Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health—A Narrative Review
Abstract
1. Introduction
2. Lactation as a Developmental Window for CKMS Programming
2.1. Maternal Malnutrition
2.2. Maternal Medical Condition and Pregnancy Complication
2.3. Environmental Pollutants Exposure
2.4. Drug Use
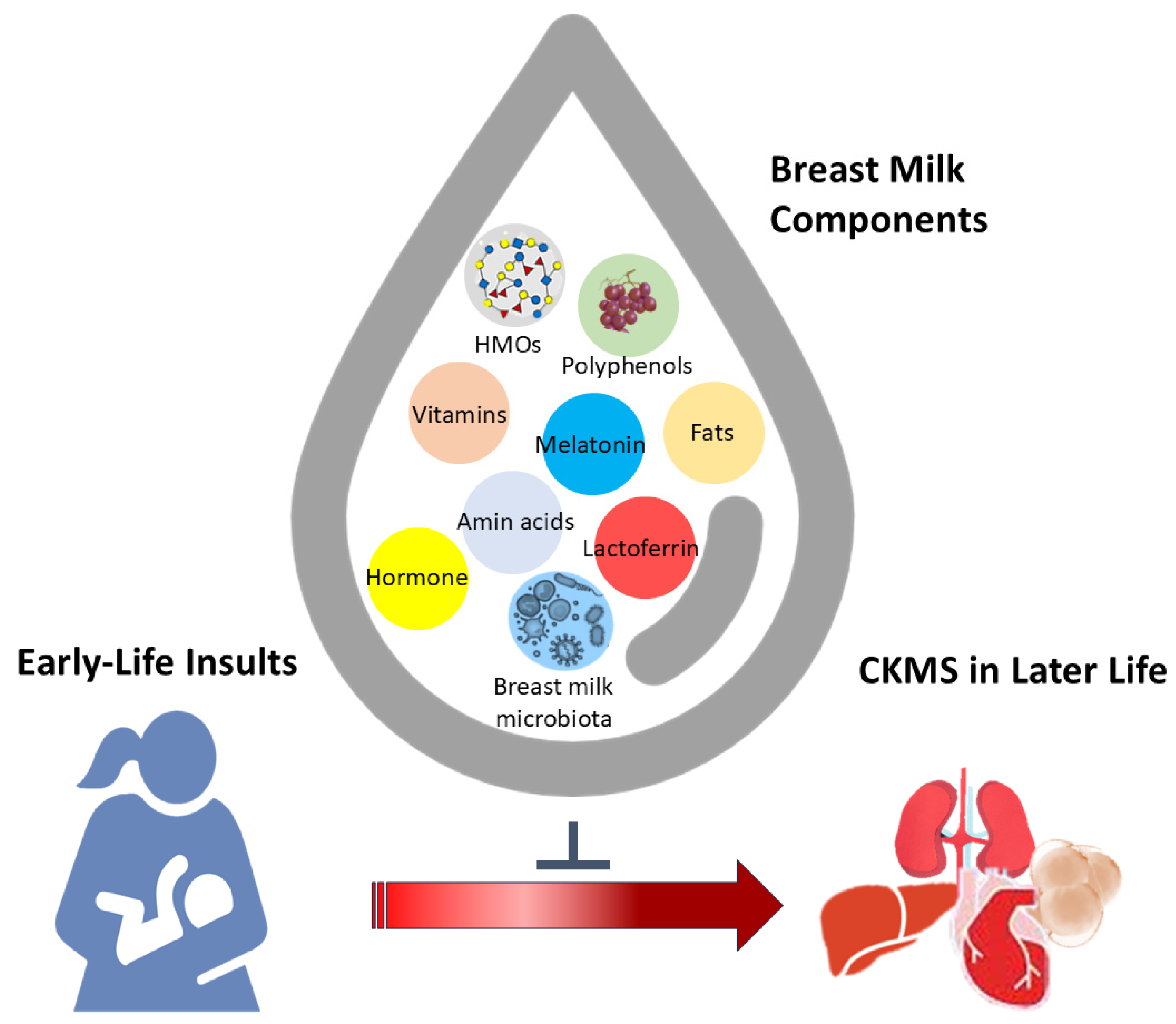
3. Breast Milk: Synthesis and Components
3.1. Macronutrients
3.2. Micronutrients
3.3. MicroRNAs
3.4. Hormones and Growth Factors
3.5. Breast Milk Microbiota
3.6. Others
4. The Link Between Breastfeeding and Mechanisms Underlying CKMS Programming
4.1. Oxidative Stress
4.2. Epigenetic Modifications
4.3. Gut Microbiota Dysbiosis
4.4. Others
5. Targeting Breast Milk as a Reprogramming Strategy
5.1. Amino Acids
5.2. Fats
5.3. Micronutrients
5.4. Bioactive Components
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.; Bland, R. The Benefits of Breastfeeding on Child Intelligence, Behavior, and Executive Function: A Review of Recent Evidence. Breastfeed Med. 2023, 18, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Womens Health 2021, 66, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, P.C.; Miranda, R.A.; Souza, L.L.; Moura, E.G. Can breastfeeding affect the rest of our life? Neuropharmacology 2021, 200, 108821. [Google Scholar] [CrossRef]
- Barker, D.J.; Eriksson, J.G.; Forsen, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A Conceptual Framework for the Developmental Origins of Health and Disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Picó, C.; Reis, F.; Egas, C.; Mathias, P.; Matafome, P. Lactation as a programming window for metabolic syndrome. Eur. J. Clin. Investig. 2021, 51, e13482. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Schellong, K.; Schulz, S.; Stupin, J.H. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 641–653. [Google Scholar] [CrossRef]
- Massy, Z.A.; Drueke, T.B. Combination of Cardiovascular, Kidney, and Metabolic Diseases in a Syndrome Named Cardiovascular-Kidney-Metabolic, With New Risk Prediction Equations. Kidney Int. Rep. 2024, 9, 2608–2618. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. American Heart Association. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Ostrominski, J.W.; Vaduganathan, M. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults, 2011–2020. JAMA 2024, 331, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Scurt, F.G.; Ganz, M.J.; Herzog, C.; Bose, K.; Mertens, P.R.; Chatzikyrkou, C. Association of metabolic syndrome and chronic kidney disease. Obes. Rev. 2024, 25, e13649. [Google Scholar] [CrossRef] [PubMed]
- Saxon, D.R.; Reiter-Brennan, C.; Blaha, M.J.; Eckel, R.H. Cardiometabolic Medicine: Development of a New Subspecialty. J.Clin. Endocrinol. Metab. 2020, 105, dgaa261. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Maternal Dietary Strategies for Improving Offspring Cardiovascular-Kidney-Metabolic Health: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 9788. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Krug, A.; Montero-Bravo, A.; Morais-Moreno, C.; Puga, A.M.; Samaniego-Vaesken, M.L.; Partearroyo, T.; Varela-Moreiras, G. Nutritional Status of Breastfeeding Mothers and Impact of Diet and Dietary Supplementation: A Narrative Review. Nutrients 2024, 16, 301. [Google Scholar] [CrossRef]
- Holemans, K.; Verhaeghe, J.; Dequeker, J.; Van Assche, F.A. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J. Soc. Gynecol. Investig. 1996, 3, 71–77. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lamana, G.L.; Ferrari, A.L.L.; Gontijo, J.A.R.; Boer, P.A. Gestational and Breastfeeding Low-Protein Intake on Blood Pressure, Kidney Structure, and Renal Function in Male Rat Offspring in Adulthood. Front. Physiol. 2021, 12, 658431. [Google Scholar] [CrossRef] [PubMed]
- Berleze, K.J.; Müller, A.P.; Schweigert, I.D.; Longoni, A.; Sordi, F.; de Assis, A.M.; Rotta, L.N.; de Souza, D.O.; Perry, M.L. Gestational and postnatal low protein diet alters insulin sensitivity in female rats. Exp. Biol. Med. 2009, 234, 1437–1444. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021, 65, e2001116. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Tsai, C.C.; Huang, L.T.; Hsu, C.N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef]
- Tsai, T.A.; Tsai, C.K.; Huang, L.T.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Chen, C.C.; Lin, I.C.; Lai, Y.J.; Tsai, C.C.; et al. Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. Int. J. Environ. Res. Public Health 2020, 17, 2780. [Google Scholar] [CrossRef]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378.e1–378.e6. [Google Scholar] [CrossRef]
- Chao, Y.M.; Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Developmental programming of the metabolic syndrome: Next generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao 2016, 68, 557–567. [Google Scholar]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef]
- Chao, Y.M.; Wu, K.L.H.; Tsai, P.C.; Tain, Y.L.; Leu, S.; Lee, W.C.; Chan, J.Y.H. Anomalous AMPK-regulated angiotensin AT1R expression and SIRT1-mediated mitochondrial biogenesis at RVLM in hypertension programming of offspring to maternal high fructose exposure. J. Biomed. Sci. 2020, 27, 68. [Google Scholar] [CrossRef] [PubMed]
- Boubred, F.; Daniel, L.; Buffat, C.; Feuerstein, J.M.; Tsimaratos, M.; Oliver, C.; Dignat-George, F.; Lelièvre-Pégorier, M.; Simeoni, U. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am. J. Physiol. Renal Physiol. 2009, 297, F943–F951. [Google Scholar] [CrossRef]
- Conceição, E.P.; Franco, J.G.; Oliveira, E.; Resende, A.C.; Amaral, T.A.; Peixoto-Silva, N.; Passos, M.C.; Moura, E.G.; Lisboa, P.C. Oxidative stress programming in a rat model of postnatal early overnutrition--role of insulin resistance. J. Nutr. Biochem. 2013, 24, 81–87. [Google Scholar] [CrossRef]
- Souza, L.L.; Moura, E.G.; Lisboa, P.C. Litter Size Reduction as a Model of Overfeeding during Lactation and Its Consequences for the Development of Metabolic Diseases in the Offspring. Nutrients 2022, 14, 2045. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Andreotti, S.; Chimin, P.; Sertié, R.A.; Farias Tda, S.; Torres-Leal, F.L.; de Proença, A.R.; Campaña, A.B.; D’Avila, L.S.; Oliveira, K.A.; et al. Neonatal streptozotocin-induced diabetes in mothers promotes metabolic programming of adipose tissue in male rat offspring. Life Sci. 2015, 136, 151–156. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar]
- O’Dowd, R.; Kent, J.C.; Moseley, J.M.; Wlodek, M.E. Effects of uteroplacental insufficiency and reducing litter size on maternal mammary function and postnatal offspring growth. Am. J. Physiol. 2008, 294, R539–R548. [Google Scholar] [CrossRef]
- Walton, S.L.; Mazzuca, M.Q.; Tare, M.; Parkington, H.C.; Wlodek, M.E.; Moritz, K.M.; Gallo, L.A. Angiotensin receptor blockade in juvenile male rat offspring: Implications for long-term cardio-renal health. Pharmacol. Res. 2018, 134, 320–331. [Google Scholar] [CrossRef]
- Wlodek, M.E.; Westcott, K.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008, 74, 187–195. [Google Scholar] [CrossRef]
- Nüsken, K.D.; Dötsch, J.; Rauh, M.; Rascher, W.; Schneider, H. Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: Impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 2008, 149, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tiao, M.M.; Huang, L.T.; Chen, C.J.; Sheen, J.M.; Tain, Y.L.; Chen, C.C.; Kuo, H.C.; Huang, Y.H.; Tang, K.S.; Chu, E.W.; et al. Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. BioMed Res. Int. 2014, 2014, 942172. [Google Scholar] [CrossRef]
- Tsai, C.C.; Tiao, M.M.; Sheen, J.M.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Lai, Y.J.; Chen, C.C.; Chang, K.A.; et al. Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue. Lipids Health Dis. 2019, 18, 19. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Lisboa, P.C.; Pires, L.; de Oliveira, E.; Lima, N.S.; Bonomo, I.T.; Reis, A.M.; Passos, M.C.; Moura, E.G. Prolactin inhibition at mid-lactation influences adiposity and thyroid function in adult rats. Horm. Metab. Res. 2010, 42, 562–569. [Google Scholar] [CrossRef]
- Passos, M.A.; Passos, M.C.; Oliveira, E.; Trotta, P.A.; Nogueira-Neto, J.F.; Bonomo, I.T.; Lisboa, P.C.; de Moura, E.G. Maternal prolactin inhibition during lactation is associated to renal dysfunction in their adult rat offspring. Horm. Metab. Res. 2011, 43, 636–641. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br. J. Pharmacol. 2011, 164, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Conceição, E.P.; Peixoto-Silva, N.; Pinheiro, C.R.; Oliveira, E.; Moura, E.G.; Lisboa, P.C. Maternal nicotine exposure leads to higher liver oxidative stress and steatosis in adult rat offspring. Food Chem. Toxicol. 2015, 78, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal Antioxidant Prevents Nicotine-Mediated Hypertensive Response in Rat Adult Offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chou, H.C.; Huang, L.T. Maternal nicotine exposure during gestation and lactation induces kidney injury and fibrosis in rat offspring. Pediatr. Res. 2015, 77, 56–63. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Nyomba, B.L. Whole body insulin resistance in rat offspring of mothers consuming alcohol during pregnancy or lactation: Comparing prenatal and postnatal exposure. J. Appl. Physiol. (1985) 2004, 96, 167–172. [Google Scholar] [CrossRef]
- Soraya, H.; Sheikholeslami, S.; Shirpoor, A.; Nezami Majd, F.; Naderi, R.; Rasmi, Y. Influence of Maternal Ethanol Exposure on Systemic Hemodynamic Variables and Histopathological Changes in the Aorta Wall of Male Rat Offspring: A Three-month Follow-up. Iran. J. Med. Sci. 2022, 47, 468–476. [Google Scholar]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef]
- Galyon, K.D.; Farshidi, F.; Han, G.; Ross, M.G.; Desai, M.; Jellyman, J.K. Maternal bisphenol A exposure alters rat offspring hepatic and skeletal muscle insulin signaling protein abundance. Am. J. Obstet. Gynecol. 2017, 216, 290.e1–290.e9. [Google Scholar] [CrossRef]
- Liao, J.X.; Chen, Y.W.; Shih, M.K.; Tain, Y.L.; Yeh, Y.T.; Chiu, M.H.; Chang, S.K.C.; Hou, C.Y. Resveratrol Butyrate Esters Inhibit BPA-Induced Liver Damage in Male Offspring Rats by Modulating Antioxidant Capacity and Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 5273. [Google Scholar] [CrossRef]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sofin, R.G.S.; Das, S. Perinatal Exposure to Bisphenol A and Developmental Programming of the Cardiovascular Changes in the Offspring. Curr. Med. Chem. 2022, 29, 4235–4250. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.C.; Kopf, P.G.; Campen, M.J.; Huwe, J.K.; Walker, M.K. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: Effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol. Sci. 2008, 101, 321–330. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hung, C.H.; Hou, C.Y.; Chang, C.I.; Tain, Y.L. Perinatal Resveratrol Therapy to Dioxin-Exposed Dams Prevents the Programming of Hypertension in Adult Rat Offspring. Antioxidants 2021, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal Resveratrol Therapy Protects Male Rat Offspring against Programmed Hypertension Induced by TCDD and Dexamethasone Exposures: Is It Relevant to Aryl Hydrocarbon Receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl) phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef]
- Rajagopal, G.; Bhaskaran, R.S.; Karundevi, B. Maternal di-(2-ethylhexyl) phthalate exposure alters hepatic insulin signal transduction and glucoregulatory events in rat F1 male offspring. J. Appl. Toxicol. 2019, 39, 751–763. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients 2023, 15, 697. [Google Scholar] [CrossRef]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Parra-Vargas, M.; Bouret, S.G.; Bruning, J.C.; de Moura, E.G.; Garland, T., Jr.; Lisboa, P.C.; Ozanne, S.E.; Patti, M.E.; Plagemann, A.; Speakman, J.R.; et al. The long-lasting shadow of litter size in rodents: Litter size is an underreported variable that strongly determines adult physiology. Mol. Metab. 2023, 71, 101707. [Google Scholar] [CrossRef]
- Nguyen, P.T.H.; Pham, N.M.; Chu, K.T.; Van Duong, D.; Van Do, D. Gestational Diabetes and Breastfeeding Outcomes: A Systematic Review. Asia Pac. J. Public Health 2019, 31, 183–198. [Google Scholar] [CrossRef]
- Singh, M. Breastfeeding and Medication Use in Kidney Disease. Adv. Chronic Kidney Dis. 2020, 27, 516–524. [Google Scholar] [CrossRef]
- Fernandez-Vaz, C.; Gonzalez-Sanz, J.D. Cortisol, Maternal Stress, and Breastfeeding Rate at Hospital Discharge: A Systematic Review. Breastfeed. Med. 2022, 17, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.; Baker, T.; Hale, T.W. Maternal medication, drug use, and breastfeeding. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, E.O.; Rahman, M.S.; Park, Y.J.; Kim, Y.J.; Pang, M.G. Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression. Int. J. Mol. Sci. 2021, 22, 3939. [Google Scholar] [CrossRef]
- LaKind, J.S.; Lehmann, G.M.; Davis, M.H.; Hines, E.P.; Marchitti, S.A.; Alcala, C.; Lorber, M. Infant Dietary Exposures to Environmental Chemicals and Infant/Child Health: A Critical Assessment of the Literature. Environ. Health Perspect. 2018, 126, 96002. [Google Scholar] [CrossRef] [PubMed]
- Slabiak-Blaz, N.; Adamczak, M.; Gut, N.; Grajoszek, A.; Nyengaard, J.R.; Ritz, E.; Wiecek, A. Administration of cyclosporine a in pregnant rats—The effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press. Res. 2015, 40, 413–423. [Google Scholar] [CrossRef]
- Gilbert, T.; Lelievre-Pegorier, M.; Merlet-Benichou, C. Immediate and long-term renal effects of fetal exposure to gentamicin. Pediatr. Nephrol. 1990, 4, 445–450. [Google Scholar] [CrossRef]
- Chang, H.Y.; Tain, Y.L. Postnatal dexamethasone-induced programmed hypertension is related to the regulation of melatonin and its receptors. Steroids 2016, 108, 1–6. [Google Scholar] [CrossRef]
- Carlson, Z.; Hafner, H.; Mulcahy, M.; Bullock, K.; Zhu, A.; Bridges, D.; Bernal-Mizrachi, E.; Gregg, B. Lactational metformin exposure programs offspring white adipose tissue glucose homeostasis and resilience to metabolic stress in a sex-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E600–E612. [Google Scholar] [CrossRef]
- Birru Talabim, M.; Clowse, M.E.B. Antirheumatic medications in pregnancy and breastfeeding. Curr. Opin. Rheumatol. 2020, 32, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hagg, S.; Spigset, O. Anticonvulsant use during lactation. Drug Saf. 2000, 22, 425–440. [Google Scholar] [CrossRef]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef]
- Viña, J.; Puertes, I.R.; Saez, G.T.; Viña, J.R. Role of prolactin in amino acid uptake by the lactating mammary gland of the rat. FEBS Lett. 1981, 126, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Immunomodulatory Effects of Human Colostrum and Milk. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 337–345. [Google Scholar] [CrossRef]
- Jenness, R. The composition of human milk. In Seminars in Perinatology; Elsevier: Amsterdam, The Netherlands, 1979; pp. 225–239. [Google Scholar]
- Saarela, T.; Kokkonen, J.; Koivisto, M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005, 94, 1176–1181. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef]
- Ross, M.G.; Kavasery, M.P.; Cervantes, M.K.; Han, G.; Horta, B.; Coca, K.P.; Costa, S.O.; Desai, M. High-Fat, High-Calorie Breast Milk in Women with Overweight or Obesity and Its Association with Maternal Serum Insulin Concentration and Triglycerides Levels. Children 2024, 11, 141. [Google Scholar] [CrossRef]
- Arthur, P.G.; Kent, J.C.; Hartmann, P.E. Metabolites of lactose synthesis in milk from women during established lactation. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 260–266. [Google Scholar]
- Berger, P.K.; Fields, D.A.; Demerath, E.W.; Fujiwara, H.; Goran, M.I. High-Fructose Corn-Syrup-Sweetened Beverage Intake Increases 5-Hour Breast Milk Fructose Concentrations in Lactating Women. Nutrients 2018, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Plows, J.F.; Demerath, E.W.; Fields, D.A. Carbohydrate composition in breast milk and its effect on infant health. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F.; Hart, G.W. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. In Personalized Nutrition for the Diverse Needs of Infants and Children; Karger Publishers: Basel, Switzerland, 2008; Volume 62, pp. 205–222. [Google Scholar]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.M.; Martínez, I.; Walter, J.; Goin, C.; Hutkins, R.W. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE 2011, 6, e25200. [Google Scholar] [CrossRef]
- Perrine, C.G.; Sharma, A.J.; Jefferds, M.E.; Serdula, M.K.; Scanlon, K.S. Adherence to vitamin D recommendations among US infants. Pediatrics 2010, 125, 627–632. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Committee on Fetus and Newborn. Controversies concerning vitamin K and the newborn. American Academy of Pediatrics Committee on Fetus and Newborn. Pediatrics 2003, 112, 191–192. [Google Scholar] [CrossRef]
- Sneed, S.M.; Zane, C.; Thomas, M.R. The effects of ascorbic acid, vitamin B6, vitamin B12, and folic acid supplementation on the breast milk and maternal nutritional status of low socioeconomic lactating women. Am. J. Clin. Nutr. 1981, 34, 1338–1346. [Google Scholar] [CrossRef]
- Domellöf, M.; Lönnerdal, B.; Dewey, K.G.; Cohen, R.J.; Hernell, O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 2004, 79, 111–115. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Lonnerdal, B. Human Milk MicroRNAs/Exosomes: Composition and Biological Effects. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 83–92. [Google Scholar] [PubMed]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells and lipids conserve numerous known and novel miRNAs, some of which are differentially expressed during lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef]
- Lowry, D.E.; Paul, H.A.; Reimer, R.A. Impact of maternal obesity and prebiotic supplementation on select maternal milk microRNA levels and correlation with offspring outcomes. Br. J. Nutr. 2022, 127, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Banderali, G.; Barberi, S.; Radaelli, G.; Lops, A.; Betti, F.; Riva, E.; Giovannini, M. Epigenetic effects of human breast milk. Nutrients 2014, 6, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Kakulas, F. Milk exosomes and microRNAs: Potential epigenetic regulators. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–28. [Google Scholar]
- Zeng, B.; Chen, T.; Luo, J.Y.; Zhang, L.; Xi, Q.Y.; Jiang, Q.Y.; Sun, J.J.; Zhang, Y.L. Biological Characteristics and Roles of Noncoding RNAs in Milk-Derived Extracellular Vesicles. Adv. Nutr. 2021, 12, 1006–1019. [Google Scholar] [CrossRef]
- Rubio, M.; Bustamante, M.; Hernandez-Ferrer, C.; Fernandez-Orth, D.; Pantano, L.; Sarria, Y.; Piqué-Borras, M.; Vellve, K.; Agramunt, S.; Carreras, R.; et al. Circulating miRNAs, isomiRs and small RNA clusters in human plasma and breast milk. PLoS ONE 2018, 13, e0193527. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Cardioprotective growth factors. Cardiovasc. Res. 2009, 83, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Caba-Flores, M.D.; Ramos-Ligonio, A.; Camacho-Morales, A.; Martínez-Valenzuela, C.; Viveros-Contreras, R.; Caba, M. Breast Milk and the Importance of Chrononutrition. Front. Nutr. 2022, 9, 867507. [Google Scholar] [CrossRef]
- Ananthan, A.; Balasubramanian, H.; Mohan, D.; Rao, S.; Patole, S. Early erythropoietin for preventing necrotizing enterocolitis in preterm neonates—An updated meta-analysis. Eur. J. Pediatr. 2022, 181, 1821–1833. [Google Scholar] [CrossRef]
- Stahl, A.; Hellstrom, A.; Smith, L.E. Insulin-like growth factor-1 and anti-vascular endothelial growth factor in retinopathy of prematurity: Has the time come? Neonatology 2014, 106, 254–260. [Google Scholar] [CrossRef]
- Fields, D.A.; Schneider, C.R.; Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity 2016, 24, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; Lewis, Z.T. MICROBIOTA. Mother’s littlest helpers. Science 2015, 348, 1427–1428. [Google Scholar] [CrossRef]
- Houghteling, P.D.; Walker, W.A. Why is initial bacterial colonization of the intestine important to infants’ and children’s health? J. Pediatr. Gastroenterol. Nutr. 2015, 60, 294–307. [Google Scholar] [CrossRef]
- Edwards, C.A.; Van Loo-Bouwman, C.A.; Van Diepen, J.A.; Schoemaker, M.H.; Ozanne, S.E.; Venema, K.; Stanton, C.; Marinello, V.; Rueda, R.; Flourakis, M.; et al. A systematic review of breast milk microbiota composition and the evidence for transfer to and colonisation of the infant gut. Benef. Microbes 2022, 13, 365–382. [Google Scholar] [CrossRef]
- Padilha, M.; Brejnrod, A.; Danneskiold-Samsoe, N.B.; Hoffmann, C.; de Melo Iaucci, J.; Cabral, V.P.; Xavier-Santos, D.; Taddei, C.R.; Kristiansen, K.; Saad, S.M.I. Response of the human milk microbiota to a maternal prebiotic intervention is individual and influenced by maternal age. Nutrients 2020, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- LeMay-Nedjelski, L.; Asbury, M.R.; Butcher, J.; Ley, S.H.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W.; Stintzi, A.; et al. Maternal Diet and Infant Feeding Practices Are Associated with Variation in the Human Milk Microbiota at 3 Months Postpartum in a Cohort of Women with High Rates of Gestational Glucose Intolerance. J. Nutr. 2021, 151, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos, M.; Prada, A.; Valdiviezo, G.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2013, 162, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef]
- Nagel, E.M.; Peña, A.; Dreyfuss, J.M.; Lock, E.F.; Johnson, K.E.; Lu, C.; Fields, D.A.; Demerath, E.W.; Isganaitis, E. Gestational Diabetes, the Human Milk Metabolome, and Infant Growth and Adiposity. JAMA Netw. Open 2024, 7, e2450467. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Piccolo, B.D.; Andres, A. Maternal adiposity alters the human milk metabolome: Associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 2020, 112, 1228–1239. [Google Scholar] [CrossRef]
- Ahern, G.J.; Hennessy, A.; Ryan, C.A.; Ross, R.P.; Stanton, C. Advances in infant formula science. Annu. Rev. Food Sci. Technol. 2019, 10, 75–102. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Grigore, D.; Alexander, B.T. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension 2008, 52, 44–50. [Google Scholar] [CrossRef]
- Chong, E.; Yosypiv, I.V. Developmental programming of hypertension and kidney disease. Int. J. Nephrol. 2012, 2012, 760580. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L. Advocacy for DOHaD research optimizing child kidney health. Pediatr. Neonatol. 2024, 66, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; South, A.M.; August, P.; Bertagnolli, M.; Ferranti, E.P.; Grobe, J.L.; Jones, E.J.; Loria, A.S.; Safdar, B.; Sequeira Lopez, M.L.S.; et al. Appraising the Preclinical Evidence of the Role of the Renin-Angiotensin-Aldosterone System in Antenatal Programming of Maternal and Offspring Cardiovascular Health Across the Life Course: Moving the Field Forward: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e75–e89. [Google Scholar]
- Tain, Y.L.; Lin, Y.J.; Hsu, C.N. Animal Models for Studying Developmental Origins of Cardiovascular-Kidney-Metabolic Syndrome. Biomedicines 2025, 13, 452. [Google Scholar] [CrossRef]
- Shoji, H.; Koletzko, B. Oxidative stress and antioxidant protection in the perinatal period. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 324–328. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. The NOS/NO System in Renal Programming and Reprogramming. Antioxidants 2023, 12, 1629. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Zempleni, J. Milk exosomes: Beyond dietary microRNAs. Genes Nutr. 2017, 12, 12. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Bedogni, G.; Brambilla, P.; Cianfarani, S.; Nobili, V.; Pietrobelli, A.; Agostoni, C. Epigenetic mechanisms elicited by nutrition in early life. Nutr. Res. Rev. 2011, 24, 198–205. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, Y.; Cheng, C.; Wang, Y.; Liao, F.; Duan, Q.; Wang, X.; Miao, C. Progress in epigenetic regulation of milk synthesis, with particular emphasis on mRNA regulation and DNA methylation. Cell Cycle 2023, 22, 1675–1693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. mBio 2014, 5, e02113-14. [Google Scholar] [CrossRef]
- Dimova, L.G.; de Boer, J.F.; Plantinga, J.; Plösch, T.; Hoekstra, M.; Verkade, H.J.; Tietge, U.J.F. Inhibiting Cholesterol Absorption During Lactation Programs Future Intestinal Absorption of Cholesterol in Adult Mice. Gastroenterology 2017, 153, 382–385.e3. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Addi, T.; Dou, L.; Burtey, S. Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins 2018, 10, 412. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, L.; Zeng, X.; Fang, Q.; Zheng, B.; Luo, C.; Rao, T.; Ouyang, D. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; De La Cochetiere, M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics-A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Zółkiewicz, J.; Marzec, A.; Ruszczyn’ski, M.; Feleszko, W. Postbiotics-A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Nuzzi, G.; Trambusti, I.; DI Cicco, M.E.; Peroni, D.G. Breast milk: More than just nutrition! Minerva Pediatr. 2021, 73, 111–114. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, S.M.; Kim, Y.G.; Lee, S.H.; Moon, J.Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. Renal Physiol. 2020, 318, F1327–F1340. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Glynn, L.M.; Davis, E.P. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013, 75, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Moritz, K.M.; Cuffe, J.S.; Wilson, L.B.; Dickinson, H.; Wlodek, M.E.; Simmons, D.G.; Denton, K.M. Review: Sex specific programming: A critical role for the renal renin-angiotensin system. Suppl. Placenta 2010, 31, S40–S46. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Castellano, J.M.; Ruiz-Pino, F.; Garcia-Galiano, D.; Manfredi-Lozano, M.; Leon, S.; Romero-Ruiz, A.; Dieguez, C.; Pinilla, L.; Tena-Sempere, M. Metabolic programming of puberty: Sexually dimorphic responses to early nutritional challenges. Endocrinology 2013, 154, 3387–3400. [Google Scholar] [CrossRef]
- Powe, C.E.; Knott, C.D.; Conklin-Brittain, N. Infant sex predicts breast milk energy content. Am. J. Hum. Biol. 2010, 22, 50–54. [Google Scholar] [CrossRef]
- Ganu, R.S.; Harris, R.A.; Collins, K.; Aagaard, K.M. Early origins of adult disease: Approaches for investigating the programmable epigenome in humans, nonhuman primates, and rodents. ILAR J. 2012, 53, 306–321. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- El-Khuffash, A.; Jain, A.; Lewandowski, A.J.; Levy, P.T. Preventing disease in the 21st century: Early breast milk exposure and later cardiovascular health in premature infants. Pediatr. Res. 2020, 87, 385–390. [Google Scholar] [CrossRef]
- Masi, A.C.; Stewart, C.J. Role of breastfeeding in disease prevention. Microb. Biotechnol. 2024, 17, e14520. [Google Scholar] [CrossRef]
- Gregg, B.; Ellsworth, L.; Pavela, G.; Shah, K.; Berger, P.K.; Isganaitis, E.; VanOmen, S.; Demerath, E.W.; Fields, D.A. Bioactive compounds in mothers milk affecting offspring outcomes: A narrative review. Pediatr. Obes. 2022, 17, e12892. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.K. Breastfeeding and chronic disease in childhood and adolescence. Pediatr. Clin. N. Am. 2001, 48, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Farajian, S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: A review of evidence. Adv. Biomed. Res. 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef]
- Mazur, D.; Satora, M.; Rekowska, A.K.; Kabała, Z.; Łomża, A.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B. Influence of Breastfeeding on the State of Meta-Inflammation in Obesity-A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 9003–9018. [Google Scholar] [CrossRef]
- Luo, K.; Chen, P.; Li, S.; Li, W.; He, M.; Wang, T.; Chen, J. Effect of L-arginine supplementation on the hepatic phosphatidylinositol 3-kinase signaling pathway and gluconeogenic enzymes in early intrauterine growth-restricted rats. Exp. Ther. Med. 2017, 14, 2355–2360. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Protective Role of Taurine on Rat Offspring Hypertension in the Setting of Maternal Chronic Kidney Disease. Antioxidants 2023, 12, 2059. [Google Scholar] [CrossRef]
- Thaeomor, A.; Tangnoi, C.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents the Adverse Effects of Maternal Dyslipidemia on Growth and Cardiovascular Control in Adult Rat Offspring. Adv. Exp. Med. Biol. 2019, 1155, 415–427. [Google Scholar]
- Kim, J.; Kim, J.; Kwon, Y.H. Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet. Lipids Health Dis. 2023, 22, 50. [Google Scholar] [CrossRef]
- Gray, C.; Vickers, M.H.; Segovia, S.A.; Zhang, X.D.; Reynolds, C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE 2015, 10, e0115994. [Google Scholar]
- Gregório, B.M.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal fish oil supplementation benefits programmed offspring from rat dams fed low-protein diet. Am. J. Obstet. Gynecol. 2008, 199, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blanco, C.; Amusquivar, E.; Bispo, K.; Herrera, E. Dietary fish oil supplementation during early pregnancy in rats on a cafeteria-diet prevents fatty liver in adult male offspring. Food Chem. Toxicol. 2019, 123, 546–552. [Google Scholar] [CrossRef]
- Cabral, E.V.; Vieira, L.D.; Sant’Helena, B.R.M.; Ribeiro, V.S.; Farias, J.S.; Aires, R.S.; Paz, S.T.; Muzi-Filho, H.; Paixão, A.D.; Vieyra, A. Alpha-Tocopherol during lactation and after weaning alters the programming effect of prenatal high salt intake on cardiac and renal functions of adult male offspring. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1151–1165. [Google Scholar] [CrossRef]
- Sun, W.X.; Shu, Y.P.; Yang, X.Y.; Huang, W.; Chen, J.; Yu, N.N.; Zhao, M. Effects of folic acid supplementation in pregnant mice on glucose metabolism disorders in male offspring induced by lipopolysaccharide exposure during pregnancy. Sci. Rep. 2023, 13, 7984. [Google Scholar] [CrossRef]
- Li, L.; Sun, L.; Liang, X.; Ou, Q.; Tan, X.; Li, F.; Lai, Z.; Ding, C.; Chen, H.; Yu, X.; et al. Maternal betaine supplementation ameliorates fatty liver disease in offspring mice by inhibiting hepatic NLRP3 inflammasome activation. Nutr. Res. Pract. 2023, 17, 1084–1098. [Google Scholar] [CrossRef]
- Gray, C.; Li, M.; Reynolds, C.M.; Vickers, M.H. Pre-weaning growth hormone treatment reverses hypertension and endothelial dysfunction in adult male offspring of mothers undernourished during pregnancy. PLoS ONE 2013, 8, e53505. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.; Pomar, C.A.; Palou, A.; Palou, M.; Picó, C. Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats. Nutrients 2023, 15, 570. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chen, W.L.; Liao, W.T.; Hsu, C.N. Lactoferrin Supplementation during Pregnancy and Lactation Protects Adult Male Rat Offspring from Hypertension Induced by Maternal Adenine Diet. Nutrients 2024, 16, 2607. [Google Scholar] [CrossRef] [PubMed]
- Paul, H.A.; Collins, K.H.; Nicolucci, A.C.; Urbanski, S.J.; Hart, D.A.; Vogel, H.J.; Reimer, R.A. Maternal prebiotic supplementation reduces fatty liver development in offspring through altered microbial and metabolomic profiles in rats. FASEB J. 2019, 33, 5153–5167. [Google Scholar] [CrossRef]
- Jia, L.; Cao, M.; Chen, H.; Zhang, M.; Dong, X.; Ren, Z.; Sun, J.; Pan, L.L. Butyrate Ameliorates Antibiotic-Driven Type 1 Diabetes in the Female Offspring of Nonobese Diabetic Mice. J. Agric. Food Chem. 2020, 68, 3112–3120. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yu, H.R.; Lin, I.C.; Tiao, M.M.; Huang, L.T.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, J.; Xu, H.; Lyv, Y.; Feng, X.; Fang, Y.; Xu, Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014, 53, 1669–1683. [Google Scholar] [CrossRef]
- Baxi, D.B.; Singh, P.K.; Vachhrajani, K.D.; Ramachandran, A.V. Neonatal corticosterone programs for thrifty phenotype adult diabetic manifestations and oxidative stress: Countering effect of melatonin as a deprogrammer. J. Matern. Fetal Neonatal Med. 2012, 25, 1574–1585. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell. Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef]
- Spevacek, A.R.; Smilowitz, J.T.; Chin, E.L.; Underwood, M.A.; German, J.B.; Slupsky, C.M. Infant Maturity at Birth Reveals Minor Differences in the Maternal Milk Metabolome in the First Month of Lactation. J. Nutr. 2015, 145, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lonnerdal, B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Amino Acids during Pregnancy and Offspring Cardiovascular-Kidney-Metabolic Health. Nutrients 2024, 16, 1263. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Physician 2009, 80, 345–350. [Google Scholar]
- Koletzko, B.; Rodriguez-Palmero, M. Polyunsaturated fatty acids in human milk and their role in early infant development. J. Mammary Gland Biol. Neoplasia 1999, 4, 269–284. [Google Scholar] [CrossRef]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
- Vahdaninia, M.; Mackenzie, H.; Dean, T.; Helps, S. The effectiveness of ω-3 polyunsaturated fatty acid interventions during pregnancy on obesity measures in the offspring: An up-to-date systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 2597–2613. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Gunn, T.R.; Rabone, D.L.; Breier, B.H.; Blum, W.F.; Gluckman, P.D. Growth hormone increases breast milk volumes in mothers of preterm infants. Pediatrics 1996, 98, 279–282. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M. Leptin and Metabolic Programming. Nutrients 2021, 14, 114. [Google Scholar] [CrossRef]
- Shini, V.S.; Udayarajan, C.T.; Nisha, P. A comprehensive review on lactoferrin: A natural multifunctional glycoprotein. Food Funct. 2022, 13, 11954–11972. [Google Scholar] [CrossRef]
- Xi, M.; Yan, Y.; Duan, S.; Li, T.; Szeto, I.M.; Zhao, A. Short-chain fatty acids in breast milk and their relationship with the infant gut microbiota. Front. Microbiol. 2024, 15, 1356462. [Google Scholar] [CrossRef]
- Saban Güler, M.; Arslan, S.; Ağagündüz, D.; Cerqua, I.; Pagano, E.; Berni Canani, R.; Capasso, R. Butyrate: A potential mediator of obesity and microbiome via different mechanisms of actions. Food Res. Int. 2025, 199, 115420. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.; Valero-Jara, V.; Thomas-Valdés, S. Phytochemicals in breast milk and their benefits for infants. Crit. Rev. Food Sci. Nutr. 2022, 62, 6821–6836. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Maternal Polyphenols and Offspring Cardiovascular-Kidney-Metabolic Health. Nutrients 2024, 16, 3168. [Google Scholar] [CrossRef]
- Berger, S.; Oesterle, I.; Ayeni, K.I.; Ezekiel, C.N.; Rompel, A.; Warth, B. Polyphenol exposure of mothers and infants assessed by LC-MS/MS based biomonitoring in breast milk. Anal. Bioanal. Chem. 2024, 416, 1759–1774. [Google Scholar] [CrossRef]
- Song, B.J.; Jouni, Z.E.; Ferruzzi, M.G. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition 2013, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; Dieckman, K.; Mota, D.; Zenner, A.J.; Schleusner, M.A.; Cecilio, J.O.; Vieira, F.V.M. Melatonin in Human Milk: A Scoping Review. Biol. Res. Nurs. 2025, 27, 142–167. [Google Scholar] [CrossRef]
- Gomes, P.R.L.; Motta-Teixeira, L.C.; Gallo, C.C.; Carmo Buonfiglio, D.D.; Camargo, L.S.; Quintela, T.; Reiter, R.J.; Amaral, F.G.D.; Cipolla-Neto, J. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen. Comp. Endocrinol. 2021, 300, 113633. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y. Transcriptional regulation of programmed hypertension by melatonin: An epigenetic perspective. Int. J. Mol. Sci. 2014, 15, 18484–18495. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Melatonin Use during Pregnancy and Lactation Complicated by Oxidative Stress: Focus on Offspring’s Cardiovascular-Kidney-Metabolic Health in Animal Models. Antioxidants 2024, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.A.; Torres, A.; Le Drean, G.; Queignec, M.; Castellano, B.; Tesson, L.; Remy, S.; Anegon, I.; Pitard, B.; Kaeffer, B. Oral Delivery of miR-320-3p with Lipidic Aminoglycoside Derivatives at Mid-Lactation Alters miR-320-3p Endogenous Levels in the Gut and Brain of Adult Rats According to Early or Regular Weaning. Int. J. Mol. Sci. 2022, 24, 191. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
| Model | Exposure Timing Gestation/Lactation | Age at Evaluation (Weeks) | Components of CKMS | References |
|---|---|---|---|---|
| Low caloric diet | Yes/Yes | 12–16 | Insulin resistance, hypertension, and kidney disease | [20,21,22] |
| Low protein diet | Yes/Yes | 16–24 | Insulin resistance, hypertension, and kidney disease | [23,24] |
| High-fat diet | Yes/Yes | 16 | Obesity, insulin resistance, dyslipidemia, hypertension, and kidney disease | [25,26,27,28] |
| High-fructose diet | Yes/Yes | 12–52 | Hypertension, obesity, insulin resistance, and dyslipidemia | [29,30,31,32] |
| Litter size reduction | No/Yes | 24–52 | Obesity, insulin resistance, hypertension, and kidney disease | [33,34,35] |
| Maternal diabetes | Yes/Yes | 12–16 | Hypertension, obesity, insulin resistance, dyslipidemia, and kidney disease | [36,37,38] |
| Uteroplacental insufficiency | Yes/No | 22–30 | Dyslipidemia, insulin resistance, hypertension, and kidney disease | [39,40,41,42,43,44] |
| Maternal chronic kidney disease | Yes/Yes | 12 | Kidney disease and hypertension | [44,45] |
| Maternal stress | Yes/Yes | 16–24 | Obesity, insulin resistance, hypertension, and kidney disease | [46,47,48,49] |
| Maternal hypoprolactinemia | No/Yes | 13–24 | Obesity, insulin resistance, and kidney disease | [50,51] |
| Nicotine exposure | Yes/Yes | 20–32 | Hypertension, kidney disease, steatosis, and hyperlipidemia, | [52,53,54,55] |
| Ethanol exposure | Yes/Yes | 12–24 | Insulin resistance and cardiovascular disease | [56,57] |
| BPA exposure | Yes/Yes | 16–24 | Hypertension, insulin resistance, steatosis, and cardiovascular disease | [58,59,60,61] |
| TCDD exposure | Yes/Yes | 12 | Hypertension, kidney disease, and cardiovascular disease | [62,63,64] |
| DEHP exposure | Yes/Yes | 12–21 | Hypertension and insulin resistance | [65,66,67] |
| Reprogramming Intervention | Period Gestation/Lactation | Experimental Model | Species | Age at Evaluation (Weeks) | Protective CKMS Phenotype | Ref |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| L-arginine (200 mg/kg/day) | No/Yes | Low protein diet | SD rat/M | 8 | Hepatic insulin signaling | [179] |
| L-citrulline (2.5 g/L in drinking water) | Yes/Yes | Maternal diabetes | SD rat/M | 12 | Hypertension and kidney disease | [36] |
| L-taurine (3% in drinking water) | Yes/Yes | Maternal CKD | SD rat/M | 12 | Hypertension and kidney disease | [180] |
| L-taurine (3% in drinking water) | Yes/Yes | Maternal dyslipidemia | Wistar rat/M and F | 16 | Obesity, dyslipidemia, and hypertension | [181] |
| L-leucine (1.5% in chow) | Yes/Yes | High-fat diet | C57BL/6 mice/M | 16 | Obesity and glucose intolerance | [182] |
| Fats | ||||||
| Conjugated linoleic acid (1% in chow) | Yes/Yes | Hight-fat diet | SD rat/M | 18 | Hypertension | [183] |
| PUFA (1.5 g/kg/day) | Yes/Yes | Low protein diet | Wistar rat/M and F | 24 | Hypertension and cardiovascular disease | [184] |
| PUFA (8.78% in chow) | Yes/Yes | Cafeteria diet | SD rat/M | 56 | Fatty liver | [185] |
| Micronutrients | ||||||
| Vitamin E (0.35 g/kg/day) | No/Yes | High-salt diet | Wistar rat/M | 30 | Kidney disease and cardiovascular disease | [186] |
| Folic acid (5 mg/kg/day) | Yes/Yes | Maternal LPS exposure | CD-1 mice/M | 16 | Glucose intolerance | [187] |
| Betaine (1% in drinking water) | Yes/Yes | High-fat diet | C57BL/6 mice/M and F | 8 | Fatty liver | [188] |
| Bioactive components | ||||||
| Growth hormone (2.5 ug/g/day) | No/Yes | Low caloric diet | SD rat/M | 23 | Hypertension and cardiovascular disease | [189] |
| Leptin 1 | No/Yes | Western diet | Wistar rat/M | 16 | Obesity and insulin resistance | [190] |
| Lactoferrin (10% in chow) | Yes/Yes | Maternal CKD | SD rat/M | 12 | Hypertension | [191] |
| Oligofructose (10% in drinking water) | Yes/Yes | High-fat/sucrose diet | SD rat/M | 24 | Diabetes and fatty liver | [192] |
| Butyrate (400 mg/kg/day) | Yes/Yes | Maternal diabetes | NOD mice/M | 16 | Diabetes | [193] |
| Butyrate (400 mg/kg/day) | Yes/Yes | Tryptophan-free diet | SD rat/M | 16 | Hypertension | [194] |
| Quercetin (50 mg/kg/day) | Yes/Yes | High-fat diet | SD rat/M and F | 14 | Obesity, hyperglycemia, hyperlipemia, and hyperinsulinemia | [195] |
| Melatonin (1 mg/kg/day at night) | No/Yes | Neonatal corticosterone exposure | SD rat/M and F | 16 | Diabetes, liver steatosis, and hyperlipidemia | [196] |
| Melatonin (0.01% in drinking water) | Yes/Yes | Low caloric diet | SD rat/M | 12 | Hypertension and kidney disease | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Lin, Y.-J.; Hsu, C.-N. Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health—A Narrative Review. Nutrients 2025, 17, 995. https://doi.org/10.3390/nu17060995
Tain Y-L, Lin Y-J, Hsu C-N. Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health—A Narrative Review. Nutrients. 2025; 17(6):995. https://doi.org/10.3390/nu17060995
Chicago/Turabian StyleTain, You-Lin, Ying-Jui Lin, and Chien-Ning Hsu. 2025. "Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health—A Narrative Review" Nutrients 17, no. 6: 995. https://doi.org/10.3390/nu17060995
APA StyleTain, Y.-L., Lin, Y.-J., & Hsu, C.-N. (2025). Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health—A Narrative Review. Nutrients, 17(6), 995. https://doi.org/10.3390/nu17060995









