Prognostic Impact of Malnutrition Evaluated via Bioelectrical Impedance Vector Analysis (BIVA) in Acute Ischemic Stroke: Findings from an Inverse Probability Weighting Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Bioelectrical Impedance Analysis
2.3. Primary Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, C.L.; Bayraktutan, U. Risk factors for ischaemic stroke. Int. J. Stroke 2008, 3, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tian, H.; Ji, T.; Zhong, T.; Gao, L.; Chen, L. Predictive Value of Malnutrition, Identified via Different Nutritional Screening or Assessment Tools, for Functional Outcomes in Patients with Stroke: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3280. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, S.; Wang, H.; Chen, J.; Zhang, X.; Xu, P.; Xie, Y.; Zhu, X.; Zhu, W.; Sun, W.; et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin. Nutr. 2021, 40, 2535–2542. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef]
- Di Vincenzo, O.; D’Elia, L.; Ballarin, G.; Pasanisi, F.; Scalfi, L. Controlling Nutritional Status (CONUT) score and the risk of mortality or impaired physical function in stroke patients: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, Y.; Wang, X.; Tian, X.; Pei, L.-L.; Wang, X.; Zhang, L.; Sun, W.; Wu, J.; Sun, S.; et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score Is Associated with Poor Outcome of Acute Ischemic Stroke. Front. Neurol. 2021, 11, 610318. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Zhu, Y.; Zhang, X.; Ni, J.; Li, Y. Malnutrition Defined by Geriatric Nutritional Risk Index Predicts Outcomes in Severe Stroke Patients: A Propensity Score-Matched Analysis. Nutrients 2022, 14, 4786. [Google Scholar] [CrossRef]
- Liu, M.; Sun, M.; Zhang, T.; Li, P.; Liu, J.; Liu, Y.; Song, Y.; Liu, S.; Yang, H.; Zhou, Z.; et al. Prognostic Nutritional Index (PNI) as a potential predictor and intervention target for perioperative ischemic stroke: A retrospective cohort study. BMC Anesthesiol. 2023, 23, 268. [Google Scholar] [CrossRef]
- McKenna, S.P.; Heaney, A. Composite outcome measurement in clinical research: The triumph of illusion over reality? J. Med. Econ. 2020, 23, 1196–1204. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (bia) and dual energy x-ray absorptiometry (dxa): A critical overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Kit-Chung Ng, J.; Lik-Fung Lau, S.; Chun-Kau Chan, G.; Tian, N.; Kam-Tao Li, P. Nutritional Assessments by Bioimpedance Technique in Dialysis Patients. Nutrients 2024, 16, 15. [Google Scholar]
- Lee, K.; Kim, J.; Lee, H.; Kang, J. Association Between Changes in Bioelectrical Impedance Analysis (BIA) Parameter and the Clinical Outcomes in Patients with Acute Heart Failure. J. Korean Med. Sci. 2023, 38, e276. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrazzoli, P.; Lobascio, F.; Masi, S.; Crotti, S.; Klersy, C.; Turri, A.; Stobäus, N.; Tank, M.; Franz, K.; et al. The prognostic impact of BIA-derived fat-free mass index in patients with cancer. Clin. Nutr. 2021, 40, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Pillon, L.; Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 2002, 18, 153–167. [Google Scholar] [CrossRef]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-valued Resource? Biol. Res. Nurs. 2023, 25, 240–249. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef]
- Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Recommendations on stroke prevention, diagnosis, and therapy. Stroke 1989, 20, 1407–1408. [Google Scholar]
- Nwosu, A.C.; Mayland, C.R.; Mason, S.; Cox, T.F.; Varro, A.; Stanley, S.; Ellershaw, J. Bioelectrical impedance vector analysis (BIVA) as a method to compare body composition differences according to cancer stage and type. Clin. Nutr. ESPEN 2019, 30, 59–66. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Liu, G.; Li, F. Predictive value of the random forest model based on bioelectrical impedance analysis parameter trajectories for short-term prognosis in stroke patients. Eur. J. Med. Res. 2024, 29, 382. [Google Scholar] [CrossRef]
- Siotto, M.; Germanotta, M.; Guerrini, A.; Pascali, S.; Cipollini, V.; Cortellini, L.; Ruco, E.; Khazrai, Y.M.; De Gara, L.; Aprile, I. Relationship between Nutritional Status, Food Consumption and Sarcopenia in Post-Stroke Rehabilitation: Preliminary Data. Nutrients 2022, 14, 4825. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, J.; Yang, Y.; Lee, J.; Jeon, G. Bioelectrical impedance analysis for severe stroke patients with upper extremity hemiplegia. J. Phys. Ther. Sci. 2016, 28, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Kim, Y.; Kim, M.-W. Correlation of Body Composition via Bioelectrical Impedance Analysis and Motor Function and Recovery of Upper Extremity in Patients Undergoing Stroke Rehabilitation. Brain Neurorehabilit. 2022, 15, e20. [Google Scholar] [CrossRef] [PubMed]
- Vanzella, L.M.; Lawand, R.; Shuaib, M.; Oh, P.; Corbett, D.; Marzolini, S. Validity of Bioelectric Impedance in Relation to Dual-Energy X-Ray Absorptiometry for Measuring Baseline and Change in Body Composition After an Exercise Program in Stroke. J. Strength Cond. Res. 2022, 36, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Mizushima, T. Assessment of changes in muscle mass, strength, and quality and activities of daily living in elderly stroke patients. Int. J. Rehabil. Res. 2022, 45, 161–167. [Google Scholar] [CrossRef]
- Bise, T.; Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Kido, Y.; Shimazu, S.; Shiraishi, A.; Matsumoto, A. Association between BIA-derived Phase Angle and Sarcopenia and Improvement in Activities of Daily Living and Dysphagia in Patients undergoing Post-Stroke Rehabilitation. J. Nutr. Heal. Aging 2022, 26, 590–597. [Google Scholar] [CrossRef]
- Abe, T.; Yoshimua, Y.; Imai, R.; Sato, Y. A Combined Assessment Method of Phase Angle and Skeletal Muscle Index to Better Predict Functional Recovery after Acute Stroke. J. Nutr. Heal. Aging 2022, 26, 445–451. [Google Scholar] [CrossRef]
- Ishiyama, D.; Toi, K.; Aoyagi, Y.; Suzuki, K.; Takayama, T.; Yazu, H.; Yoshida, M.; Kimura, K. The extracellular-to-total body water ratio reflects improvement in the activities of daily living in patients who experienced acute stroke. J. Stroke Cerebrovasc. Dis. 2024, 33, 107810. [Google Scholar] [CrossRef]
- Gheri, C.F.; Luisi, M.L.E.; Scalfi, L.; Di Vincenzo, O. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2024, 43, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C.; Brantlov, S. Bioimpedance basics and phase angle fundamentals. Rev. Endocr. Metab. Disord. 2023, 24, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A. Identification of operational clues to dry weight prescription in hemodialysis using bioimpedance vector analysis. Kidney Int. 1998, 53, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Bellido, D.; García-García, C.; Talluri, A.; Lukaski, H.C.; García-Almeida, J.M. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef]
- Flegal, K.M.; A Shepherd, J.; Looker, A.C.; I Graubard, B.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; E Everhart, J.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef]
- Wellens, R.I.; Roche, A.F.; Khamis, H.J.; Jackson, A.S.; Pollock, M.L.; Siervogel, R.M. Relationships between the body mass index and body composition. Obes. Res. 1996, 4, 35–44. [Google Scholar] [CrossRef]
- Xie, H.; Yuan, K.; Ruan, G.; Wei, L.; Zhang, H.; Ge, Y.; Lin, S.; Song, M.; Wang, Z.; Liu, C.; et al. Improving the assessment of malnutrition in cancer: Using systemic inflammation markers as a supplement to the inflammation items of the GLIM criteria. Clin. Nutr. 2023, 42, 2036–2044. [Google Scholar] [CrossRef]
- Patterson, G.T.; Osorio, E.Y.; Peniche, A.; Dann, S.M.; Cordova, E.; Preidis, G.A.; Suh, J.H.; Ito, I.; Saldarriaga, O.A.; Loeffelholz, M.; et al. Pathologic Inflammation in Malnutrition Is Driven by Proinflammatory Intestinal Microbiota, Large Intestine Barrier Dysfunction, and Translocation of Bacterial Lipopolysaccharide. Front. Immunol. 2022, 13, 846155. [Google Scholar] [CrossRef]
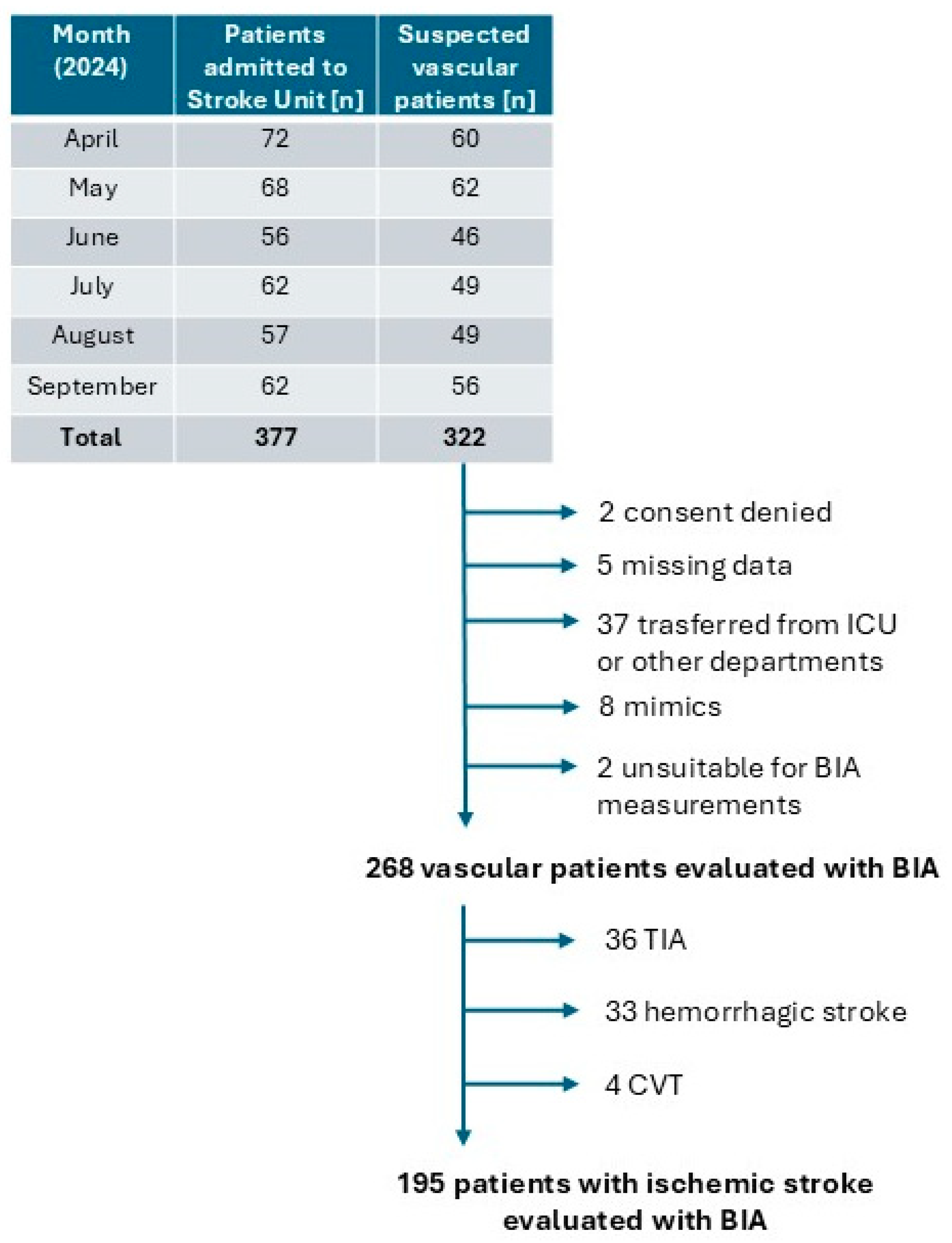
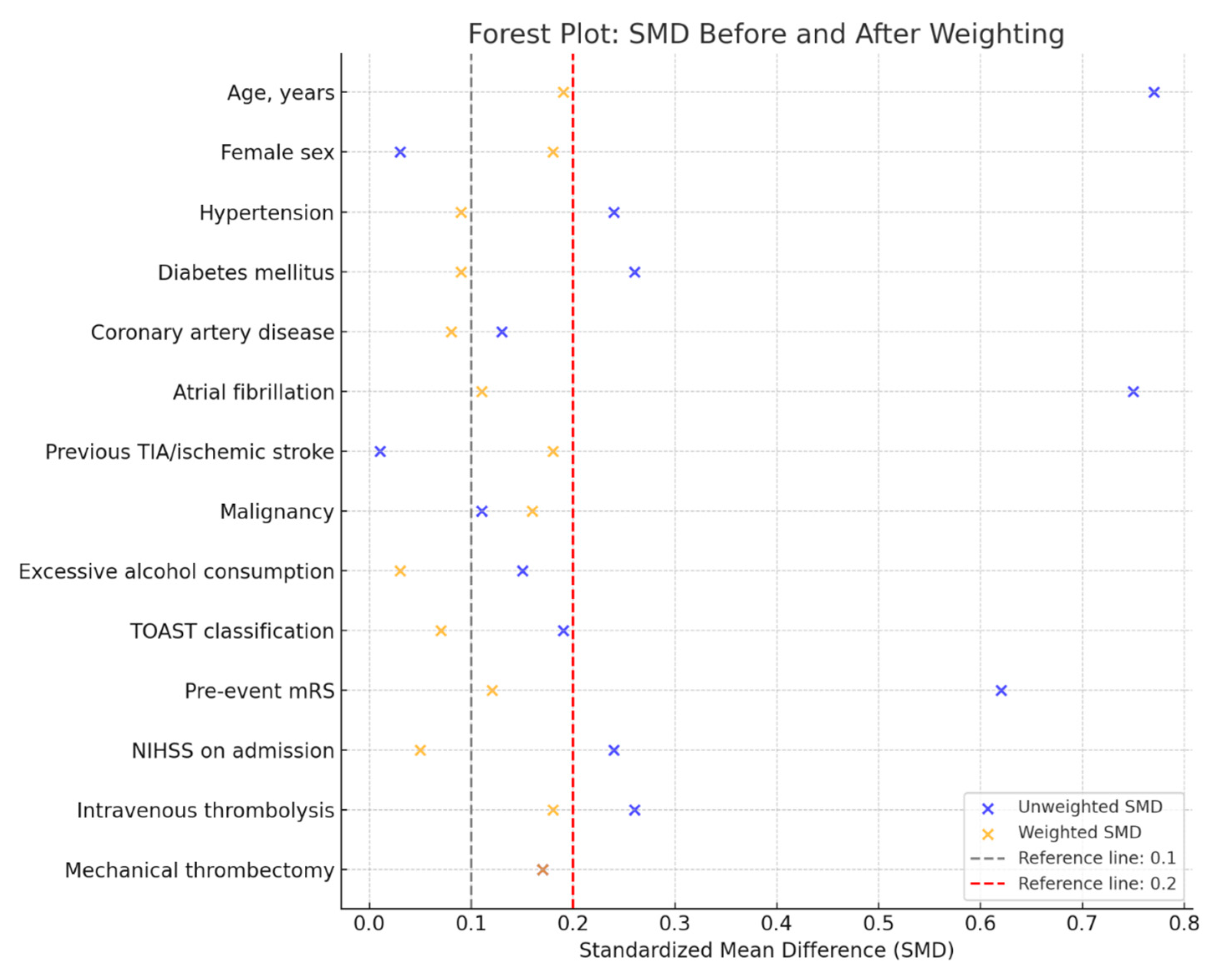

| Variables | Overall Population (N = 195) | Presence of Malnutrition (N = 37) | Absence of Malnutrition (N = 158) | SMD Unweighted | SMD Weighted |
|---|---|---|---|---|---|
| Age, years [median (IQR)] | 76 (65–82) | 80 (75–86) | 75 (63–82) | 0.77 | 0.19 |
| Female sex [n, (%)] | 87 (44.6) | 17 (45.9) | 70 (44.3) | 0.03 | 0.18 |
| Hypertension [n, (%)] | 148 (75.9) | 31 (83.8) | 117 (74.1) | 0.24 | 0.09 |
| Diabetes mellitus [n, (%)] | 36 (18.5) | 10 (27.0) | 26 (16.4) | 0.26 | 0.09 |
| Coronary artery disease [n, (%)] | 20 (10.2) | 5 (13.5) | 15 (9.5) | 0.13 | 0.08 |
| Atrial fibrillation [n, (%)] | 61 (31.3) | 22 (59.5) | 39 (24.7) | 0.75 | 0.11 |
| Previous TIA/ischemic stroke [n, (%)] | 26 (13.3) | 5 (13.5) | 21 (13.3) | 0.01 | 0.18 |
| Malignancy [n, (%)] | 35 (17.9) | 8 (21.6) | 27 (17.1) | 0.11 | 0.16 |
| Excessive alcohol consumption † [n, (%)] | 23 (11.8) | 3 (8.1) | 20 (12.7) | 0.15 | 0.03 |
| TOAST classification | 0.19 | 0.07 | |||
| Large artery atherosclerosis [n, (%)] | 25 (12.8) | 4 (10.8) | 21 (13.3) | ||
| Cardioembolism [n, (%)] | 62 (31.8) | 19 (51.3) | 43 (27.2) | ||
| Small vessel occlusion [n, (%)] | 30 (15.4) | 1 (2.7) | 29 (18.3) | ||
| Other determined cause [n, (%)] | 4 (2.1) | 0 (0.0) | 4 (2.5) | ||
| Undetermined cause [n, (%)] | 74 (37.9) | 13 (35.1) | 61 (38.6) | ||
| Pre-event mRS [median (IQR)] | 0 (0–1) | 1 (0–3) | 0 (0–1) | 0.62 | 0.12 |
| NIHSS on admission [median (IQR)] | 4 (2–9) | 5 (2–13) | 4 (2–8) | 0.24 | 0.05 |
| Intravenous thrombolysis [n, (%)] | 89 (45.6) | 13 (35.1) | 76 (48.1) | 0.26 | 0.18 |
| Mechanical thrombectomy [n, (%)] | 27 (13.8) | 7 (18.9) | 20 (12.7) | 0.17 | 0.17 |
| Variables | Overall Population (N = 195) | Presence of Malnutrition (N = 37) | Absence of Malnutrition (N = 158) | p |
|---|---|---|---|---|
| Lymphocytes, μL [median (IQR)] | 1440 (1110–1820) | 1210 (870–1720) | 1490 (1157–1835) | 0.013 |
| C-reactive protein, mg/L [median (IQR)] | 3 (1–11) | 5 (1–14) | 3 (1–10) | 0.582 |
| HbA1c, % [median (IQR)] | 5.9 (5.6–6.2) | 5.8 (5.6–6.1) | 5.9 (5.6–6.2) | 0.782 |
| Total protein, g/L [median (IQR)] | 65 (61–68) | 64 (60–67) | 65 (61–68) | 0.311 |
| Albumin, g/L [median (IQR)] | 40 (37.7–42) | 38 (36.7–40.2) | 40 (38–42.2) | 0.007 |
| Total cholesterol, mg/dL [median (IQR)] | 168 (135–204) | 147 (122–159) | 172 (138–211) | 0.001 |
| HDL, mg/dL [median (IQR)] | 48 (42–57) | 46 (43–59) | 49 (40–57) | 0.755 |
| LDL, mg/dL [median (IQR)] | 92 (67–125) | 67 (53–92) | 99 (74–128) | 0.001 |
| Triglycerides, mg/dL [median (IQR)] | 91 (72–123) | 88 (69–118) | 91 (74–127) | 0.171 |
| mRS Shift (Univariate) | |||
|---|---|---|---|
| Predictors | Common Odds Ratio | CI | p |
| Presence of malnutrition | 3.34 | 1.74–6.41 | 0.001 |
| mRS Shift (Multivariate) | |||
| Predictors | Adjusted Common Odds Ratio | CI | p |
| Presence of malnutrition | 2.79 | 1.37–5.70 | 0.005 |
| Female sex | 0.85 | 0.48–1.51 | 0.580 |
| NIHSS at admission (per unitary increase) | 1.19 | 1.11–1.28 | <0.001 |
| IVT | 0.28 | 0.15–0.52 | <0.001 |
| Mechanical thrombectomy | 0.41 | 0.14–1.18 | 0.097 |
| Lymphocytes (per unitary increase) | 1.01 | 1.00–1.02 | 0.027 |
| Albumin (per unitary increase) | 0.82 | 0.75–0.89 | <0.001 |
| Total cholesterol (per unitary increase) | 0.99 | 0.97–1.02 | 0.875 |
| LDL (per unitary increase) | 1.01 | 0.98–1.03 | 0.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bello, S.; Ceccarelli, L.; Tereshko, Y.; Gigli, G.L.; D’Anna, L.; Valente, M.; Merlino, G. Prognostic Impact of Malnutrition Evaluated via Bioelectrical Impedance Vector Analysis (BIVA) in Acute Ischemic Stroke: Findings from an Inverse Probability Weighting Analysis. Nutrients 2025, 17, 919. https://doi.org/10.3390/nu17050919
Dal Bello S, Ceccarelli L, Tereshko Y, Gigli GL, D’Anna L, Valente M, Merlino G. Prognostic Impact of Malnutrition Evaluated via Bioelectrical Impedance Vector Analysis (BIVA) in Acute Ischemic Stroke: Findings from an Inverse Probability Weighting Analysis. Nutrients. 2025; 17(5):919. https://doi.org/10.3390/nu17050919
Chicago/Turabian StyleDal Bello, Simone, Laura Ceccarelli, Yan Tereshko, Gian Luigi Gigli, Lucio D’Anna, Mariarosaria Valente, and Giovanni Merlino. 2025. "Prognostic Impact of Malnutrition Evaluated via Bioelectrical Impedance Vector Analysis (BIVA) in Acute Ischemic Stroke: Findings from an Inverse Probability Weighting Analysis" Nutrients 17, no. 5: 919. https://doi.org/10.3390/nu17050919
APA StyleDal Bello, S., Ceccarelli, L., Tereshko, Y., Gigli, G. L., D’Anna, L., Valente, M., & Merlino, G. (2025). Prognostic Impact of Malnutrition Evaluated via Bioelectrical Impedance Vector Analysis (BIVA) in Acute Ischemic Stroke: Findings from an Inverse Probability Weighting Analysis. Nutrients, 17(5), 919. https://doi.org/10.3390/nu17050919







