Cannabis: Zone Aspects of Raw Plant Components in Sport—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Main Components Found in Cannabis sativa Plant
3.1. Polyketide (PK) Pathway
3.2. Methyl-Erythritol Phosphate (MEP) Pathway
3.3. Mevalonate (MV) Pathway
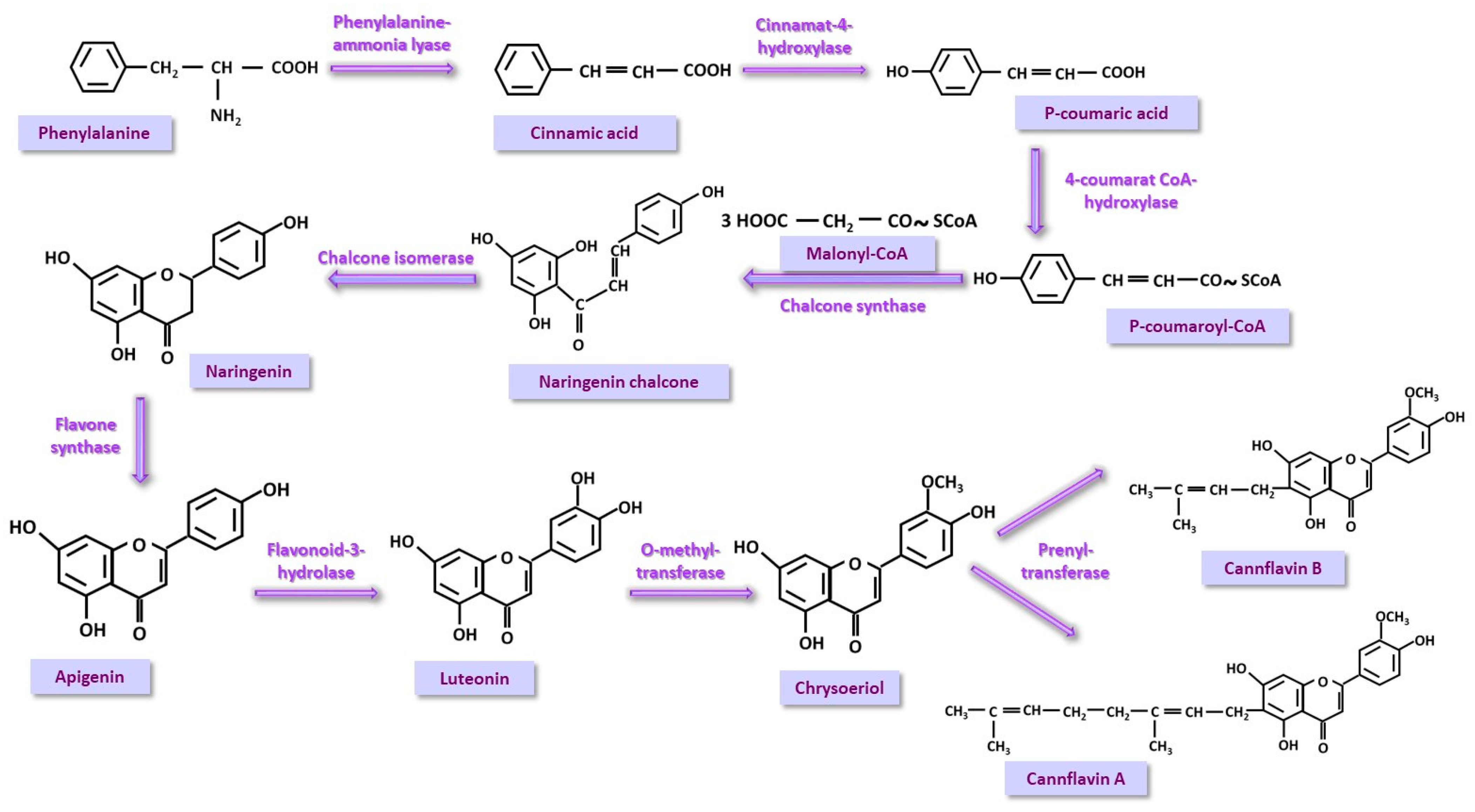
3.4. Phenyl-Propanoid (PP) Pathway
4. Dark Zone: Abuse and Prohibition in Sport
4.1. A Brief Discussion of Current Worldwide Legislative Implications
4.2. Important Pharmacokinetic Processes in Cannabinoid Detection
4.3. Detection Methods for Abuse
5. Light/Beneficial Zone: Therapeutic Effects for Athletes
5.1. THC
5.2. CBD
5.3. Cannabigerol
5.4. Monoterpenes
5.5. Sesquiterpenoids
5.6. Triterpenoids
5.7. Sterols
5.8. Cannflavins
6. Grey Zone: Discussions and Future Perspectives
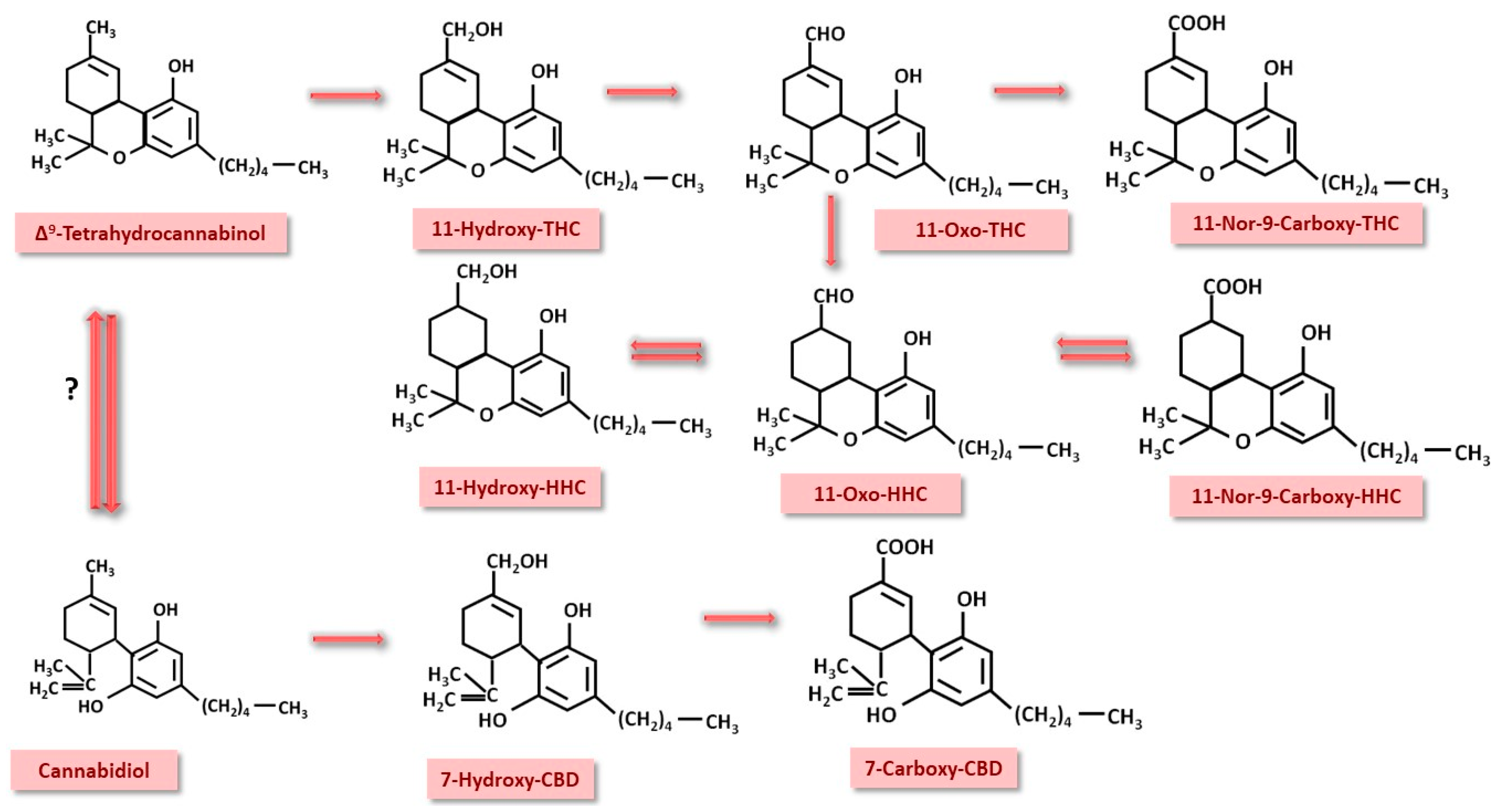
- If only CBD consumption is permitted, the use of CBD by a given athlete may generate traces of THC through the conversion of CBD to THC [90]. Furthermore, there is the possibility that 7-carboxy-CBD can be converted to 11-nor-9-carboxy-THC [174], generating false positive results. Studies on athletes on this subject are not yet complete and leave room for further research.
- Another discussion would be in the context of which beneficial effects occur in the case of natural extracts and to what extent they are attributed to the entourage effect [38,139]. Clinical results could be studied in the context of extraction and administration together and separately, and comparing the two aspects versus placebo in athletes. It is possible that the effect of CBD is enhanced by low doses of THC [38].
- Another aspect that needs to be considered is the perception of the sports physician about cannabinoids, both when and how they recommend them, knowing that THC accumulates in adipose tissue from where it is gradually released [78,80]. Here, too, it has been observed that young male physicians are more likely to accept the use of cannabinoids, not associating cannabis with the improvement of sports performance [175]. This aspect is of course in accordance with the legislation of different countries.
- Last but not least, when we talk about natural compounds, we are talking about extraction methods. The hypothetical discussion raised is related to the possibility of eliminating the THC compound from Cannabis sativa extract, which could make it possible to use the plant safely without interfering with legislative aspects.
- From another point of view, some athletes may find it convenient to cheat by relying on this legislative relaxation by consuming cannabis in order to achieve superior results in competitions. A recent study [176] aimed at finding out to what extent elite athletes (national, international competitions, Olympic or Paralympic games) dope, identified a percentage of 9.2% of athletes who consume prohibited substances during competitions. Of these, 4.2% are cannabinoid users. Of the cannabinoid users, 66.7% declared that they use two or more doping substances or methods. One of the limitations of this study lies in the honesty of the responses provided; the authors suspect that these data are actually underreported.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WADA | World Anti-doping Agency |
| THC | Δ9-tetrahydrocannabinol |
| CBD | cannabidiol |
| PK | polyketide |
| MEP | methyl-erythritol phosphate |
| MV | mevalonate |
| PP | phenyl-propanoid |
| TKS | tetraketide synthase |
| OAC | olivetolic acid cyclase |
| CNGAS | cannabigerolic acid synthase |
| THCS | tetrahydrocannabinol synthase |
| CNDAS | cannabidiolic acid synthase |
| HMBPP | hydroxyl-methyl-butenyl-diphosphate |
| DADP | dimethyl-allyl-diphosphate |
| IPDP | isopentenyl-diphosphate |
| HHC | hydrocannabinol |
| TRPV1 | Transient Receptor Potential Ion Channel 1 |
| TLR | toll-like receptor |
References
- World Anti-Doping Code International Standard Prohibited List. 2024. Available online: https://www.wada-ama.org (accessed on 16 December 2024).
- World Anti-Doping Agency. Summary of Major Modifications and Explanatory Notes. 2023 Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/2022-09/2023list_explanatory_list_en_final_26_september_2022.pdf (accessed on 16 December 2024).
- Maurer, G.E.; Mathews, N.M.; Schleich, K.T.; Slayman, T.G.; Marcussen, B.L. Understanding Cannabis-Based Therapeutics in Sports Medicine. Sports Health 2020, 12, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S., Jr. Cannabis Is Not Doping. Cannabis Cannabinoid Res. 2023, 6, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Hudzik, T.J.; Huestis, M.A.; Rossi, S.S.; Schumacher, Y.O.; Harcourt, P.; Budgett, R.; Stuart, M.; Tettey, J.; Mazzoni, I.; Rabin, O.; et al. Cannabis and sport: A World Anti-Doping perspective. Addiction 2023, 118, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Docter, S.; Khan, M.; Gohal, C.; Ravi, B.; Bhandari, M.; Gandhi, R.; Leroux, T. Cannabis Use and Sport: A Systematic Review. Sports Health 2020, 12, 189–199. [Google Scholar] [CrossRef]
- Benoy, R.; Ramirez, C.; Hitchcock, M.; Reardon, C. Cannabis Use in Adolescent and Young Adult Athletes: A Clinical Review. Sports Health 2024, 16, 279–284. [Google Scholar] [CrossRef]
- Zeiger, J.S.; Silvers, W.S.; Fleegler, E.M.; Zeiger, R.S. Attitudes about cannabis mediate the relationship between cannabis knowledge and use in active adult athletes. J. Cannabis Res. 2020, 2, 18. [Google Scholar] [CrossRef]
- Marques Azzini, G.O.; Marques Azzini, V.O.; Santos, G.S.; Visoni, S.; Fusco, M.A.; Beker, N.S.; Mahmood, A.; Bizinotto Lana, J.V.; Jeyaraman, M.; Nallakumarasamy, A.; et al. Cannabidiol for musculoskeletal regenerative medicine. Exp. Biol. Med. 2023, 248, 445–455. [Google Scholar] [CrossRef]
- Gibson, L.P.; Bryan, A.D. Running High: Cannabis Users’ Subjective Experience of Exercise During Legal Market Cannabis Use Versus No Use in a Naturalistic Setting. Cannabis Cannabinoid Res. 2024, 9, e1122–e1131. [Google Scholar] [CrossRef]
- Engeli, B.E.; Lachenmeier, D.W.; Diel, P.; Guth, S.; Villar Fernandez, M.A.; Roth, A.; Lampen, A.; Cartus, A.T.; Wätjen, W.; Hengstler, J.G.; et al. Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims. Nutrients 2025, 17, 489. [Google Scholar] [CrossRef]
- Bartončíková, M.; Lapčíková, B.; Lapčík, L.; Valenta, T. Hemp-Derived CBD Used in Food and Food Supplements. Molecules 2023, 28, 8047. [Google Scholar] [CrossRef]
- Rajapakse, T.; Gantenbein, A.R. Nutraceuticals in migraine. Handb. Clin. Neurol. 2024, 199, 125–144. [Google Scholar] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Storz, M.A.; Calapai, G. The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods 2023, 12, 3505. [Google Scholar] [CrossRef] [PubMed]
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods—A Review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef]
- Lookfong, N.A.; Raup-Konsavage, W.M.; Silberman, Y. Potential Utility of Cannabidiol in Stress-Related Disorders. Cannabis Cannabinoid Res. 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profled in Cannabis Inforescences, Leaves, Stem Barks, and Roots for Medicinal Purpose. Sci. Rep. 2020, 10, 330. [Google Scholar]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Mishra, A.K.; Cho, K.H.; Kim, K.H.; Yoon, K.M.; Baek, K.H. Biosynthesis of Phytocannabinoids and Structural Insights: A Review. Metabolites 2023, 13, 442. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Fulvio, F.; Mandolino, G.; Citti, C.; Pecchioni, N.; Cannazza, G.; Paris, R. Phytocannabinoids biosynthesis during early stages of development of young Cannabis sativa L. seedlings: Integrating biochemical and transcription data. Phytochemistry 2023, 214, 113793. [Google Scholar] [CrossRef]
- Blatt-Janmaat, K.; Qu, Y. The Biochemistry of Phytocannabinoids and Metabolic Engineering of Their Production in Heterologous Systems. Int. J. Mol. Sci. 2021, 22, 2454. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, F.N. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Zandkarimi, F.; Decatur, J.; Casali, J.; Gordon, T.; Skibola, C.; Nuckolls, C. Comparison of the Cannabinoid and Terpene Profiles in Commercial Cannabis from Natural and Artificial Cultivation. Molecules 2023, 28, 833. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Brown, P.N.; Murch, S.J. The Terroir of Cannabis: Terpene Metabolomics as a Tool to Understand Cannabis sativa Selections. Planta Med. 2019, 85, 781–796. [Google Scholar] [CrossRef]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, J.E.; Bohlmann, J. Terpene Synthases and Terpene Variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Zager, J.J.; Lange, I.; Srividya, N.; Smith, A.; Lange, B.M. Gene Networks Underlying Cannabinoid and Terpenoid Accumulation in Cannabis. Plant Physiol. 2019, 180, 1877–1897. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.J.; Kwon, E.; Oh, S.J.; Cho, M.; Kim, C.M.; Lee, W.; Hong, J. Identification of Terpene Compositions in the Leaves and Inflorescences of Hybrid Cannabis Species Using Headspace-Gas Chromatography/Mass Spectrometry. Molecules 2023, 28, 8082. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef]
- Durairaj, J.; Melillo, E.; Bouwmeester, H.J.; Beekwilder, J.; de Ridder, D.; van Dijk, A.D.J. Integrating structure-based machine learning andco-evolution to investigate specificity in plantsesquiterpene synthases. PLoS Comput. Biol. 2021, 17, e1008197. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview. Plants 2021, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.; Yin, L.; Duong, J.; Benamar, K. Cannabidiol and Beta-Caryophyllene in Combination: A Therapeutic Functional Interaction. Int. J. Mol. Sci. 2022, 23, 15470. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef]
- Kobtrakul, K.; Rani, D.; Binalee, A.; Udomlarp, P.; Srichai, T.; De-Eknamkul, W.; Vimolmangkang, S. Elicitation enhances the production of friedelin and epifriedelanol in hairy root cultures of Cannabis sativa L. Front Plant Sci. 2023, 14, 1242584. [Google Scholar] [CrossRef]
- André, R.; Gomes, A.P.; Pereira-Leite, C.; Marques-da-Costa, A.; Monteiro Rodrigues, L.; Sassano, M.; Rijo, P.; Costa, M.D.C. The Entourage Effect in Cannabis Medicinal Products: A Comprehensive Review. Pharmaceuticals 2024, 17, 1543. [Google Scholar] [CrossRef]
- Almeida, N.J.; Amando, N.D.; Simoni Bezerra, L.K.; Gomes da Cruz Silva, E.M.; de Lima Araújo, C.T.; Carvalho de Souza, A.N.; Nishimura, H.V.R.; de Souza Araújo, C.; de Oliveira, P.A.; Guedes da Silva Almeida, R.J.; et al. Phytochemical Characterization of Cannabis sativa L. Roots from Northeastern Brazil. Chem. Biodivers. 2023, 20, e202201039. [Google Scholar] [CrossRef]
- Ferrini, F.; Fraternale, D.; Donati Zeppa, S.; Verardo, G.; Gorassini, A.; Carrabs, V.; Albertini, M.C.; Sestili, P. Yield, Characterization, and Possible Exploitation of Cannabis sativa L. Roots Grown under Aeroponics Cultivation. Molecules 2021, 26, 4889. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Wiles, D.; Shanbhag, B.K.; O’Brien, M.; Doblin, M.S.; Bacic, A.; Beddoe, T. Heterologous production of Cannabis sativa-derived specialised metabolites of medicinal significance—Insights into engineering strategies. Phytochemistry 2022, 203, 113380. [Google Scholar] [CrossRef]
- Desaulniers Brousseau, V.; Wu, B.S.; MacPherson, S.; Morello, V.; Lefsrud, M. Cannabinoids and Terpenes: How Production of Photo-Protectants Can Be Manipulated to Enhance Cannabis sativa L. Phytochemistry. Front. Plant Sci. 2021, 12, 620021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Mi, Y.; Meng, X.; Zhang, Y.; Chen, W.; Cao, X.; Wan, H.; Yang, W.; Li, J.; Wang, S.; et al. Genome-wide identification of key enzyme-encoding genes and the catalytic roles of two 2-oxoglutarate-dependent dioxygenase involved in flavonoid biosynthesis in Cannabis sativa L. Microb. Cell Fact. 2022, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Laaboudi, F.Z.; Rejdali, M.; Amhamdi, H.; Salhi, A.; Elyoussfi, A.; Ahari, M.H. In the weeds: A comprehensive review of cannabis; its chemical complexity, biosynthesis, and healing abilities. Toxicol. Rep. 2024, 13, 101685. [Google Scholar] [CrossRef]
- Huestis, M.A.; Mazzoni, I.; Rabin, O. Cannabis in sport: Anti-doping perspective. Sports Med. 2011, 41, 949–966. [Google Scholar] [CrossRef]
- Kennedy, M. Cannabis, cannabidiol and tetrahydrocannabinol in sport: An overview. Int. Med. J. 2022, 52, 1471–1477. [Google Scholar] [CrossRef]
- McCartney, D.; Benson, M.J.; Desbrow, B.; Irwin, C.; Suraev, A.; McGregor, I.S. Cannabidiol and Sports Performance: A Narrative Review of Relevant Evidence and Recommendations for Future Research. Sports Med.-Open 2020, 6, 27. [Google Scholar] [CrossRef]
- Lichenstein, S.D. THC, CBD, and Anxiety: A Review of Recent Findings on the Anxiolytic and Anxiogenic Effects of Cannabis’ Primary Cannabinoids. Curr. Addict. Rep. 2022, 9, 473–485. [Google Scholar] [CrossRef]
- Martin, E.L.; Strickland, J.C.; Schlienz, N.J.; Munson, J.; Jackson, H.; Bonn-Miller, M.O.; Vandrey, R. Antidepressant and Anxiolytic Effects of Medicinal Cannabis Use in an Observational Trial. Front. Psychiatry 2021, 12, 729800. [Google Scholar] [CrossRef]
- Saugy, M.; Avois, L.; Saudan, N.; Robinson, C.; Giroud, C.; Mangin, P.; Dvorak, J. Cannabis and sport. Br. J. Sports Med. 2006, 40, 13–15. [Google Scholar] [CrossRef]
- Urits, I.; Charipova, K.; Gress, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; Viswanath, O. Adverse Effects of Recreational and Medical Cannabis. Psychopharmacol. Bull. 2021, 51, 94–109. [Google Scholar] [PubMed]
- Zimmermann, K.; Yao, S.; Heinz, M.; Zhou, F.; Dau, W.; Banger, M.; Weber, B.; Hurlemann, R.; Becker, B. Altered orbitofrontal activity and dorsal striatal connectivity during emotion processing in dependent marijuana users after 28 days of abstinence. Psychopharmacology 2018, 235, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Burns, J.; Kure Liu, C.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis Addiction and the Brain: A Review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- West, M.L.; Sharif, S. Cannabis and Psychosis. Psychiatr. Clin. N. Am. 2023, 46, 703–717. [Google Scholar] [CrossRef]
- Testai, F.D.; Gorelick, P.B.; Aparicio, H.J.; Filbey, F.M.; Gonzalez, R.; Gottesman, R.F.; Melis, M.; Piano, M.R.; Rubino, T.; Song, S.Y. Use of Marijuana: Effect on Brain Health: A Scientific Statement from the American Heart Association. Stroke 2022, 53, e176–e187. [Google Scholar] [CrossRef]
- Mascherbauer, J. Cardiovascular (side) effects of cannabis. Wien Klin. Wochenschr. 2024, 136, 529–532. [Google Scholar] [CrossRef]
- Chandy, M.; Nishiga, M.; Wei, T.T.; Hamburg, N.M.; Nadeau, K.; Wu, J.C. Adverse Impact of Cannabis on Human Health. Annu. Rev. Med. 2024, 75, 353–367. [Google Scholar] [CrossRef]
- Kaplan, A.G. Cannabis and Lung Health: Does the Bad Outweigh the Good? Pulm. Ther. 2021, 7, 395–408. [Google Scholar] [CrossRef]
- de Oliveira e Silva, R.F.; Figueiredo, E.N. Current legislation on medical cannabis in the European Union: Historical background, movements, trends, and counter-trends lessons for Brazil. BrJP 2023, 6, S90–S94. [Google Scholar]
- Lipnik-Štangelj, M.; Razinger, B. A regulatory take on cannabis and cannabinoids for medicinal use in the European Union. Arch. Ind. Hyg. Toxicol. 2020, 71, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, J.; Bhatia, D.; Ellingson, J.; Molinero, K.; Hopfer, C. The impact of recreational cannabis legalization on youth: The Colorado experience. Eur. Child. Adolesc. Psychiatry 2024, 33, 637–650. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.A.; Iverson, M.G.; Suleiman, A.O.; Rhee, T.G. Is legalization of recreational cannabis associated with levels of use and cannabis use disorder among youth in the United States? A rapid systematic review. Eur. Child. Adolesc. Psychiatry 2024, 33, 701–723. [Google Scholar] [CrossRef] [PubMed]
- European Parliamentary Research Service. Recreational Use of Cannabis Laws and Policies in Selected EU Member States. 2024. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2024/762307/EPRS_BRI(2024)762307_EN.pdf (accessed on 17 February 2025).
- Tavabi, N.; Raza, M.; Singh, M.; Golchin, S.; Singh, H.; Hogue, G.D.; Kiapour, A.M. Disparities in cannabis use and documentation in electronic health records among children and young adults. NPJ Digit Med. 2023, 6, 138. [Google Scholar] [CrossRef]
- Knottnerus, A.J.; Blom, T.; van Eerden, S.; Mans, J.H.H.; van de Mheen, D.; de Neeling, J.N.D.; Schelfhout, D.C.L.; Seidell, J.C.; van Wijk, A.H.; van Wingerde, C.G.K.; et al. Cannabis policy in The Netherlands: Rationale and design of an experiment with a controlled legal (‘closed’) cannabis supply chain. Health Policy 2023, 129, 104699. [Google Scholar] [CrossRef]
- Palmiere, C.; Scarpelli, M.P. Cannabis-based medicines and medical fitness-to-drive: Current legal issues in Switzerland. Clin. Ter. 2024, 175, 113–116. [Google Scholar]
- Klosterkötter, J.; Kuhn, J. Die neue Cannabis-Regulierung in Deutschland: Desaster oder Chance? [Germany’s cannabis act: Disaster or opportunity?]. Fortschr. Neurol. Psychiatr. 2024, 92, 336–339. [Google Scholar]
- Thomasius, R. Zur Diskussion / Up for Discussion. Gefährdet die Cannabislegalisierung Kinder und Jugendliche?/Does Cannabis Legalization Endanger Children and Adolescents? Prax. Kinderpsychol. Kinderpsychiatr. 2024, 73, 652–664. [Google Scholar] [CrossRef]
- Armstrong, M.J. Canada’s Recreational Cannabis Legalization and Medical Cannabis Patient Activity, 2017–2022. Am. J. Public Health 2024, 114, S673–S680. [Google Scholar] [CrossRef]
- Feltmann, K.; Hauspie, B.; Dirkx, N.; Elgán, T.H.; Beck, O.; Van Havere, T.; Gripenberg, J. Prevalence and Misreporting of Illicit Drug Use among Electronic Dance Music Festivals Attendees: A Comparative Study between Sweden and Belgium. Toxics 2024, 12, 635. [Google Scholar] [CrossRef]
- Helander, A.; Johansson, M.; Villén, T.; Andersson, A. Appearance of hexahydrocannabinols as recreational drugs and implications for cannabis drug testing—focus on HHC, HHC-P, HHC-O and HHC-H. Scandinavian J. Clin. Lab. Investig. 2024, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tihăuan, B.M.; Onisei, T.; Slootweg, W.; Gună, D.; Iliescu, C.; Chifiriuc, M.C. Cannabidiol—A friend or a foe? Eur. J. Pharm. Sci. 2025, 8, 107036. [Google Scholar] [CrossRef] [PubMed]
- Ransing, R.; de la Rosa, P.A.; Pereira-Sanchez, V.; Handuleh, J.I.M.; Jerotic, S.; Gupta, A.K.; Karaliuniene, R.; de Filippis, R.; Peyron, E.; Sönmez Güngör, E.; et al. Current state of cannabis use, policies, and research across sixteen countries: Cross-country comparisons and international perspectives. Trends Psychiatry Psychother. 2022, 44, e20210263. [Google Scholar] [CrossRef] [PubMed]
- Kalayasiri, R.; Boonthae, S. Trends of cannabis use and related harms before and after legalization for recreational purpose in a developing country in Asia. BMC Public Health 2023, 23, 911. [Google Scholar] [CrossRef]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Polizio, A.H.; Abbate, A.; Toldo, S.; Mezzaroma, E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules 2024, 29, 473. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2020, 25, 1–3. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Varadi, G.; Zhu, Z.; Crowley, H.D.; Moulin, M.; Dey, R.; Lewis, E.D.; Evans, M. Examining the Systemic Bioavailability of Cannabidiol and Tetrahydrocannabinol from a Novel Transdermal Delivery System in Healthy Adults: A Single-Arm, Open-Label, Exploratory Study. Adv. Ther. 2023, 40, 282–293. [Google Scholar] [CrossRef]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef]
- Cobo-Golpe, M.; de-Castro-Ríos, A.; Cruz, A.; López-Rivadulla, M.; Lendoiro, E. Determination and Distribution of Cannabinoids in Nail and Hair Samples. J. Anal. Toxicol. 2021, 45, 969–975. [Google Scholar] [CrossRef]
- Zhu, J.; Peltekian, K.M. Cannabis and the liver: Things you wanted to know but were afraid to ask. Canadian Liver. J. 2019, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Falck Jørgensen, C.; Schou Rasmussen, B.; Linnet, K.; Thomsen, R. Evidence of 11-Hydroxy-hexahydrocannabinol and 11-Nor-9-carboxy-hexahydrocannabinol as Novel Human Metabolites of Δ9-Tetrahydrocannabinol. Metabolites 2023, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Yabut, K.C.B.; Wen, Y.W.; Simon, K.T.; Isoherranen, N. CYP2C9, CYP3A and CYP2C19 metabolize Δ9-tetrahydrocannabinol to multiple metabolites but metabolism is affected by human liver fatty acid binding protein (FABP1). Biochem. Pharmacol. 2024, 228, 116191. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Beers, J.L.; Jackson, K.D.; Zhou, Z. CBD and THC in Special Populations: Pharmacokinetics and Drug–Drug Interactions. Pharmaceutics 2024, 16, 484. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef]
- Chen, S.; Kim, J.-K. The Role of Cannabidiol in Liver Disease: A Systemic Review. Int. J. Mol. Sci. 2024, 25, 2370. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Goodwin, R.S.; Darwin, W.D.; Chiang, C.N.; Shih, M.; Li, S.H.; Huestis, M.A. Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J. Anal. Toxicol. 2008, 32, 562–569. [Google Scholar] [CrossRef]
- Schlienz, N.J.; Cone, E.J.; Herrmann, E.S.; Lembeck, N.A.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; LoDico, C.P.; Hayes, E.D.; Vandrey, R. Pharmacokinetic Characterization of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in Urine Following Acute Oral Cannabis Ingestion in Healthy Adults. J. Anal. Toxicol. 2018, 42, 232–247. [Google Scholar] [CrossRef]
- Sholler, D.J.; Spindle, T.R.; Cone, E.J.; Goffi, E.; Kuntz, D.; Mitchell, J.M.; Winecker, R.E.; Bigelow, G.E.; Flegel, R.R.; Vandrey, R. Urinary Pharmacokinetic Profile of Cannabidiol (CBD), Δ9-Tetrahydrocannabinol (THC) and Their Metabolites following Oral and Vaporized CBD and Vaporized CBD-Dominant Cannabis Administration. J. Anal. Toxicol. 2022, 46, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.R.; Garg, N.K. Electrochemical Oxidation of Δ9-Tetrahydrocannabinol: A Simple Strategy for Marijuana Detection. Org. Lett. 2020, 22, 3951–3955. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Bala, C. Affinity Assays for Cannabinoids Detection: Are They Amenable to On-Site Screening? Biosensors 2022, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Deenin, W.; Wenninger, N.; Schmid, M.G.; Kalcher, K.; Ortner, A.; Chaiyo, S. Rapid electrochemical lateral flow device for the detection of Δ9-tetrahydrocannabinol. Anal. Chim. Acta. 2023, 1279, 341768. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Perry, J.M.; Little, S.R.; Llorens, O.Y.; Shih, S.C.C. An electrochemical aptasensor for Δ9-tetrahydrocannabinol detection in saliva on a microfluidic platform. Biosens. Bioelectron. 2023, 222, 114998. [Google Scholar] [CrossRef]
- Xie, Y.; She, J.P.; Zheng, J.X.; Salminen, K.; Sun, J.J. Rapid nanomolar detection of Δ9-tetrahydrocannabinol in biofluids via electrochemical aptamer-based biosensor. Anal. Chim. Acta 2024, 1295, 342304. [Google Scholar] [CrossRef]
- Vohra, V.; Marraffa, J.M.; Wojcik, S.M.; Eggleston, W. An assessment of urine THC immunoassay in healthy volunteers receiving an oral proton-pump inhibitor. Clin. Toxicol. 2019, 58, 498–500. [Google Scholar] [CrossRef]
- Gomila, I.; Barceló, B.; Rosell, A.; Avella, S.; Sahuquillo, L.; Dastis, M. Cross-Reactivity of Pantoprazole with Three Commercial Cannabinoids Immunoassays in Urine. J. Anal. Toxicol. 2017, 41, 760–764. [Google Scholar] [CrossRef]
- Możdżeń, K.; Kaleta, K.; Murawska, A.; Pośpiech, J.; Panek, P.; Lorkowska-Zawicka, B.; Bujak-Giżycka, B. Guilty or not guilty?—False positive results of common medicines in drug tests: Review and practical guide. Folia Med. Cracov. 2023, 63, 107–134. [Google Scholar]
- Nahar, L.; Guo, M.; Sarker, S.D. Gas chromatographic analysis of naturally occurring cannabinoids: A review of literature published during the past decade. Phytochem. Anal. 2020, 31, 135–146. [Google Scholar] [CrossRef]
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for quantification of cannabinoids: A narrative review. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Rosendo, L.M.; Rosado, T.; Oliveira, P.; Simão, A.Y.; Margalho, C.; Costa, S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. The Determination of Cannabinoids in Urine Samples Using Microextraction by Packed Sorbent and Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 5503. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, S.; Jokar, F.; Kazem Koohi, M.; Ghadipasha, M.; Hassan, J.; Akhgari, M.; Forouzesh, M. Enhancement and validation of a quantitative GC–MS method for the detection of ∆9-THC and THCCOOH in postmortem blood and urine samples. MethodsX 2024, 13, 102962. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Freire, I.; Valeiras-Fernández, A.; Cabarcos-Fernández, P.; Bermejo-Barrera, A.M.; Tabernero-Duque, M.J. Simple Method for the Determination of THC and THC-COOH in Human Postmortem Blood Samples by Gas Chromatography—Mass Spectrometry. Molecules 2023, 28, 3586. [Google Scholar] [CrossRef]
- Sempio, C.; Almaraz-Quinones, N.; Jackson, M.; Zhao, W.; Wang, G.S.; Liu, Y.; Leehey, M.; Knupp, K.; Klawitter, J.; Christians, U.; et al. Simultaneous Quantification of 17 Cannabinoids by LC–MS-MS in Human Plasma. J. Anal. Toxicol. 2022, 46, 383–392. [Google Scholar] [CrossRef]
- Hubbard, J.A.; Smith, B.E.; Sobolesky, P.M.; Kim, S.; Hoffman, M.A.; Stone, J.; Huestis, M.A.; Grelotti, D.J.; Grant, I.; Marcotte, T.D.; et al. Validation of a liquid chromatography tandem mass spectrometry (LC-MS/MS) method to detect cannabinoids in whole blood and breath. Clin. Chem. Lab. Med. 2020, 58, 673–681. [Google Scholar] [CrossRef]
- Pires da Silva, C.; Pugen Dalpiaz, L.P.; Gerbase, F.E.; Vendramini Muller, V.; Cezimbra da Silva, A.; Feltraco Lizot, L.; Zilles Hahn, R.; Luiz da Costa, J.; Venzon Antunes, M.; Linden, R. Determination of cannabinoids in plasma using salting-out-assisted liquid–liquid extraction followed by LC–MS/MS analysis. Biomed. Chromatogr. 2020, 34, e4952. [Google Scholar]
- Manca, A.; Chiara, F.; Mula, J.; Palermiti, A.; Maiese, D.; Zeaiter, S.; De Nicolò, A.; Imperiale, D.; De Filippis, G.; Vischia, F.; et al. A new UHPLC-MS/MS method for cannabinoids determination in human plasma: A clinical tool for therapeutic drug monitoring. Biomed. Pharmacother. 2022, 156, 113899. [Google Scholar] [CrossRef]
- Sim, Y.E.; Kim, J.W.; Ko, B.J.; Kim, J.Y.; Cheong, J.C.; Pyo, J. Determination of urinary metabolites of cannabidiol, Δ8-tetrahydrocannabinol, and Δ9-tetrahydrocannabinol by automated online μSPE-LC-MS/MS method. J. Chromatogr. B 2023, 1214, 123568. [Google Scholar] [CrossRef]
- Reber, J.D.; Karschner, E.L.; Seither, J.Z.; Knittel, J.L.; Walterscheid, J.P. Screening and confirmation methods for the qualitative identification of nine phytocannabinoids in urine by LC-MS/MS. Clin. Biochem. 2021, 98, 54–62. [Google Scholar] [CrossRef]
- Cho, H.S.; Cho, B.; Sim, J.; Baeck, S.K.; In, S.; Kim, E. Detection of 11-nor-9-carboxy-tetrahydrocannabinol in the hair of drug abusers by LC-MS/MS analysis. Forensic. Sci. Int. 2019, 295, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.; Madry, M.M.; Kraemer, T.; Baumgartner, M.R. LC-MS-MS Analysis of Δ9-THC, CBN and CBD in Hair: Investigation of Artifacts. J. Anal. Toxicol. 2022, 46, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.S.; Lanaro, R.; Dolores, R.C.; Morais, D.R.; Arantes, A.C.F.; Oliveira, K.D.; Costa, J.L. Determination of Drugs of Abuse in Hair by LC-MS-MS: Application to Suicide Attempts Investigation. J. Anal. Toxicol. 2022, 46, 577–581. [Google Scholar] [CrossRef]
- Hehet, P.; Franz, T.; Kunert, N.; Musshoff, F. Fast and highly sensitive determination of tetrahydrocannabinol (THC) metabolites in hair using liquid chromatography-multistage mass spectrometry (LC-MS3). Drug Test Anal. 2022, 14, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, V.D.; Müller, V.V.; Feltraco Lizot, L.L.; Hahn, R.Z.; Schneider, A.; Antunes, M.V.; Linden, R. Sensitive determination of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol and complementary cannabinoids in hair using alkaline digestion and mixed-mode solid phase extraction followed by liquid-chromatography-tandem mass spectrometry. Forensic. Sci. Int. 2021, 328, 111047. [Google Scholar] [CrossRef]
- Angeli, I.; Casati, S.; Ravelli, A.; Minoli, M.; Orioli, M. A novel single-step GC-MS/MS method for cannabinoids and 11-OH-THC metabolite analysis in hair. J. Pharm. Biomed. Anal. 2018, 155, 1–6. [Google Scholar] [CrossRef]
- Rodrigues, A.; Yegles, M.; Van Elsué, N.; Schneider, S. Determination of cannabinoids in hair of CBD rich extracts consumers using gas chromatography with tandem mass spectrometry (GC/MS-MS). Forensic. Sci. Int. 2018, 292, 163–166. [Google Scholar] [CrossRef]
- Rojas-Valverde, D. Potential Role of Cannabidiol on Sports Recovery: A Narrative Review. Front. Physiol. 2021, 12, 722550. [Google Scholar] [CrossRef]
- Weizman, L.; Sharon, H.; Dayan, L.; Espaniol, J.; Brill, S.; Nahman-Averbuch, H.; Hendler, T.; Jacob, G. Oral Delta-9-Tetrahydrocannabinol (THC) Increases Parasympathetic Activity and Supraspinal Conditioned Pain Modulation in Chronic Neuropathic Pain Male Patients: A Crossover, Double-Blind, Placebo-Controlled Trial. CNS Drugs 2024, 38, 375–385. [Google Scholar] [CrossRef]
- Safi, K.; Sobieraj, J.; Błaszkiewicz, M.; Żyła, J.; Salata, B.; Dzierżanowski, T. Tetrahydrocannabinol and Cannabidiol for Pain Treatment-An Update on the Evidence. Biomedicines 2024, 12, 307. [Google Scholar] [CrossRef]
- Thapa, D.; Patil, M.; Warne, L.N.; Carlessi, R.; Falasca, M. Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011. Pharmaceuticals 2025, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Pereira, A.T.; Dias, M.J.; Sousa, C.; Vinha, A.F.; Moutinho, C.; Carvalho, M. Cannabis for Chronic Pain: Mechanistic Insights and Therapeutic Challenges. Stresses 2025, 5, 7. [Google Scholar] [CrossRef]
- Al-Husinat, L.; Obeidat, S.; Azzam, S.; Al-Gwairy, Y.; Obeidat, F.; Al Sharie, S.; Haddad, D.; Haddad, F.; Rekatsina, M.; Leoni, M.L.G.; et al. Role of Cannabis in the Management of Chronic Non-Cancer Pain: A Narrative Review. Clin. Pract. 2025, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Glaser, T.; Villegas, C.; Burgos, V.; Ulrich, H.; Paz, C. Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment. Life 2022, 12, 2117. [Google Scholar] [CrossRef]
- Mohammed, A.; FK Alghetaa, H.; Miranda, K.; Wilson, K.; Singh, N.P.; Cai, G.; Putluri, N.; Nagarkatti, P.; Nagarkatti, M. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int. J. Mol. Sci. 2020, 21, 6244. [Google Scholar] [CrossRef]
- Alvarez, X.; Sestak, K.; Byrareddy, S.N.; Mohan, M. Long Term Delta-9-tetrahydrocannabinol Administration Inhibits Proinflammatory Responses in Minor Salivary Glands of Chronically Simian Immunodeficieny Virus Infected Rhesus Macaques. Viruses 2020, 12, 713. [Google Scholar] [CrossRef]
- McCartney, D.; Irwin, C.; Bawa, Z.; Palmer, B.; Sahinovic, A.; Delang, N.; Cox, G.R.; Desbrow, B.; Lau, N.S.; McGregor, I.S. The Effect of Cannabidiol on Subjective Responses to Endurance Exercise: A Randomised Controlled Trial. Sports Med.-Open 2024, 10, 61. [Google Scholar] [CrossRef]
- Sahinovic, A.; Irwin, C.; Doohan, P.T.; Kevin, R.C.; Cox, A.J.; Lau, N.S.; Desbrow, B.; Johnson, N.A.; Sabag, A.; Hislop, M.; et al. Effects of Cannabidiol on Exercise Physiology and Bioenergetics: A Randomised Controlled Pilot Trial. Sports Med.-Open 2022, 8, 27. [Google Scholar] [CrossRef]
- Bezuglov, E.; Achkasov, E.; Rudiakova, E.; Shurygin, V.; Malyakin, G.; Svistunov, D.; Butovskiy, M.; Fedorova, A.; Kapralova, E. The Effect of Cannabidiol on Performance and Post-Load Recovery among Healthy and Physically Active Individuals: A Systematic Review. Nutrients 2024, 16, 2840. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Jensen, S.S.; Nikolajsen, G.N.; Ziegler Bruun, H.; Hoeng, R.B.J. The therapeutic potential of purified cannabidiol. J. Cannabis Res. 2023, 5, 21. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Fallas-Campos, A. Cannabidiol in sports: Insights on how CBD could improve performance and recovery. Front. Pharmacol. 2023, 14, 1210202. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–041. [Google Scholar] [CrossRef] [PubMed]
- Moltke, J.; Hindocha, C. Reasons for cannabidiol use: A cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J. Cannabis Res. 2021, 3, 5. [Google Scholar] [CrossRef]
- Stone, W.J.; Tolusso, D.V.; Pancheco, G.; Brgoch, S.; Nguyen, V.T. A Pilot Study on Cannabidiol (CBD) and Eccentric Exercise: Impact on Inflammation, Performance, and Pain. Int. J. Exerc. Sci. 2023, 16, 109–117. [Google Scholar] [CrossRef]
- Zlatanova-Tenisheva, H.; Georgieva-Kotetarova, M.; Vilmosh, N.; Kandilarov, I.; Delev, D.; Dermendzhiev, T.; Kostadinov, I.D. Exploring the Anxiolytic, Antidepressant, and Immunomodulatory Effects of Cannabidiol in Acute Stress Rat Models. Appl. Biosci. 2025, 4, 4. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta 2020, 1866, 165771. [Google Scholar] [CrossRef]
- Thompson, E.S.; Alcorn, J.; Neary, J.P. Cannabinoid Therapy in Athletics: A Review of Current Cannabis Research to Evaluate Potential Real-World Cannabinoid Applications in Sport. Sports Med. 2024, 54, 2743–2769. [Google Scholar] [CrossRef]
- Klinsang, T.; Charoensit, P.; Phimnuan, P.; Luangpraditkun, K.; Ross, G.M.; Viennet, C.; Ross, S.; Viyoch, J. In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex. Pharmaceutics 2023, 15, 2682. [Google Scholar] [CrossRef]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef]
- Shah, P.; Holmes, K.; Chibane, F.; Wang, P.; Chagas, P.; Salles, E.; Jones, M.; Palines, P.; Masoumy, M.; Baban, B.; et al. Cutaneous Wound Healing and the Effects of Cannabidiol. Int. J. Mol. Sci. 2024, 25, 7137. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.C.; Jeffery, C.S.; Sandhu, Z.; Brownlee, B.P.; Queimado, L.; Mims, M.M. The effect of cannabinoids on wound healing: A review. Health Sci. Rep. 2024, 7, e1908. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Malhi, N.K.; Huang, J.; Li, Q.; Zhou, Z.; Wang, R.; Peng, J.; Yin, T.; Wang, H. Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules 2024, 29, 5471. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Karuppagounder, V.; Nowak, I.; Sepulveda, D.E.; Lewis, G.S.; Norbury, C.C.; Raup-Konsavage, W.M.; Vrana, K.E.; Kamal, F.; Elbarbary, R.A. Cannabidiol and Cannabigerol, Nonpsychotropic Cannabinoids, as Analgesics that Effectively Manage Bone Fracture Pain and Promote Healing in Mice. J. Bone Mineral Res. 2023, 38, 1560–1576. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Chung, J.; Abdeen, A.; Thompson, A.; Bouboukas, A.; Pinamont, W.J.; Yoshioka, N.K.; Sepulveda, D.E.; Raup-Konsavage, W.M.; Graziane, N.M.; et al. Therapeutic Effects of Non-Euphorigenic Cannabis Extracts in Osteoarthritis. Cannabis Cannabinoid Res. 2023, 8, 1030–1044. [Google Scholar] [CrossRef]
- Carone, M.; Premoli, M.; Bonini, S.A.; Latsi, R.; Maccarinelli, G.; Memo, M. Behavioral effects of two cannabidiol and cannabigerol-rich formulas on mice. Heliyon 2024, 10, e39938. [Google Scholar] [CrossRef]
- Cuttler, C.; Stueber, A.; Cooper, Z.D.; Russo, E. Acute effects of cannabigerol on anxiety, stress, and mood: A double-blind, placebo-controlled, crossover, field trial. Sci. Rep. 2024, 14, 16163. [Google Scholar] [CrossRef]
- Gillham, S.H.; Starke, L.; Welch, L.; Mather, E.; Whitelegg, T.; Chester, N.; Owens, D.J.; Bampouras, T.; Close, G.L. Does a broad-spectrum cannabidiol supplement improve performance in a 10-min cycle ergometer performance-test? Eur. J. Sport Sci. 2024, 24, 870–877. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.M.; Largent-Milnes, T.M. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 98–126. [Google Scholar] [CrossRef]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and terpene regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Eyal, A.M.; Davidson, E.M. Optimal Treatment with Cannabis Extracts Formulations Is Gained via Knowledge of Their Terpene Content and via Enrichment with Specifically Selected Monoterpenes and Monoterpenoids. Molecules 2022, 27, 6920. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Johnson, A.; Stewart, A.; El-Hakim, I.; Hamilton, T.J. Effects of super-class cannabis terpenes beta-caryophyllene and alpha-pinene on zebrafish behavioural biomarkers. Sci. Rep. 2022, 12, 17250. [Google Scholar] [CrossRef]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Raz, N.; Eyal, A.M.; Berneman Zeitouni, D.; Hen-Shoval, D.; Davidson, E.M.; Danieli, A.; Tauber, M.; Ben-Chaim, Y. Selected cannabis terpenes synergize with THC to produce increased CB1 receptor activation. Biochem. Pharmacol. 2023, 212, 115548. [Google Scholar]
- Blevins, L.K.; Bach, A.P.; Crawford, R.B.; Zhou, J.; Henriquez, J.E.; Rizzo, M.D.; Sermet, S.; Khan, D.M.I.O.; Turner, H.; Small-Howard, A.L.; et al. Evaluation of the anti-inflammatory effects of selected cannabinoids and terpenes from Cannabis sativa employing human primary leukocytes. Food Chem. Toxicol. 2022, 170, 113458. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Tory, R.; Spindle, C.; Zamarripa, A.; Russo, E.; Pollak, L.; Bigelow, G.; Ward, A.M.; Tompson, B.; Sempio, C.; Shokati, T.; et al. Vaporized D-limonene selectively mitigates the acute anxiogenic effects of Δ9-tetrahydrocannabinol in healthy adults who intermittently use cannabis. Drug Alcohol. Depend. 2024, 257, 111267. [Google Scholar]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Kaminsky, N.; Hubert, J.; Guerin, C.; Mazlani, M.; Kotland, A.; Pozzobon, V.; Marant, B.; Mailhac, H.; Poigny, S. Deciphering the Phytochemical Potential of Hemp Hairy Roots: A Promising Source of Cannabisins and Triterpenes as Bioactive Compounds. Molecules 2024, 29, 5792. [Google Scholar] [CrossRef] [PubMed]
- Mekarunothai, A.; Bacher, M.; Buathong, R.; Intarasam, S.; Tayana, N.; Kongkiatpaiboon, S.; Charoenrat, T.; Napiroon, T. β-sitosterol isolated from the leaves of Trema orientalis (Cannabaceae) promotes viability and proliferation of BF-2 cells. PeerJ 2024, 12, e16774. [Google Scholar] [CrossRef]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From plant to patient: A scoping review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Radwan, M.M.; Metwaly, A.M.; Eissa, I.H.; Hazekamp, A.; Sohly, M.A. Chemistry and Biological Activities of Cannflavins of the Cannabis Plant. Cannabis Cannabinoid Res. 2023, 8, 974–985. [Google Scholar] [CrossRef]
- Fitzpatrick, J.K.; O’Riordan, D.; Downer, E.J. Cannflavin A inhibits TLR4-induced chemokine and cytokine expression in human macrophages. Nat. Prod. Res. 2024, 23, 1–7. [Google Scholar] [CrossRef]
- Chuanphongpanich, S.; Racha, S.; Saengsitthisak, B.; Pirakitikulr, P.; Racha, K. Computational Assessment of Cannflavin A as a TAK1 Inhibitor: Implication as a Potential Therapeutic Target for Anti-Inflammation. Sci. Pharm. 2023, 91, 36. [Google Scholar] [CrossRef]
- Li, H.; Deng, N.; Puopolo, T.; Jiang, X.; Seeram, N.P.; Liu, C.; Ma, H. Cannflavins A and B with Anti-Ferroptosis, Anti-Glycation, and Antioxidant Activities Protect Human Keratinocytes in a Cell Death Model with Erastin and Reactive Carbonyl Species. Nutrients 2023, 15, 4565. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Cuvelier, G.; Mendes, A.; Aucouturier, J.; Berthoin, S.; Di Marzo, V.; Heyman, E. Cannabidiol in sport: Ergogenic or else? Pharmacol. Res. 2020, 156, 104764. [Google Scholar] [CrossRef]
- Hart, E.D.; Vikingsson, S.; Mitchell, J.M.; Winecker, R.E.; Flegel, R.; Hayes, E.D. Conversion of 7-Carboxy-Cannabidiol (7-COOH-CBD) to 11-Nor-9-Carboxy-Tetrahydrocannabinol (THC-COOH) during Sample Preparation for GC-MS Analysis. J. Anal. Toxicol. 2022, 46, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Trojian, T.; Cushman, D.M. Physician Perceptions of Cannabidiol (CBD) and Cannabis in Sports Medicine and Performance. Transl. Sports Med. 2023, 2023, 8824466. [Google Scholar] [CrossRef] [PubMed]
- Davoren, A.K.; Rulison, K.; Milroy, J.; Grist, P.; Fedoruk, M.; Lewis, L.; Wyrick, D. Doping Prevalence among U.S. Elite Athletes Subject to Drug Testing under the World Anti-Doping Code. Sports Med. Open. 2024, 10, 57. [Google Scholar] [CrossRef] [PubMed]



| Country | Legalization, Comments | Study |
|---|---|---|
| United States | 38 states for medicinal use 23 states for recreational use | Tavabi N et al., 2023 [67] |
| Nederlands | Recreational since 1967 | Knottnerus JA 2023 [68] |
| Switzerland | Medical use since 2022 | Palmiere C et al., 2024 [69] |
| Germany | Medical use since 2017 Recreational since 2024 | Klosterkotter J et al., 2024 [70] Thomasius R et al., 2024 [71] |
| Canada | Medical use since 2001 Recreational since 2018 | Armstrong MJ, 2020 [72] |
| Sweden | Medical: CBD only Recreational: forbidden | Feltmann K et al., 2024 [73] Helander, A et al., 2024 [74] |
| Serbia Bulgaria Nepal Malaysia Iran Kenya Ethiopia | Medical and Recreational: forbidden | Tihauan BM et al., 2025 [75] Ransing R et al., 2023 [76] |
| France Poland UK Romania Hungary Norway Finland | Medical use with prescription: legal Recreational use: illegal | Tihauan BM et al., 2025 [75] Ransing R et al., 2023 [76] |
| Thailand | Medical use since 2019 Recreational since 2021 | Kalayasiri, R et al., 2023 [77] |
| Spain | Medical use since 2010 Recreational: allowed only for personal use | Ransing R et al., 2023 [76] |
| France | Medical use since 2020 Recreational: illegal | Ransing R et al., 2023 [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flangea, C.; Vlad, D.; Popescu, R.; Dumitrascu, V.; Rata, A.L.; Tryfon, M.E.; Balasoiu, B.; Vlad, C.S. Cannabis: Zone Aspects of Raw Plant Components in Sport—A Narrative Review. Nutrients 2025, 17, 861. https://doi.org/10.3390/nu17050861
Flangea C, Vlad D, Popescu R, Dumitrascu V, Rata AL, Tryfon ME, Balasoiu B, Vlad CS. Cannabis: Zone Aspects of Raw Plant Components in Sport—A Narrative Review. Nutrients. 2025; 17(5):861. https://doi.org/10.3390/nu17050861
Chicago/Turabian StyleFlangea, Corina, Daliborca Vlad, Roxana Popescu, Victor Dumitrascu, Andreea Luciana Rata, Maria Erika Tryfon, Bogdan Balasoiu, and Cristian Sebastian Vlad. 2025. "Cannabis: Zone Aspects of Raw Plant Components in Sport—A Narrative Review" Nutrients 17, no. 5: 861. https://doi.org/10.3390/nu17050861
APA StyleFlangea, C., Vlad, D., Popescu, R., Dumitrascu, V., Rata, A. L., Tryfon, M. E., Balasoiu, B., & Vlad, C. S. (2025). Cannabis: Zone Aspects of Raw Plant Components in Sport—A Narrative Review. Nutrients, 17(5), 861. https://doi.org/10.3390/nu17050861







