Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
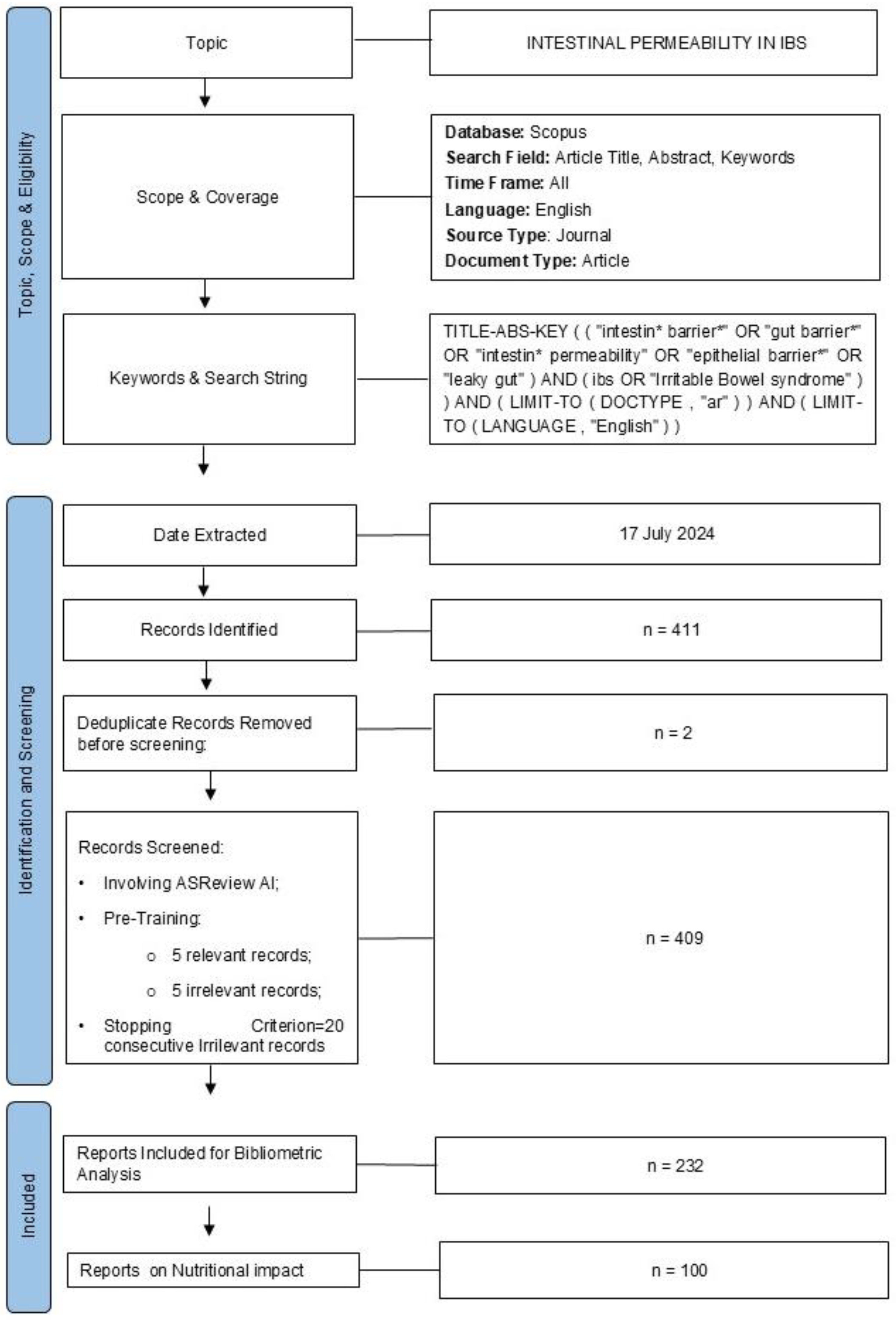
2.1. Research Criteria and Record Identification
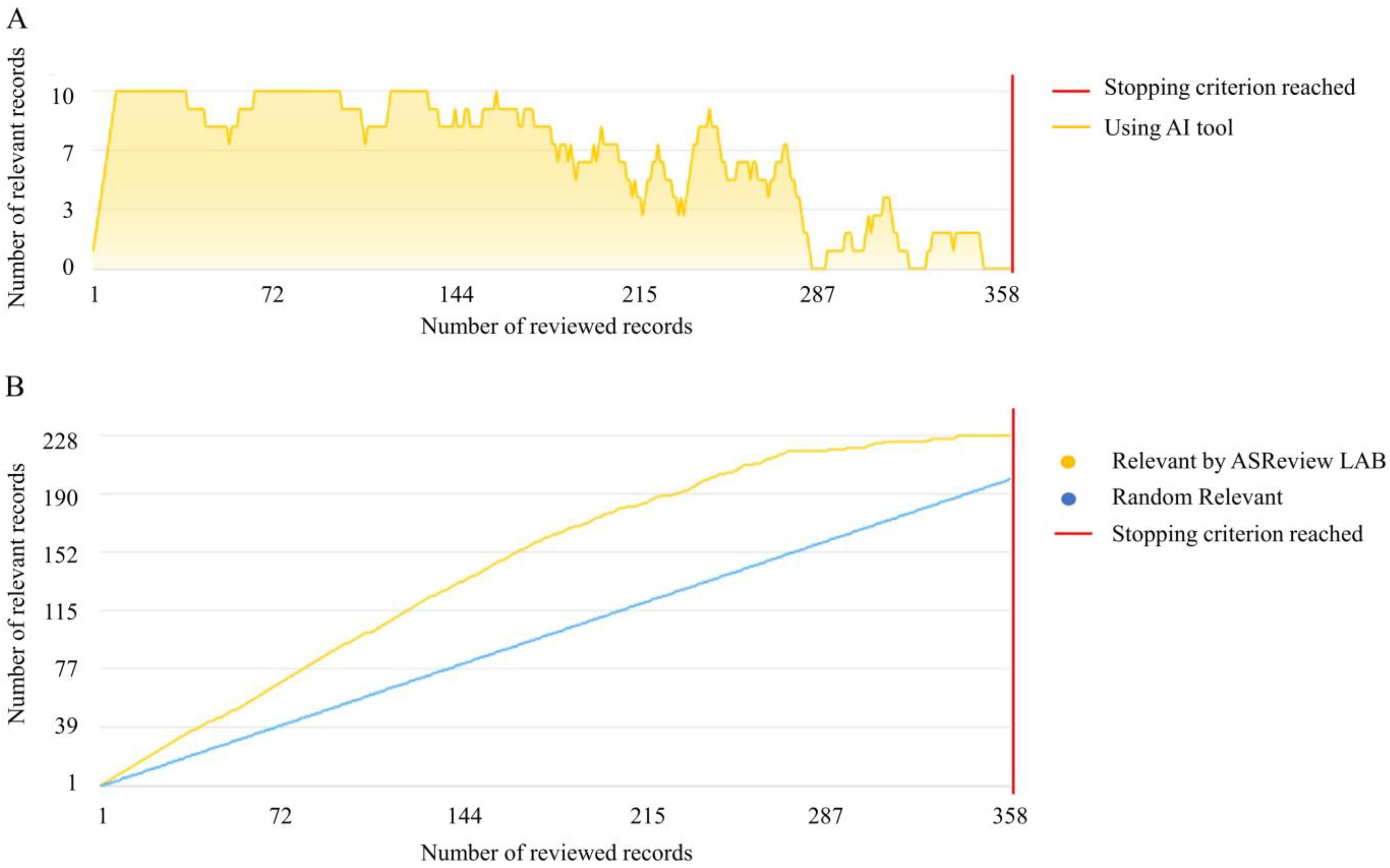
2.2. Record Screening
2.3. Bibliometric Analysis
2.4. Nutritional Impact Analysis
3. Results
3.1. Dataset Download and Screening
3.2. Dataset General Description
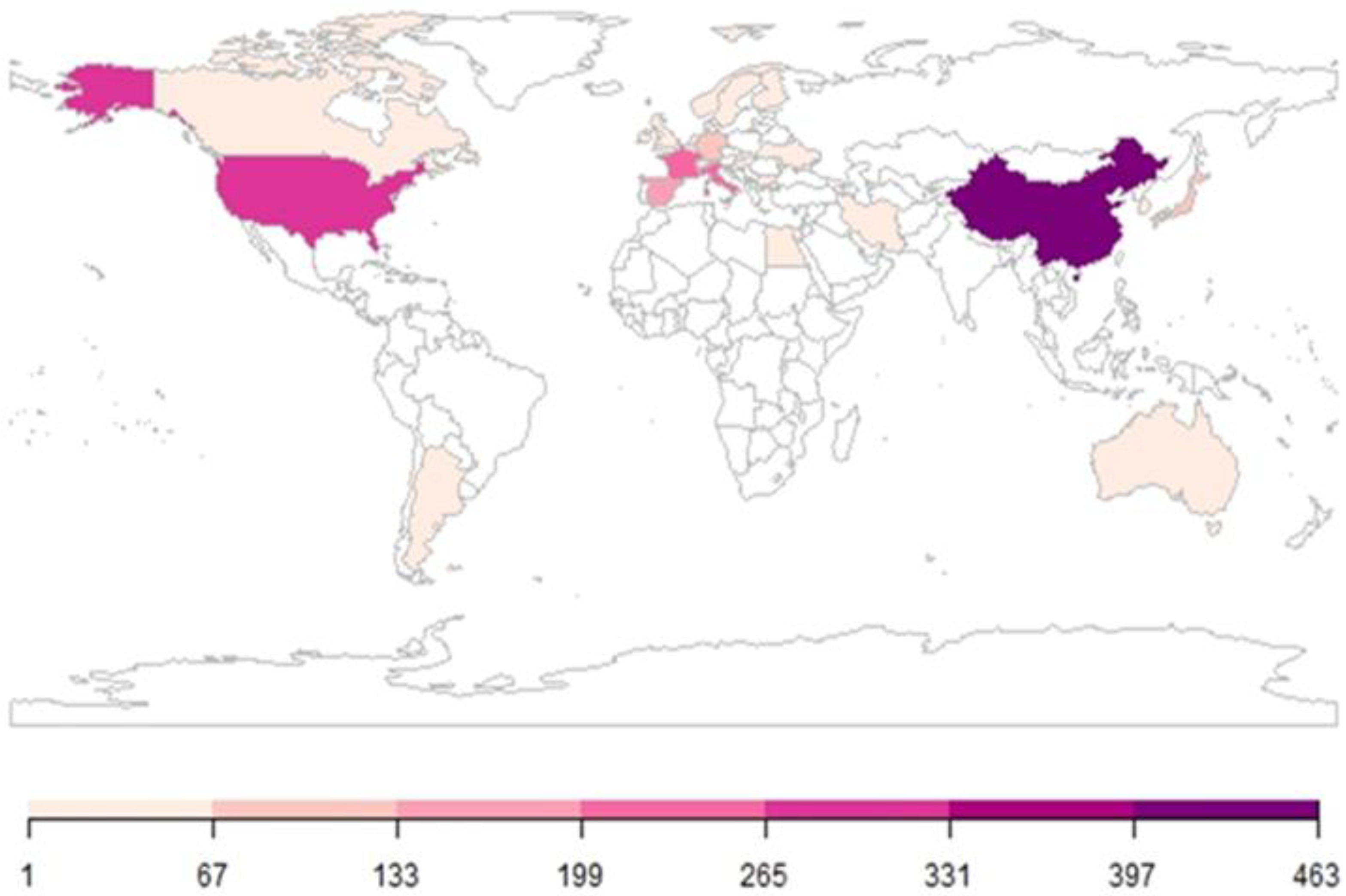
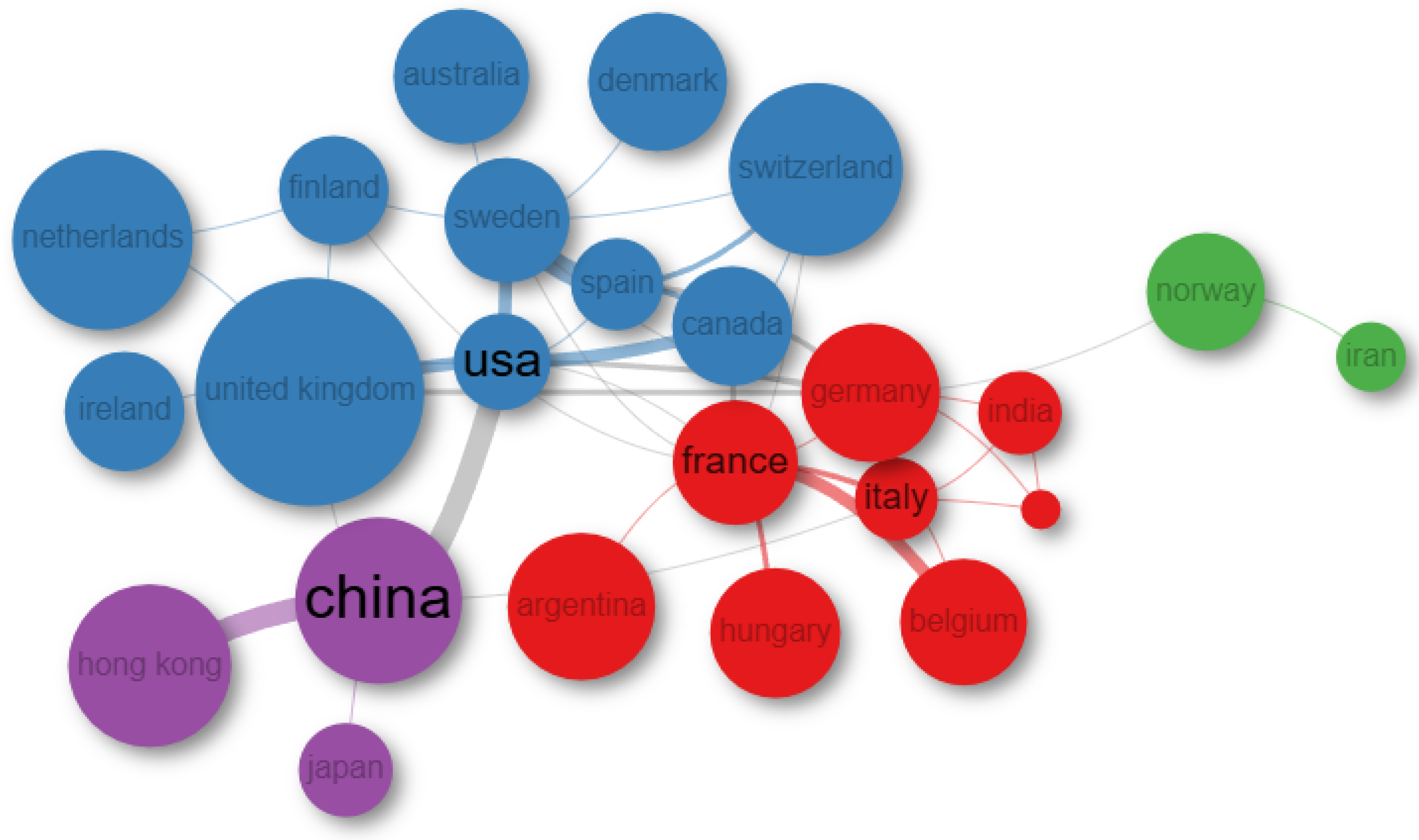
3.3. Geographical Distribution and International Collaboration Network
3.4. Most Productive Journals
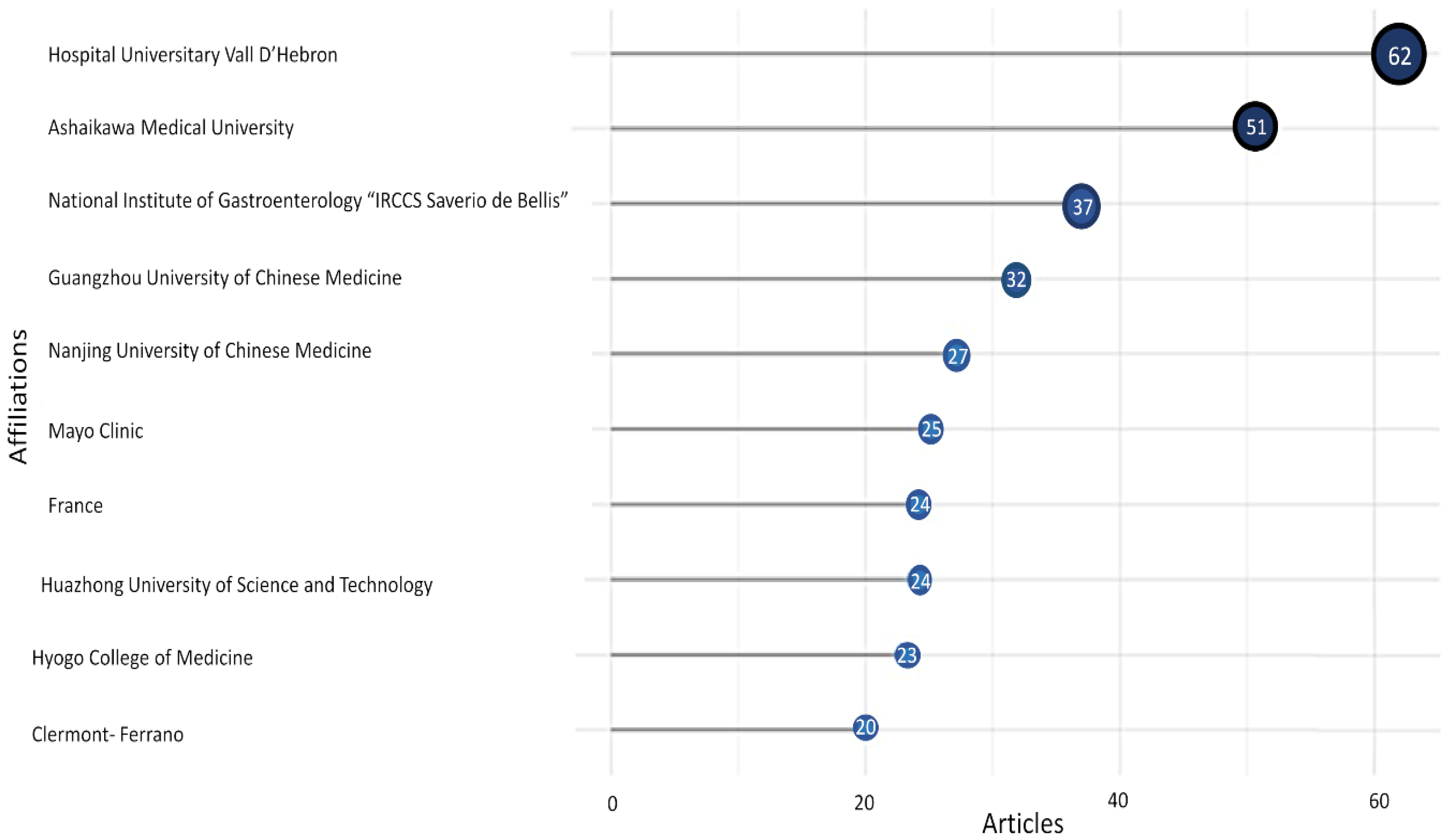
3.5. Authors and Institutions
3.6. Most Global Cited Documents
3.7. Three-Fields Plot
3.8. Word Cloud Frequency
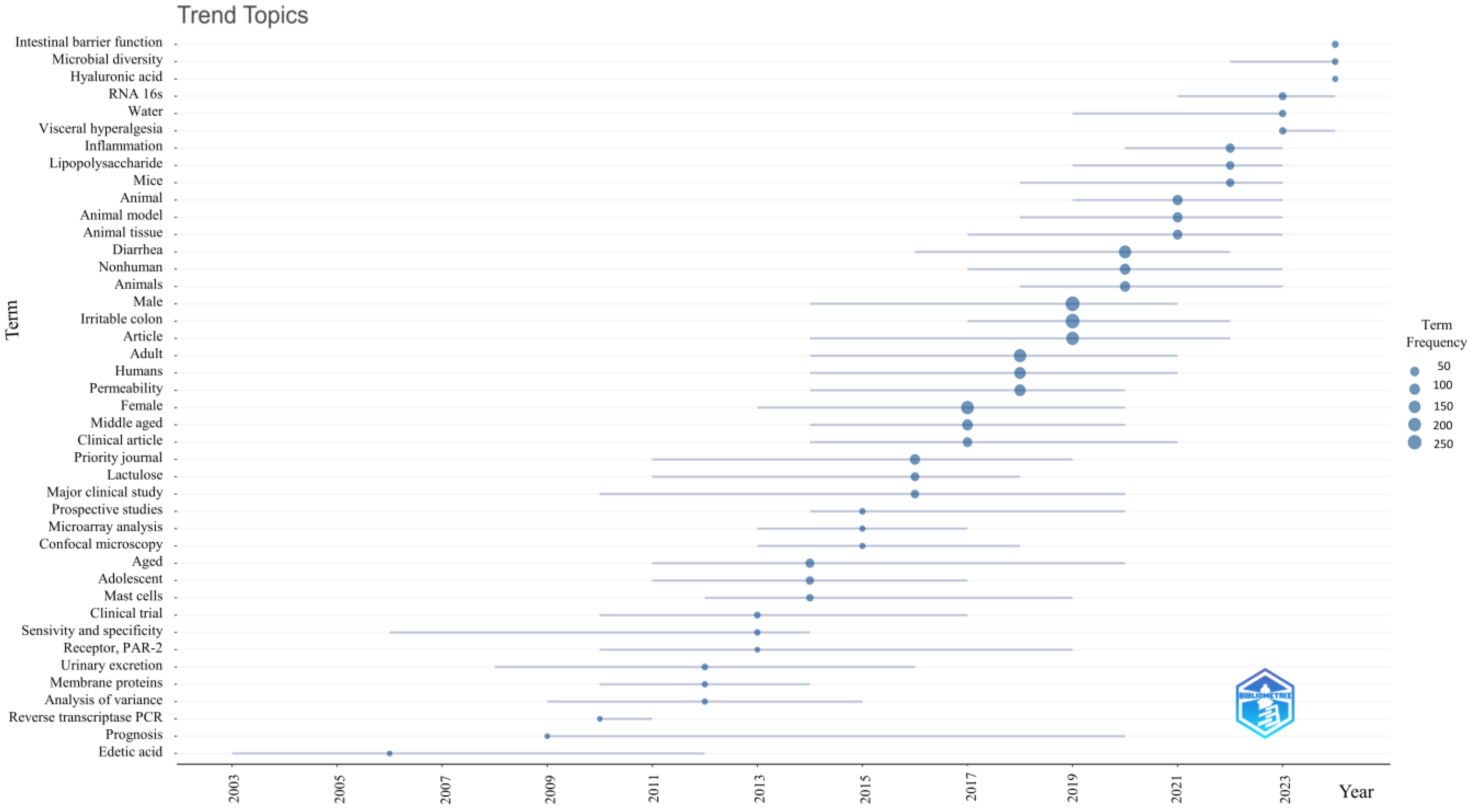
3.9. Trending Topics About Intestinal Permeability in IBS
3.10. Nutritional Impact Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Jiang, A.J.; Wang, X.Y.; Wang, H.; Guan, Y.Y.; Li, F.; Shen, G.M. Uncovering the pathophysiology of irritable bowel syndrome by exploring the gut-brain axis: A narrative review. Ann. Transl. Med. 2021, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.J.; Jarrett, M.E.; Cain, K.C.; Broussard, E.K.; Heitkemper, M.M. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 2014, 49, 1467–1476. [Google Scholar] [CrossRef]
- Li, L.; Xiong, L.; Yao, J.; Zhuang, X.; Zhang, S.; Yu, Q.; Xiao, Y.; Cui, Y.; Chen, M. Increased small intestinal permeability and RNA expression profiles of mucosa from terminal ileum in patients with diarrhoea-predominant irritable bowel syndrome. Dig. Liver Dis. 2016, 48, 880–887. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Therap Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Ringel, Y.; Maharshak, N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G529–G541. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Khoshbin, K.; Camilleri, M. Effects of dietary components on intestinal permeability in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef]
- Inczefi, O.; Bacsur, P.; Resal, T.; Keresztes, C.; Molnar, T. The Influence of Nutrition on Intestinal Permeability and the Microbiome in Health and Disease. Front. Nutr. 2022, 9, 718710. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Linsalata, M.; Riezzo, G.; Clemente, C.; D’Attoma, B.; Russo, F. Noninvasive Biomarkers of Gut Barrier Function in Patients Suffering from Diarrhea Predominant-IBS: An Update. Dis. Markers 2020, 2020, 2886268. [Google Scholar] [CrossRef]
- Russo, F.; Chimienti, G.; Riezzo, G.; Linsalata, M.; D’Attoma, B.; Clemente, C.; Orlando, A. Adipose Tissue-Derived Biomarkers of Intestinal Barrier Functions for the Characterization of Diarrhoea-Predominant IBS. Dis. Markers 2018, 2018, 1827937. [Google Scholar] [CrossRef]
- Linsalata, M.; Riezzo, G.; D’Attoma, B.; Clemente, C.; Orlando, A.; Russo, F. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: A case-control study. BMC Gastroenterol. 2018, 18, 167. [Google Scholar] [CrossRef]
- Prospero, L.; Riezzo, G.; Linsalata, M.; Orlando, A.; D’Attoma, B.; Di Masi, M.; Martulli, M.; Russo, F. Somatization in patients with predominant diarrhoea irritable bowel syndrome: The role of the intestinal barrier function and integrity. BMC Gastroenterol. 2021, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Manoj Kumar, L.; George, R.J.; S, A.P. Bibliometric Analysis for Medical Research. Indian. J. Psychol. Med. 2023, 45, 277–282. [Google Scholar] [CrossRef]
- Stefanis, C.; Stavropoulou, E.; Giorgi, E.; Voidarou, C.C.; Constantinidis, T.C.; Vrioni, G.; Tsakris, A. Honey’s Antioxidant and Antimicrobial Properties: A Bibliometric Study. Antioxidants 2023, 12, 414. [Google Scholar] [CrossRef]
- Stefanis, C.; Giorgi, E.; Tselemponis, G.; Voidarou, C.; Skoufos, I.; Tzora, A.; Tsigalou, C.; Kourkoutas, Y.; Constantinidis, T.C.; Bezirtzoglou, E. Terroir in View of Bibliometrics. Stats 2023, 6, 956–979. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- van de Schoot, R.; de Bruin, J.; Schram, R.; Zahedi, P.; de Boer, J.; Weijdema, F.; Kramer, B.; Huijts, M.; Hoogerwerf, M.; Ferdinands, G.; et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat. Mach. Intell. 2021, 3, 125–133. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Infantino, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Perna, S.; Iannello, G.; Peroni, G.; Gasparri, C.; Rondanelli, M. The Use of Berberine in Diabetes and Metabolic Syndrome: Two Sides of the Same Coin. A Bibliometric Analysis. Curr. Nutr. Food Sci. 2021, 17, 240–247. [Google Scholar] [CrossRef]
- Morandi, G.; Guido, D.; Tagliabue, A. A bibliometric study of scientific literature on the dietary therapies for epilepsy in Scopus. Nutr. Neurosci. 2015, 18, 201–209. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Peroni, G.; Guido, D. A bibliometric study of scientific literature in Scopus on botanicals for treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Awad, K.; Barmeyer, C.; Bojarski, C.; Nagel, O.; Lee, I.M.; Schweiger, M.R.; Schulzke, J.D.; Bucker, R. Impaired Intestinal Permeability of Tricellular Tight Junctions in Patients with Irritable Bowel Syndrome with Mixed Bowel Habits (IBS-M). Cells 2023, 12, 236. [Google Scholar] [CrossRef]
- Linsalata, M.; Riezzo, G.; Orlando, A.; D’Attoma, B.; Prospero, L.; Ignazzi, A.; Losurdo, G.; Di Leo, A.; Giannelli, G.; Russo, F. The Role of Intestinal Barrier Function in Overweight Patients with IBS with Diarrhea Undergoing a Long-Term Low Fermentable Oligo-, Di-, and Monosaccharide and Polyol Diet. Nutrients 2023, 15, 4683. [Google Scholar] [CrossRef]
- Awad, K.; Barmeyer, C.; Bojarski, C.; Nagel, O.; Lee, I.M.; Schweiger, M.R.; Schulzke, J.D.; Bucker, R. Epithelial Barrier Dysfunction in Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) via Downregulation of Claudin-1. Cells 2023, 12, 2846. [Google Scholar] [CrossRef]
- Filippone, A.; Ardizzone, A.; Bova, V.; Lanza, M.; Casili, G.; Cuzzocrea, S.; Esposito, E.; Campolo, M.; Paterniti, I. A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome. J. Clin. Med. 2022, 11, 7073. [Google Scholar] [CrossRef]
- Ceren Akgor, M.; Vuralli, D.; Sucu, D.H.; Gokce, S.; Tasdelen, B.; Gultekin, F.; Bolay, H. Distinct Food Triggers for Migraine, Medication Overuse Headache and Irritable Bowel Syndrome. J. Clin. Med. 2023, 12, 6488. [Google Scholar] [CrossRef]
- Ammar, R.M.; Pferschy-Wenzig, E.M.; Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Thumann, T.; Bauer, R. Possible role of the gut microbiome in mediating the beneficial effects of the six-herbal formulation STW 5-II on digestive health. Phytomedicine 2023, 119, 154996. [Google Scholar] [CrossRef]
- van Orten-Luiten, A.B.; de Roos, N.M.; Majait, S.; Witteman, B.J.M.; Witkamp, R.F. Effects of Cannabidiol Chewing Gum on Perceived Pain and Well-Being of Irritable Bowel Syndrome Patients: A Placebo-Controlled Crossover Exploratory Intervention Study with Symptom-Driven Dosing. Cannabis Cannabinoid Res. 2022, 7, 436–444. [Google Scholar] [CrossRef]
- Zimmermann, J.; Longin, F.H.; Schweinlin, A.; Basrai, M.; Bischoff, S.C. No Difference in Tolerance between Wheat and Spelt Bread in Patients with Suspected Non-Celiac Wheat Sensitivity. Nutrients 2022, 14, 2800. [Google Scholar] [CrossRef]
- van Dijk, S.H.B.; Brusse-Keizer, M.G.J.; Bucsan, C.C.; van der Palen, J.; Doggen, C.J.M.; Lenferink, A. Artificial intelligence in systematic reviews: Promising when appropriately used. BMJ Open 2023, 13, e072254. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, G.; Schram, R.; de Bruin, J.; Bagheri, A.; Oberski, D.L.; Tummers, L.; Teijema, J.J.; van de Schoot, R. Performance of active learning models for screening prioritization in systematic reviews: A simulation study into the Average Time to Discover relevant records. Syst. Rev. 2023, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kang, S.B.; Marchelletta, R.R.; Penrose, H.M.; Ruiter-Visser, R.; Jung, B.; Docherty, M.J.; Boland, B.S.; Sandborn, W.J.; McCole, D.F. The ClC-2 Chloride Channel Activator, Lubiprostone, Improves Intestinal Barrier Function in Biopsies from Crohn’s Disease but Not Ulcerative Colitis Patients. Pharmaceutics 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; Barbara, G.; Aubert, P.; Bruley des Varannes, S.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut 2009, 58, 196–201. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef]
- De Palma, G.; Lynch, M.D.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Tibble, J.A.; Sigthorsson, G.; Foster, R.; Forgacs, I.; Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002, 123, 450–460. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009, 146, 41–46. [Google Scholar] [CrossRef]
- Gecse, K.; Roka, R.; Ferrier, L.; Leveque, M.; Eutamene, H.; Cartier, C.; Ait-Belgnaoui, A.; Rosztoczy, A.; Izbeki, F.; Fioramonti, J.; et al. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008, 57, 591–599. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salojarvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; de Vos, W.M. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.; Meddings, J.; Collins, S.M.; Investigators, W.E.L. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 2004, 20, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Bertiaux-Vandaele, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Leroi, A.M.; Dechelotte, P.; Menard, J.F.; Ducrotte, P.; et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 2011, 106, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gao, J.; Gillilland, M., 3rd; Wu, X.; Song, I.; Kao, J.Y.; Owyang, C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014, 146, 484–496.e4. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, K. Effects of the digital economy on carbon emissions in China: An analysis based on different innovation paths. Environ. Sci. Pollut. Res. Int. 2023, 30, 79451–79468. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20; quiz 21–22. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef]
- Fortea, M.; Albert-Bayo, M.; Abril-Gil, M.; Ganda Mall, J.P.; Serra-Ruiz, X.; Henao-Paez, A.; Exposito, E.; Gonzalez-Castro, A.M.; Guagnozzi, D.; Lobo, B.; et al. Present and Future Therapeutic Approaches to Barrier Dysfunction. Front. Nutr. 2021, 8, 718093. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, J.S. Irritable bowel syndrome in China: A review on the epidemiology, diagnosis, and management. Chin. Med. J. 2021, 134, 1396–1401. [Google Scholar] [CrossRef]
- Hungin, A.P.; Chang, L.; Locke, G.R.; Dennis, E.H.; Barghout, V. Irritable bowel syndrome in the United States: Prevalence, symptom patterns and impact. Aliment. Pharmacol. Ther. 2005, 21, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Arie, H.; Nozu, T.; Miyagishi, S.; Ida, M.; Izumo, T.; Shibata, H. Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats. Nutrients 2019, 11, 2646. [Google Scholar] [CrossRef] [PubMed]
- Maqoud, F.; Orlando, A.; Tricarico, D.; Antonacci, M.; Di Turi, A.; Giannelli, G.; Russo, F. Anti-Inflammatory Effects of a Novel Acetonitrile-Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells. Int. J. Mol. Sci. 2024, 25, 3802. [Google Scholar] [CrossRef]
- Russo, F.; Riezzo, G.; Linsalata, M.; Orlando, A.; Tutino, V.; Prospero, L.; D’Attoma, B.; Giannelli, G. Managing Symptom Profile of IBS-D Patients With Tritordeum-Based Foods: Results From a Pilot Study. Front. Nutr. 2022, 9, 797192. [Google Scholar] [CrossRef]
- Linsalata, M.; Riezzo, G.; Orlando, A.; D’Attoma, B.; Prospero, L.; Tutino, V.; Notarnicola, M.; Russo, F. The Relationship between Low Serum Vitamin D Levels and Altered Intestinal Barrier Function in Patients with IBS Diarrhoea Undergoing a Long-Term Low-FODMAP Diet: Novel Observations from a Clinical Trial. Nutrients 2021, 13, 1011. [Google Scholar] [CrossRef]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef]
- Cui, L.; Zou, S.; Liu, J.; Lv, H.; Li, H.; Zhang, Z. Potential effects of sodium hyaluronate on constipation-predominant irritable bowel syndrome. Int. Immunopharmacol. 2024, 127, 111404. [Google Scholar] [CrossRef]
- Valibouze, C.; Dubuquoy, C.; Chavatte, P.; Genin, M.; Maquet, V.; Modica, S.; Desreumaux, P.; Rousseaux, C. Chitin-glucan improves important pathophysiological features of irritable bowel syndrome. World J. Gastroenterol. 2024, 30, 2258–2271. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Carvalho, F.A.; Holowacz, S.; Delannoy, J.; Lenoir, L.; Jacouton, E.; Barbut, F.; Langella, P.; Bermudez-Humaran, L.G.; Waligora-Dupriet, A.J. Screening of probiotic strains to improve visceral hypersensitivity in irritable bowel syndrome by using in vitro and in vivo approaches. Benef. Microbes 2024, 15, 293–310. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W. A tale of two databases: The use of Web of Science and Scopus in academic papers. Scientometrics 2020, 123, 321–335. [Google Scholar] [CrossRef]
- Öztürk, O.; Kocaman, R.; Kanbach, D.K. How to design bibliometric research: An overview and a framework proposal. Rev. Manag. Sci. 2024, 18, 3333–3361. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Chu, T.N.; Sanford, D.I.; Abreu, A.; Duddalwar, V.; Oberai, A.; Kuo, C.C.J.; Liu, X.; Denniston, A.K.; Vasey, B.; et al. PRISMA AI reporting guidelines for systematic reviews and meta-analyses on AI in healthcare. Nat. Med. 2023, 29, 14–15. [Google Scholar] [CrossRef]
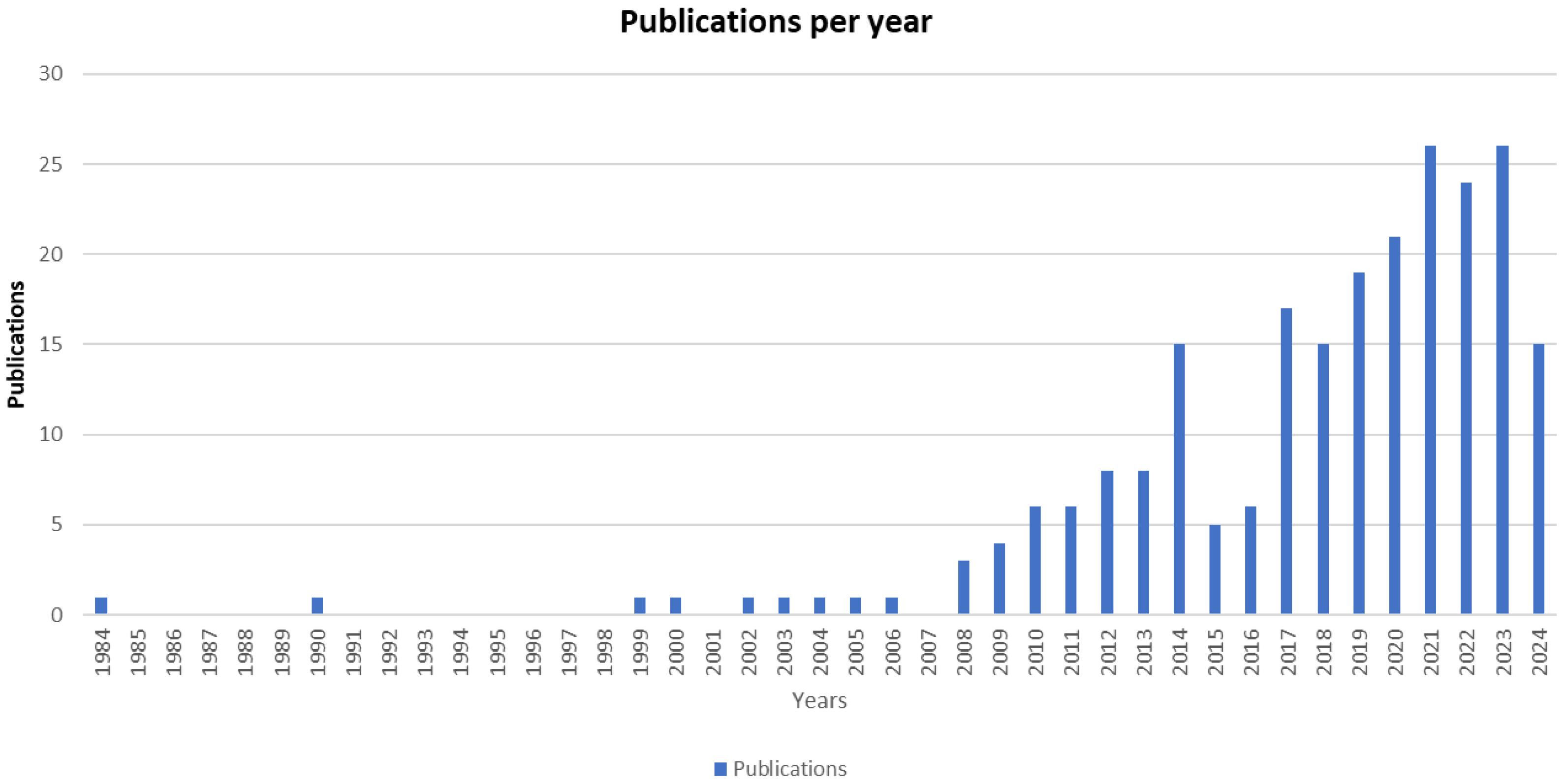




| Year | NP | TC | TC/NP | Percentage (%) |
|---|---|---|---|---|
| 1984 | 1 | 111 | 111.00 | 0.4 |
| 1990 | 1 | 64 | 64.00 | 0.4 |
| 1999 | 1 | 129 | 129.00 | 0.4 |
| 2000 | 1 | 88 | 88.00 | 0.4 |
| 2002 | 1 | 361 | 361.00 | 0.4 |
| 2003 | 1 | 171 | 171.00 | 0.4 |
| 2004 | 1 | 248 | 248.00 | 0.4 |
| 2005 | 1 | 20 | 20.00 | 0.4 |
| 2006 | 1 | 406 | 406.00 | 0.4 |
| 2008 | 3 | 588 | 196.00 | 1.3 |
| 2009 | 4 | 955 | 238.75 | 1.7 |
| 2010 | 6 | 777 | 129.50 | 2.6 |
| 2011 | 6 | 343 | 57.17 | 2.6 |
| 2012 | 8 | 665 | 83.12 | 3.4 |
| 2013 | 7 | 562 | 104.75 | 3.0 |
| 2014 | 15 | 1250 | 83.33 | 6.5 |
| 2015 | 5 | 286 | 57.20 | 2.2 |
| 2016 | 6 | 250 | 41.67 | 2.6 |
| 2017 | 17 | 1135 | 66.76 | 7.3 |
| 2018 | 15 | 567 | 37.80 | 6.5 |
| 2019 | 19 | 855 | 45.00 | 8.2 |
| 2020 | 21 | 518 | 24.67 | 9.1 |
| 2021 | 26 | 440 | 16.92 | 11.2 |
| 2022 | 24 | 153 | 6.38 | 10.3 |
| 2023 | 26 | 69 | 2.65 | 11.2 |
| 2024 ^ | 15 | 11 | 0.73 | 6.5 |
| SCR | First Author and Year | Title | Journal (IF-2023) | TC | TC/Y |
|---|---|---|---|---|---|
| 1 | Piche T, 2009 [44] | Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators | Gut (23, Q1) | 454 | 28.38 |
| 2 | Dunlop SP, 2006 [45] | Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes | American Journal of Gastroenterology (8, Q1) | 406 | 21.37 |
| 3 | De Palma G, 2017 [46] | Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice | Science Translational Medicine (15.8, Q1) | 374 | 46.75 |
| 4 | Tibble JA, 2002 [47] | Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease | Gastroenterology (25.7, Q1) | 361 | 15.70 |
| 5 | Zhou Q, 2009 [48] | Intestinal membrane permeability and hypersensitivity in irritable bowel syndrome | Pain (5.9, Q1) | 320 | 20.00 |
| 6 | Gecse K, 2008 [49] | Increased fecal serine protease activity in diarrhea IBS patients: a colonic luminal factor impairing colonic permeability and sensitivity | Gut (23, Q1) | 280 | 16.47 |
| 7 | Jalanka-Tuovinen J, 2014 [50] | Fecal microbiota composition and host-microbe cross-talk following gastroenteritis and in post infectious irritable bowel syndrome | Gut (23, Q1) | 252 | 22.91 |
| 8 | Marshall JK, 2004 [51] | Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario | Alimentary Pharmacology & Therapeutics (6.6, Q1) | 248 | 11.81 |
| 9 | Bertiaux-Vandaële N, 2011 [52] | The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype | American Journal of Gastroenterology (8, Q1) | 237 | 16.93 |
| 10 | Xu D, 2014 [53] | Rifaximin Alters Intestinal Bacteria and Prevents Stress-Induced Gut Inflammation and Visceral Hyperalgesia in Rats | Gastroenterology (25.7, Q1) | 233 | 21.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallardi, D.; Maqoud, F.; Guido, D.; Aloisio, M.; Linsalata, M.; Russo, F. Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis. Nutrients 2025, 17, 1064. https://doi.org/10.3390/nu17061064
Mallardi D, Maqoud F, Guido D, Aloisio M, Linsalata M, Russo F. Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis. Nutrients. 2025; 17(6):1064. https://doi.org/10.3390/nu17061064
Chicago/Turabian StyleMallardi, Domenica, Fatima Maqoud, Davide Guido, Michelangelo Aloisio, Michele Linsalata, and Francesco Russo. 2025. "Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis" Nutrients 17, no. 6: 1064. https://doi.org/10.3390/nu17061064
APA StyleMallardi, D., Maqoud, F., Guido, D., Aloisio, M., Linsalata, M., & Russo, F. (2025). Mapping Research Trends on Intestinal Permeability in Irritable Bowel Syndrome with a Focus on Nutrition: A Bibliometric Analysis. Nutrients, 17(6), 1064. https://doi.org/10.3390/nu17061064








