Benefits of Dietary Supplementation with Specific Silicon-Enriched Spirulina on Arterial Function in Healthy Elderly Individuals: A Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Ethics
2.4. Dietary Supplement
2.5. Vascular Parameter Assessments
2.6. Biological Assays
2.7. Data Analysis
3. Results
3.1. Subject Characteristics
3.2. Effect of SpSi Supplementation in All Subjects
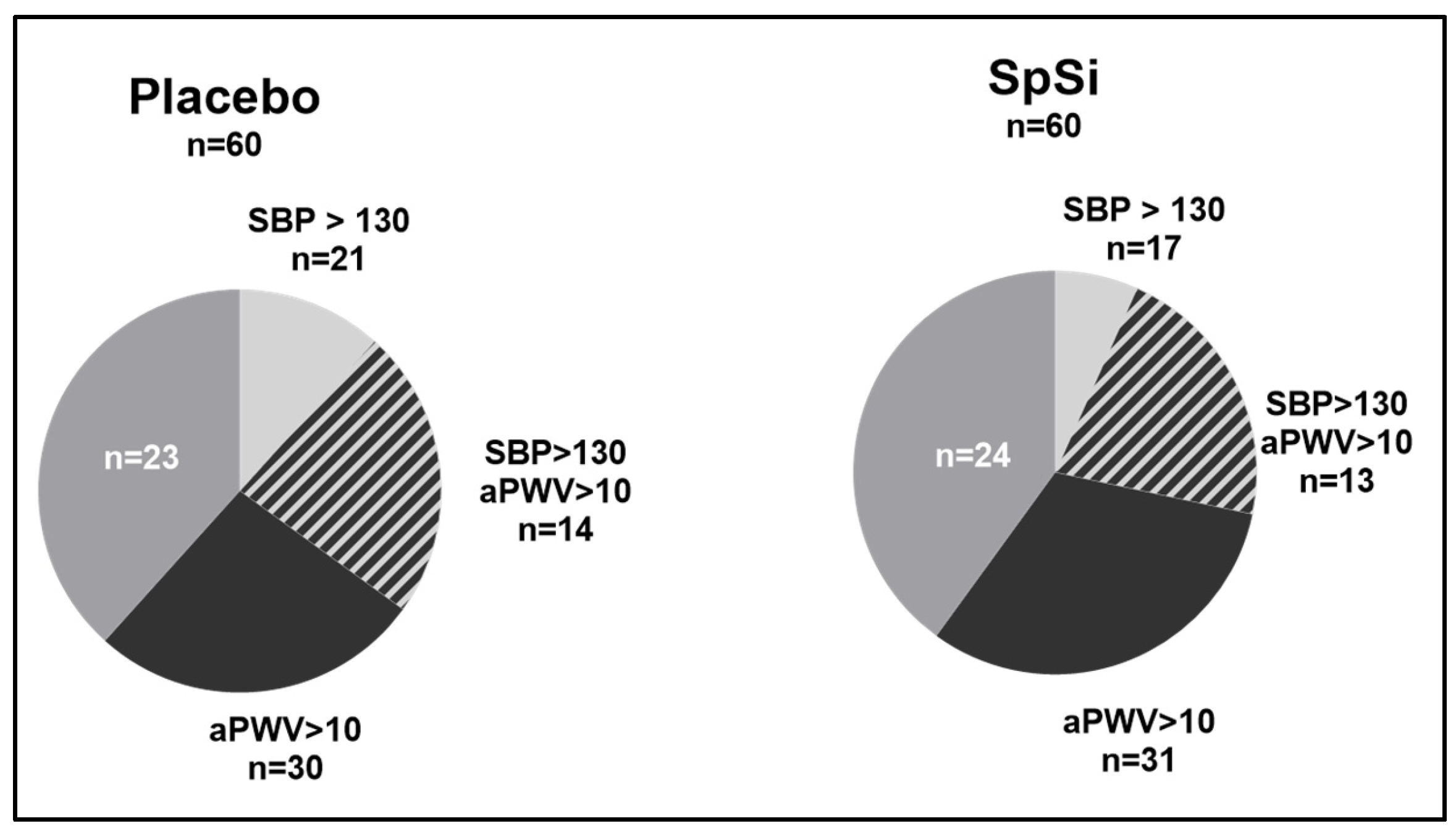
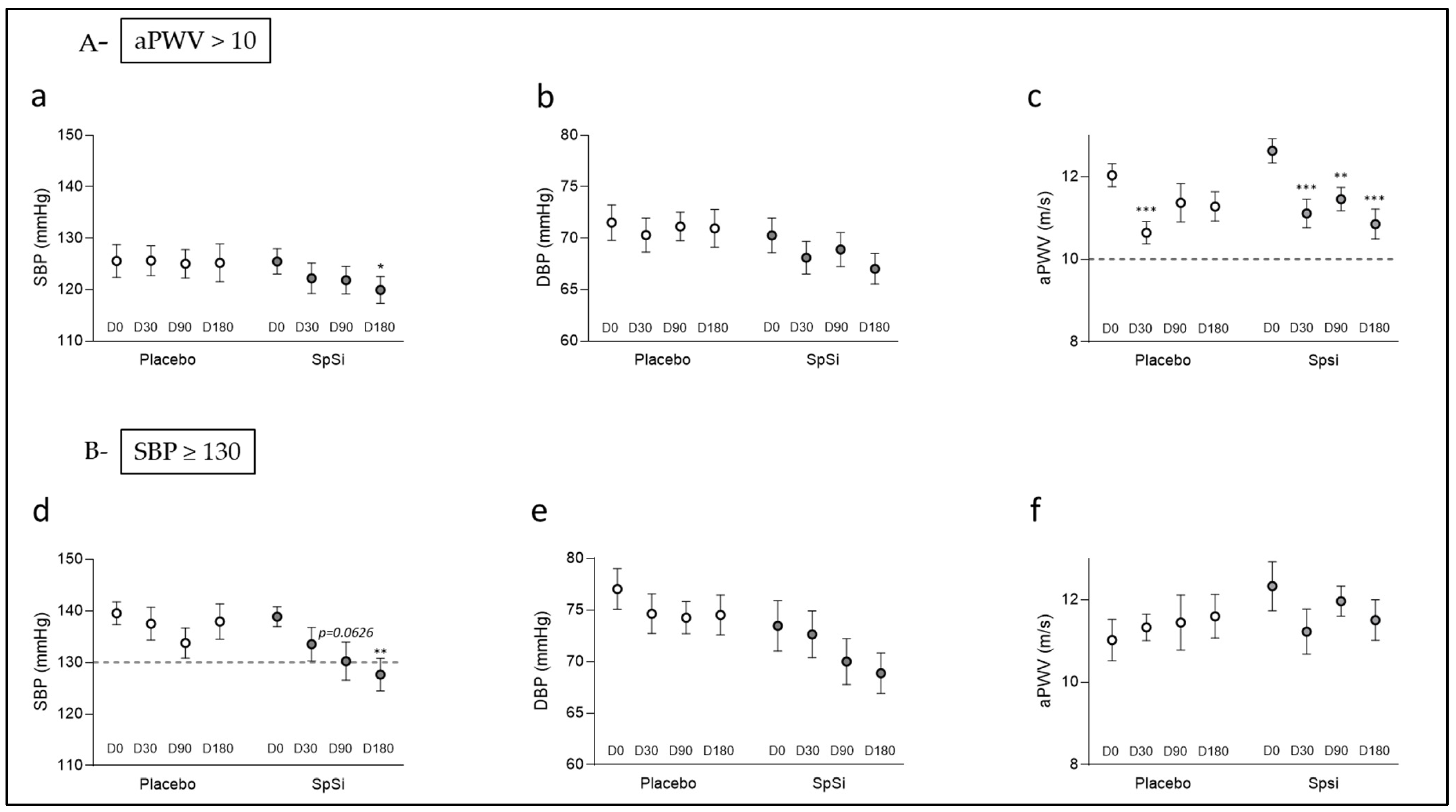
3.3. Effects of SpSi Supplementation in Subjects with Elevated Aortic Pulse Wave Velocity
3.4. Effects of SpSi Supplementation in Subjects with Elevated Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vakka, A.; Warren, J.S.; Drosatos, K. Cardiovascular Aging: From Cellular and Molecular Changes to Therapeutic Interventions. J. Cardiovasc. Aging 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.-M.; Charlton, P.H.; Grillo, A.; et al. Vascular Ageing: Moving from Bench towards Bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. Elastin in Large Artery Stiffness and Hypertension. J. Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, Arterial Mechanics, and Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Powell, J.T. Arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1025–1027. [Google Scholar] [CrossRef]
- Franklin, S.S.; Gustin, W.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic Patterns of Age-Related Changes in Blood Pressure. The Framingham Heart Study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. The Age-Related Hemodynamic Changes of Blood Pressure and Their Impact on the Incidence of Cardiovascular Disease and Stroke: New Evidence. J. Clin. Hypertens. 2014, 16, 87–90. [Google Scholar] [CrossRef]
- Sesso, H.D.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Gaziano, J.M.; Manson, J.E.; Glynn, R.J. Systolic and Diastolic Blood Pressure, Pulse Pressure, and Mean Arterial Pressure as Predictors of Cardiovascular Disease Risk in Men. Hypertension 2000, 36, 801–807. [Google Scholar] [CrossRef]
- Kannel, W.B.; Gordon, T.; Schwartz, M.J. Systolic versus Diastolic Blood Pressure and Risk of Coronary Heart Disease: The Framingham Study. Am. J. Cardiol. 1971, 27, 335–346. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Abridged Version of the Expert Consensus Document on Arterial Stiffness. Artery Res. 2007, 1, 2–12. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S.; et al. Elevated Aortic Pulse Wave Velocity, a Marker of Arterial Stiffness, Predicts Cardiovascular Events in Well-Functioning Older Adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef] [PubMed]
- Tripp, F. The Use of Dietary Supplements in the Elderly: Current Issues and Recommendations. J. Am. Diet. Assoc. 1997, 97, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hamidu, S.; Yang, X.; Wang, Q.; Li, L.; Oduro, P.K.; Li, Y. Dietary Supplements and Natural Products: An Update on Their Clinical Effectiveness and Molecular Mechanisms of Action During Accelerated Biological Aging. Front. Genet. 2022, 13, 880421. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; LaRocca, T.J.; Martens, C.R.; Seals, D.R. Healthy Lifestyle-Based Approaches for Successful Vascular Aging. J. Appl. Physiol. 2018, 125, 1888–1900. [Google Scholar] [CrossRef]
- Carlisle, E.M. The Nutritional Essentiality of Silicon. Nutr. Rev. 1982, 40, 193–198. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the Possible Nutritional Importance of Silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon. In Biochemistry of the Essential Ultratrace Elements; Biochemistry of the Elements; Springer: Boston, MA, USA, 1984; Volume 3, pp. 257–261. [Google Scholar] [CrossRef]
- Martin, K.R. Sigel, A., Sigel, H., Sigel, R., Eds.; Silicon: The Health Benefits of a Metalloid. In Interrelations Between Essential Metal Ions and Human Diseases; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 451–473. [Google Scholar] [CrossRef]
- Schwarz, K. Silicon, fibre, and atherosclerosis. Lancet 1977, 309, 454–457. [Google Scholar] [CrossRef]
- Subrahmanyam, G.; Pathapati, R.M.; Ramalingam, K.; Indira, S.A.; Kantha, K.; Soren, B. Arterial Stiffness and Trace Elements in Apparently Healthy Population- A Cross-Sectional Study. J. Clin. Diagn. Res. 2016, 10, LC12–LC15. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake Is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef]
- Schwarz, K.; Ricci, B.A.; Punsar, S.; Karvonen, M.J. Inverse relation of silicon in drinking water and atherosclerosis in Finland. Lancet 1977, 309, 538–539. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Reported Anti Atherosclerotic Activity of Silicon May Reflect Increased Endothelial Synthesis of Heparan Sulfate Proteoglycans. Med. Hypotheses 1997, 49, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Maehira, F.; Motomura, K.; Ishimine, N.; Miyagi, I.; Eguchi, Y.; Teruya, S. Soluble Silica and Coral Sand Suppress High Blood Pressure and Improve the Related Aortic Gene Expressions in Spontaneously Hypertensive Rats. Nutr. Res. 2011, 31, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Loeper, J.; Goy, J.; Fragny, M.; Troniou, R.; Bedu, O. Study of Fatty Acids in Atheroma Induced in Rabbits by an Atherogenic Diet with or Withoit Silicon I.V. Treatment. Life Sci. 1988, 42, 2105–2112. [Google Scholar] [CrossRef]
- Vidé, J.; Virsolvy, A.; Romain, C.; Ramos, J.; Jouy, N.; Richard, S.; Cristol, J.-P.; Gaillet, S.; Rouanet, J.-M. Dietary Silicon-Enriched Spirulina Improves Early Atherosclerosis Markers in Hamsters on a High-Fat Diet. Nutrition 2015, 31, 1148–1154. [Google Scholar] [CrossRef]
- Arthur-Ataam, J.; Bideaux, P.; Charrabi, A.; Sicard, P.; Fromy, B.; Liu, K.; Eddahibi, S.; Pasqualin, C.; Jouy, N.; Richard, S.; et al. Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats. Nutrients 2019, 11, 2574. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Gershwin, M.E.; Belay, A. (Eds.) Spirulina in Human Nutrition and Health; CRC press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Majewski, M.; Klett-Mingo, M.; Verdasco-Martín, C.M.; Otero, C.; Ferrer, M. Spirulina Extract Improves Age-Induced Vascular Dysfunction. Pharm. Biol. 2022, 60, 627–637. [Google Scholar] [CrossRef]
- de Freitas Brito, A.; Silva, A.S.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.L.; da Cunha Araujo, L.C.; da Silva Félix, G.; de Souza Sampaio, R.; da Conceição Correia Silva, M.; Tavares, R.L.; et al. Supplementation with Spirulina platensis Modulates Aortic Vascular Reactivity through Nitric Oxide and Antioxidant Activity. Oxidative Med. Cell. Longev. 2019, 2019, e7838149. [Google Scholar] [CrossRef]
- Vidé, J.; Romain, C.; Feillet-Coudray, C.; Bonafos, B.; Cristol, J.P.; Fouret, G.; Rouanet, J.-M.; Gaillet, S. Assessment of Potential Toxicological Aspects of Dietary Exposure to Silicon-Rich Spirulina in Rats. Food Chem. Toxicol. 2015, 80, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). An Update on Behalf of the Advisory Board of the 3rd, 4th and 5th Watching the Risk Symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Asmar, R.; Benetos, A.; Topouchian, J.; Laurent, P.; Pannier, B.; Brisac, A.M.; Target, R.; Levy, B.I. Assessment of Arterial Distensibility by Automatic Pulse Wave Velocity Measurement. Validation and Clinical Application Studies. Hypertension 1995, 26, 485–490. [Google Scholar] [CrossRef]
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of Pulse Wave Velocity in Healthy People and in the Presence of Cardiovascular Risk Factors: “Establishing Normal and Reference Values”. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Benjamin, E.J. Assessment of Endothelial Function Using Digital Pulse Amplitude Tonometry. Trends Cardiovasc. Med. 2009, 19, 6–11. [Google Scholar] [CrossRef]
- Faizi, A.K.; Kornmo, D.W.; Agewall, S. Evaluation of Endothelial Function Using Finger Plethysmography. Clin. Physiol. Funct. Imaging 2009, 29, 372–375. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M. The 2017 Clinical Practice Guideline for High Blood Pressure. JAMA 2017, 318, 2073–2074. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction: An Individual Participant Meta-Analysis of Prospective Observational Data From 17,635 Subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Chen, X.; Barywani, S.B.; Hansson, P.-O.; Rosengren, A.; Thunström, E.; Zhong, Y.; Ergatoudes, C.; Mandalenakis, Z.; Caidahl, K.; Fu, M. High-Normal Blood Pressure Conferred Higher Risk of Cardiovascular Disease in a Random Population Sample of 50-Year-Old Men: A 21-Year Follow-Up. Medicine 2020, 99, e19895. [Google Scholar] [CrossRef]
- Vasan, R.S.; Larson, M.G.; Leip, E.P.; Evans, J.C.; O’Donnell, C.J.; Kannel, W.B.; Levy, D. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease. N. Engl. J. Med. 2001, 345, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Loeper, J.; Loeper, J.; Fragny, M. The Physiological Role of the Silicon and Its AntiAtheromatous Action. In Biochemistry of Silicon and Related Problems; Bendz, G., Lindqvist, I., Runnström-Reio, V., Eds.; Nobel Foundation Symposia; Springer: New York, NY, USA, 1978; pp. 281–296. ISBN 978-1-4613-4020-1. [Google Scholar]
- Martin, K.R. The Chemistry of Silica and Its Potential Health Benefits. J. Nutr. Health Aging 2007, 11, 94–97. [Google Scholar] [PubMed]
- Calomme, M.R.; Vanden Berghe, D.A. Supplementation of Calves with Stabilized Orthosilicic Acid. Effect on the Si, Ca, Mg, and P Concentrations in Serum and the Collagen Concentration in Skin and Cartilage. Biol. Trace Elem. Res. 1997, 56, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Dudek, Ł.; Kochman, W.; Dziedzic, E. Silicon in Prevention of Atherosclerosis and Other Age-Related Diseases. Front. Cardiovasc. Med. 2024, 11, 1370536. [Google Scholar] [CrossRef]
- Loeper, J.; Goy-Loeper, J.; Rozensztajn, L.; Fragny, M. The Antiatheromatous Action of Silicon. Atherosclerosis 1979, 33, 397–408. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid. Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Zdziebłowska, S.; Czarnecki, M.; Ciosek-Skibińska, P.; Ruzik, L. The Microalgae’s Ability to Accumulate Selected Trace Elements Studied by ICP-MS/MS and Chemometric Methods. J. Trace Elem. Med. Biol. 2024, 81, 127351. [Google Scholar] [CrossRef]
- Mazo, V.K.; Gmoshinski, I.V.; Zorin, S.N. New Food Sources of Essential Trace Elements Produced by Biotechnology Facilities. Biotechnol. J. 2007, 2, 1297–1305. [Google Scholar] [CrossRef]
- Aimaretti, E.; Porchietto, E.; Mantegazza, G.; Gargari, G.; Collotta, D.; Einaudi, G.; Ferreira Alves, G.; Marzani, E.; Algeri, A.; Dal Bello, F.; et al. Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model. Nutrients 2024, 16, 552. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Spirulina—An Invaluable Source of Macro- and Micronutrients with Broad Biological Activity and Application Potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Low Dog, T.; Sarma, N.D.; Giancaspro, G.I.; et al. United States Pharmacopeia Safety Evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A. Framingham Study Insights on the Hazards of Elevated Blood Pressure. JAMA 2008, 300, 2545–2547. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redán, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Rabi, D.M.; McBrien, K.A.; Sapir-Pichhadze, R.; Nakhla, M.; Ahmed, S.B.; Dumanski, S.M.; Butalia, S.; Leung, A.A.; Harris, K.C.; Cloutier, L.; et al. Hypertension Canada’s 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2020, 36, 596–624. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Braun, L.T.; Ndumele, C.E.; Smith, S.C.; Sperling, L.S.; Virani, S.S.; Blumenthal, R.S. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation 2019, 139, e1162–e1177. [Google Scholar] [CrossRef]
- Tanaka, H.; Safar, M.E. Influence of Lifestyle Modification on Arterial Stiffness and Wave Reflections. Am. J. Hypertens. 2005, 18, 137–144. [Google Scholar] [CrossRef]
- Jennings, A.; Berendsen, A.M.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Brzozowska, A.; Sicinska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef]

| Variable | Placebo (n = 60) | SpSi (n = 60) | p-Value |
|---|---|---|---|
| Age (year) | 65.9 ± 0.6 | 65.3 ± 0.5 | 0.6169 |
| BMI (kg/m2) | 24.2 ± 0.5 | 24.5 ± 0.4 | 0.3728 |
| Systolic blood pressure (mmHg) | 121.8 ± 1.8 | 120.8 ± 2.1 | 0.4455 |
| Diastolic blood pressure (mmHg) | 68.3 ± 1.1 | 69.3 ± 1.2 | 0.5537 |
| Heart rate (bpm) | 61.3 ± 1.5 | 62.1 ± 1.2 | 0.3721 |
| Aortic pulse wave velocity (m/s) | 10.48 ± 0.27 | 11.01 ± 0.29 | 0.1565 |
| Reactive hyperemia index | 2.27 ± 0.08 | 2.46 ± 0.11 | 0.4055 |
| Hemoglobin | 14.24 ± 0.14 | 14.23 ± 0.14 | 0.9646 |
| Glucose (mmol/L) | 5.57 ± 0.07 | 5.43 ± 0.07 | 0.1606 |
| Insulin (pmol/L) | 72.2 ± 6.1 | 64.5 ± 4.2 | 0.4441 |
| HOMA-IR | 3.34 ± 0.69 | 2.27 ± 0.16 | 0.2886 |
| Total cholesterol (mmol/L) | 5.70 ± 0.11 | 5.55 ± 0.11 | 0.3141 |
| HDL-cholesterol (mmol/L) | 1.80 ± 0.07 | 1.81 ± 0.06 | 0.6939 |
| LDL-cholesterol (mmol/L) | 3.41 ± 0.10 | 3.22 ± 0.09 | 0.1117 |
| Triglycerides (mmol/L) | 1.09 ± 0.05 | 1.19 ± 0.07 | 0.5224 |
| Vitamin C (µmol/L) | 67.5 ± 3.2 | 59.9 ± 3.2 | 0.2143 |
| Vitamin E (µg/L) | 0.52 ± 0.05 | 0.66 ± 0.08 | 0.4087 |
| Variable | Placebo | SpSi | ||||
|---|---|---|---|---|---|---|
| D0 | D180 | p-Value | D0 | D180 | p-Value | |
| Subjects (n) | 58 | 59 | ||||
| Men (n) | 28 | 28 | ||||
| Women (n) | 30 | 31 | ||||
| Age (y) | 65.2 ± 0.9 | 66 ± 0.6 | 0.6169 # | |||
| BMI (kg/m2) | 24.6 ± 0.5 | 24.1 ± 0.5 | 0.6241 # | |||
| Systolic blood pressure (mmHg) | 120.8 ± 2.1 | 121.5 ± 2.1 | 0.8995 | 121.7 ± 1.8 | 118.9 ± 1.7 | 0.1165 |
| Diastolic blood pressure (mmHg) | 69.6 ± 1.2 | 68.8 ± 1.1 | 0.8302 | 68.2 ± 1.1 | 67.0 ± 1.2 | 0.4589 |
| Mean blood pressure (mmHg) | 95.2 ± 1.5 | 95.2 ± 1.5 | >0.9999 | 95 ± 1.3 | 92.9 ± 1.2 | 0.1423 |
| Pulse pressure (mmHg) | 51.2 ±1.6 | 52.6 ±1.6 | 0.5892 | 53.5 ±1.6 | 51.9 ±1.7 | 0.4269 |
| aPWV (m/s) | 10.5 ± 0.3 | 10.5 ± 0.2 | 0.9987 | 11.0 ± 0.3 | 10.4 ± 0.2 | 0.0864 |
| RHI (%) | 2.25 ± 0.08 | 2.39 ± 0.09 | 0.2989 | 2.43 ± 0.11 | 2.19 ± 0.09 | 0.0437 |
| Right carotid IMT (mm) | 0.72 ± 0.01 | 0.70 ± 0.01 | 0.3514 | 0.73 ± 0.01 | 0.71 ± 0.02 | 0.2208 |
| Left carotid IMT (mm) | 0.76 ± 0.02 | 0.74 ± 0.01 | 0.2228 | 0.75 ± 0.02 | 0.74 ± 0.01 | 0.9398 |
| Variable | Placebo (aPWV > 10) | SpSi (aPWV > 10) | ||||
|---|---|---|---|---|---|---|
| D0 | D180 | p-Value | D0 | D180 | p-Value | |
| Subjects (n) | 28 | 28 | ||||
| Men (n) | 17 | 12 | ||||
| Women (n) | 12 | 16 | ||||
| Age (y) | 65.7 ± 0.7 | 66.5 ± 0.9 | 0.6710 # | |||
| BMI (kg/m2) | 24.7 ± 0.6 | 24.3 ± 0.7 | 0.3111 # | |||
| Systolic blood pressure (mmHg) | 126.8 ± 3.1 | 125.1 ± 3.5 | 0.7482 | 125.5 ± 2.5 | 119.9 ± 2.6 | 0.0233 |
| Diastolic blood pressure (mmHg) | 72.1 ± 1.6 | 70.7 ± 1.8 | 0.6491 | 70.2 ± 1.7 | 67.9 ± 1.5 | 0.0538 |
| Mean blood pressure (mmHg) | 99.4 ± 2.1 | 97.9 ± 2.5 | 0.6211 | 97.8 ± 1.8 | 94.3 ± 1.6 | 0.0318 |
| Pulse pressure (mmHg) | 54.8 ±2.3 | 54.4 ±2.5 | 0.9908 | 55.3 ±2.3 | 52.9 ±2.4 | 0.3677 |
| aPWV (m/s) | 12.1 ± 0.3 | 11.4 ± 0.3 | 0.0920 | 12.6 ± 0.3 | 10.9 ± 0.4 | <0.0001 |
| RHI (%) | 2.36 ± 0.11 | 2.31 ± 0.10 | 0.8642 | 2.54 ± 0.10 | 2.20 ± 0.13 | 0.0192 |
| Right carotid IMT (mm) | 0.72 ± 0.02 | 0.71 ± 0.02 | 0.8156 | 0.74 ± 0.02 | 0.73 ± 0.03 | 0.6998 |
| Left carotid IMT (mm) | 0.76 ± 0.02 | 0.76 ± 0.02 | 0.9023 | 0.75 ±0.02 | 0.75 ± 0.02 | 0.9796 |
| Variable | Placebo (SBP ≥ 130) | SpSi (SBP ≥ 130) | ||||
|---|---|---|---|---|---|---|
| D0 | D180 | p-Value | D0 | D180 | p-Value | |
| Subjects (n) | 20 | 17 | ||||
| Men (n) | 14 | 6 | ||||
| Women (n) | 6 | 11 | ||||
| Age (y) | 66.1 ± 1.0 | 67.9 ± 1.1 | 0.1859 * | |||
| BMI (kg/m2) | 24.8 ± 0.7 | 23.8 ± 0.9 | 0.2344 * | |||
| Systolic blood pressure (mmHg) | 139.5 ± 2.1 | 137.1 ± 3.3 | 0.7482 | 138.9 ± 1.9 | 127.6 ± 3.2 | 0.0004 |
| Diastolic blood pressure (mmHg) | 76.9 ± 1.9 | 73.9 ± 1.9 | 0.6491 | 73.5 ± 2.4 | 68.9 ± 2.0 | 0.0721 |
| Mean blood pressure (mmHg) | 108.2 ± 1.9 | 105.5 ± 2.6 | 0.6211 | 106.2 ± 1.7 | 98.3 ± 2.1 | 0.0005 |
| Pulse pressure (mmHg) | 62.6 ±2.4 | 63.2 ±2.8 | 0.9908 | 65.4 ± 2.7 | 58.7 ± 3.3 | 0.0530 |
| aPWV (m/s) | 11.2 ± 0.5 | 11.7 ± 0.5 | 0.0920 | 12.3 ± 0.6 | 11.5 ± 0.5 | 0.3726 |
| RHI (%) | 2.5 ± 0.2 | 2.3 ± 0.1 | 0.8642 | 2.7 ± 0.3 | 2.5 ± 0.2 | 0.7084 |
| Right carotid IMT (mm) | 0.75 ± 0.03 | 0.75 ± 0.02 | 0.8156 | 0.74 ± 0.02 | 0.75 ± 0.03 | 0.3868 |
| Left carotid IMT (mm) | 0.82 ± 0.03 | 0.79 ± 0.03 | 0.9023 | 0.74 ±0.03 | 0.74 ± 0.03 | 0.9980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virsolvy, A.; Benmira, A.M.; Allal, S.; Demattei, C.; Sutra, T.; Cristol, J.-P.; Jouy, N.; Richard, S.; Perez-Martin, A. Benefits of Dietary Supplementation with Specific Silicon-Enriched Spirulina on Arterial Function in Healthy Elderly Individuals: A Randomized, Placebo-Controlled Trial. Nutrients 2025, 17, 864. https://doi.org/10.3390/nu17050864
Virsolvy A, Benmira AM, Allal S, Demattei C, Sutra T, Cristol J-P, Jouy N, Richard S, Perez-Martin A. Benefits of Dietary Supplementation with Specific Silicon-Enriched Spirulina on Arterial Function in Healthy Elderly Individuals: A Randomized, Placebo-Controlled Trial. Nutrients. 2025; 17(5):864. https://doi.org/10.3390/nu17050864
Chicago/Turabian StyleVirsolvy, Anne, Amir Mokhfi Benmira, Salim Allal, Christophe Demattei, Thibault Sutra, Jean-Paul Cristol, Nicolas Jouy, Sylvain Richard, and Antonia Perez-Martin. 2025. "Benefits of Dietary Supplementation with Specific Silicon-Enriched Spirulina on Arterial Function in Healthy Elderly Individuals: A Randomized, Placebo-Controlled Trial" Nutrients 17, no. 5: 864. https://doi.org/10.3390/nu17050864
APA StyleVirsolvy, A., Benmira, A. M., Allal, S., Demattei, C., Sutra, T., Cristol, J.-P., Jouy, N., Richard, S., & Perez-Martin, A. (2025). Benefits of Dietary Supplementation with Specific Silicon-Enriched Spirulina on Arterial Function in Healthy Elderly Individuals: A Randomized, Placebo-Controlled Trial. Nutrients, 17(5), 864. https://doi.org/10.3390/nu17050864






