From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Nutrients
3.2. Fatty Acids
3.3. Microbiota
3.4. Immunological Factors
3.5. Hormones
3.6. Ions
3.7. microRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| AAs | amino acids |
| ACT1 | actinobacteria |
| BDI | Beck Depression Inventory |
| BSSS | Berlin Social Support Scale |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| CTQ | Childhood Trauma Questionnaire |
| DHA | docosahexaenoic acid |
| EGF | epidermal growth factor |
| ELSQ | Early Life Stress Questionnaire |
| EPA | Eicosapentaenoic acid |
| EPDS | Edinburgh Postpartum Depression Scale |
| EVs | extracellular vesicles |
| FA | fatty acids |
| GC | gas chromatography |
| GROα | chemokine (C-X-C motif) ligand 1 |
| HM | human milk |
| HMOs | human milk oligosaccharides |
| HPA | hypothalamic–pituitary–adrenocortical |
| HPLC | high-performance liquid chromatography |
| ICP | ion-coupled plasma |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IL | interleukin |
| IR | infra-red |
| JTV | Jeugd Trauma Vragenlijst |
| LAB | lactic acid bacteria |
| LC | liquid chromatography |
| LSCR | Life Stressor Checklist—Revised |
| LSC-r | Life Stressor Checklist—Revised |
| MCP1 | monocyte chemotactic protein-1 |
| MID-IR | mid-infra-red transmission spectroscopy |
| MIP1β | Macrophage Inflammatory Protein-1-beta |
| MS | mass spectrometry |
| MUFA | monounsaturated fatty acid |
| POMS | Profile of Mood States |
| PPD | postpartum depression |
| PRAQ | Pregnancy-Related Anxiety Questionnaire—Revised |
| PRO1 | proteobacteria |
| PSS | perceived Stress Scale |
| PUFA | polyunsaturated fatty acid |
| RID | radial immunodiffusion |
| RLCQ | Recent Life Changes Questionnaire |
| SIgA | secretory immunoglobulin A |
| SSRI | selective serotonin reuptake inhibitor |
| STAI | State-Trait Anxiety Inventory |
| TGFβ2 | transforming growth factor beta two |
| TNFα | tumor necrosis factor alpha |
| TPSS | Tennessee Postpartum Stress Scale |
References
- Eisha, S.; Joarder, I.; Wijenayake, S.; McGowan, P.O. Non-Nutritive Bioactive Components in Maternal Milk and Offspring Development: A Scoping Review. J. Dev. Orig. Health Dis. 2022, 13, 665–673. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human Breast Milk: A Review on Its Composition and Bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. MECHANISMS IN ENDOCRINOLOGY: White, Brown and Pink Adipocytes: The Extraordinary Plasticity of the Adipose Organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 2019, 8, 662. [Google Scholar] [CrossRef]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Kim, S. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, S.; Ozkan, Y.; Kumru, S. Ghrelin Is Present in Human Colostrum, Transitional and Mature Milk. Peptides 2006, 27, 878–882. [Google Scholar] [CrossRef]
- Pang, W.W.; Hartmann, P.E. Initiation of Human Lactation: Secretory Differentiation and Secretory Activation. J. Mammary Gland Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef]
- El-Loly, M.M. Colostrum Ingredients, Its Nutritional and Health Benefits—An Overview. Clin. Nutr. Open Sci. 2022, 44, 126–143. [Google Scholar] [CrossRef]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The Bioactivity of Colostrum and Milk Exosomes of High, Average, and Low Immune Responder Cows on Human Intestinal Epithelial Cells. J. Dairy Sci. 2021, 104, 2499–2510. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Martz, J.; Lapp, H.E.; Storm, J.A.; Champagne, F.A.; Kentner, A.C. The Contributions of Parental Lactation on Offspring Development: It’s Not Udder Nonsense! Horm. Behav. 2023, 153, 105375. [Google Scholar] [CrossRef]
- Godhia, M.; Patel, N. Colostrum—Its Composition, Benefits as a Nutraceutical: A Review. Curr. Res. Nutr. Food Sci. J. 2013, 1, 37–47. [Google Scholar] [CrossRef]
- Kawano, A.; Emori, Y.; Miyagawa, S. Association Between Stress-Related Substances in Saliva and Immune Substances in Breast Milk in Puerperae. Biol. Res. Nurs. 2009, 10, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.; Cooper, N.R.; Sel, A.; Russo, R. The Social Readjustment Rating Scale: Updated and Modernised. PLoS ONE 2023, 18, e0295943. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J. Postpartum Depression. JAMA 2002, 287, 762. [Google Scholar] [CrossRef]
- Agrawal, I.; Mehendale, A.M.; Malhotra, R. Risk Factors of Postpartum Depression. Cureus 2022, 14, e30898. [Google Scholar] [CrossRef]
- Darcy, J.M.; Grzywacz, J.G.; Stephens, R.L.; Leng, I.; Clinch, C.R.; Arcury, T.A. Maternal Depressive Symptomatology: 16-Month Follow-up of Infant and Maternal Health-Related Quality of Life. J. Am. Board Fam. Med. 2011, 24, 249–257. [Google Scholar] [CrossRef]
- Vesga-López, O.; Blanco, C.; Keyes, K.; Olfson, M.; Grant, B.F.; Hasin, D.S. Psychiatric Disorders in Pregnant and Postpartum Women in the United States. Arch. Gen. Psychiatry 2008, 65, 805. [Google Scholar] [CrossRef]
- de Avilla, J.C.; Giugliani, C.; Bizon, A.M.B.L.; Martins, A.C.M.; de Senna, A.F.K.; Giugliani, E.R.J. Association between Maternal Satisfaction with Breastfeeding and Postpartum Depression Symptoms. PLoS ONE 2020, 15, e0242333. [Google Scholar] [CrossRef] [PubMed]
- Konjevod, M.; Gredicak, M.; Vuic, B.; Tudor, L.; Nikolac Perkovic, M.; Milos, T.; Svob Strac, D.; Pivac, N.; Nedic Erjavec, G. Overview of Metabolomic Aspects in Postpartum Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 127, 110836. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Ein-Dor, T. A Unified Model of the Biology of Peripartum Depression. Transl. Psychiatry 2023, 13, 138. [Google Scholar] [CrossRef]
- Mehdi, S.; Wani, S.U.D.; Krishna, K.L.; Kinattingal, N.; Roohi, T.F. A Review on Linking Stress, Depression, and Insulin Resistance via Low-Grade Chronic Inflammation. Biochem. Biophys. Rep. 2023, 36, 101571. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S. New Insights into Perinatal Depression: Pathogenesis and Treatment during Pregnancy and Postpartum. Dialogues Clin. Neurosci. 2011, 13, 89–100. [Google Scholar] [CrossRef]
- Kivlighan, K.T.; Schneider, S.S.; Browne, E.P.; Pentecost, B.T.; Anderton, D.L.; Arcaro, K.F. Mammary Epithelium Permeability during Established Lactation: Associations with Cytokine Levels in Human Milk. Front. Nutr. 2024, 11, 1258905. [Google Scholar] [CrossRef]
- Cabello, M.; Miret, M.; Caballero, F.F.; Chatterji, S.; Naidoo, N.; Kowal, P.; D’Este, C.; Ayuso-Mateos, J.L. The Role of Unhealthy Lifestyles in the Incidence and Persistence of Depression: A Longitudinal General Population Study in Four Emerging Countries. Glob. Health 2017, 13, 18. [Google Scholar] [CrossRef]
- Hechler, C.; Beijers, R.; Riksen-Walraven, J.M.; de Weerth, C. Are Cortisol Concentrations in Human Breast Milk Associated with Infant Crying? Dev. Psychobiol. 2018, 60, 639–650. [Google Scholar] [CrossRef]
- Grey, K.R.; Davis, E.P.; Sandman, C.A.; Glynn, L.M. Human Milk Cortisol Is Associated with Infant Temperament. Psychoneuroendocrinology 2013, 38, 1178–1185. [Google Scholar] [CrossRef]
- Netzer-Tomkins, H.; Rubin, L.; Ephros, M. Breastfeeding Is Associated with Decreased Hospitalization for Neonatal Fever. Breastfeed. Med. 2016, 11, 218–221. [Google Scholar] [CrossRef]
- Dagvadorj, A.; Ota, E.; Shahrook, S.; Baljinnyam Olkhanud, P.; Takehara, K.; Hikita, N.; Bavuusuren, B.; Mori, R.; Nakayama, T. Hospitalization Risk Factors for Children’s Lower Respiratory Tract Infection: A Population-Based, Cross-Sectional Study in Mongolia. Sci. Rep. 2016, 6, 24615. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, B.M.; Patro, B.; Veldhorst, M.; Kouwenhoven, S.; Crespo Escobar, P.; Calvo Lerma, J.; Koletzko, B.; van Goudoever, J.B.; Szajewska, H. Nutrition of Infants and Young Children (One to Three Years) and Its Effect on Later Health: A Systematic Review of Current Recommendations (EarlyNutrition Project). Crit. Rev. Food Sci. Nutr. 2017, 57, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.A.; Pike, C.J. Obesity and Sex Interact in the Regulation of Alzheimer’s Disease. Neurosci. Biobehav. Rev. 2016, 67, 102–118. [Google Scholar] [CrossRef]
- Poton, W.L.; Soares, A.L.G.; de Oliveira, E.R.A.; Gonçalves, H. Breastfeeding and Behavior Disorders among Children and Adolescents: A Systematic Review. Rev. Saude Publica 2018, 52, 9. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Machado-Vieira, R.; Anderson, G.; Solmi, M.; Sanz, Y.; Berk, M.; Köhler, C.; Carvalho, A. Intestinal Dysbiosis, Gut Hyperpermeability and Bacterial Translocation: Missing Links Between Depression, Obesity and Type 2 Diabetes. Curr. Pharm. Des. 2016, 22, 6087–6106. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; O’Callaghan, M.J.; Bor, W.; Williams, G.M.; Najman, J.M. Association of Breastfeeding and Adolescents’ Psychopathology: A Large Prospective Study. Breastfeed. Med. 2012, 7, 480–486. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Chen, Y.-W.; Stubbs, B.; Carvalho, A.F.; Whiteley, P.; Tang, C.-H.; Yang, W.-C.; Chen, T.-Y.; Li, D.-J.; Chu, C.-S.; et al. Maternal Breastfeeding and Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Nutr. Neurosci. 2019, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Krol, K.M.; Grossmann, T. Psychological Effects of Breastfeeding on Children and Mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61, 977–985. [Google Scholar] [CrossRef]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breastfeeding and the Gut-Brain Axis: Is There a Role for Melatonin? Biomol. Concepts 2017, 8, 185–195. [Google Scholar] [CrossRef]
- Anderson, G.; Seo, M.; Berk, M.; Carvalho, A.; Maes, M. Gut Permeability and Microbiota in Parkinson’s Disease: Role of Depression, Tryptophan Catabolites, Oxidative and Nitrosative Stress and Melatonergic Pathways. Curr. Pharm. Des. 2016, 22, 6142–6151. [Google Scholar] [CrossRef]
- Rodriguez, M.; Wootla, B.; Anderson, G. Multiple Sclerosis, Gut Microbiota and Permeability: Role of Tryptophan Catabolites, Depression and the Driving Down of Local Melatonin. Curr. Pharm. Des. 2016, 22, 6134–6141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Morris, G.; Carvalho, A.F.; Anderson, G.; Galecki, P.; Maes, M. The Many Neuroprogressive Actions of Tryptophan Catabolites (TRYCATs) That May Be Associated with the Pathophysiology of Neuro-Immune Disorders. Curr. Pharm. Des. 2016, 22, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, J.E.; Zhou, Y.; McGeachie, M.J.; Ziniti, J.; Lange, N.; Laranjo, N.; Savage, J.R.; Carey, V.; O’Connor, G.; Sandel, M.; et al. Factors Influencing the Infant Gut Microbiome at Age 3–6 Months: Findings from the Ethnically Diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol. 2017, 139, 482–491.e14. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and Immunological Factors in Breast Milk: A Role in the Intergenerational Transmission from Maternal Psychopathology to Child Development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRlSMA 2020 statement. an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial Stress and Cortisol Stress Reactivity Predict Breast Milk Composition. Sci. Rep. 2021, 11, 11576. [Google Scholar] [CrossRef]
- Dombrowska-Pali, A.; Chrustek, A.; Gebuza, G.; Kaźmierczak, M. Composition of Human Milk in Women with the Risk of Postpartum Depression Symptoms. J. Health Inequalities 2023, 9, 58–64. [Google Scholar] [CrossRef]
- Juncker, H.G.; Naninck, E.F.G.; van Keulen, B.J.; Harinck, J.E.; Schipper, L.; Lucassen, P.J.; van Goudoever, J.B.; de Rooij, S.R.; Korosi, A. Maternal Stress Is Associated with Higher Protein-Bound Amino Acid Concentrations in Human Milk. Front. Nutr. 2023, 10, 1165764. [Google Scholar] [CrossRef]
- Palnizky Soffer, G.; Siri, M.; Mangel, L.; Mandel, D.; Lubetzky, R. Impact of Maternal Anxiety on Human Milk Macronutrients Content: A Observational Study. Breastfeed. Med. 2020, 15, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Riedy, H.; Bertrand, K.; Chambers, C.; Bandoli, G. The Association Between Maternal Psychological Health and Human Milk Oligosaccharide Composition. Breastfeed. Med. 2024, 19, 837–847. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Seafood Consumption, the DHA Content of Mothers’ Milk and Prevalence Rates of Postpartum Depression: A Cross-National, Ecological Analysis. J. Affect. Disord. 2002, 69, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wei, Q.; Zou, L.; Jiang, S.; Deng, H.; Jiang, C.; Cui, N.; Huang, S.; Ge, Y.; Li, Y.; et al. Postpartum Dietary Intake, Depression and the Concentration of Docosahexaenoic Acid in Mature Breast Milk in Wuhan, China. Food Funct. 2023, 14, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Juncker, H.G.; Naninck, E.F.G.; Schipper, L.; Lucassen, P.J.; van Goudoever, J.B.; de Rooij, S.R.; Korosi, A. Maternal Stress in the Postpartum Period Is Associated with Altered Human Milk Fatty Acid Composition. Clin. Nutr. 2022, 41, 2517–2528. [Google Scholar] [CrossRef]
- Keim, S.A.; Daniels, J.L.; Siega-Riz, A.M.; Dole, N.; Herring, A.H.; Scheidt, P.C. Depressive Symptoms during Pregnancy and the Concentration of Fatty Acids in Breast Milk. J. Hum. Lact. 2012, 28, 189–195. [Google Scholar] [CrossRef]
- Rodriguez, N.; Tun, H.M.; Field, C.J.; Mandhane, P.J.; Scott, J.A.; Kozyrskyj, A.L. Prenatal Depression, Breastfeeding, and Infant Gut Microbiota. Front. Microbiol. 2021, 12, 664257. [Google Scholar] [CrossRef]
- Zijlmans, M.A.C.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal Prenatal Stress Is Associated with the Infant Intestinal Microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Fernández-Tuñas, M.d.C.; Pérez-Muñuzuri, A.; Trastoy-Pena, R.; Pérez del Molino, M.L.; Couce, M.L. Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants. Nutrients 2023, 15, 4006. [Google Scholar] [CrossRef]
- Groër, M.W.; Humenick, S.; Hill, P.D. Characterizations and Psychoneuroimmunologic Implications of Secretory Immunoglobulin A and Cortisol in Preterm and Term Breast Milk. J. Perinat. Neonatal Nurs. 1994, 7, 42–51. [Google Scholar] [CrossRef]
- Groer, M.; Davis, M.; Steele, K. Associations between Human Milk SIgA and Maternal Immune, Infectious, Endocrine, and Stress Variables. J. Hum. Lact. 2004, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Suda, Y.; Nakao, A.; Oh-Oka, K.; Suzuki, K.; Ishimaru, K.; Sato, M.; Tanaka, T.; Nagai, A.; Yamagata, Z. Maternal Psychosocial Factors Determining the Concentrations of Transforming Growth Factor-Beta in Breast Milk. Pediatr. Allergy Immunol. 2011, 22, 853–861. [Google Scholar] [CrossRef]
- Kim, E.S.; Jeong, M.J.; Kim, S.; Shin, H.-A.; Lee, H.K.; Shin, K.; Han, J.H. Maternal Psychosocial Factors That Affect Breastfeeding Adaptation and Immune Substances in Human Milk. Korean J. Women Health Nurs. 2014, 20, 14. [Google Scholar] [CrossRef]
- Kawano, A.; Emori, Y. The Relationship Between Maternal Postpartum Psychological State and Breast Milk Secretory Immunoglobulin A Level. J. Am. Psychiatr. Nurses Assoc. 2015, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shariat, M.; Abedinia, N.; Rezaei, N.; Farrokhzad, N. Increase Concentration of Transforming Growth Factor Beta (TGF-β) in Breast Milk of Mothers With Psychological Disorders. Acta Med. Iran. 2017, 55, 429–436. [Google Scholar] [PubMed]
- Moirasgenti, M.; Doulougeri, K.; Panagopoulou, E.; Theodoridis, T. Psychological Stress Reduces the Immunological Benefits of Breast Milk. Stress Health 2019, 35, 681–685. [Google Scholar] [CrossRef]
- Aparicio, M.; Browne, P.D.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; de Weerth, C.; Fernández, L. Human Milk Cortisol and Immune Factors over the First Three Postnatal Months: Relations to Maternal Psychosocial Distress. PLoS ONE 2020, 15, e0233554. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Apanasewicz, A.; Danel, D.P.; Babiszewska, M.; Piosek, M.; Orczyk-Pawiłowicz, M. Maternal Distress and Social Support Are Linked to Human Milk Immune Properties. Nutrients 2021, 13, 1857. [Google Scholar] [CrossRef]
- Thibeau, S.; D’Apolito, K.; Minnick, A.F.; Dietrich, M.S.; Kane, B.; Cooley, S.; Groer, M. Relationships of Maternal Stress with Milk Immune Components in African American Mothers of Healthy Term Infants. Breastfeed. Med. 2016, 11, 6–14. [Google Scholar] [CrossRef]
- Juncker, H.G.; Ruhé, E.J.M.; Korosi, A.; van Goudoever, J.B.; van Gils, M.J.; van Keulen, B.J. Maternal Stress and Human Milk Antibodies During the COVID-19 Pandemic. Front. Nutr. 2022, 9, 923501. [Google Scholar] [CrossRef]
- Rosen-Carole, C.B.; Greenman, S.; Wang, H.; Sonawane, S.; Misra, R.; O’Connor, T.; Järvinen, K.; D’Angio, C.; Young, B.E. Association between Maternal Stress and Premature Milk Cortisol, Milk IgA, and Infant Health: A Cohort Study. Front. Nutr. 2024, 11, 1270523. [Google Scholar] [CrossRef]
- Zagoory-Sharon, O.; Yirmiya, K.; Peleg, I.; Shimon-Raz, O.; Sanderlin, R.; Feldman, R. Breast Milk Oxytocin and S-IgA Modulate Infant Biomarkers and Social Engagement; The Role of Maternal Anxiety. Compr. Psychoneuroendocrinol. 2024, 17, 100219. [Google Scholar] [CrossRef]
- Hart, S.; Boylan, L.M.; Border, B.; Carroll, S.R.; McGunegle, D.; Lampe, R.M. Breast Milk Levels of Cortisol and Secretory Immunoglobulin A (SIgA) Differ with Maternal Mood and Infant Neuro-Behavioral Functioning. Infant. Behav. Dev. 2004, 27, 101–106. [Google Scholar] [CrossRef]
- Matyas, M.; Apanasewicz, A.; Krzystek-Korpacka, M.; Jamrozik, N.; Cierniak, A.; Babiszewska-Aksamit, M.; Ziomkiewicz, A. The Association between Maternal Stress and Human Milk Concentrations of Cortisol and Prolactin. Sci. Rep. 2024, 14, 28115. [Google Scholar] [CrossRef] [PubMed]
- Groër, M.; Davis, M.; Casey, K.; Short, B.; Smith, K.; Groër, S. Neuroendocrine & Immune Relationships in Postpartum Fatigue. MCN Am. J. Matern./Child Nurs. 2005, 30, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Apanasewicz, A.; Matyas, M.; Piosek, M.; Jamrozik, N.; Winczowska, P.; Krzystek-Korpacka, M.; Ziomkiewicz, A. Infant Temperament Is Associated with Milk Cortisol but Not with Maternal Childhood Trauma. Am. J. Hum. Biol. 2024, 36, e24150. [Google Scholar] [CrossRef] [PubMed]
- Ten-Doménech, I.; Moreno-Giménez, A.; Campos-Berga, L.; Zapata de Miguel, C.; López-Nogueroles, M.; Parra-Llorca, A.; Quintás, G.; García-Blanco, A.; Gormaz, M.; Kuligowski, J. Impact of Maternal Health and Stress on Steroid Hormone Profiles in Human Milk: Implications for Infant Development. J. Lipid Res. 2024, 65, 100688. [Google Scholar] [CrossRef] [PubMed]
- Vacaru, S.V.; Brett, B.E.; Eckermann, H.; de Weerth, C. Determinants of Maternal Breast Milk Cortisol Increase: Examining Dispositional and Situational Factors. Psychoneuroendocrinology 2023, 158, 106385. [Google Scholar] [CrossRef]
- Zanardo, S.; Nicolussi, F.; Favaro, D.; Faggian, M.; Plebani, F.; Marzari, F.; Freato, V. Effect of Postpartum Anxiety on the Colostral Milk β-Endorphin Concentrations of Breastfeeding Mothers. J. Obstet. Gynaecol. 2001, 21, 130–134. [Google Scholar] [CrossRef]
- Tekgündüz, S.E.; Lazoglu, M.; Nailoglu, M.; Apay, S.E.; Tekgündüz, K.S. The Relationship of Preterm, Term, and Post-Term Births to Maternal Stress and Human Milk Cortisol Levels. Breastfeed. Med. 2023, 18, 462–468. [Google Scholar] [CrossRef]
- Zielinska-Pukos, M.A.; Brys, J.; Kucharz, N.; Chrobak, A.; Wesolowska, A.; Grabowicz-Chastrzyriska, I.; Hamulka, J. Factors Influencing Cortisol Concentrations in Breastmilk and Its with Breastmilk Composition and Infant Development in the First Six Months of Lactation. Int. J. Environ. Res. Public Health 2022, 19, 14809. [Google Scholar] [CrossRef]
- Noh, S.; Lee, E. Relationship between Selected Trace Elements in Human Milk and Psychosocial Characteristics in Korean Early Postpartum Women. Int. J. Environ. Res. Public Health 2021, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, A.; Kumral, A.; Guvenir, T.; Tas, F.V.; Gencer, O.; Duman, N.; Ozkan, H. Maternal Psychosocial Aspects in Hypernatremic Dehydration with High Sodium Concentrations in Breast Milk: A Case–Control Study. J. Paediatr. Child Health 2008, 44, 38–43. [Google Scholar] [CrossRef]
- Serim Demirgoren, B.; Ozbek, A.; Ormen, M.; Kavurma, C.; Ozer, E.; Aydın, A. Do Mothers with High Sodium Levels in Their Breast Milk Have High Depression and Anxiety Scores? J. Int. Med. Res. 2017, 45, 843–848. [Google Scholar] [CrossRef]
- Flores-Quijano, M.E.; Córdova, A.; Contreras-Ramírez, V.; Farias-Hernández, L.; Cruz Tolentino, M.; Casanueva, E. Risk for Postpartum Depression, Breastfeeding Practices, and Mammary Gland Permeability. J. Hum. Lact. 2008, 24, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Colicino, E.; Rodosthenous, R.; Bloomquist, T.R.; Baccarelli, A.A.; Wright, R.O.; Wright, R.J.; Lee, A.G. Associations between Maternal Lifetime Stressors and Negative Events in Pregnancy and Breast Milk-Derived Extracellular Vesicle MicroRNAs in the Programming of Intergenerational Stress Mechanisms (PRISM) Pregnancy Cohort. Epigenetics 2021, 16, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Whaites Heinonen, E.; Bertrand, K.; Chambers, C. Macronutrients in Human Milk Exposed to Antidepressant and Anti-Inflammatory Medications. JAMA Netw. Open 2025, 8, e2453332. [Google Scholar] [CrossRef]
- Santos, J.; Maran, P.L.; Rodríguez-Urrutia, A. Stress, Microbiota, and the Gut–Brain Axis in Mental and Digestive Health. Med. Clin. 2025, in press. [Google Scholar] [CrossRef]
- Sudo, N. Role of Gut Microbiota in Brain Function and Stress-Related Pathology. Biosci. Microbiota Food Health 2019, 38, 75–80. [Google Scholar] [CrossRef]
- Castro-Quintas, Á.; Palma-Gudiel, H.; San Martín-González, N.; Caso, J.R.; Leza, J.C.; Fañanás, L. Salivary Secretory Immunoglobulin A as a Potential Biomarker of Psychosocial Stress Response during the First Stages of Life: A Systematic Review. Front. Neuroendocrinol. 2023, 71, 101083. [Google Scholar] [CrossRef]
- Campos-Rodríguez, R.; Godínez-Victoria, M.; Abarca-Rojano, E.; Pacheco-Yépez, J.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Stress Modulates Intestinal Secretory Immunoglobulin A. Front. Integr. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Sasahara, A.; Yoshida, T.; Sira, M.M.; Futatani, T.; Kanegane, H.; Miyawaki, T. Role of Transforming Growth Factor-β in Breast Milk for Initiation of IgA Production in Newborn Infants. Early Hum. Dev. 2004, 77, 67–75. [Google Scholar] [CrossRef]
- Calthorpe, R.J.; Poulter, C.; Smyth, A.R.; Sharkey, D.; Bhatt, J.; Jenkins, G.; Tatler, A.L. Complex Roles of TGF-β Signaling Pathways in Lung Development and Bronchopulmonary Dysplasia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L285–L296. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Filteau; Lietz; Mulokozi, G.; Bilotta, S.; Henry. Tomkins Milk Cytokines and Subclinical Breast Inflammation in Tanzanian Women: Effects of Dietary Red Palm Oil or Sunflower Oil Supplementation. Immunology 1999, 97, 595–600. [Google Scholar] [CrossRef]
- Chockalingam, A.; Paape, M.J.; Bannerman, D.D. Increased Milk Levels of Transforming Growth Factor-α, Β1, and Β2 During Escherichia Coli-Induced Mastitis. J. Dairy Sci. 2005, 88, 1986–1993. [Google Scholar] [CrossRef]
- Creswell, D.; Brown, K.W.; Cohen, S.; Creswell, K.; Zoccola, P.; Dickerson, S.; Dutcher, J.; Wu, S.; Chin, B. Does High Perceived Stress over the Past Month Alter Cortisol Reactivity to the Trier Social Stress Test? Psychoneuroendocrinology 2025, 172, 107256. [Google Scholar] [CrossRef]
- Diaz-Stransky, A.; Tierney, E. Cognitive and Behavioral Aspects of Smith–Lemli–Opitz Syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160, 295–300. [Google Scholar] [CrossRef]
- Kumar, S.; Suthar, R.; Panigrahi, I. Hypercortisolism and Hypothyroidism in an Infant with Smith-Lemli-Opitz Syndrome. J. Pediatr. Endocrinol. Metab. 2012, 25, 1001–1005. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If It Goes up, Must It Come down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef]
- Booij, S.H.; Bos, E.H.; de Jonge, P.; Oldehinkel, A.J. The Temporal Dynamics of Cortisol and Affective States in Depressed and Non-Depressed Individuals. Psychoneuroendocrinology 2016, 69, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, C.; Bascuas, P.J.; Piedrafita, E. Effects of Melatonin Administration on Physical Performance and Biochemical Responses Following Exhaustive Treadmill Exercise. Curr. Issues Mol. Biol. 2024, 46, 13647–13661. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Kholghi, G.; Eskandari, M.; Shokouhi Qare Saadlou, M.-S.; Zarrindast, M.-R.; Vaseghi, S. Night Shift Hormone: How Does Melatonin Affect Depression? Physiol. Behav. 2022, 252, 113835. [Google Scholar] [CrossRef]
- Elgellaie, A.; Larkin, T.; Kaelle, J.; Mills, J.; Thomas, S. Plasma Prolactin Is Higher in Major Depressive Disorder and Females, and Associated with Anxiety, Hostility, Somatization, Psychotic Symptoms and Heart Rate. Compr. Psychoneuroendocrinol. 2021, 6, 100049. [Google Scholar] [CrossRef]
- Kim, Y.J. Pivotal Roles of Prolactin and Other Hormones in Lactogenesis and the Nutritional Composition of Human Milk. Clin. Exp. Pediatr. 2020, 63, 312–313. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef]
- Rothenberg, S.J.; Chicz-DeMet, A.; Schnaas, L.; Karchmer, S.; Salinas, V.; Guzmán, L.A. Umbilical Cord β-Endorphin and Early Childhood Motor Development. Early Hum. Dev. 1996, 46, 83–95. [Google Scholar] [CrossRef]
- Osborne, L.M.; Payne, J.L.; Sherer, M.L.; Sabunciyan, S. Altered Extracellular MRNA Communication in Postpartum Depression Is Associated with Decreased Autophagy. Mol. Psychiatry 2022, 27, 4526–4535. [Google Scholar] [CrossRef]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Del Rosso, L.; Mendoza, S.P.; Capitanio, J.P. Cortisol in Mother’s Milk across Lactation Reflects Maternal Life and Predicts Infant Temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef]
- Van De Rest, O.; Van Hooijdonk, L.W.A.; Doets, E.; Schiepers, O.J.G.; Eilander, A.; De Groot, L.C.P.G.M. B Vitamins and N-3 Fatty Acids for Brain Development and Function: Review of Human Studies. Ann. Nutr. Metab. 2012, 60, 272–292. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Jafarirad, S.; Amani, R. Postpartum Depression and Vitamin D: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Ogiji, J.; Rich, W. An Exploratory Study of Vitamin D Levels during Pregnancy and Its Association with Postpartum Depression. Psychiatry Res. Commun. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Bernardi, J.R.; Escobar, R.D.S.; Ferreira, C.F.; Silveira, P.P. Fetal and Neonatal Levels of Omega-3: Effects on Neurodevelopment, Nutrition, and Growth. Sci. World J. 2012, 2012, 202473. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children. Children 2024, 11, 796. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]


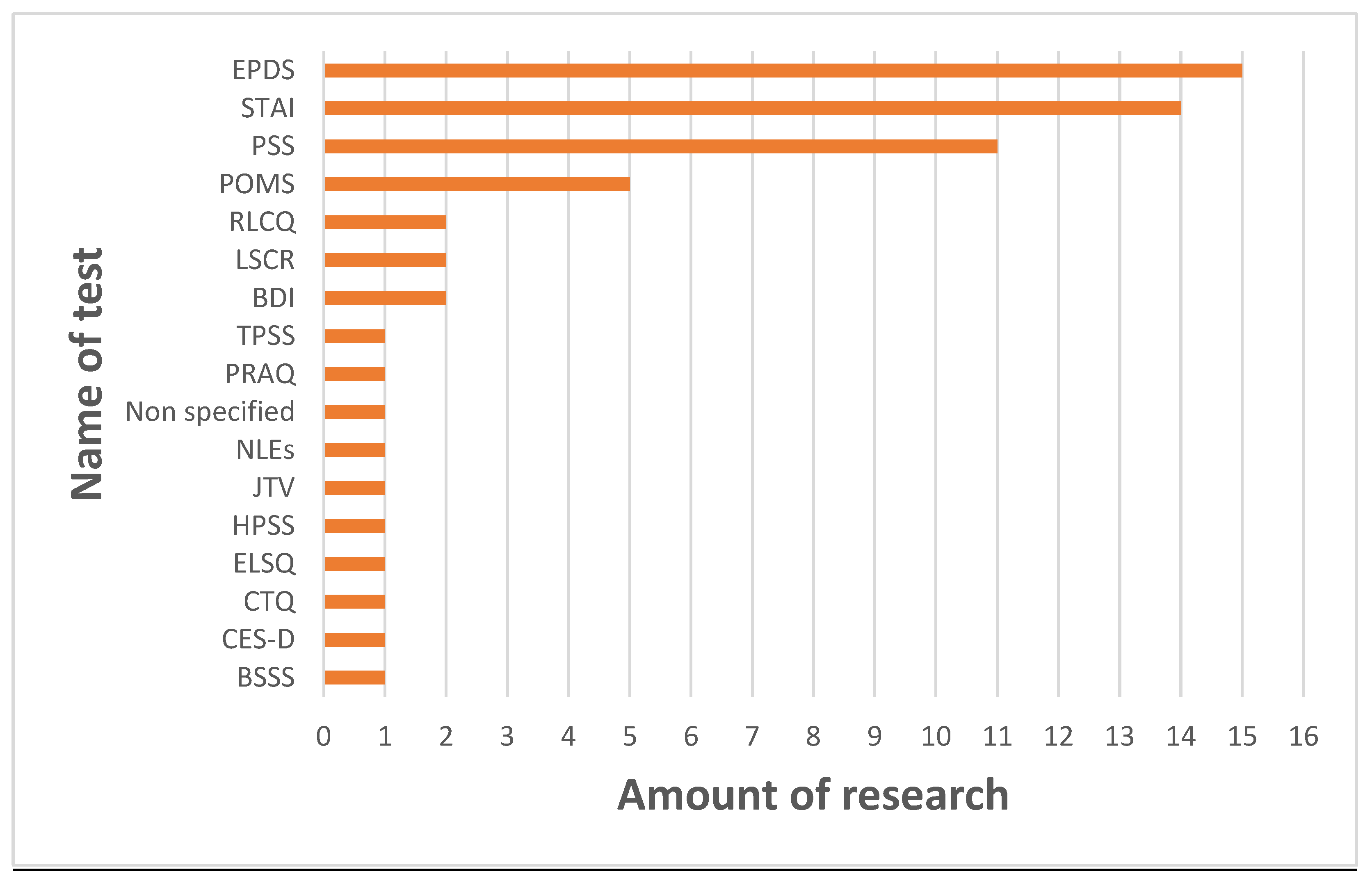
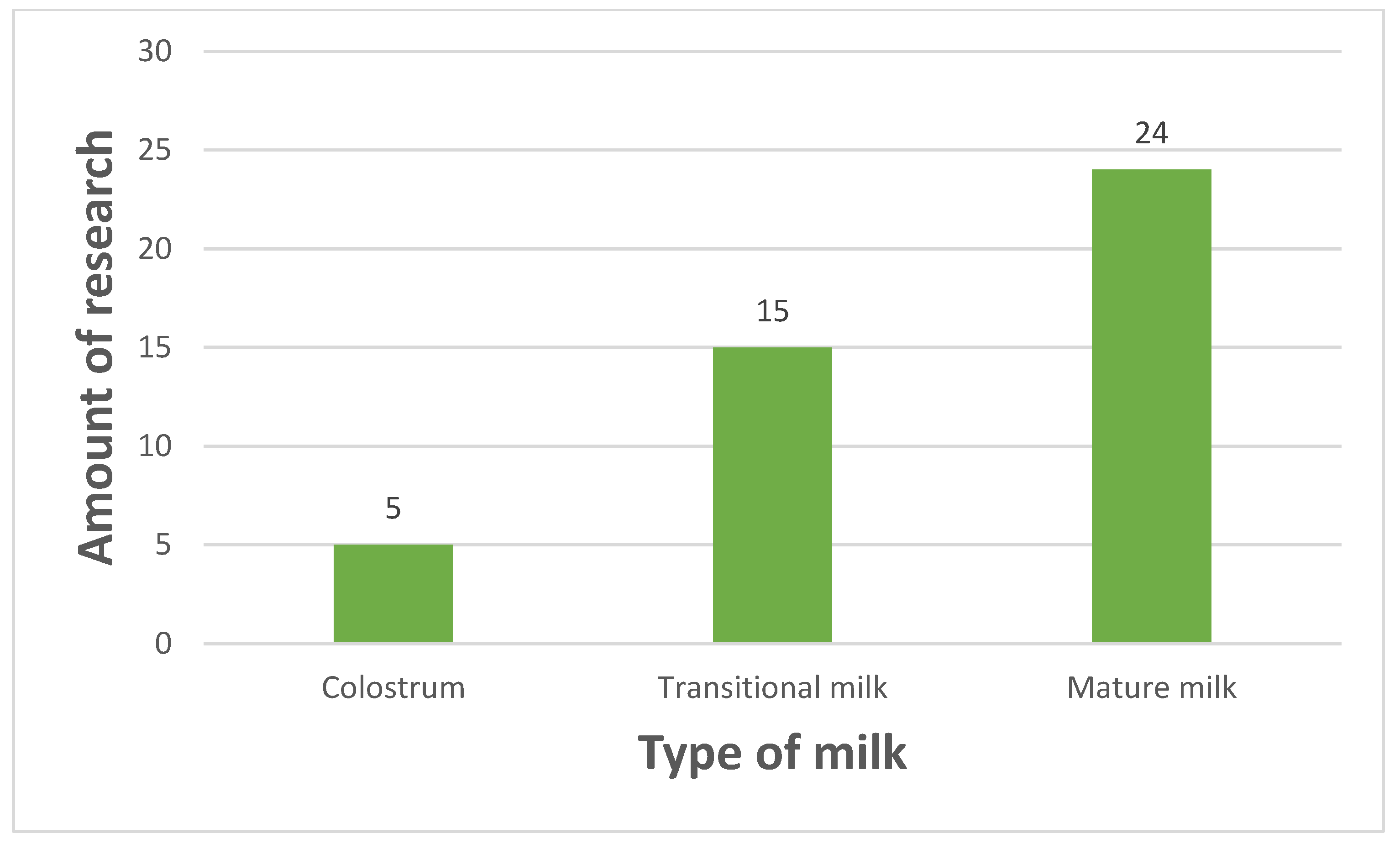
| Ref. | Year | Sample Size | Method of Evaluating Mental State | Time of Evaluating Mental State (Days/Weeks/Months After Giving Birth) | Method of Analysis | Time of Milk Sampling (Days/Weeks/Months After Giving Birth) | Results | Additional Information |
|---|---|---|---|---|---|---|---|---|
| General nutritional value (n = 6) | ||||||||
| [48] | 2021 | 146 | RLCQ | 5 months | MID-IR | 5 months | ↓ energy density | |
| [49] | 2023 | 75 | EPDS | 4 weeks | MID-IR | no longer than 6 months | No correlation: total protein and carbohydrates concentration, calorie content | |
| [50] | 2023 | 116 | LSCR + EPDS + STAI + JTV | 10 days | LC-MS/MS | 10, 17, 24 days | ↑ bound AAs, ↑ Free methionine | BAAs positively associated with cortisol No correlation found for bound methionine |
| No correlation: free AAs | ||||||||
| [51] | 2020 | 21 | STAI-T + STAI-S | STAI-T + STAI-S—1 day after admission | IR transmission spectroscopy | 1 and 2 days after admission and 1 week after discharge | Energy content ↑ on day 7 compared with admission | No significant differences in fat and energy content between days (1 to 2 and 2 to 7) |
| STAI-S—1 week after discharge | ||||||||
| [52] | 2024 | 926 | EPDS, STAI, PSS | 60 days | HPLC | 60 days | Change in oligosaccharides: lacto-N-fucopentaose III, lacto-N-hexaose, disialyl-lacto-N-hexaose | |
| Fatty acids (n = 6) | ||||||||
| [49] | 2023 | 75 | EPDS | 4 weeks | MID-IR | no longer than 6 months | No correlation: total FA content | |
| [51] | 2020 | 21 | STAI-T + STAI-S | STAI-T + STAI-S—1 day after admission | IR transmission spectroscopy | 1, 2 days after admission and 1 week after discharge | FA ↑ on day 7 compared with admission | No significant differences in fat and energy content between days (1 to 2 and 2 to 7) |
| STAI-S—1 week after discharge | ||||||||
| [53] | 2002 | 14 532 | EPDS | 4–240 days | varies depending on source * | varies depending on source * | ↓ DHA | AA and EPA content in HM were unrelated to PPD prevalence |
| [54] | 2023 | 115 | EPDS | 30–120 days | GC-MS | 30–120 days | ↓ DHA | |
| [55] | 2022 | 116 | PSS, EPDS, STAI | 10 days | GC-FID | 10, 17 and 24 days | ↓ FA, PUFA, ω-6 PUFA | Changes observed only in mature milk and not in transitional |
| [56] | 2012 | 287 | CES-D Scale | before 20 weeks and at 24–29 weeks of pregnancy | chromatography with standards comparison | 4 months | ↓ DHA | |
| No correlation: other than DHA FA | ||||||||
| Microbiota ** (n = 3) | ||||||||
| [57] | 2021 | 996 | Non-specified | prenatal | QIAamp DNA Stool Mini Kit | 3–4 months | changes in microbiota in infants who are not exclusively breastfed | Microbiota composition was not affected by mood in group infants breastfed at 3–4 months |
| [58] | 2015 | 192 | STAI + PRAQ | prenatal (35 week) | Human Intestinal Tract Chip | 7, 13, 25, 84, 112 days | low stress = ↑ LAB and ACT1 | |
| prenatal stress = ↑ PRO1, ↓ LAB, ACT1 | ||||||||
| [59] | 2023 | 52 newborns and 45 mothers | parental stress scale PSS:NICU | 3, 7 and 15 days | MilkoSkanTM Mars, FOSS, Hilleroed, Denmark | 3, 7 and 15 days | ↑ microbiota diversity = ↓ stress | Results were not statistically significant |
| Immunological factors (n = 14) | ||||||||
| [60] | 1994 | 34 preterm + 29 terms | POMS | 5 days | RID | 5 days | Preterm: ↑ SIgA (anger) | There is no significant correlation between value and anxiety score |
| Term: no correlation: SIgA | ||||||||
| [61] | 2004 | 50 | PSS, POMS | 10 weeks | ELISA | 10 weeks | ↑ SIgA | |
| [62] | 2011 | 139 | EPDS | 3 months | ELISA | 3 months | ↑ TGFβ2 | |
| [63] | 2014 | 84 | POMS | 6 weeks | ELISA | 2–4 days and 6 weeks | ↑ SIgA | Significant, positive correlation between colostrum SIgA and anger (subcategory of mood state) was reported |
| No correlation: TGFβ2 | ||||||||
| [64] | 2015 | 81 | POMS | 2 weeks | ELISA | 2 weeks | ↓ SIgA | |
| [65] | 2017 | 110 | STAI, BDI | 4–6 months | ELISA | 4–6 months PP | ↑ TGFβ2 | |
| [66] | 2019 | 89 | Hung Postpartum Stress Scale +PSS | 4–6 weeks | ELISA | 4–6 weeks | ↓ SIgA | |
| [67] | 2020 | 51 | STAI-S, EPDS | 6 weeks | ELISA | 2, 6, 12 weeks | ↑ IL-8 * ↑ IL-7 * ↓ TGFβ2 | |
| No correlation: IgA, IgG, IgM, IL-1, TNFα, MCP1, MIP1β, GROα, IL-5, EGF, | ||||||||
| [68] | 2021 | 103 | STAI + BSSS | 5 months | ELISA | >5 months | ↓ Lactoferrin ↓ SIgA | |
| No correlation: IgG, IgM | ||||||||
| [69] | 2016 | 85 | PSS | 3 and 9 days | ELISA (SIgA), Luminex | 3, 9, 14 days | ↓ EGF ↓ MIP1α ↓ TNFα | |
| No correlation: SIgA, IL-4, IL-6, IL-8, IL-10, MCP-1, IP-10 | ||||||||
| 14 days | ↑ IL-8 ↑ MIP1α | |||||||
| No correlation: SIgA, EGF, IL-4, IL-6, IL-10, MCP-1, IP-10, TNFα | ||||||||
| [70] | 2022 | 2310 | PSS, LSCR | 0–12 months | ELISA | up to 12 months | ↓ SIgA (SARS-CoV-2-specific antibodies) | |
| [71] | 2024 | 26 | EPDS, STAI | 5 weeks | Immunoassay | 1 and 5 weeks | ↑ SIgA (only week 5) | |
| [72] | 2004 | 55 | STAI | 1–8 months | ELISA | 1–8 months ^ | No correlation: SIgA | |
| [73] | 2004 | 32 | BDI, POMS | 7–11 days | Nephelometry | 7–11 days | ↑ SIgA | |
| Hormones (n = 13) | ||||||||
| [60] | 1994 | 34 preterm + 29 terms | POMS | 5 days | RID | 5 days | Preterm: no correlation: cortisol | |
| Term: no correlation: cortisol | ||||||||
| [67] | 2020 | 51 | STAI-S, EPDS | 6 weeks | LC-MS | 2, 6, 12 weeks | ↑ Cortisol | |
| [74] | 2024 | 116 | RLCQ | 5 months | ELISA | 5 months | No correlation: cortisol, prolactin | Study additionally shows negative association between salivary cortisol and milk cortisol + prolactine (potential association between long-term stress) |
| [73] | 2004 | 32 | BDI + POMS | 7–11 days | double enzyme fluorometric assay | 7–11 days | ↑ Cortisol (POMS-Anger) | |
| [75] | 2005 | 119 | TPSS, PSS | 4–6 weeks | ELISA | 4–6 weeks | ↓ Prolactin, ↑ melatonin | SIgA was also correlated with milk prolactin, mutual relation between hormones were observed: lower milk prolactin was associated with higher milk melatonin |
| [71] | 2024 | 26 | EPDS, STAI | 5 weeks | chemiluminescent immunoassay | 1 and 5 weeks | ↓ Cortisol (only week 1) | |
| [76] | 2024 | 90 | EPDS, ELSQ | 5 and 12 months | ELISA | 5 months | No correlation: cortisol | |
| [77] | 2024 | 17 preterm 25 term | PSS, STAI, EPDS | 6 months and at specific point *** | RP-UPLC MS/MS | 6 months and at specific point *** | ↑ Pregnenolone | |
| No correlation: cortisol | ||||||||
| [78] | 2023 | 73 | CTQ, STAI, EPDS | 3 trimester, 2, 6, 12 weeks | LC-MS/MS | 2, 6, 12 weeks | ↑ Cortisol | week 2—state anxiety week 6—adverse childhood experiences |
| [79] | 2001 | 42 | STAI | 4 days | 125-I RIA | 4 days | ↓ β-endorphin (state anxiety only) | No correlation for trait anxiety |
| [80] | 2023 | 30 preterm 38 term 22 post-term | PSS | 7 days | ELISA | 7 days | ↑ Cortisol | |
| [81] | 22 | 38 | PSS, EPDS | 1, 3, 6 months | ELISA | 1, 3, 6 months | No correlation: cortisol | |
| [72] | 2024 | 55 | STAI | 1–8 months ^ | ELISA | 1–8 months ^ | No correlation: oxytocin | |
| Ions (n = 5) | ||||||||
| [71] | 2024 | 26 | EPDS, STAI | 5 weeks | ion selective electrodes (Na+ and K+) | 1 and 5 weeks | No correlation: Na:K ratio | |
| [82] | 2021 | 40 | EPDS | 7 days | ICP-MS | 7 days | No correlation: Ca, Na, Fe, Se | |
| [83] | 2008 | 64 | STAI, EPDS | 10 days | Hiatchi Modular Analytics ISE—ion selective method | 10 days | ↑ Na (only state anxiety) | |
| [84] | 2017 | 150 | STAI, EPDS | 8–15 days | ion selective electrode | 8–15 days | ↑ Na (only state anxiety), Na:K ratio | |
| [85] | 2008 | 163 | EPDS | 6 weeks | SPSS | 2–12 weeks | ↑ mammary gland permeability (Na:K ratio) | |
| RNA (n = 1) | ||||||||
| [86] | 2021 | 80 | NLEs, LSCR | 27 ± 8 weeks of gestation | exoEasy Maxi Kit | 6.1 ± 5.9 weeks | ↑ negative events during pregnancy = ↓ microRNA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Lichocki, I.; Godziszewski, F.; Orczyk-Pawiłowicz, M. From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk. Nutrients 2025, 17, 1093. https://doi.org/10.3390/nu17061093
Skowron K, Lichocki I, Godziszewski F, Orczyk-Pawiłowicz M. From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk. Nutrients. 2025; 17(6):1093. https://doi.org/10.3390/nu17061093
Chicago/Turabian StyleSkowron, Krystian, Igor Lichocki, Filip Godziszewski, and Magdalena Orczyk-Pawiłowicz. 2025. "From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk" Nutrients 17, no. 6: 1093. https://doi.org/10.3390/nu17061093
APA StyleSkowron, K., Lichocki, I., Godziszewski, F., & Orczyk-Pawiłowicz, M. (2025). From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk. Nutrients, 17(6), 1093. https://doi.org/10.3390/nu17061093







