Evaluating the Components, Nutrients, and Antioxidant and Anti-Inflammatory Properties of Centranthera grandiflora Benth Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Crude C. Grandiflora Benth Extract (CGE) Preparation
2.3. Characterization of Phytochemical Composition
2.3.1. UPLC-ESI-Q-TOF/MS
2.3.2. Proximate Composition Analyses
2.3.3. Mineral Analysis
2.3.4. Analyses of Amino Acid Composition
2.4. Acute Toxicity Analyses
2.5. Network Pharmacology Analyses
2.6. Molecular Docking
2.7. Assessment of Antioxidant Activities
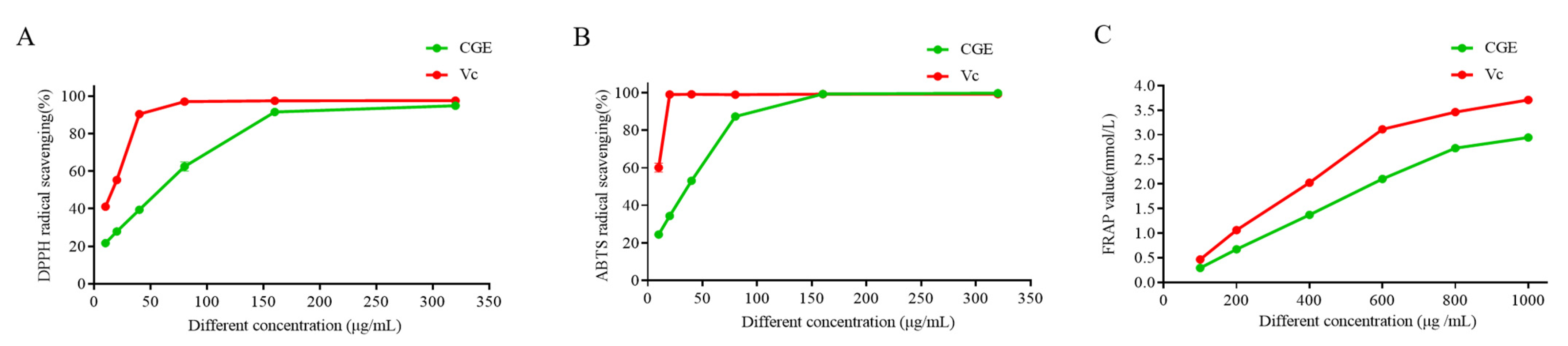
2.7.1. DPPH Scavenging Assay
2.7.2. ABTS Assays
2.7.3. FRAP Assay
2.8. Cellular Analysis of the Antioxidant and Anti-Inflammatory Properties of CGE
2.8.1. Cells
2.8.2. Cell Viability
2.8.3. Intracellular ROS Analyses
2.8.4. MDA, SOD, and GSH-Px Analyses
2.8.5. Analyses of NO and Inflammatory Cytokine Production
2.8.6. Western Immunoblotting
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analysis
3.1.1. UPLC-ESI-Q-TOF/MS Analysis
3.1.2. Proximate Compositional Analyses
3.1.3. Mineral Composition Analyses
3.1.4. Amino Acid Composition Analyses
3.1.5. Toxicity
3.2. Network Pharmacology
3.3. Molecular Docking
3.4. Antioxidant Activity Analyses
3.5. CGE Protects Against Oxidative Injury in LPS-Treated RAW264.7 Cells
3.5.1. Evaluation of the Effects of CGE on RAW264.7 Viability
3.5.2. Analyses of LPS-Induced ROS Biogenesis
3.5.3. Analyses of MDA, SOD, and GSH-Px Concentrations in LPS-Stimulated RAW264.7 Cells
3.6. CGE Exhibits Anti-Inflammatory Activity
3.6.1. CGE Alters LPS-Induced NO and Inflammatory Mediator Production in RAW264.7 Cells
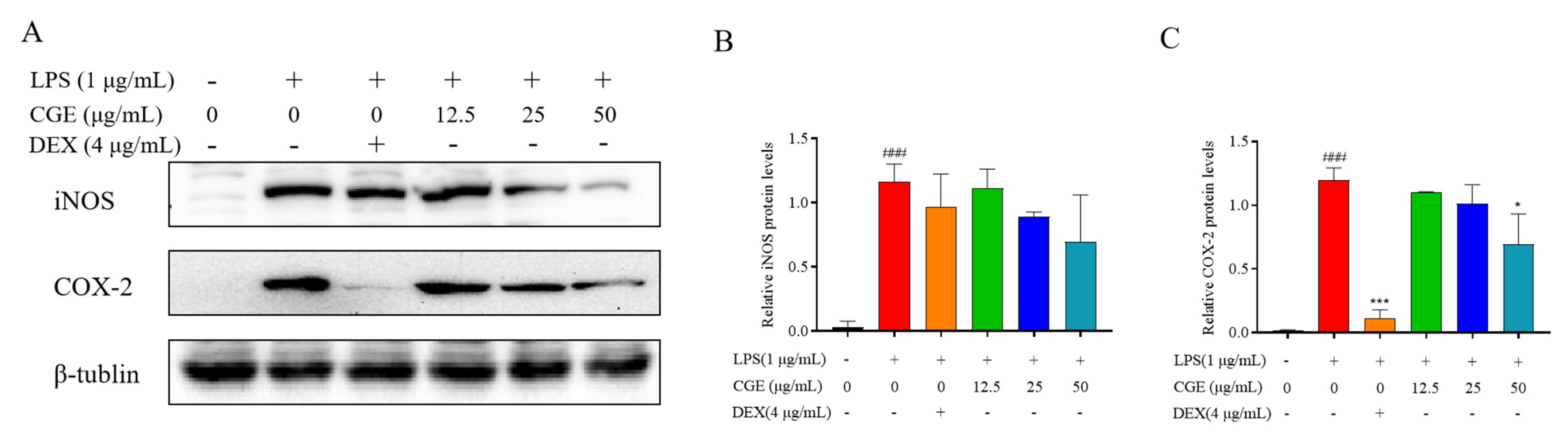
3.6.2. CGE Affects Inflammation-Associated Levels of iNOS and COX-2
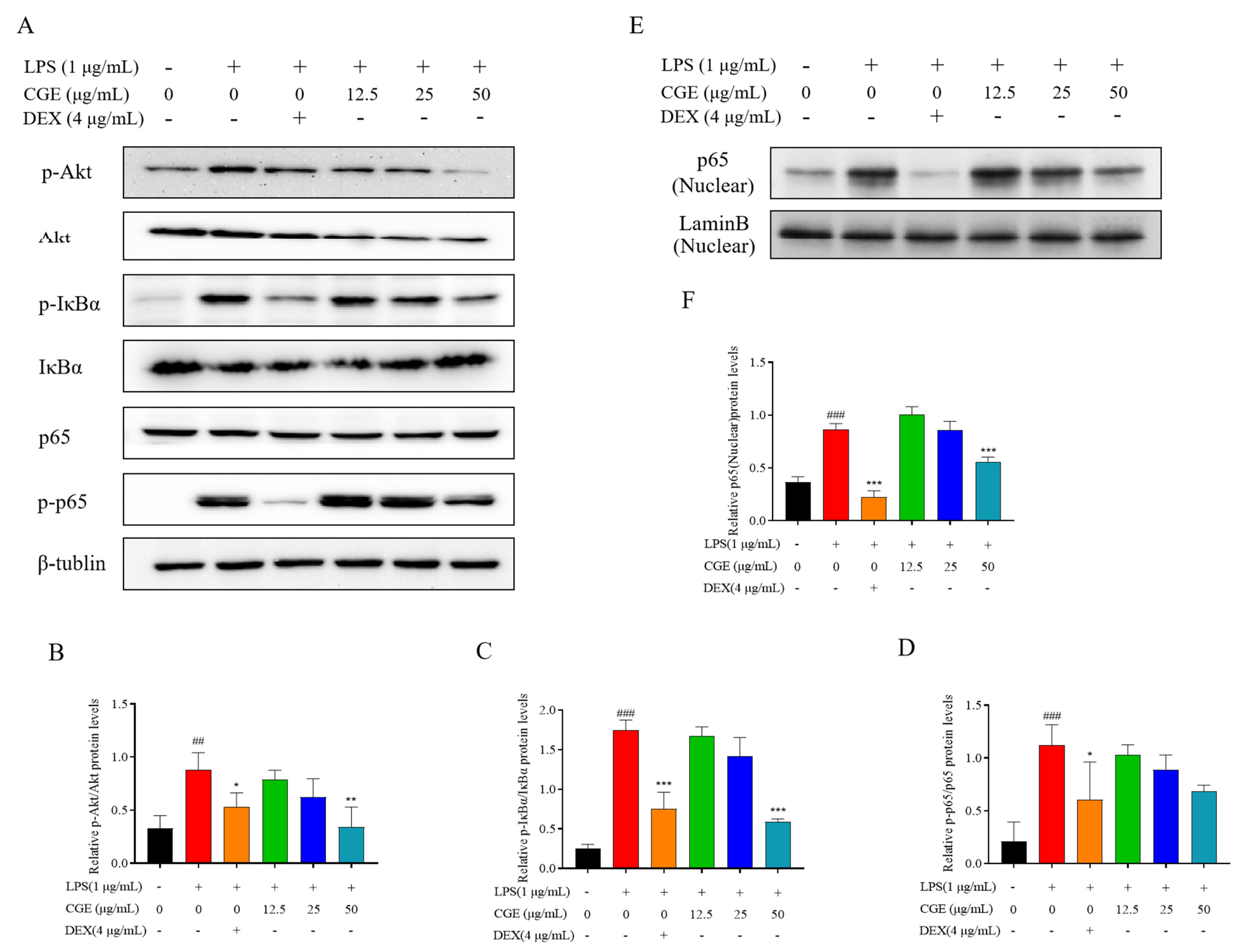
3.6.3. CGE Modulates the NF-κB Axis in RAW264.7 Cells
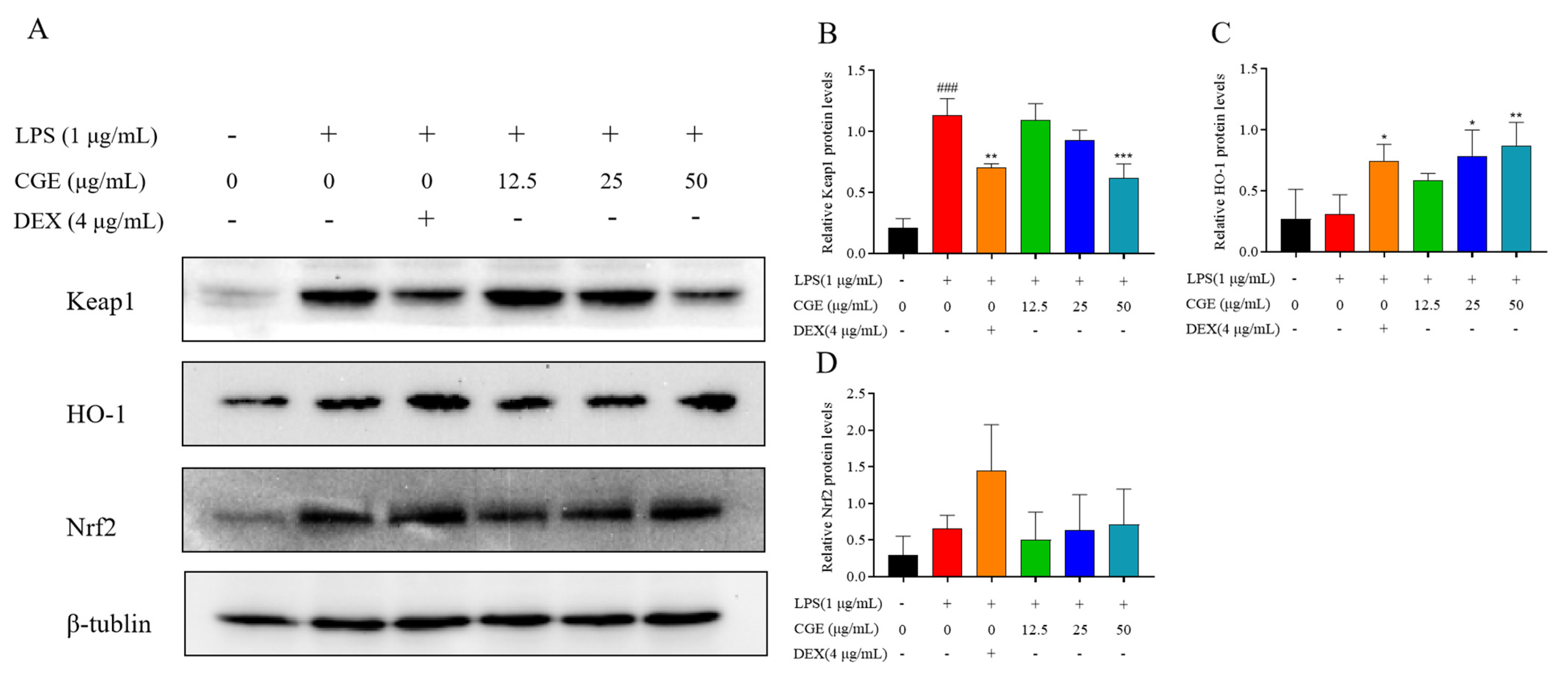
3.6.4. CGE Also Modulates Keap1-Nrf2 Pathway Signaling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.B.; Wang, Y.F.; Huang, J.L.; Dong, Z.Q.; Xiao, P.G. Analysis on patents of health care products with substances of medicine food homology in China. Chin. Herb. Med. 2024, 16, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1979; pp. 344–346. [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Liao, L.P.; Zhang, Z.J.; Hu, Z.B.; Chou, G.X.; Wang, Z.T. Iridoid glycosides from Centranthera grandiflora. Chin. Tradit. Herb. Drugs 2012, 43, 2369–2371. [Google Scholar]
- Liang, J.Z.; Zhang, J.S.; Ma, X.B.; Wang, G.L.; Chen, M.Q.; Wen, Y.C.; Gan, L.X. Identification of chemical constituents from Centranthera grandiflora. Chin. Bull. Bot. 1984, Z1, 43–47. [Google Scholar]
- Zhang, X.D.; Li, C.X.; Wang, L.C.; Fei, Y.H.; Qin, W.S. Analysis of Centranthera grandiflora Benth Transcriptome Explores Genes of Catalpol, Acteoside and Azafrin Biosynthesis. Int. J. Mol. Sci. 2019, 20, 6034. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chou, G.X.; Li, Q.L. Cardioprotective role of azafrin in against myocardial injury in rats via activation of the Nrf2-ARE pathway. Phytomedicine 2018, 47, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shan, R.; Chen, Y.; Zhu, Y.; Du, B.; Li, H.L.; Yao, Z. The effect of total Iridoid glycosides of Centranthera grandiflora Benth on the liver function of nonalcoholic fatty liver rats. Chin. J. Ethnomed. Ethnopharm. 2021, 30, 25–28. [Google Scholar]
- Liao, L.P. Investigation into the Bioactive Components and Chemical Constituents from the Roots of Centranthera Grandiflora Benth; Shanghai University of Traditional Chinese Medicine: Shanghai, China, 2014. [Google Scholar]
- Gao, X.M.; Dong, W.H.; Xia, C.L.; Ma, Z.Y.; Wang, Y.; Sarra, S.; Xu, H.M.; Qi, W.Y. Centranthera grandiflore alleviates alcohol-induced oxidative stress and cell apoptosis. Chin. J. Nat. Med. 2022, 20, 572–579. [Google Scholar] [CrossRef]
- Li, L.; Li, H.L.; Yan, H.L.; Ma, X.L.; Xiang, S.Q.; He, W.X.; Yao, Z. Response surface optimization of extraction process and antioxidant activity of total Iridoid glycosides from Centranthera grandijlora Benth. Chin. Food Addit. 2021, 32, 18–25. [Google Scholar] [CrossRef]
- Liang, X.X.; Li, Q.; Li, H.J.; Ning, Y.M.; Zhang, R.H.; Zhang, X.J.; Li, X.L.; Xiao, W.L. Centrantheroside F, a new ionone glycoside from Centranthera grandiflora. J. Asian Nat. Prod. 2022, 24, 777–783. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; Zhang, G.Y.; Tan, G.F.; Zhang, M. Pharmacognosy of Centranthera grandiflora Benth. Chin. Tradit. Pat. Med. 2021, 43, 540–543. [Google Scholar]
- Hang, M.F.; Zhang, L.Q.; Li, Y.M. Research progress on the chemical structure and pharmacological effects of aucubin and their derivatives. Chin. Tradit. Herb. Drugs 2017, 19, 4105–4113. [Google Scholar] [CrossRef]
- Tang, C.C.; Huang, H.P.; Lee, Y.J.; Tang, Y.H. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem. Toxicol. 2013, 62, 786–796. [Google Scholar] [CrossRef]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2018, 14, 76–85. [Google Scholar] [CrossRef]
- Yang, Y.J.; Tian, T.; Wang, Y.P.; Li, Z.; Xing, K.; Tian, G. SIRT6 protects vascular endothelial cells from angiotensin II-induced apoptosis and oxidative stress by promoting the activation of Nrf2/ARE signaling. Eur. J. Pharmacol. 2019, 859, 172516. [Google Scholar] [CrossRef]
- Wang, F.G.; Chen, S.X.; Deng, L.; Chen, L.; Huang, Y.W.; Tian, M.; Li, C.J.; Zhou, X. Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways. Molecules 2019, 24, 373. [Google Scholar] [CrossRef]
- GB/T 6435-2014; Determination of Moisture in Feedstuffs. National Standardization Administration, State Administration for Market Regulation, National Standards of the People’s Republic of China: Beijing, China, 2015.
- GB/T 6438-2007; Animal Feeding Stuffs—Determination of Crude Ash. National Standardization Administration, State Administration for Market Regulation, National Standardsof the People’s Republic of China: Beijing, China, 2007.
- GB/T 5009.88-2023; Determination of Dietary Fibre in Food. National Standardization Administration, State Administration for Market Regulation, National Standardsof the People’s Republic of China: Beijing, China, 2023.
- AOAC International. Official Methods of Analysis of AOAC International (AOAC), 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Z.S.; Cao, S.J.; Chang, Y.F.; Cao, Y.Q.; Li, J.B.; Yao, Z.X.; Wen, Y.P.; Huang, X.B.; Wu, R.; et al. Acute oral toxicity test and assessment of combined toxicity of cadmium and aflatoxin B1 in kunming mice. Food Chem. 2019, 131, 110577. [Google Scholar] [CrossRef]
- OECD. Acute Oral Toxicity—Fixed Dose Procedure (Chptr). In OECD Guideline for Testing of Chemical; OECD Publishing: Paris, France, 2001. [Google Scholar]
- Heon, T.K.; Jung, W.K.; Yeong, S.P.; Kim, H.; Chung, D.K. In Vitro Anti-Wrinkle and Skin-Moisturizing Effects of Evening Primrose (Oenothera biennis) Sprout and Identification of Its Active Components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, X.Y.; Shi, N.W.; Zhang, Z.Y.; Chen, Y.M.; Yan, M.Y.; Li, Y.P. Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.D.; Lee, S.H.; Kwak, C.H.; Chang, Y.C.; Lee, Y.C.; Chung, T.W.; Magae, J.; Kim, C.H. Ascochlorin induces caspase-independent necroptosis in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 239, 111898. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, Y.M.; Sun, X.; Guo, N.; Li, J.; Wang, W.; Yao, L.P.; Fu, Y.J. Hepatoprotective effect of 2′-O-galloylhyperin against oxidative stress-induced liver damage through induction of Nrf2/ARE-mediated antioxidant pathway. Food Chem. Toxicol. 2017, 102, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Tian, X.X.; Zhang, W.; Zheng, P.G.; Huang, F.F.; Ding, G.F.; Yang, Z.S. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nfr-Mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.C.; Chen, L.; Liu, R.J.; Chang, M.; Wang, X.G. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Pagire, S.H.; Lee, E.; Pagire, H.S.; Bae, E.J.; Ryu, S.J.; Lee, D.; Kim, M.H.; Kim, G.R.; Hwang, K.S.; Ahn, S.; et al. Design, synthesis and biological evaluation of glutamic acid derivatives as anti-oxidant and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2018, 28, 529–532. [Google Scholar] [CrossRef]
- Cesar, V.F.B.; Jan, S.; Robert, V.; Sandra, B.R.; Amelia, T.H. Indole alkaloids from Rauwolfia sellowii. Phytochemistry 1996, 41, 969–973. [Google Scholar] [CrossRef]
- Afzan, A.; Marcourt, L.; Abd, K.H.A.; Kasim, N.; Queiroz, E.F.; Osman, C.P.; Ismail, N.H.; Wolfender, J.L. Chemical characterizations dataset of flavonoid glycoside isomers and other constituents from Ficus deltoidea Jack. Data Brief. 2024, 54, 110414. [Google Scholar] [CrossRef]
- Gao, L.J.; De, J.S.; Daelemans, D.; Herdewijn, P. L-Aspartic and l-glutamic acid ester-based ProTides of anticancer nucleosides: Synthesis and antitumoral evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 2142–2146. [Google Scholar] [CrossRef]
- Hiroaki, N.; Hiroshi, S.; Takashi, M.; Masao, C.; Hiroshi, M. Six glycosides from Rehmannia glutinosa var. Purpurea. Phytochemisry 1990, 29, 3303–3306. [Google Scholar]
- Hiroshi, S.; Takashi, M.; Hiroaki, N.; Tatsunori, O.; Takao, K.; Kô, S.; Masao, C.; Hiroshi, M. Norcarotenoids of Rehmannia glutinosa var. Hueichingensis. Phytochemistry 1991, 30, 1997–2001. [Google Scholar] [CrossRef]
- Panida, Y.Y.; Suchada, S.K.; Muenduen, P. Isolation, separation and purification of rutin from Banana leaves (Musa balbisiana). Ind. Crops Prod. 2020, 149, 112307. [Google Scholar] [CrossRef]
- Jorda, R.; Magar, P.; Hendrychová, D.; Pauk, K.; Dibus, M.; Pilařová, E.; Imramovský, A.; Kryštof, V. Novel modified leucine and phenylalanine dipeptides modulate viability and attachment of cancer cells. Eur. J. Med. Chem. 2020, 188, 112036. [Google Scholar] [CrossRef]
- Wu, J.G.; Yang, X.L.; Ge, J.; Zhang, Y.P.; Wu, L.; Liu, J.M.; Zhang, X.Y. Biotransformation of sophoricoside in Fructus sophorae by the fungus Schizophyllum commune. Bioresour. Technol. 2012, 111, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.; Freire, C.S.; Pascoal, N.C.; Silvestre, A.J.; Silva, A.M. Chemical composition of the epicuticular wax from the fruits of Eucalyptus globulus. Phytochem. Anal. 2005, 16, 364–369. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Xu, J.J.; Guo, J.X.; Peng, H.L.; Zhang, Y. A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress. Molecules 2023, 29, 215. [Google Scholar] [CrossRef]
- Park, J.S.; Park, E.M.; Kim, D.Y.; Jung, K.; Jung, J.S.; Lee, E.J.; Hyun, J.W.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [CrossRef]
- He, S.Q.; Xu, Z.Y.; Li, J.; Guo, Y.P.; Lin, Q.X.; Jin, H.X. Peptides from Harpadon nehereus protect against hyperglycemia-induced HepG2 via oxidative stress and glycolipid metabolism regulation. J. Funct. Foods 2023, 108, 105723. [Google Scholar] [CrossRef]
- Chidambaram, B.S.; Anand, N.; Varma, R.S.; Ramamurthy, S.V.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.; Musthafa, M.E. Superoxide dismutase and neurological disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.; Yerizel, E.; Rifa, H. Moringa Oleifera Leaf Extract as an Antioxidant on Malondialdehyde (MDA) and Glutathione Peroxidase (GSH-Px) Levels in Rats with Hyperglycemia: A Systematic Literature Review. Int. J. Res. Rev. 2023, 10, 26–34. [Google Scholar] [CrossRef]
- Huang, K.K.; Liang, Y.L.; Wang, K.; Luo, H.D.; Yi, B. Influence of circulating nesfatin-1, GSH and SOD on insulin secretion in the development of T2DM. Front. Public Health. 2022, 10, 882686. [Google Scholar] [CrossRef]
- Rosenfeld, M.E. Inflammation and atherosclerosis: Direct versus indirect mechanisms. Curr. Opin. Pharmacol. 2013, 13, 154–160. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P.; Allavena. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Liu, T.C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef]
- Maituoheti, R.; Rouzimaimaiti, R.; Xu, N.N.; Zhao, J.; Aisa, H.A. seco-iridoid glycosides and flavonoid glycosides from the Gentiana olivieri Griseb and their anti-inflammatory activities. Fitoterapia 2024, 177, 106049. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, L.; Olatunde, O.O.; Singh, S.; Ovatlarnporn, C.; Basit, A.; Olatunji, O.J. UPLC–ESI–QTOF–MS profiling, antioxidant, antidiabetic, antibacterial, anti-inflammatory, antiproliferative activities and in silico molecular docking analysis of Barleria strigose. Chem. Biol. Technol. Agric. 2023, 10, 73. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.A.; Wang, Y.M.; Yang, Y.; Huo, T.; Yang, S.M.; Ju, Y.J.; Quan, Q.H. Aloe Extracts Inhibit Skin Inflammatory Responses by Regulating NF-κB, ERK, and JNK Signaling Pathways in an LPS-Induced RAW264.7 Macrophages Model. Clin. Cosmet. Investig. Dermatol. 2023, 16, 267–278. [Google Scholar] [CrossRef]
- Xue, Q.J.; Yan, Y.C.; Zhang, R.H.; Xiong, H.B. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Liang, J.; Yuan, S.W.; Wang, X.H.; Lei, Y.; Zhang, X.M.; Huang, M.C.; Ouyang, H. Attenuation of pristimerin on TNF-α-induced endothelial inflammation. Int. Immunopharmacol. 2020, 82, 106326. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, J.; Guo, L.C.; Xu, Y.; Hu, L.M.; Mao, H.P.; Miao, L.; Zhang, H.; Chai, L.J. Neobavaisoflavone ameliorates LPS-induced RAW264.7 cell inflammations by suppressing the activation of NF-κB and MAPKs signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 1021–1027. [Google Scholar] [CrossRef]
- Lee, E.H.; Cho, J.H.; Kim, D.H.; Hong, S.H.; Kim, N.H.; Park, M.J.; Hong, E.J.; Cho, Y.J. Anti-inflammatory activity of manassantin A from ultra-fine ground Saururus chinensis in lipopolysaccharide-stimulated RAW 264.7 cells. Appl. Biol. Chem. 2017, 60, 63–71. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Morsy, M.A.; Eladaly, M.; Kamel, B.A.; AbdelGaber, S.A. Pregnenolone protects the liver against doxorubicin-induced cellular injury by anti-inflammatory, antioxidant, and antiapoptotic mechanisms: Role of Keap1/Nrf2/HO-1 and P-glycoprotein. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4718–4734. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Munhoz, C.D. ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front. Pharmacol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.X.Y.; Li, R.L.; Peng, W.; Wu, C.J. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
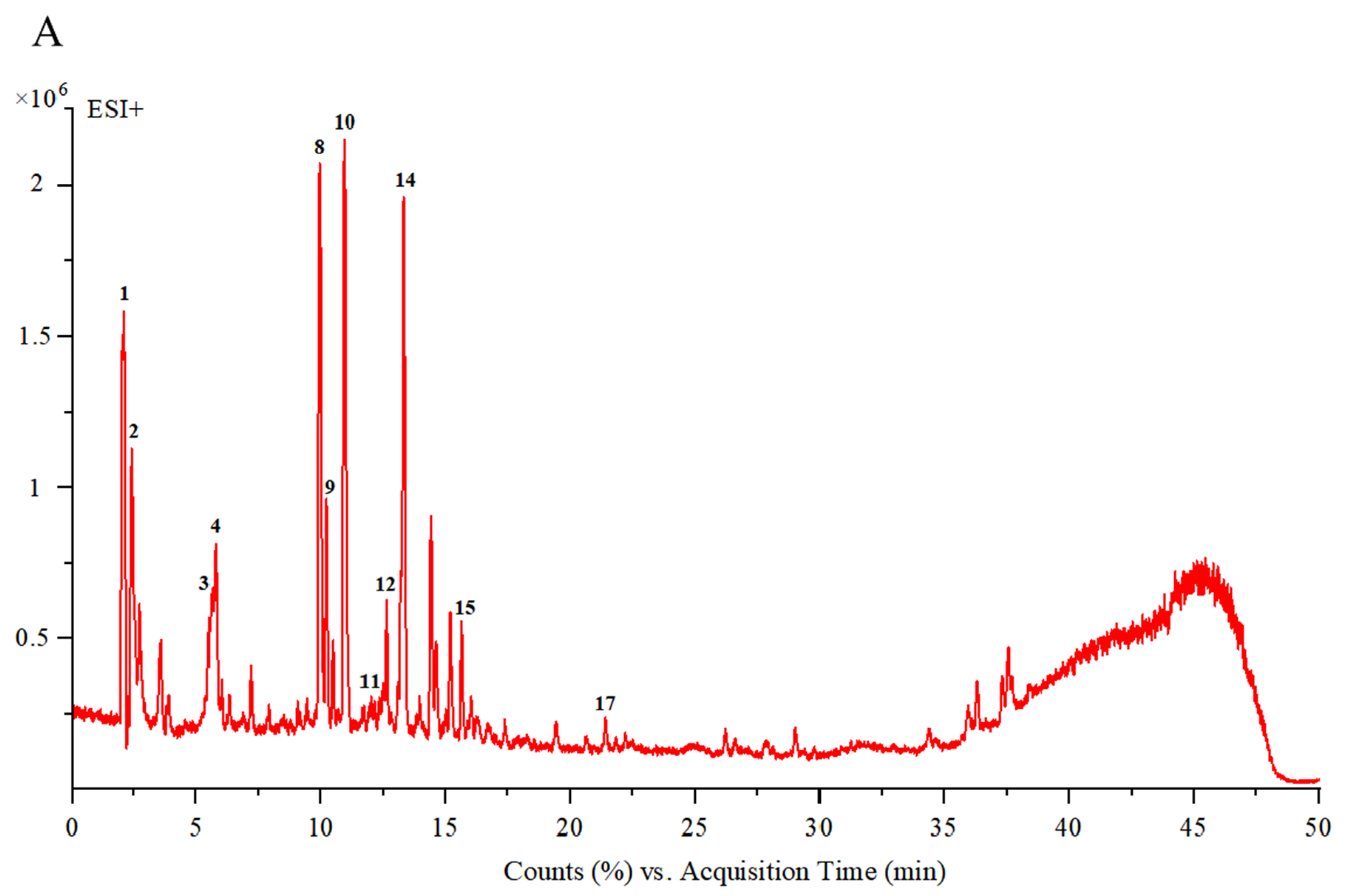
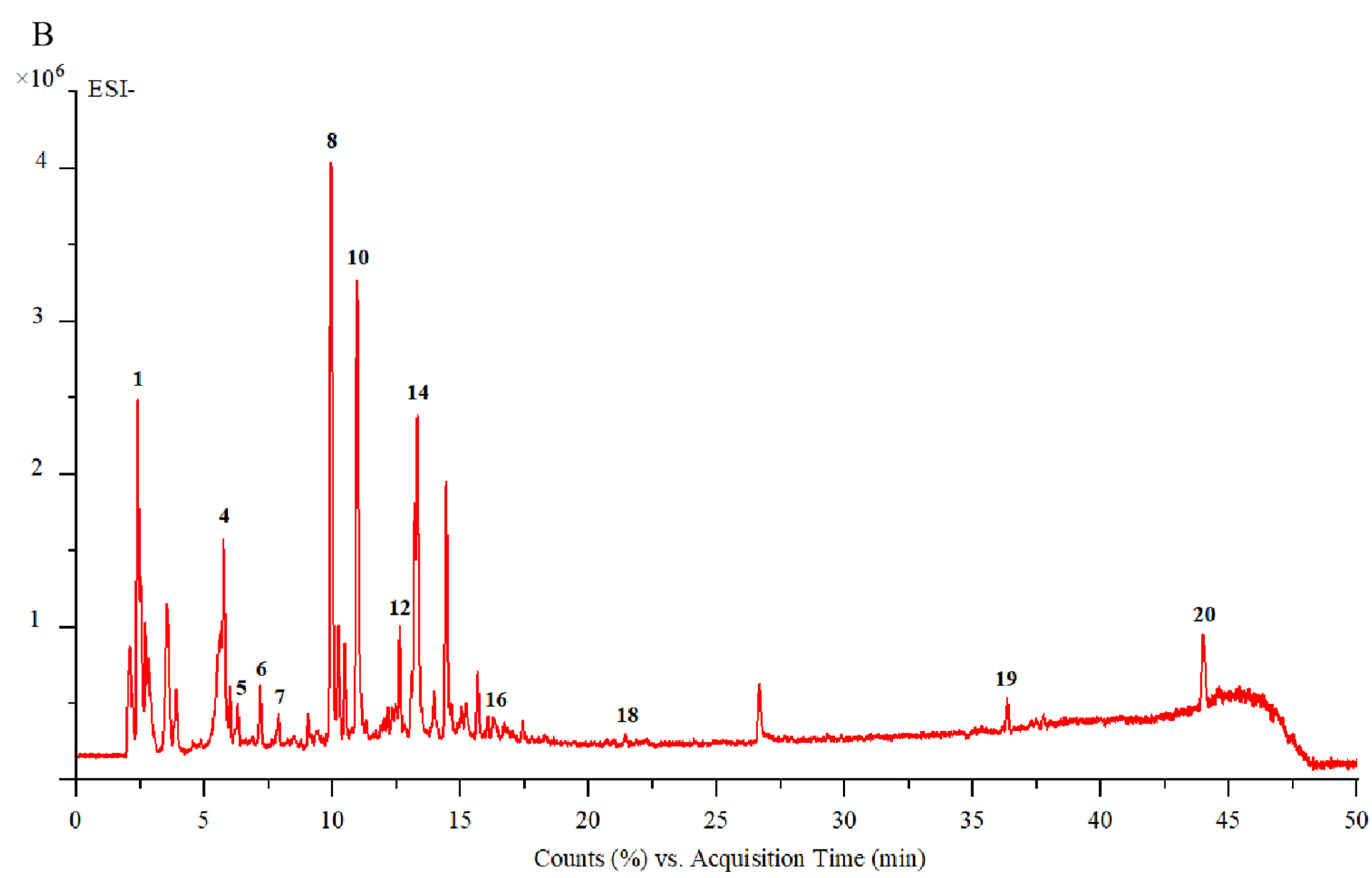




| NO. | RT (Min) | Ionization Mode | Experimental m/z | MS/MS Fragments | Tentative Compound | Molecular Formula | Molecular Weight | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.07 | [M−H]+ | 146.9962 | 146,129,101,84 | Glutamate | C5H9NO4 | 147 | [33] |
| 2 | 2.39 | [M+H]+ | 183.0862 | 129,116,104,61 | Mannitol | C6H14O6 | 182 | [9] |
| 3 | 5.69 | [M+H]+ | 347.1256 | 347,311,268,168,61 | Aucubin | C15H22O9 | 346 | [4] |
| 4 | 5.74 | [M+K]+ | 389.1240 | 327,293,167,131,103 | Vomilenine | C21H22N2O3 | 350 | [34] |
| 5 | 6.02 | [M−H]− | 577.1559 | 577,533,473,327,298 | Vitexin 2″-O-rhamnoside | C27H30O14 | 578 | [35] |
| 6 | 7.15 | [M−H]− | 373.1134 | 373,248,198,149,105 | Geniposidic acid | C16H24O10 | 374 | [4] |
| 7 | 7.86 | [M−H]− | 375.4306 | 375,220,162,84 | Mussaenosidic acid | C16H24O10 | 376 | [4] |
| 8 | 9.95 | [M+H]+ | 405.2121 | 405,287,225,207 | Melasmoside | C19H32O9 | 404 | [9] |
| 9 | 10.21 | [M+H]+ | 134.1559 | 134,116,105,88,70 | Aspartic acid | C4H7NO4 | 133 | [36] |
| 10 | 10.95 | [M+H]+ | 391.1619 | 391,373,229,193,179 | Mussaenoside | C17H26O10 | 390 | [4] |
| 11 | 12.01 | [M+Na]+ | 455.4532 | 455,413,121 | 2-methoxy-4-methy-lphenyl-O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside | C19H28O11 | 432 | [37] |
| 12 | 12.65 | [M+H]+ | 243.1581 | 225,127,189,165 | Trihydroxy-β-violetone | C13H22O4 | 242 | [38] |
| 13 | 13.21 | [M+CH3COO]− | 669.1829 | 609,520,491,367,248 | Rutin | C27H30O16 | 610 | [39] |
| 14 | 13.30 | [M+H]+ | 132.1016 | 132,129,86 | Leucine | C5H9NO4 | 131 | [40] |
| 15 | 15.70 | [M+Na−H2O]+ | 437.2151 | 415,397,305,281,217 | Centrantheroside A | C21H36O9 | 432 | [9] |
| 16 | 15.90 | [M+CH3COO-H2O]− | 401.1457 | 248,187,154 | Catalpol | C16H24O9 | 360 | [4] |
| 17 | 21.46 | [M+H]+ | 227.1641 | 227,209,188,141 | Dihydroxy-β-violetone | C13H22O3 | 226 | [38] |
| 18 | 22.24 | [M+CH3COO]− | 491.1205 | 491,403,316,197 | Sophoricoside | C21H20O10 | 432 | [41] |
| 19 | 26.21 | [M−H]− | 425.2707 | 425,384,355,311 | Azafrin | C27H38O4 | 426 | [5] |
| 20 | 44.27 | [M−H]− | 455.3541 | 455,397,339,275 | Ursolic acid | C30H48O3 | 456 | [42] |
| Proximate Composition (%) | Minerals (mg per 100 g) | Amino Acids (mg per 100 g) | |||
|---|---|---|---|---|---|
| Name | Content | Name | Content | Name | Content |
| Carbohydrates | 43.00 | Fe | 292.72 | Asp | 3.79 |
| Fiber | 35.94 | Mg | 443.14 | Thr | 1.87 |
| Ash | 12.26 | Na | 61.74 | Ser | 2.93 |
| Protein | 8.77 | P | 151.89 | Glu | 4.08 |
| Moisture | 8.67 | Ca | 276.76 | Gly | 2.09 |
| Lipids | 0.04 | K | 760.02 | Ala | 1.51 |
| Cu | 2.52 | Val | 1.74 | ||
| Zn | 5.61 | Met | 1.01 | ||
| Cr | 1.63 | Ile | 2.05 | ||
| Total | 2601.03 | Leu | 3.19 | ||
| Tyr | 2.18 | ||||
| Phe | 1.82 | ||||
| His | 0.14 | ||||
| Lys | 0.54 | ||||
| Total | 28.92 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Liu, X.; Wang, X.; Nian, Z.; Wu, X.; Zi, C.; Xu, S.; Shen, X.; Wang, X. Evaluating the Components, Nutrients, and Antioxidant and Anti-Inflammatory Properties of Centranthera grandiflora Benth Extracts. Nutrients 2025, 17, 925. https://doi.org/10.3390/nu17050925
Yuan W, Liu X, Wang X, Nian Z, Wu X, Zi C, Xu S, Shen X, Wang X. Evaluating the Components, Nutrients, and Antioxidant and Anti-Inflammatory Properties of Centranthera grandiflora Benth Extracts. Nutrients. 2025; 17(5):925. https://doi.org/10.3390/nu17050925
Chicago/Turabian StyleYuan, Wenjuan, Xinlan Liu, Xinting Wang, Zejin Nian, Xiaoyun Wu, Chengting Zi, Sha Xu, Xiaojing Shen, and Xuanjun Wang. 2025. "Evaluating the Components, Nutrients, and Antioxidant and Anti-Inflammatory Properties of Centranthera grandiflora Benth Extracts" Nutrients 17, no. 5: 925. https://doi.org/10.3390/nu17050925
APA StyleYuan, W., Liu, X., Wang, X., Nian, Z., Wu, X., Zi, C., Xu, S., Shen, X., & Wang, X. (2025). Evaluating the Components, Nutrients, and Antioxidant and Anti-Inflammatory Properties of Centranthera grandiflora Benth Extracts. Nutrients, 17(5), 925. https://doi.org/10.3390/nu17050925






