1.21) |
| Tertile 3 (>0.42) | 0.99 (0.54–1.83) | 0.98 (0.51–1.86) | 0.97 (0.45–2.08) |
| Omega-3 (g) | |||
| Tertile 1 (≤0.92) | Reference | Reference | Reference |
| Tertile 2 (>0.92, ≤1.60) | 0.67 (0.41–1.11) | 0.65 (0.38–1.13) | 0.59 (0.30–1.17) |
| Tertile 3 (>1.60) | 0.62 (0.40–0.96) | 0.55 (0.35–0.86) | 0.43 (0.19–0.96) |
| Decanoic: omega 3 PUFAs ratio | |||
| Tertile 1 (≤0.14) | Reference | Reference | Reference |
| Tertile 2 (>0.14, ≤0.33) | 1.20 (0.74–1.95) | 1.16 (0.73–1.84) | 1.14 (0.73–1.78) |
| Tertile 3 (>0.33) | 1.57 (0.78–3.17) | 1.70 (0.81–3.58) | 1.64 (0.75–3.60) |
| Omega-6 (g) | |||
| Tertile 1 (≤8.79) | Reference | Reference | Reference |
| Tertile 2 (>8.79, ≤15.13) | 1.03 (0.67–1.59) | 1.01 (0.63–1.64) | 1.04 (0.57–1.89) |
| Tertile 3 (>15.13) | 0.87 (0.52–1.45) | 0.89 (0.51–1.53) | 0.82 (0.36–1.85) |
| Omega 3: omega 6 PUFAs ratio | |||
| Tertile 1 (≤0.09) | Reference | Reference | Reference |
| Tertile 2 (>0.09, ≤0.12) | 1.21 (0.76–1.94) | 1.11 (0.70–1.74) | 1.22 (0.75–1.98) |
| Tertile 3 (>0.12) | 0.78 (0.49–1.25) | 0.66 (0.43–1.03) | 0.71 (0.45–1.13) |
| SFAs (g) | |||
| Tertile 1 (≤15.75) | Reference | Reference | Reference |
| Tertile 2 (>15.75, ≤25.41) | 0.98 (0.60–1.62) | 0.92 (0.57–1.49) | 0.97 (0.53–1.78) |
| Tertile 3 (>25.41) | 0.98 (0.55–1.75) | 0.97 (0.55–1.72) | 1.07 (0.41–2.75) |
| MUFAs (g) | |||
| Tertile 1 (≤17.89) | Reference | Reference | Reference |
| Tertile 2 (>17.89, ≤29.67) | 1.14 (0.68–1.91) | 1.31 (0.82–2.09) | 1.38 (0.81–2.35) |
| Tertile 3 (>29.67) | 0.86 (0.48–1.56) | 0.90 (0.49–1.67) | 0.93 (0.40–2.20) |
| PUFAs (g) | |||
| Tertile 1 (≤9.90) | Reference | Reference | Reference |
| Tertile 2 (>9.90, ≤17.15) | 1.01 (0.64–1.59) | 1.00 (0.60–1.67) | 1.01 (0.53–1.91) |
| Tertile 3 (>17.15) | 0.85 (0.52–1.41) | 0.86 (0.51–1.45) | 0.75 (0.34–1.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yu, Y.; Su, J.; Ren, F.; Chen, J. Dietary Fatty Acids and Antinuclear Antibodies Among Adults with Arthritis in the United States: NHANES 1999–2004. Nutrients 2025, 17, 934. https://doi.org/10.3390/nu17060934
Guo J, Yu Y, Su J, Ren F, Chen J. Dietary Fatty Acids and Antinuclear Antibodies Among Adults with Arthritis in the United States: NHANES 1999–2004. Nutrients. 2025; 17(6):934. https://doi.org/10.3390/nu17060934
Chicago/Turabian StyleGuo, Jie, Yifei Yu, Jiaqi Su, Fazheng Ren, and Juan Chen. 2025. "Dietary Fatty Acids and Antinuclear Antibodies Among Adults with Arthritis in the United States: NHANES 1999–2004" Nutrients 17, no. 6: 934. https://doi.org/10.3390/nu17060934
APA StyleGuo, J., Yu, Y., Su, J., Ren, F., & Chen, J. (2025). Dietary Fatty Acids and Antinuclear Antibodies Among Adults with Arthritis in the United States: NHANES 1999–2004. Nutrients, 17(6), 934. https://doi.org/10.3390/nu17060934






