Socio-Demographic, Nutritional, and Lifestyle Factors Influencing Perceived Sleep Quality in Spain, with a Particular Focus on Women and Young People
Abstract
1. Introduction
1.1. Study Hypotheses
1.2. Study Objective
2. Materials and Methods
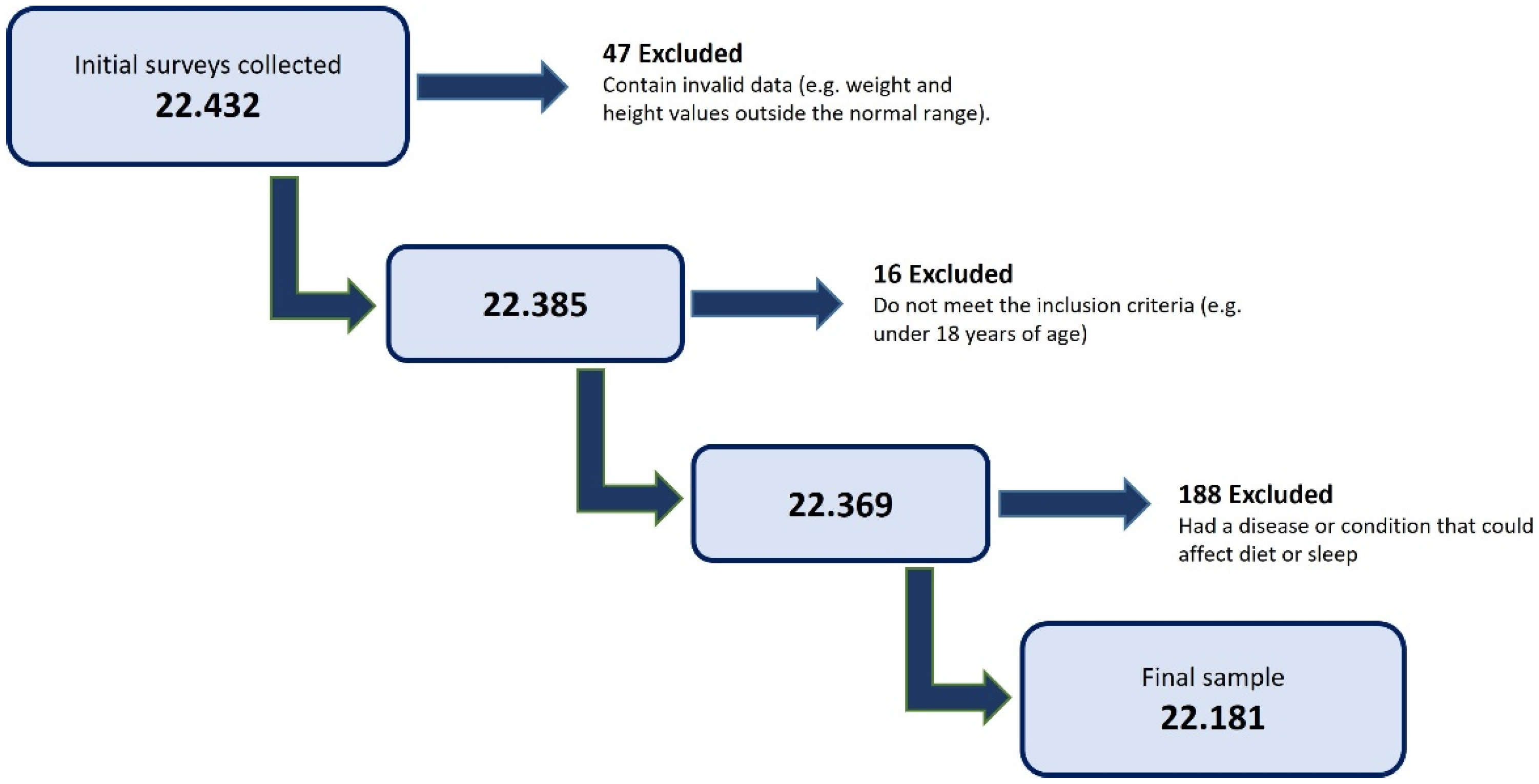
2.1. Study Design and Participant Selection
2.2. Sample Size Calculation
2.3. Ethical Considerations
2.4. Measurement Tool
- F1: Mediterranean Diet
- F2: Healthy and Unhealthy Foods
- F3: Meat and Dairy Products
- F4: Eating Disorders
- F5: Sleep Habits
- F6: Alcohol Consumption
- NUTRI (encompassing F2 and F3)
- HH (Healthy Habits, encompassing F4 and F5)
2.5. Data Collection
2.6. Variables
2.6.1. IASE—Spanish Healthy Eating Index
- Healthy: 58.4–73
- Needs Changes: 36.5–58.4
- Unhealthy: <36.5
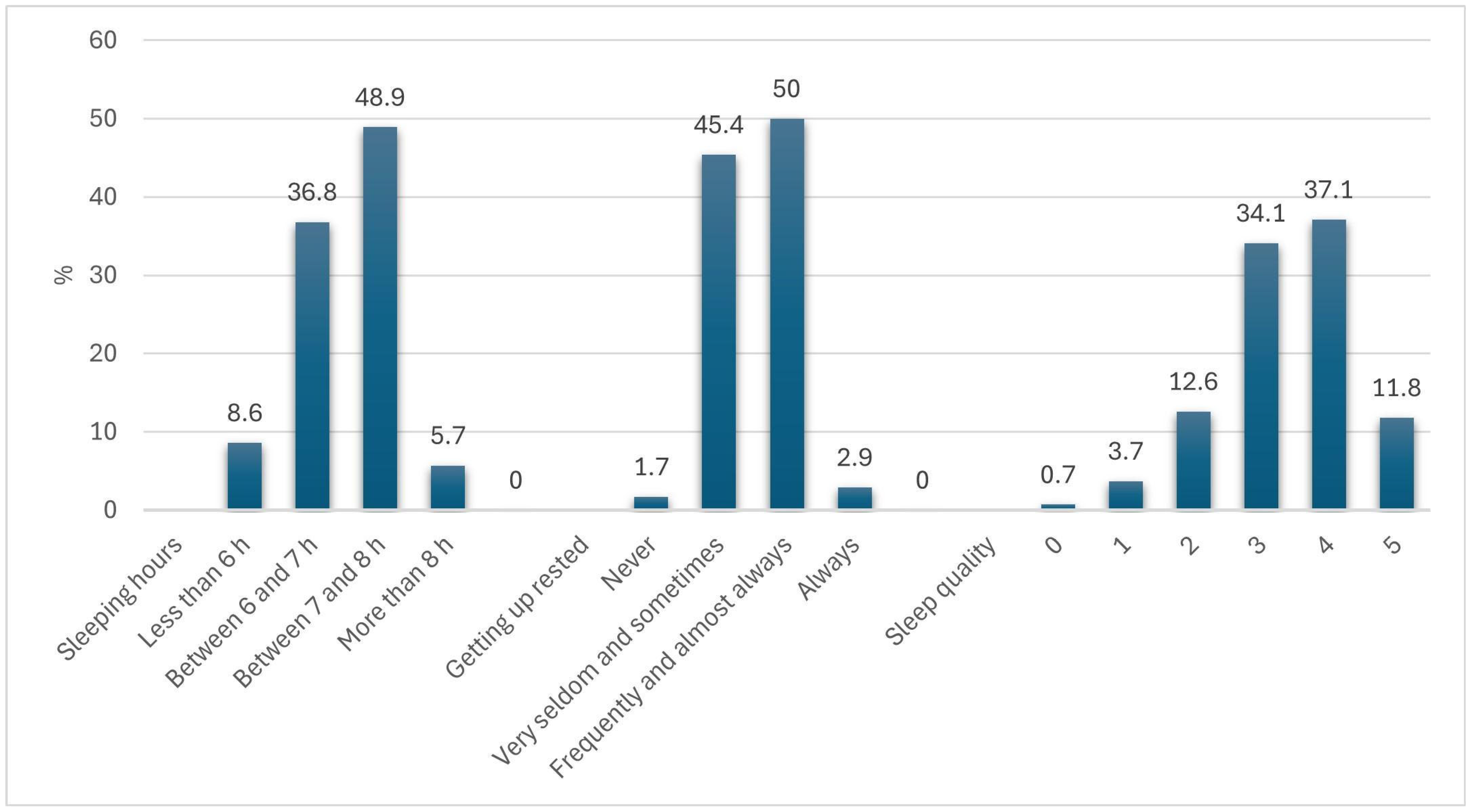
2.6.2. Sleep Variables
- -
- Sleeping hours: <6 h = 1; 6–7 h = 2; 7–8 h = 3; >8 h = 4
- -
- Getting up rested: Never = 1; Very seldom/sometimes = 2; Frequently/almost always = 3; Always = 4
- -
- Sleep quality: Rated on a Likert scale from 0 (worst) to 5 (best).
- Sleeping hours:
- ∘
- Insufficient Sleep Hours (ISH): <6 h or 6–7 h
- ∘
- Sufficient Sleep Hours (SSH): 7–8 h or >8 h
- Getting up rested:
- ∘
- NO Get Up Rested (NO_GUR): Never, very seldom, or sometimes
- ∘
- YES Get Up Rested (YES_GUR): Frequently, almost always, or always
- Sleep quality:
- ∘
- Low Sleep Quality (LSQ): Scores of 0 or 1
- ∘
- Medium Sleep Quality (MSQ): Scores of 2 or 3
- ∘
- High Sleep Quality (HSQ): Scores of 4 or 5
2.7. Data Analysis
3. Results
3.1. Sample Description
3.2. Socio-Demographic Differences in Sleep Variables
3.3. Relationship Between Nutrition, Lifestyle, and Sleep Variables
3.4. Analysis of the Relationship Between Nutrition, Lifestyle Variables, and Sleep Quality
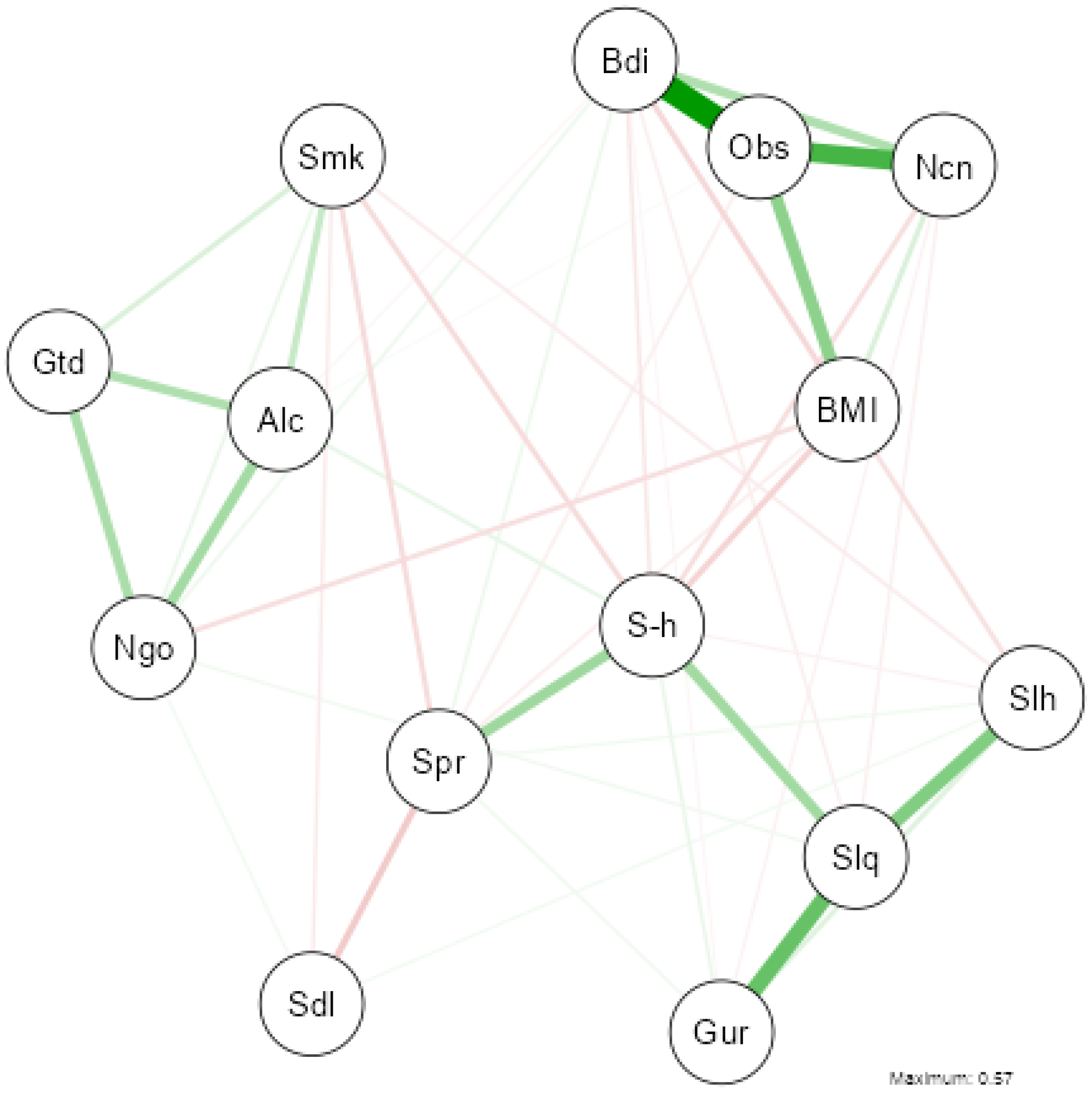
3.5. Network Analysis of Health, Lifestyle, and Sleep Variables
3.6. Network Analysis of Nutritional and Sleep Variables
4. Discussion
4.1. Strengths and Limitations of the Study
4.2. Public Health Implications of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISH | Insufficient Sleep Hours |
| SSH | Sufficient Sleep Hours |
| NO_GUR | NO Get Up Rested |
| YES_GUR | YES Get Up Rested |
| LSQ | Low Sleep Quality |
| MSQ | Medium Sleep Quality |
| HSQ | High Sleep Quality |
| Bdi | Body image |
| Obs | Obesophobia |
| Ncn | No control |
| Slh | Sleeping hours |
| Slq | Sleep quality |
| Gur | Get up rested |
| Alc | Alcohol |
| Smk | Smoking |
| Ngo | Night outings |
| Spr | Sport |
| Fsf | Fast food |
| Frf | Fried food |
| U-f | Ultra-processed food |
| Fsh | Fish consumption |
| Sfd | Soft drinks consumption |
| IAS | IASE |
| Wtr | Water intake |
| Cff | Coffee consumption |
Appendix A
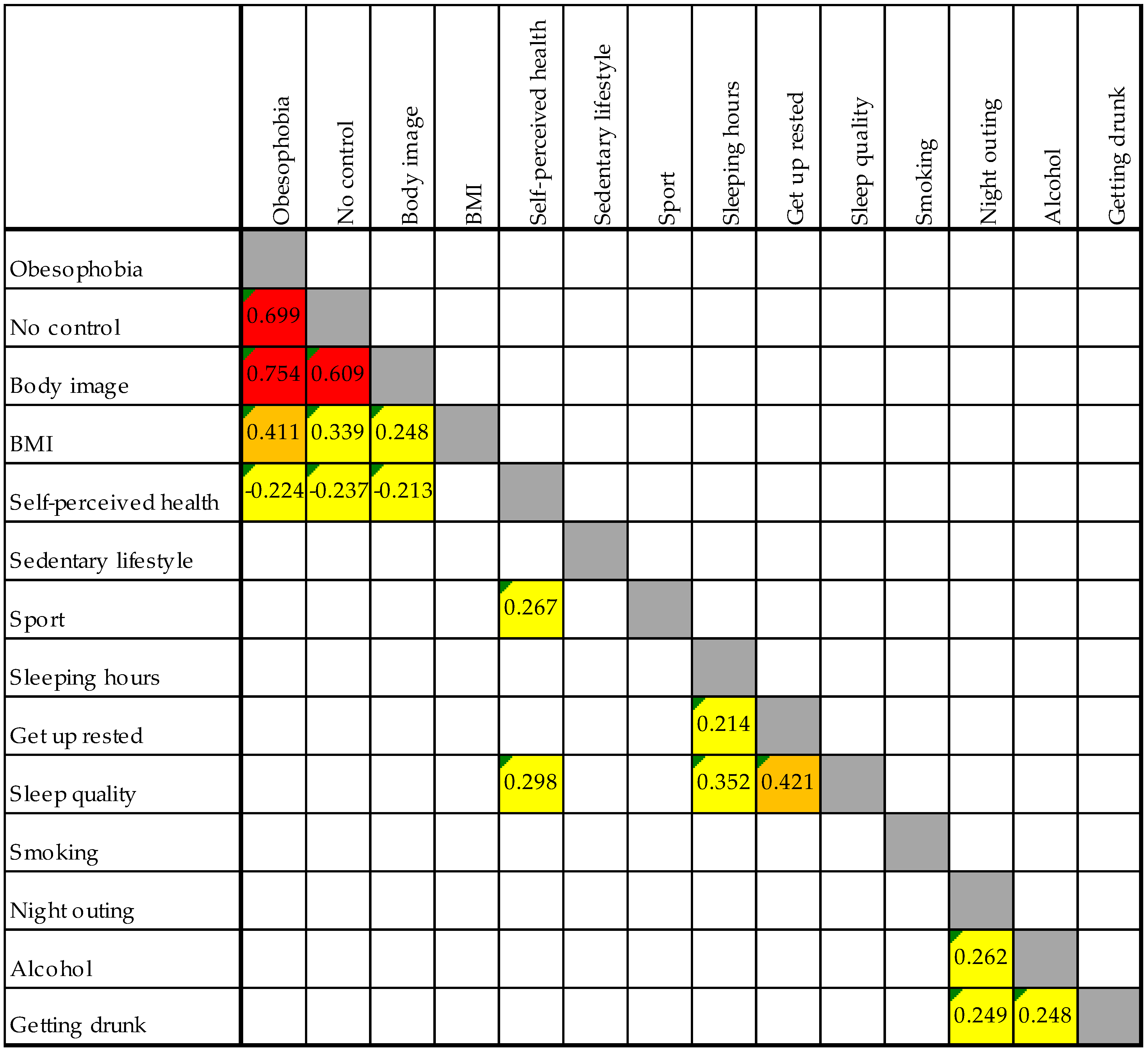

Appendix B
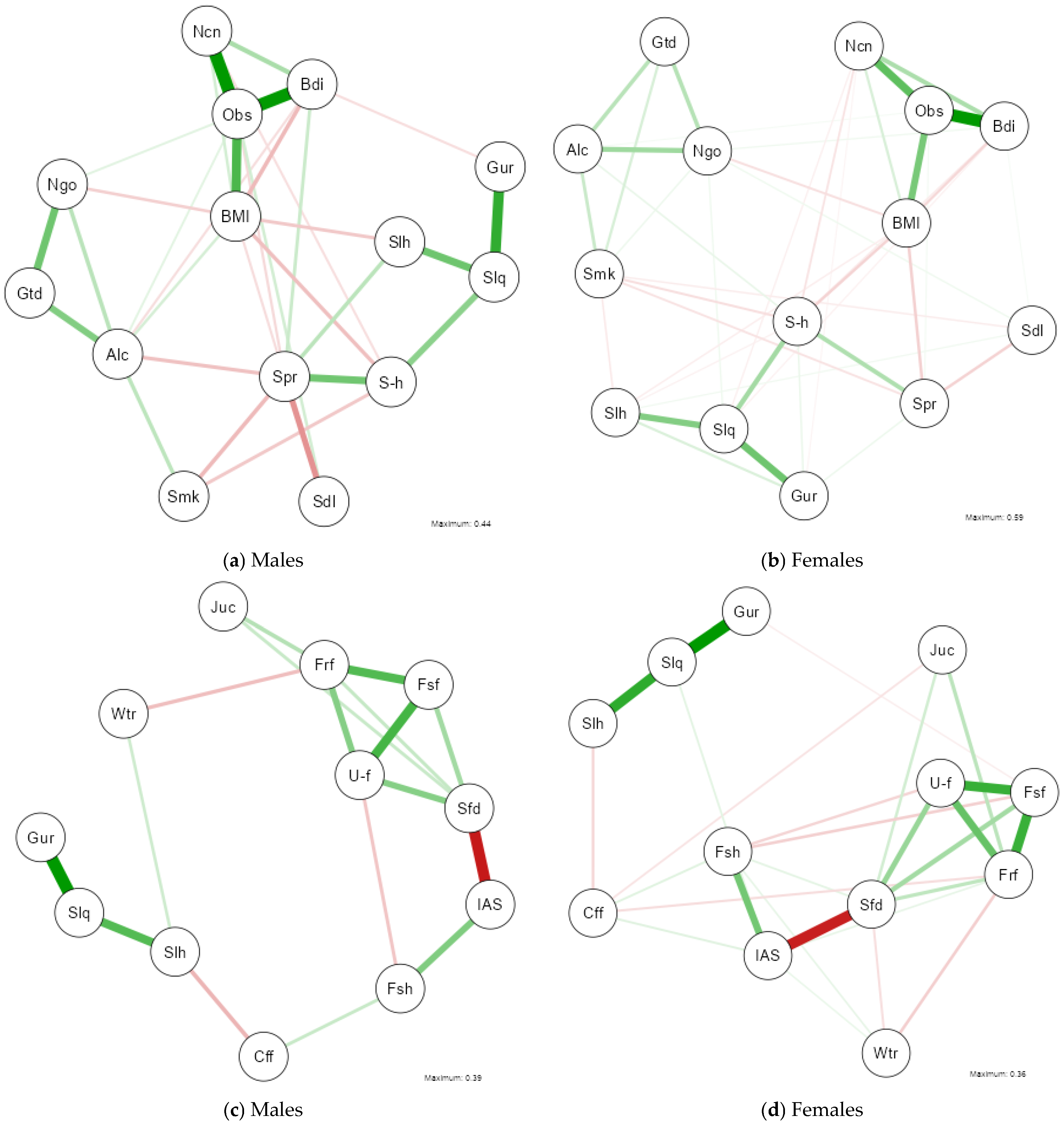
References
- Placidi, F.; Romigi, A. Neurofisiologia del sonno e tecniche di analisi. Nóos 2004, 10, 7–14. [Google Scholar]
- Chaput, J.-P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping Hours: What Is the Ideal Number and How Does Age Impact This? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Sleep Function: An Evolutionary Perspective. Lancet Neurol. 2022, 21, 937–946. [Google Scholar] [CrossRef]
- Lanza, G.; DelRosso, L.M.; Ferri, R. Sleep and Homeostatic Control of Plasticity. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 184, pp. 53–72. ISBN 978-0-12-819410-2. [Google Scholar]
- Radwan, B.; Yanez Touzet, A.; Hammami, S.; Chaudhury, D. Prolonged Exposure to Social Stress Impairs Homeostatic Sleep Regulation. Front. Neurosci. 2021, 15, 633955. [Google Scholar] [CrossRef]
- Dudai, Y. The Neurobiology of Consolidations, Or, How Stable Is the Engram? Annu. Rev. Psychol. 2004, 55, 51–86. [Google Scholar] [CrossRef]
- Van Cauter, E.; Plat, L. Physiology of Growth Hormone Secretion during Sleep. J. Pediatr. 1996, 128, S32–S37. [Google Scholar] [CrossRef]
- Lim, A.S.P.; Kowgier, M.; Yu, L.; Buchman, A.S.; Bennett, D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013, 36, 1027–1032. [Google Scholar] [CrossRef]
- Eldevik, M.F.; Flo, E.; Moen, B.E.; Pallesen, S.; Bjorvatn, B. Insomnia, Excessive Sleepiness, Excessive Fatigue, Anxiety, Depression and Shift Work Disorder in Nurses Having Less than 11 Hours in-Between Shifts. PLoS ONE 2013, 8, e70882. [Google Scholar] [CrossRef]
- Jemere, T.; Mossie, A.; Berhanu, H.; Yeshaw, Y. Poor Sleep Quality and Its Predictors among Type 2 Diabetes Mellitus Patients Attending Jimma University Medical Center, Jimma, Ethiopia. BMC Res. Notes 2019, 12, 488. [Google Scholar] [CrossRef]
- Harris, L.M.; Huang, X.; Linthicum, K.P.; Bryen, C.P.; Ribeiro, J.D. Sleep Disturbances as Risk Factors for Suicidal Thoughts and Behaviours: A Meta-Analysis of Longitudinal Studies. Sci. Rep. 2020, 10, 13888. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008552. [Google Scholar] [CrossRef] [PubMed]
- Dilixiati, D.; Kadier, K.; Laihaiti, D.; Lu, J.-D.; Rezhake, R.; Azhati, B.; Rexiati, M. The Relationship between Sleep Disorders, Quality, and Duration and Sexual Dysfunction: A Systematic Review and Meta-Analysis. J. Sex. Med. 2023, 20, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring Sleep Quality in Older Adults: A Comparison Using Subjective and Objective Methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Giganti, F.; Arzilli, C.; Cerasuolo, M.; Ficca, G. La percezione del sonno e dei suoi segnali nel soggetto insonne. Cogn. Clin. 2016, 13, 41–53. [Google Scholar]
- Harvey, A.G.; Tang, N.K.Y. (Mis)Perception of Sleep in Insomnia: A Puzzle and a Resolution. Psychol. Bull. 2012, 138, 77–101. [Google Scholar] [CrossRef]
- Morin, C.M.; Blais, F.; Savard, J. Are Changes in Beliefs and Attitudes about Sleep Related to Sleep Improvements in the Treatment of Insomnia? Behav. Res. Ther. 2002, 40, 741–752. [Google Scholar] [CrossRef]
- Baglioni, C.; Regen, W.; Teghen, A.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Riemann, D. Sleep Changes in the Disorder of Insomnia: A Meta-Analysis of Polysomnographic Studies. Sleep Med. Rev. 2014, 18, 195–213. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short Sleep Duration and Health Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Knutson, K.L. Sociodemographic and Cultural Determinants of Sleep Deficiency: Implications for Cardiometabolic Disease Risk. Soc. Sci. Med. 2013, 79, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewski, J.M.; Sabet, S.M.; Ghose, S.M.; Perez, E.; Soto, P.; Ravyts, S.G.; Dautovich, N.D. Lifestyle Factors and Sleep Health across the Lifespan. Int. J. Environ. Res. Public Health 2021, 18, 6626. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between Diet and Sleep Quality: A Systematic Review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, E.L.; Gershon, A.; Eidelman, P.; Kaplan, K.A.; Harvey, A.G. Physical Activity and Sleep: Day-to-Day Associations among Individuals with and without Bipolar Disorder. Ment. Health Phys. Act. 2014, 7, 183–190. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estadística. Available online: https://www.ine.es/ (accessed on 4 March 2025).
- World Medical Association. World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2025, 333, 71–74. [Google Scholar] [CrossRef]
- Sandri, E.; Piredda, M.; De Maria, M.; Mancin, S.; Sguanci, M.; Cabo, A.; Cerdá Olmedo, G. Development and Psychometric Testing of the Nutritional and Social Health Habits Scale (NutSo-HH): A Methodological Review of Existing Tools. MethodsX 2024, 12, 102768. [Google Scholar] [CrossRef]
- Biernacki, P.; Waldorf, D. Snowball Sampling: Problems and Techniques of Chain Referral Sampling. Sociol. Methods Res. 1981, 10, 141–163. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Sandri, E.; Sguanci, M.; Cantín Larumbe, E.; Cerdá Olmedo, G.; Werner, L.U.; Piredda, M.; Mancin, S. Plant-Based Diets versus the Mediterranean Dietary Pattern and Their Socio-Demographic Determinants in the Spanish Population: Influence on Health and Lifestyle Habits. Nutrients 2024, 16, 1278. [Google Scholar] [CrossRef]
- Sandri, E.; Borghesi, D.; Cantín Larumbe, E.; Cerdá Olmedo, G.; Vega-Bello, M.J.; Bernalte Martí, V. Intermittent Fasting: Socio-Economic Profile of Spanish Citizens Who Practice It and the Influence of This Dietary Pattern on the Health and Lifestyle Habits of the Population. Nutrients 2024, 16, 2028. [Google Scholar] [CrossRef] [PubMed]
- Sandri, E.; Cantín Larumbe, E.; Capoferri, M.; Cerdá Olmedo, G.; Werner, L.U.; Vega-Bello, M.J. Socio-Demographic Determinants of Dietary Choices and Their Impact on Health in Spanish Adults. Front. Public Health 2024, 12, 1417925. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Trabajo y Economía Social. Real Decreto 231/2020, de 4 de Febrero, Por El Que Se Fija El Salario Mínimo Interprofesional Para 2020. 2020, pp. 10814–10818. Available online: https://www.boe.es/eli/es/rd/2020/02/04/231 (accessed on 4 March 2025).
- Norte Navarro, A.I.; Ortiz Moncada, R. Spanish diet quality according to the healthy eating index. Nutr. Hosp. 2011, 26, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Aranceta Bartrina, J. Guías Alimentarias Para La Población Española (SENC, 2016); La Nueva Pirámide de La Alimentación Saludable. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar] [CrossRef]
- DATAtab Team. DATAtab Statistics Software. Available online: https://datatab.net/statistics-software (accessed on 4 March 2025).
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. Qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Soft. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Mao, Y.; Raju, G.; Zabidi, M.A. Association Between Occupational Stress and Sleep Quality: A Systematic Review. Nat. Sci. Sleep 2023, 15, 931–947. [Google Scholar] [CrossRef]
- Tähkämö, L.; Partonen, T.; Pesonen, A.-K. Systematic Review of Light Exposure Impact on Human Circadian Rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Sun, L.; Zhang, J.; Wang, T.; Wang, J. The Association of Sedentary Time with Sleep Disturbances among the US Population, 2005 to 2014. BMC Public Health 2024, 24, 2565. [Google Scholar] [CrossRef]
- Yasugaki, S.; Okamura, H.; Kaneko, A.; Hayashi, Y. Bidirectional Relationship between Sleep and Depression. Neurosci. Res. 2025, 211, 57–64. [Google Scholar] [CrossRef]
- Mencacci, C.; Ullo, A.; Larcan, R.; Orthmann, N.; Merzagora, F.; Aguglia, E. Genere e disturbi del sonno. Studio multicentrico e ricerca qualitativa nella popolazione italiana. J. Psychopathol. 2013, 19, 375–382. [Google Scholar]
- Schmidt, C.; Peigneux, P.; Cajochen, C. Age-Related Changes in Sleep and Circadian Rhythms: Impact on Cognitive Performance and Underlying Neuroanatomical Networks. Front. Neurol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.E.; Lugo, A.; Ghislandi, S.; Colombo, P.; Pacifici, R.; Gallus, S. Sleep Dissatisfaction and Insufficient Sleep Duration in the Italian Population. Sci. Rep. 2020, 10, 17943. [Google Scholar] [CrossRef]
- Magee, C.A.; Iverson, D.C.; Caputi, P. Factors Associated with Short and Long Sleep. Prev. Med. 2009, 49, 461–467. [Google Scholar] [CrossRef]
- Ursin, R.; Bjorvatn, B.; Holsten, F. Sleep Duration, Subjective Sleep Need, and Sleep Habits of 40- to 45-Year-Olds in the Hordaland Health Study. Sleep 2005, 28, 1260–1269. [Google Scholar] [CrossRef]
- Moore, P.J.; Adler, N.E.; Williams, D.R.; Jackson, J.S. Socioeconomic Status and Health: The Role of Sleep. Psychosom. Med. 2002, 64, 337–344. [Google Scholar] [CrossRef]
- Hall, M.; Buysse, D.J.; Nofzinger, E.A.; Reynolds, C.F.; Thompson, W.; Mazumdar, S.; Monk, T.H. Financial Strain Is a Significant Correlate of Sleep Continuity Disturbances in Late-Life. Biol. Psychol. 2008, 77, 217–222. [Google Scholar] [CrossRef]
- Khan, A.; Uddin, R. Is Consumption of Fast-Food and Carbonated Soft Drink Associated with Anxiety-Induced Sleep Disturbance among Adolescents? A Population-Based Study. Clin. Nutr. ESPEN 2020, 36, 162–165. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Ha, J.; Gillman, J.; Zambrano, M.; Castillo, P.R. Dietary Fish Intake and Sleep Quality: A Population-Based Study. Sleep Med. 2016, 17, 126–128. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Cauter, E.V. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The Effects of Physical Activity on Sleep: A Meta-Analytic Review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Cardinal, B.J. Association between Objectively-Measured Physical Activity and Sleep, NHANES 2005–2006. Ment. Health Phys. Act. 2011, 4, 65–69. [Google Scholar] [CrossRef]
- Dolezal, B.A.; Neufeld, E.V.; Boland, D.M.; Martin, J.L.; Cooper, C.B. Interrelationship between Sleep and Exercise: A Systematic Review. Adv. Prev. Med. 2017, 2017, 1364387. [Google Scholar] [CrossRef] [PubMed]
- Matthew, W. Why We Sleep: Unlocking the Power of Sleep and Dreams; First Scribner Trade Paperback Edition; Scribner: New York, NY, USA; London, UK; Toronto, ON, Canada; Sydney, Australia; New Delhi, India, 2018; ISBN 978-1-5011-4431-8. [Google Scholar]
- Carney, A.E.; Wescott, D.L.; Carmona, N.E.; Carney, C.E.; Roecklein, K.A. The Role of Beliefs about Sleep in Nightly Perceptions of Sleep Quality across a Depression Continuum. J. Affect. Disord. 2022, 311, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.K.Y.; Harvey, A.G. Altering Misperception of Sleep in Insomnia: Behavioral Experiment versus Verbal Feedback. J. Consult. Clin. Psychol. 2006, 74, 767–776. [Google Scholar] [CrossRef]
- Scott, A.J.; Webb, T.L.; Martyn-St James, M.; Rowse, G.; Weich, S. Improving Sleep Quality Leads to Better Mental Health: A Meta-Analysis of Randomised Controlled Trials. Sleep Med. Rev. 2021, 60, 101556. [Google Scholar] [CrossRef]
- Salzano, G.; Maglitto, F.; Bisogno, A.; Vaira, L.A.; De Riu, G.; Cavaliere, M.; Di Stadio, A.; Mesolella, M.; Motta, G.; Ionna, F.; et al. Obstructive Sleep Apnoea/Hypopnoea Syndrome: Relationship with Obesity and Management in Obese Patients. Acta Otorhinolaryngol. Ital. 2021, 41, 120–130. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef]
- Het, S.; Vocks, S.; Wolf, J.M.; Hammelstein, P.; Herpertz, S.; Wolf, O.T. Blunted Neuroendocrine Stress Reactivity in Young Women with Eating Disorders. J. Psychosom. Res. 2015, 78, 260–267. [Google Scholar] [CrossRef]
- Lo Martire, V.; Caruso, D.; Palagini, L.; Zoccoli, G.; Bastianini, S. Stress & Sleep: A Relationship Lasting a Lifetime. Neurosci. Biobehav. Rev. 2020, 117, 65–77. [Google Scholar] [CrossRef]
- Tyagi, M.; Shah, U.; Patel, G.; Toshniwal, V.; Bhongade, R.; Sharma, P. The impact of sleep on physical and mental health: Importance of healthy sleep habits. Georgian Med. News 2023, 339, 89–94. [Google Scholar]
- Nooijen, C.F.J.; Möller, J.; Forsell, Y.; Ekblom, M.; Galanti, M.R.; Engström, K. Do Unfavourable Alcohol, Smoking, Nutrition and Physical Activity Predict Sustained Leisure Time Sedentary Behaviour? A Population-Based Cohort Study. Prev. Med. 2017, 101, 23–27. [Google Scholar] [CrossRef]
- Yang, Y.; Shin, J.C.; Li, D.; An, R. Sedentary Behavior and Sleep Problems: A Systematic Review and Meta-Analysis. Int. J. Behav. Med. 2017, 24, 481–492. [Google Scholar] [CrossRef]
- Ebrahim, I.O.; Shapiro, C.M.; Williams, A.J.; Fenwick, P.B. Alcohol and Sleep I: Effects on Normal Sleep. Alcohol. Clin. Exp. Res. 2013, 37, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, S. Exploring the Relationship between Smoking and Poor Sleep Quality: A Cross-Sectional Study Using NHANES. Front. Psychiatry 2024, 15, 1407741. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Devarshi, P.P.; Mun, J.G.; Marshall, K.; Mitmesser, S.H. Association of Omega-3 Levels and Sleep in US Adults, National Health and Nutrition Examination Survey, 2011-2012. Sleep Health 2022, 8, 294–297. [Google Scholar] [CrossRef]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Middleton, B.; Khan, J.; Forster, J.; Jackson, P.A. Differential Effects of DHA- and EPA-Rich Oils on Sleep in Healthy Young Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 248. [Google Scholar] [CrossRef]
- Roehrs, T.; Roth, T. Caffeine: Sleep and Daytime Sleepiness. Sleep Med. Rev. 2008, 12, 153–162. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Kacprowski, T.; Menni, C.; Gustafsson, S.; Pivin, E.; Adamski, J.; Artati, A.; Eap, C.B.; Ehret, G.; Friedrich, N.; et al. Genome-Wide Association Study of Caffeine Metabolites Provides New Insights to Caffeine Metabolism and Dietary Caffeine-Consumption Behavior. Hum. Mol. Genet. 2016, 25, 5472–5482. [Google Scholar] [CrossRef]
- Sun, Y.-H.C. Health Concern, Food Choice Motives, and Attitudes toward Healthy Eating: The Mediating Role of Food Choice Motives. Appetite 2008, 51, 42–49. [Google Scholar] [CrossRef]
- Karam, J.; Haddad, C.; Sacre, H.; Serhan, M.; Salameh, P.; Jomaa, L. Financial Wellbeing and Quality of Life Among a Sample of the Lebanese Population: The Mediating Effect of Food Insecurity. Front. Nutr. 2022, 9, 906646. [Google Scholar] [CrossRef]
- Urhan, M.; Okut Aysin, E. Nutritional and Health Behaviour Predictors of the Weight Gain during the COVID-19 Pandemic. Eur. J. Nutr. 2022, 61, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep Physiology, Pathophysiology, and Sleep Hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef]
- Team, E.E. Estrategia Española de Promoción y Prevención de la Salud y su Implementación Local. EuroHealthNet Magazine, 17 June 2017. [Google Scholar]
- Office of Disease Prevention and Health Promotion. Healthy People 2030; U.S. Department of Health and Human Services: Washington, DC, USA, 2020. [Google Scholar]

| Mean (SD) or N (%) | ||
|---|---|---|
| Sex | Male | 4251 (19.2%) |
| Female | 17,930 (80.8%) | |
| Age (years) | Age total | 34.9 (11.7) |
| Age male | 36.5 (13.4) | |
| Age female | 34.5 (11.2) | |
| Young (≤30 years) | 9692 (43.7%) | |
| Adults (>30 years) | 12,489 (56.3%) | |
| Education level | Basic Education | 7027 (31.7%) |
| Higher Education | 15,154 (68.3%) | |
| Income level | Low | 9727 (43.9%) |
| Medium–high | 10,616 (47.9%) | |
| No answer | 1839 (8.3%) | |
| Municipality size | <2000 | 1014 (4.6%) |
| 2000–10,000 | 3587 (16.2%) | |
| >10,000 | 17,580 (79.3%) | |
| Living arrangement | Living alone | 2202 (9.9%) |
| Not living alone | 19,979 (90.1%) | |
| Family life | Living with family | 16,732 (75.4%) |
| Living without family | 5449 (24.6%) |
| Socio-Demographic Variables | Sleep Hours | Get Up Rested | Sleep Quality | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | p-Value $ | Mean (SD) | p-Value $ | Mean (SD) | p-Value $ | ||
| Sex | Male | 2.48 (0.73) | <0.001 | 2.61 (0.58) | <0.001 | 3.48 (1.03) | <0.001 |
| Female | 2.53 (0.73) | 2.53 (0.59) | 3.36 (0.96) | ||||
| Age | Young (18–30 years) | 2.66 (0.70) | <0.001 | 2.54 (0.57) | 0.406 | 3.51 (0.98) | <0.001 |
| Adults (>30 years) | 2.41 (0.74) | 2.54 (0.59) | 3.29 (1.04) | ||||
| Education | Basic Education | 2.49 (0.76) | <0.001 | 2.50 (0.60) | <0.001 | 3.30 (1.05) | <0.001 |
| Higher Education | 2.53 (0.72) | 2.56 (0.58) | 3.42 (1.00) | ||||
| Incomes | Low | 2.51 (0.75) | 0.562 | 2.51 (0.59) | <0.001 | 3.32 (1.03) | <0.001 |
| Medium–high | 2.51 (0.71) | 2.57 (0.57) | 3.43 (1.00) | ||||
| Municipality size | <2000 | 2.53 (0.73) | 0.076 & | 2.52 (0.58) | 0.061 & | 3.35 (1.02) | 0.065 & |
| 2000–10,000 | 2.56 (0.77) | 2.52 (0.62) | 3.36 (1.06) | ||||
| >10,000 | 2.51 (0.73) | 2.55 (0.58) | 3.39 (1.01) | ||||
| Living arrangement | Living alone | 2.52 (0.73) | <0.001 | 2.54 (0.58) | 0.458 | 3.39 (1.01) | 0.253 |
| Not living alone | 2.45 (0.75) | 2.55 (0.59) | 3.36 (1.02) | ||||
| Family life | Living with family | 2.54 (0.72) | 0.001 | 2.55 (0.58) | 0.207 | 3.44 (0.99) | <0.001 |
| Living without family | 2.51 (0.74) | 2.54 (0.58) | 3.37 (1.02) | ||||
| (a) | |||
| Numerical Variable | Sleep Hours | ||
| Insufficient Sleep Hours (ISH) | Sufficient Sleep Hours (SSH) | ||
| Median (IQR) | Median (IQR) | p-Value $ | |
| IASE | 55.0 (13.5) | 56.0 (12.6) | <0.001 |
| BMI (kg/m2) | 23.5 (5.66) | 22.6 (4.72) | <0.001 |
| Fried food | 2.00 (1.00) | 2.00 (1.00) | 0.286 |
| Fast food | 2.00 (1.00) | 2.00 (1.00) | 0.399 |
| Ultra-processed food | 2.00 (1.00) | 2.00 (1.00) | 0.172 |
| Fish | 2.00 (0.50) | 2.00 (0.50) | 0.901 |
| Water | 3.00 (1.00) | 3.00 (1.00) | <0.001 |
| Sugary soft drinks | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Juice | 1.00 (1.00) | 1.00 (1.00) | 0.887 |
| Coffee and energy drinks | 2.00 (1.00) | 2.00 (1.00) | <0.001 |
| Sedentary lifestyle | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Self-perceived health | 4.00 (1.00) | 4.00 (1.00) | <0.001 |
| Sport (minuts/week) | 90.0 (225) | 135.0 (225) | <0.001 |
| Smoking | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Alcohol | 2.00 (1.00) | 2.00 (1.00) | 0.017 |
| Getting drunk | 1.00 (1.00) | 1.00 (1.00) | 0.556 |
| Night outings | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Obesophobia | 4.00 (2.00) | 3.00 (1.00) | <0.001 |
| No control | 3.00 (2.00) | 3.00 (1.00) | <0.001 |
| Body image | 4.00 (2.00) | 3.00 (1.00) | <0.001 |
| (b) | |||
| Numerical Variable | Get Up Rested | ||
| NO Get Up Rested (NO_GUR) | YES Get Up Rested (YES_GUR) | ||
| Median (IQR) | Median (IQR) | p-Value $ | |
| IASE | 55.0 (12.5) | 56.0 (12.5) | <0.001 |
| BMI (kg/m2) | 23.2 (5.57) | 22.9 (4.80) | <0.001 |
| Fried food | 2.00 (1.00) | 2.00 (1.00) | <0.001 |
| Fast food | 2.00 (1.00) | 2.00 (1.00) | <0.001 |
| Ultra-processed food | 2.00 (1.00) | 2.00 (1.00) | <0.001 |
| Fish | 2.00 (0.50) | 2.00 (0.50) | <0.001 |
| Water | 3.00 (1.00) | 3.00 (1.00) | 0.008 |
| Sugary soft drinks | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Juice | 1.00 (1.00) | 1.00 (1.00) | 0.954 |
| Coffee and energy drinks | 2.00 (1.00) | 2.00 (1.00) | 0.704 |
| Sedentary lifestyle | 1.00 (1.00) | 1.00 (1.00) | <0.001 |
| Self-perceived health | 4.00 (1.00) | 4.00 (0.00) | <0.001 |
| Sport (minutes/week) | 90.0 (210) | 135.0 (225) | <0.001 |
| Smoking | 1.00 (0.00) | 1.00 (0.00) | <0.001 |
| Alcohol | 1.00 (1.00) | 2.00 (1.00) | <0.001 |
| Getting drunk | 1.00 (0.00) | 1.00 (0.00) | 0.732 |
| Night outings | 1.00 (0.00) | 1.00 (0.00) | 0.005 |
| Obesophobia | 4.00 (3.00) | 4.00 (2.00) | <0.001 |
| No control | 3.00 (2.00) | 2.00 (1.00) | <0.001 |
| Body image | 4.00 (2.00) | 3.00 (1.00) | <0.001 |
| Numerical Variable | Low Sleep Quality (LSQ) | Medium Sleep Quality (MSQ) | High Sleep Quality (HSQ) | p-Value & |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| IASE | 52.0 (15.0) | 55.0 (13.5) | 56.0 (12.5) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| BMI | 24.0 (6.96) | 23.2 (5.39) | 22.8 (4.73) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Fried food | 2.00 (1.00) | 2.00 (1.00) | 2.00 (1.00) | LSQ-MSQ (p = 0.101) |
| LSQ-HSQ (p = 0.013) | ||||
| MSQ-HSQ (p = 0.124) | ||||
| Fast food | 2.00 (1.00) | 2.00 (1.00) | 2.00 (1.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Ultra-processed food | 2.00 (1.00) | 2.00 (1.00) | 2.00 (1.00) | LSQ-MSQ (p = 0.033) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Fish | 2.00 (0.50) | 2.00 (0.50) | 2.00 (0.50) | LSQ-MSQ (p = 0.003) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p = 0.011) | ||||
| Water | 3.00 (1.00) | 3.00 (1.00) | 3.00 (1.00) | LSQ-MSQ (p = 0.148) |
| LSQ-HSQ (p = 0.002) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Sugary soft drinks | 1.00 (1.00) | 1.00 (1.00) | 1.00 (1.00) | LSQ-MSQ (p = 0.005) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Juice | 1.00 (1.00) | 1.00 (1.00) | 1.00 (1.00) | LSQ-MSQ (p = 0.984) |
| LSQ-HSQ (p = 0.235) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Coffee and energy drinks | 2.00 (1.00) | 2.00 (1.00) | 2.00 (1.00) | p = 0.212 |
| Sedentary lifestyle | 1.00 (1.00) | 1.00 (1.00) | 1.00 (1.00) | p = 0.089 |
| Self-perceived health | 3.00 (2.00) | 4.00 (1.00) | 4.00 (1.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Sport | 30.0 (203) | 105.0 (225) | 135.0 (225) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Smoking | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Alcohol | 1.00 (1.00) | 2.00 (1.00) | 2.00 (1.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Getting drunk | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | p = 0.505 |
| Night outings | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Obesophobia | 4.00 (2.00) | 4.00 (2.00) | 3.00 (2.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| No control | 3.00 (3.00) | 3.00 (2.00) | 2.00 (1.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) | ||||
| Body image | 4.00 (2.00) | 4.00 (2.00) | 3.00 (1.00) | LSQ-MSQ (p < 0.001) |
| LSQ-HSQ (p < 0.001) | ||||
| MSQ-HSQ (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandri, E.; Broccolo, A.; Piredda, M. Socio-Demographic, Nutritional, and Lifestyle Factors Influencing Perceived Sleep Quality in Spain, with a Particular Focus on Women and Young People. Nutrients 2025, 17, 1065. https://doi.org/10.3390/nu17061065
Sandri E, Broccolo A, Piredda M. Socio-Demographic, Nutritional, and Lifestyle Factors Influencing Perceived Sleep Quality in Spain, with a Particular Focus on Women and Young People. Nutrients. 2025; 17(6):1065. https://doi.org/10.3390/nu17061065
Chicago/Turabian StyleSandri, Elena, Agnese Broccolo, and Michela Piredda. 2025. "Socio-Demographic, Nutritional, and Lifestyle Factors Influencing Perceived Sleep Quality in Spain, with a Particular Focus on Women and Young People" Nutrients 17, no. 6: 1065. https://doi.org/10.3390/nu17061065
APA StyleSandri, E., Broccolo, A., & Piredda, M. (2025). Socio-Demographic, Nutritional, and Lifestyle Factors Influencing Perceived Sleep Quality in Spain, with a Particular Focus on Women and Young People. Nutrients, 17(6), 1065. https://doi.org/10.3390/nu17061065







