Genome-Wide Impact of Folic Acid on DNA Methylation and Gene Expression in Lupus Adipocytes: An In Vitro Study on Obesity
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Patients with SLE Donors of Subcutaneous Adipose Tissue
2.3. Experiment Design: Cell Culture
2.4. DNA Methylation Analysis
2.5. Gene Expression Analysis by RT-qPCR
3. Results
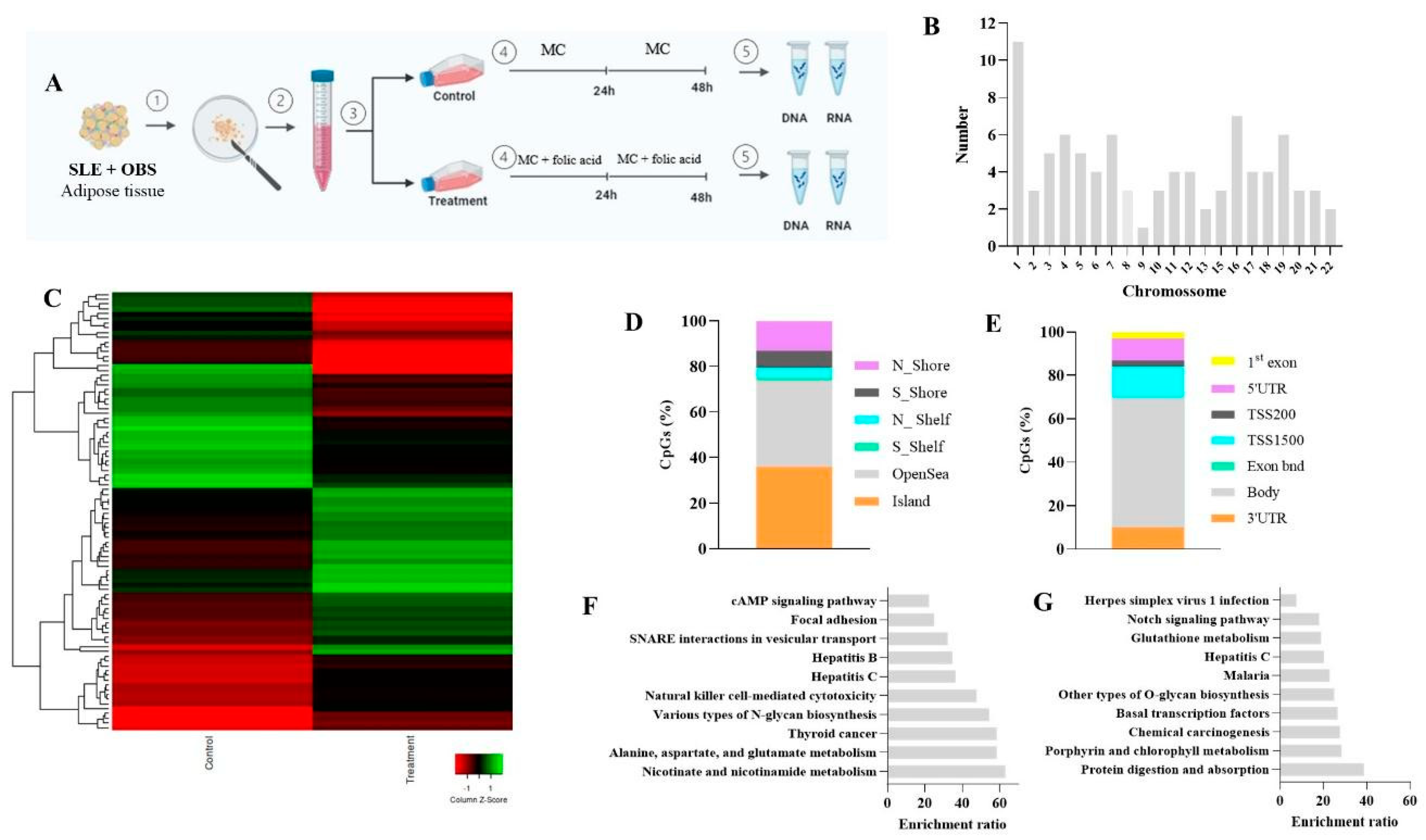
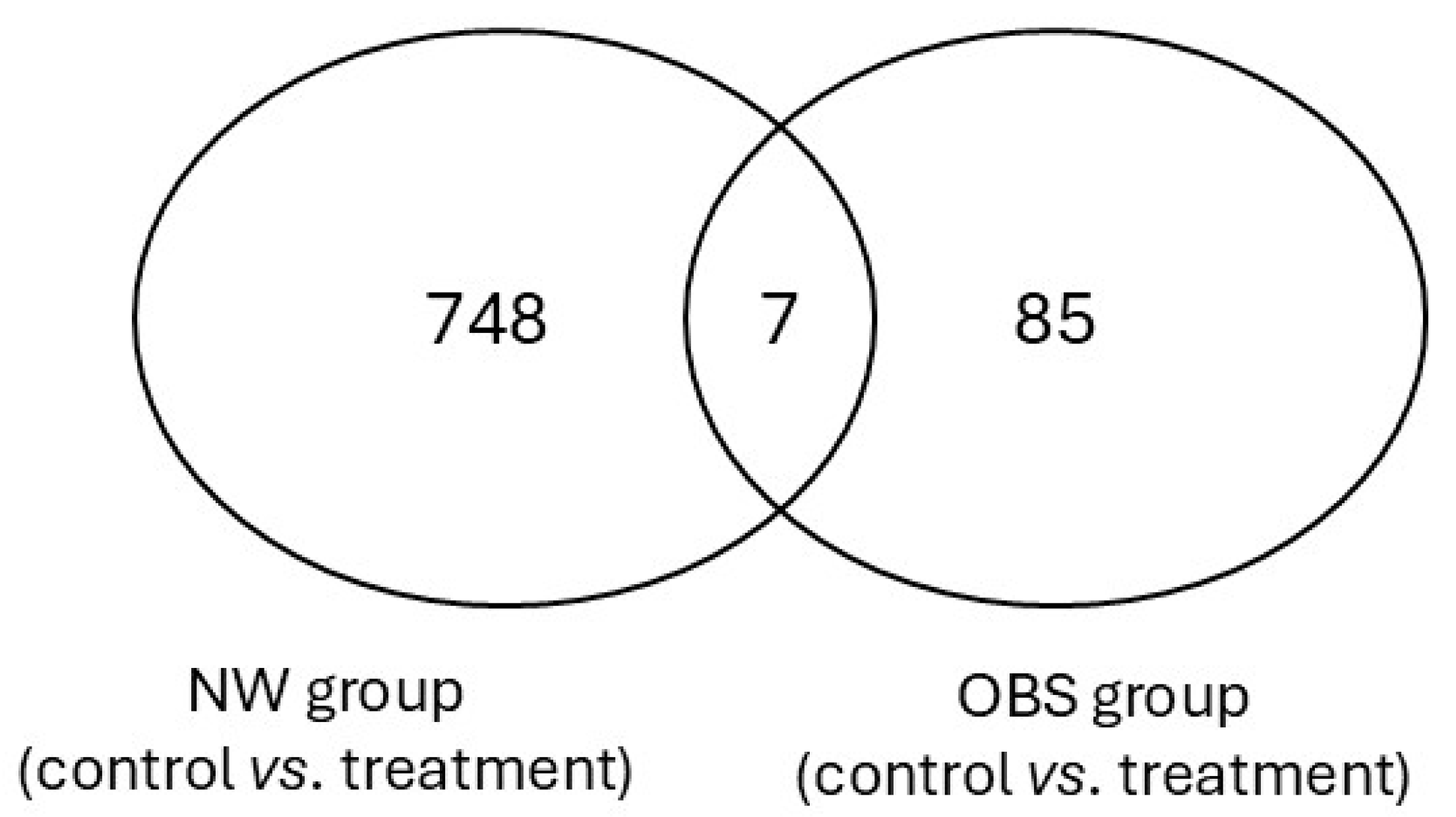
3.1. Folic Acid Effects on DNA Methylation Profile
3.2. Folic Acid Effects on DNMT1, IL-6, CREM2, LEP, and ADIPOQ Expression
4. Discussion
4.1. Limitations
4.2. Perspectives for Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef] [PubMed]
- Lvovich, S.; Goldsmith, D.P. Neurological Complications of Rheumatic Disease. Semin. Pediatr. Neurol. 2017, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Arcelus, M.; Rich, S.S.; Raychaudhuri, S. Autoimmune diseases-connecting risk alleles with molecular traits of the immune system. Nat. Rev. Genet. 2016, 17, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef]
- Almutairi, K.B.; Nossent, J.C.; Preen, D.B.; Keen, H.I.; Inderjeeth, C.A. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-based Studies. J. Rheumatol. 2021, 48, 669–676. [Google Scholar] [CrossRef]
- Iaremenko, O.; Koliadenko, D.; Stepanova, N.; Matiyashchuk, I. Prediction of atherosclerosis development and modification of its risk factors in patients with systemic lupus erythematosus. J. Hypertens. 2023, 41, e139–e140. [Google Scholar] [CrossRef]
- Iaremenko, O.; Koliadenko, D.; Iaremenko, K.; Matiyashchuk, I. Lupus nephritis: Clinical characteristics, serological associations, pattern of pro- and anti-inflammatory markers. Ukr. J. Nephrol. Dial. 2023, 2, 69–80. [Google Scholar] [CrossRef]
- Hernández-Negrín, H.; Ricci, M.; Mancebo-Sevilla, J.J.; Sanz-Cánovas, J.; López-Sampalo, A.; Cobos-Palacios, L.; Romero-Gómez, C.; de Pedro, I.P.; Ayala-Gutiérrez, M.d.M.; Gómez-Huelgas, R.; et al. Obesity, Diabetes, and Cardiovascular Risk Burden in Systemic Lupus Erythematosus: Current Approaches and Knowledge Gaps—A Rapid Scoping Review. Int. J. Env. Res. Public Health 2022, 19, 14768. [Google Scholar] [CrossRef]
- Mok, C.C. Metabolic syndrome and systemic lupus erythematosus: The connection. Expert. Rev. Clin. Immunol. 2019, 15, 765–775. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Barbhaiya, M.; Castro-Webb, N.; Conte, C.; Tedeschi, S.; Leatherwood, C.; Costenbader, K.H.; Rosenberg, L. A prospective study of obesity and risk of systemic lupus erythematosus (SLE) among Black women. Semin. Arthritis Rheum. 2019, 48, 1030–1034. [Google Scholar] [CrossRef]
- Islam, A.N.M.S.; Sultana, H.; Nazmul Hassan Refat Md Farhana, Z.; Abdulbasah Kamil, A.; Meshbahur Rahman, M. The global burden of overweight-obesity and its association with economic status, benefiting from STEPs survey of WHO member states: A meta-analysis. Prev. Med. Rep. 2024, 46, 102882. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.; Carvalho, B.G.; Souza, L.L.; da Mota, J.C.; Ribeiro, A.A.; Nicoletti, C.F. Obesity as an aggravating factor of systemic lupus erythematosus disease: What we already know and what we must explore?—A Rapid Scoping Review. Nutrition 2024, 128, 112559. [Google Scholar] [CrossRef]
- Huang, A.; Zhou, L.; Xu, W. The causal associations of inflammatory cytokines with obesity and systemic lupus erythematosus: A Mendelian randomization study. Int. J. Rheum. Dis. 2024, 27, e15214. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Castejón, M.L.; Muñoz-García, R.; Alarcón-De-la-Lastra, C. Epigenetic linkage of systemic lupus erythematosus and nutrition. Nutr. Res. Rev. 2021, 36, 39–59. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Luo, Q.; Sui, H.; Du, X.-N.; Zhao, Y.-J.; Liu, L.; Guan, Q.; Zhou, Y.; Wen, Q.-S.; Shi, Y.; et al. Recent advances in the involvement of epigenetics in the pathogenesis of systemic lupus erythematosus. Clin. Immunol. 2024, 258, 109857. [Google Scholar] [CrossRef]
- Jiang, Y.; Bressler, J.; Beaudet, A.L. Epigenetics and human disease. Annu. Rev. Genom. Hum. Genet. 2004, 5, 479–510. [Google Scholar] [CrossRef] [PubMed]
- Gibson, F.; Hanly, A.; Grbic, N.; Grunberg, N.; Wu, M.; Collard, M.; Alani, R.M. Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Clin. Rev. Allergy Immunol. 2022, 63, 447–471. [Google Scholar] [CrossRef]
- Finnell, R.H.; Spiegelstein, O.; Wlodarczyk, B.; Triplett, A.; Pogribny, I.P.; Melnyk, S.; James, J.S. Trans-HHS Workshop: Diet, DNA Methylation Processes and Health DNA Methylation in Folbp1 Knockout Mice Supplemented with Folic Acid during Gestation. J. Nutr. 2002, 132, 2457S–2461S. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Epigenetic Dysregulation in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 1019. [Google Scholar] [CrossRef]
- Barreto, G.; Schäfer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Döderlein, G.; Maltry, N.; Wu, W.; Lyko, F.; et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, C.M.; Tsokos, G.C. Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol. Med. 2011, 17, 714–724. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, E.; Gómez-Viais, Y.I.; Cruz-Aranda, M.M.; Martínez-Razo, L.D.; Reyes-Mayoral, C.; Ibarra-González, L.; Montoya-Estrada, A.; Osorio-Caballero, M.; Perichart-Perera, O.; Camacho-Arroyo, I.; et al. The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2023, 24, 6857. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.; Zhang, N.; van der Touw, W.; Ding, Y.; Ju, W.; Bottinger, E.P.; Reid, S.P.; Levy, D.E.; Bromberg, J.S. Epigenetic Regulation of Foxp3 Expression in Regulatory T Cells by DNA Methylation. J. Immunol. 2009, 182, 259–273. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, J.; Gao, F.; Wu, X.; Liang, Y.; Yin, H.; Lu, Q. Hypomethylation of IL10 and IL13 Promoters in CD4+ T Cells of Patients with Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 931018. [Google Scholar] [CrossRef]
- Hedrich, C.M.; Crispin, J.C.; Rauen, T.; Ioannidis, C.; Apostolidis, S.A.; Lo, M.S.; Kyttaris, V.C.; Tsokos, G.C. cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc. Natl. Acad. Sci. USA 2012, 109, 16606–16611. [Google Scholar] [CrossRef]
- Rauen, T.; Hedrich, C.M.; Tenbrock, K.; Tsokos, G.C. CAMP responsive element modulator: A critical regulator of cytokine production. Trends Mol. Med. 2013, 19, 262–269. [Google Scholar] [CrossRef]
- Ly, A.; Hoyt, L.; Crowell, J.; Kim, Y.I. Folate and DNA methylation. Antioxid. Redox Signal 2012, 17, 302–326. [Google Scholar] [CrossRef]
- da Mota, J.C.N.L.; Ribeiro, A.A.; Carvalho, L.M.; Esteves, G.P.; Sieczkowska, S.M.; Goessler, K.F.; Gualano, B.; Nicoletti, C.F. Impact of Methyl-Donor Micronutrient Supplementation on DNA Methylation Patterns: A Systematic Review and Meta-Analysis of in vitro, Animal, and Human Studies. Lifestyle Genom. 2023, 16, 192–213. [Google Scholar] [CrossRef]
- Nikolova-Ganeva, K.; Tchorbanov, A. Folic acid in systemic lupus erythematosus—A new aspect. Clin. Rheumatol. 2023, 42, 1729–1730. [Google Scholar] [CrossRef]
- Nikolova-Ganeva, K.; Vasilev, V.; Kerezieva, S.; Tchorbanov, A. Impact of folic acid on regulatory B lymphocytes from patients with systemic lupus erythematosus in vitro. Int. J. Rheum. Dis. 2023, 26, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, C.; Elson-Schwab, I.; Mashiyama, S.T.; Kerry, N.; Ames, B.N. Folate Deficiency Inhibits the Proliferation of Primary Human CD8+ T Lymphocytes In Vitro. J. Immunol. 2004, 173, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Haase, N.; Low, P.S. Depletion of Folate-Receptor-Positive Macrophages Leads to Alleviation of Symptoms and Prolonged Survival in Two Murine Models of Systemic Lupus Erythematosus. Mol. Pharm. 2007, 4, 679–685. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Peres, C.M.C.R. Como Cultivar Células, 1st ed.; Guanabara-Koogan: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Gorelik, G.; Richardson, B. Key role of ERK pathway signaling in lupus. Autoimmunity 2010, 43, 17–22. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Orfanidou, T.; Liaskos, C.; Smyk, D.S.; Billinis, C.; Blank, M.; Rigopoulou, E.I.; Bogdanos, D.P. p38 mitogen-activated protein kinase (p38 MAPK)-mediated autoimmunity: Lessons to learn from ANCA vasculitis and pemphigus vulgaris. Autoimmun. Rev. 2013, 12, 580–590. [Google Scholar] [CrossRef]
- Yougbare, I. Alterations of cAMP/cGMP Signaling Pathways in Lupus Nephritis. J. Nephrol. Sci. 2021, 3, 8–12. [Google Scholar] [CrossRef]
- Solomou, E.E.; Juang, Y.-T.; Gourley, M.F.; Kammer, G.M.; Tsokos, G.C. Molecular Basis of Deficient IL-2 Production in T Cells from Patients with Systemic Lupus Erythematosus. J. Immunol. 2001, 166, 4216–4222. [Google Scholar] [CrossRef]
- Tenbrock, K.; Juang, Y.-T.; Gourley, M.F.; Nambiar, M.P.; Tsokos, G.C. Antisense Cyclic Adenosine 5′-Monophosphate Response Element Modulator Up-Regulates IL-2 in T Cells from Patients with Systemic Lupus Erythematosus. J. Immunol. 2002, 169, 4147–4152. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, R.; Ma, F.; Ouyang, S.; Liu, Z.; Wu, J. Folic acid supplementation alters the DNA methylation profile and improves insulin resistance in high-fat-diet-fed mice. J. Nutr. Biochem. 2018, 59, 76–83. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Sun, Y.; Zhang, Y.; Xu, J.; Yang, Y.; Chen, J. Folic Acid Attenuates High-Fat Diet-Induced Osteoporosis Through the AMPK Signaling Pathway. Front. Cell Dev. Biol. 2022, 9, 791880. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Kaur, G.; Haider, Z.; Rodriquez, E.; Beatson, C.; Snell-Bergeon, J. Efficacy of Semaglutide in Overweight and Obese Patients with Type 1 Diabetes. Diabetes Technol. Ther. 2024, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Sguanci, M.; Mancin, S.; Piredda, M.; Cordella, F.; Tagliamonte, N.L.; Zollo, L.; De Marinis, M.G. Nursing-engineering interdisciplinary research: A synthesis of methodological approach to perform healthcare-technology integrated projects. MethodsX 2024, 12, 102525. [Google Scholar] [CrossRef]
- Sosa-Holwerda, A.; Park, O.-H.; Albracht-Schulte, K.; Niraula, S.; Thompson, L.; Oldewage-Theron, W. The Role of Artificial Intelligence in Nutrition Research: A Scoping Review. Nutrients 2024, 16, 2066. [Google Scholar] [CrossRef]


| NW Patient | OBS Patient | |
|---|---|---|
| Age (years) | 37 | 34 |
| SLEDAI-2K | 0 | 0 |
| Hydroxychloroquine use (mg/day) | 400 | 400 |
| Associated comorbidities | - | - |
| BMI (kg/m2) | 21.0 | 31.1 |
| Fat mass (%) | 28.7 | 38.4 |
| CpG | Gene (Localization) | Function |
|---|---|---|
| cg00912518 | MBP (body) | Encodes myelin proteins essential for the myelin sheath in the nervous system, with variants also acting in bone marrow and the immune system. |
| cg07342845 | TAF4 (body) | Encodes a transcription factor subunit for transcription initiation and modulation of the response to regulatory signals, with implications in neurodegenerative diseases. |
| cg20478468 | EIF4E (TSS1500) | Part of the translation initiation factor complex 4F to start translation. Acts as a proto-oncogene, with its expression and activation associated with tumorigenesis. |
| cg03689146 | Unknown (island) | - |
| cg18605377 | Unknown (island) | - |
| cg26669806 | COMP (body) | Non-collagenous extracellular matrix protein. Structural functions. |
| cg15319704 | KLF5 (TSS1500; 5’UTR) | Involved in the promotion and suppression of cell proliferation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, L.L.; da Mota, J.C.N.L.; Carvalho, L.M.; Ribeiro, A.A.; Caponi, C.A.; Pinhel, M.A.S.; Costa-Fraga, N.; Diaz-Lagares, A.; Izquierdo, A.G.; Nonino, C.B.; et al. Genome-Wide Impact of Folic Acid on DNA Methylation and Gene Expression in Lupus Adipocytes: An In Vitro Study on Obesity. Nutrients 2025, 17, 1086. https://doi.org/10.3390/nu17061086
Souza LL, da Mota JCNL, Carvalho LM, Ribeiro AA, Caponi CA, Pinhel MAS, Costa-Fraga N, Diaz-Lagares A, Izquierdo AG, Nonino CB, et al. Genome-Wide Impact of Folic Acid on DNA Methylation and Gene Expression in Lupus Adipocytes: An In Vitro Study on Obesity. Nutrients. 2025; 17(6):1086. https://doi.org/10.3390/nu17061086
Chicago/Turabian StyleSouza, Leticia L., Jhulia C. N. L. da Mota, Lucas M. Carvalho, Amanda A. Ribeiro, Cesar A. Caponi, Marcela A. S. Pinhel, Nicolas Costa-Fraga, Angel Diaz-Lagares, Andrea G. Izquierdo, Carla B. Nonino, and et al. 2025. "Genome-Wide Impact of Folic Acid on DNA Methylation and Gene Expression in Lupus Adipocytes: An In Vitro Study on Obesity" Nutrients 17, no. 6: 1086. https://doi.org/10.3390/nu17061086
APA StyleSouza, L. L., da Mota, J. C. N. L., Carvalho, L. M., Ribeiro, A. A., Caponi, C. A., Pinhel, M. A. S., Costa-Fraga, N., Diaz-Lagares, A., Izquierdo, A. G., Nonino, C. B., Crujeiras, A. B., & Nicoletti, C. F. (2025). Genome-Wide Impact of Folic Acid on DNA Methylation and Gene Expression in Lupus Adipocytes: An In Vitro Study on Obesity. Nutrients, 17(6), 1086. https://doi.org/10.3390/nu17061086







