Endothelial Homeostasis Under the Influence of Alcohol—Relevance to Atherosclerotic Cardiovascular Disease
Abstract
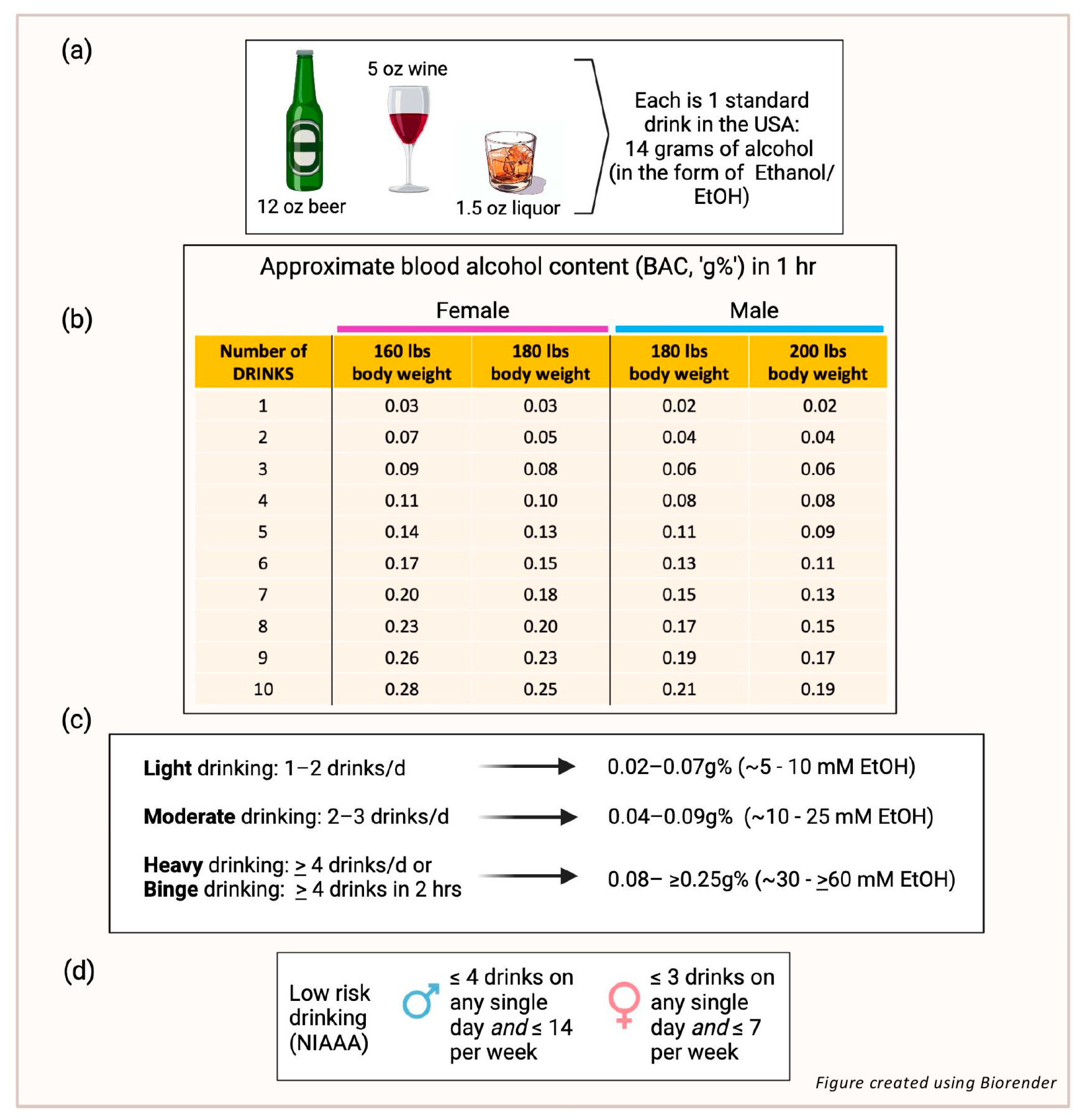
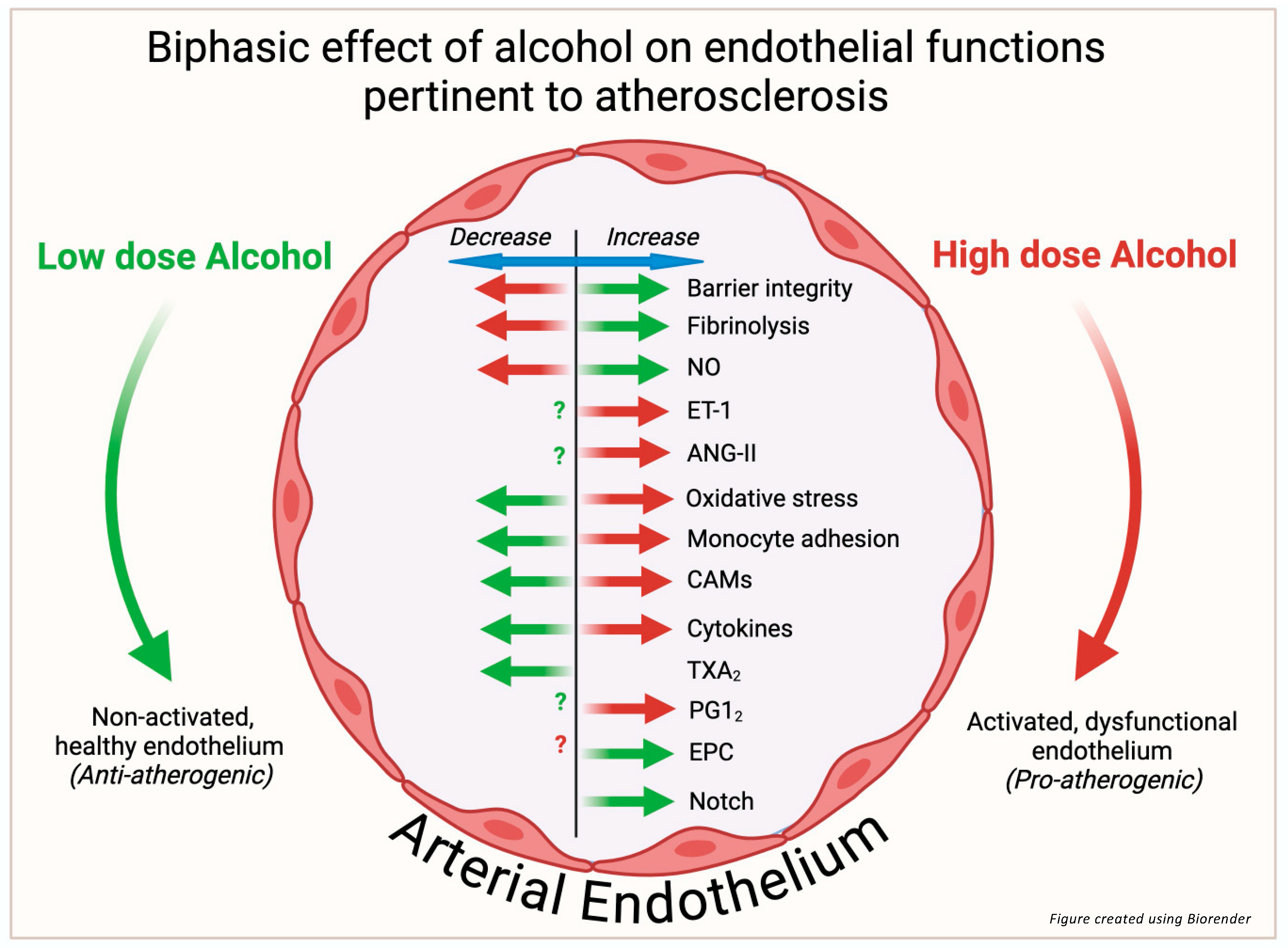
1. Alcohol and Atherosclerotic Cardiovascular Disease
2. Endothelium: Role in Atherogenesis
3. Endothelial Barrier Function
4. Vasoactive Substances
5. Nitric Oxide (NO)
6. Prostacyclin and Thromboxane A2
7. Endothelin-1
8. Angiotensin-II
9. ROS/Oxidative Stress
10. Monocyte Recruitment and Adhesion
11. Cell Adhesion Molecules
12. Inflammatory Cytokines and CRP
13. Endothelial Control of Thrombosis and Fibrinolysis
14. Endothelial Repair/Endothelial Progenitor Cells
15. Endothelial Notch Signaling
16. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9887164 (accessed on 21 February 2025). [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the global burden of disease study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Alcohol and cardiovascular disease—Modulation of vascular cell function. Nutrients 2012, 4, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Roerecke, M. Alcohol’s impact on the cardiovascular system. Nutrients 2021, 13, 3419. [Google Scholar] [CrossRef]
- Masip, J.; Lluch, J.R.G. Alcohol, health and cardiovascular disease. Rev. Clin. Esp. 2021, 211, 359–368. Available online: https://www.ncbi.nlm.nih.gov/pubmed/34059235 (accessed on 21 February 2025). [CrossRef] [PubMed]
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolainen, K. Alcohol and coronary heart disease: A meta-analysis. Addiction 2000, 95, 1505–1523. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11070527 (accessed on 21 February 2025). [CrossRef] [PubMed]
- Zhao, J.; Stockwell, T.; Roemer, A.; Naimi, T.; Chikritzhs, T. Alcohol consumption and mortality from coronary heart disease: An updated meta-analysis of cohort studies. J. Stud. Alcohol. Drugs 2017, 78, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Redmond, E.M.; Morrow, D.; Cullen, J.P. Differential effects of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development in mice. Atherosclerosis 2011, 219, 448–454. [Google Scholar] [CrossRef]
- Liu, W.; Harman, S.; DiLuca, M.; Burtenshaw, D.; Corcoran, E.; Cahill, P.A.; Redmond, E.M. Moderate alcohol consumption targets s100β + vascular stem cells and attenuates injury-induced neointimal hyperplasia. Alcohol. Clin. Exp. Res. 2020, 44, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef]
- Li, H.; Xia, N. Alcohol and the vasculature: A love-hate relationship? Pflügers Arch. Eur. J. Physiol. 2023, 475, 867–875. [Google Scholar] [CrossRef]
- Hendriks, H.F. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.E.; Nicholson, E.R.; Fischer, S.M.; Zimmer, R.; Froehlich, J.C. Alcohol Drinking and Blood Alcohol Concentration Revisited. Alcohol. Clin. Exp. Res. 2017, 42, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Thorin, E.; Shatos, M.A.; Shreeve, S.M.; Walters, C.L.; Bevan, J.A. Human vascular endothelium heterogeneity. Stroke 1997, 28, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2008, 6, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924. [Google Scholar] [CrossRef]
- Haorah, J.; Knipe, B.; Leibhart, J.; Ghorpade, A.; Persidsky, Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 2005, 78, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.G.; Yuan, S.Y.; Breslin, J.W. Sphingosine-1-phosphate protects against brain microvascular endothelial junctional protein disorganization and barrier dysfunction caused by alcohol. Microcirculation 2018, 26, e12506. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, G.; Fu, W.; Liao, M.; Frank, J.A.; Bower, K.A.; Fang, S.; Zhang, Z.; Shi, X.; Luo, J. Ethanol disrupts vascular endothelial barrier: Implication in cancer metastasis. Toxicol. Sci. 2012, 127, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Muneer, P.M.A.; Alikunju, S.; Szlachetka, A.M.; Haorah, J. The mechanisms of cerebral vascular dysfunction and neuroinflammation by mmp-mediated degradation of vegfr-2 in alcohol ingestion. Arter. Thromb. Vasc. Biol. 2012, 32, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Araiz, A.; Porcu, F.; Pérez-Hernández, M.; García-Gutiérrez, M.S.; Aracil-Fernández, M.A.; Gutierrez-López, M.D.; Guerri, C.; Manzanares, J.; O’Shea, E.; Colado, M.I. Disruption of blood–brain barrier integrity in postmortem alcoholic brain: Preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict. Biol. 2016, 22, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Liu, W.; Cahill, P.A.; Redmond, E.M. Alcohol and vascular endothelial function: Biphasic effect highlights the importance of dose. Alcohol. Clin. Exp. Res. 2023, 47, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Liu, W.; Chu, C.C.; Cahill, P.A.; Redmond, E.M. Moderate dose alcohol protects against serum amyloid protein A1-induced endothelial dysfunction via both notch-dependent and notch-independent pathways. Alcohol. Clin. Exp. Res. 2021, 45, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Lüscher, T.F. Human endothelial dysfunction: EDRFs. Pflügers Arch. Eur. J. Physiol. 2010, 459, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. The 1998 Nobel prize in Medicine: Clinical implications for 1999 and beyond. Vasc. Med. 1999, 4, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Doursout, M.; Murad, F. Vascular system: Role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. 2008, 10, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Davda, R.K.; Chandler, L.J.; Crews, F.T.; Guzman, N.J. Ethanol enhances the endothelial nitric oxide synthase response to agonists. Hypertension 1993, 21, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.J.; Cahill, P.A.; Sitzmann, J.V.; Redmond, E.M. Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J. Pharmacol. Exp. Ther. 1999, 289, 1293–1300. [Google Scholar] [CrossRef]
- Abou-Agag, L.H.; Khoo, N.K.; Binsack, R.; White, C.R.; Darley-Usmar, V.; Grenett, H.E.; Booyse, F.M.; Digerness, S.B.; Zhou, F.; Parks, D.A. Evidence of cardiovascular protection by moderate alcohol: Role of nitric oxide. Free. Radic. Biol. Med. 2005, 39, 540–548. Available online: https://journals.viamedica.pl/folia_morphologica/article/download/FM.a2016.0024/36838 (accessed on 21 February 2025). [CrossRef]
- Matsuo, S.; Nakamura, Y.; Takahashi, M.; Ouchi, Y.; Hosoda, K.; Nozawa, M.; Kinoshita, M. Effect of red wine and ethanol on production of nitric oxide in healthy subjects. Am. J. Cardiol. 2001, 87, 1029–1031. [Google Scholar] [CrossRef]
- Kuhlmann, C.R.; Li, F.; Ludders, D.W.; Schaefer, C.A.; Most, A.K.; Backenkohler, U.; Neumann, T.; Tillmanns, H.; Waldecker, B.; Erdogan, A.; et al. Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol. Clin. Exp. Res. 2024, 28, 1005–1011. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15252286 (accessed on 21 February 2025). [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Dogné, J.-M. Vascular biology of eicosanoids and atherogenesis. Expert. Rev. Cardiovasc. Ther. 2009, 7, 1079–1089. [Google Scholar] [CrossRef]
- Mikhailidis, D.; Barradas, M.; Jeremy, J. The effect of ethanol on platelet function and vascular prostanoids. Alcohol 1990, 7, 171–180. [Google Scholar] [CrossRef]
- Guivernau, M.; Baraona, E.; Lieber, C. Acute and chronic effects of ethanol and its metabolites on vascular production of prostacyclin in rats. J. Pharmacol. Exp. Ther. 1987, 240, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, R.; Steiner, M. Ethanol raises prostacyclin in vivo and in vitro. Blood 1984, 64, 679–682. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6380621 (accessed on 21 February 2025). [CrossRef]
- Karanian, J.; Stojanov, M.; Salem, N. Effect of ethanol on prostacyclin and thromboxane A2 synthesis in rat aortic rings in vitro. Prostaglandins Leukot. Med. 1985, 20, 175–186. [Google Scholar] [CrossRef] [PubMed]
- A Jakubowski, J.; Vaillancourt, R.; Deykin, D. Interaction of ethanol, prostacyclin, and aspirin in determining human platelet reactivity in vitro. Arteriosclerosis 1988, 8, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Kontula, K.; Viinikka, L.; Ylikorkala, O.; Ylikahri, R. Effect of acute ethanol intake on thromboxane and prostacyclin in human. Life Sci. 1982, 31, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Thorin, E.; Webb, D.J. Endothelium-derived endothelin-1. Pflügers Arch. Eur. J. Physiol. 2009, 459, 951–958. [Google Scholar] [CrossRef]
- Corder, R.; Douthwaite, J.A.; Lees, D.M.; Khan, N.Q.; dos Santos, A.C.V.; Wood, E.G.; Carrier, M.J. Endothelin-1 synthesis reduced by red wine. Nature 2001, 414, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.Q.; Lees, D.M.; Douthwaite, J.A.; Carrier, M.J.; Corder, R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin. Sci. 2002, 103, 72S–75S. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T.O.; Saraste, A.; Lehtimäki, T.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. Decreased endothelin-1 levels after acute consumption of red wine and de-alcoholized red wine. Atherosclerosis 2010, 211, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Karatzis, E.; Papamichael, C.; Lekakis, J.; Zampelas, A. Effects of red wine on endothelial function: Postprandial studies vs clinical trials. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health effects of red wine consumption: A narrative review of an issue that still deserves debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.R.; Casolari, D.A.; Montezano, A.C.; Yogi, A.; Tostes, R.C.; Legros, E.; D’orléans-Juste, P.; Lanchote, V.L.; Uyemura, S.A.; de Oliveira, A.M. Ethanol consumption enhances endothelin-1-induced contraction in the isolated rat carotid. J. Pharmacol. Exp. Ther. 2006, 318, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.R.; Legros, E.; Brochu, I.; Honoré, J.-C.; Lanchote, V.L.; A Uyemura, S.; De Oliveira, A.M.; D’Orléans-Juste, P. Chronic ethanol intake modulates vascular levels of endothelin-1 receptor and enhances the pressor response to endothelin-1 in anaesthetized rats. Br. J. Pharmacol. 2008, 154, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Zilkens, R.R.; Rich, L.; Burke, V.; Beilin, L.J.; Watts, G.F.; Puddey, I.B. Effects of alcohol intake on endothelial function in men. J. Hypertens. 2003, 21, 97–103. [Google Scholar] [CrossRef]
- Di Gennaro, C.; Biggi, A.; Barilli, A.L.; Fasoli, E.; Carra, N.; Novarini, A.; Delsignore, R.; Montanari, A. Endothelial dysfunction and cardiovascular risk profile in long-term withdrawing alcoholics. J. Hypertens. 2007, 25, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; França-Falcão, M.S.; Calzerra, N.T.M.; Luz, M.S.; Gadelha, D.D.A.; Balarini, C.M.; Queiroz, T.M. Role of renin-angiotensin system components in atherosclerosis: Focus on ang-ii, ace2, and ang-1–7. Front. Physiol. 2020, 11, 1067. [Google Scholar] [CrossRef]
- van Thiel, B.S.; van der Pluijm, I.; Riet, L.T.; Essers, J.; Danser, A.J. The renin–angiotensin system and its involvement in vascular disease. Eur. J. Pharmacol. 2015, 763, 3–14. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin ii in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Vazquez, M.; Ansari, R.A.; Malafa, M.P.; Lalla, J. Chronic alcohol-induced oxidative endothelial injury relates to angiotensin II levels in the rat. Mol. Cell Biochem. 2007, 307, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Passaglia, P.; Ceron, C.S.; Mecawi, A.S.; Antunes-Rodrigues, J.; Coelho, E.B.; Tirapelli, C.R. Angiotensin type 1 receptor mediates chronic ethanol consumption-induced hypertension and vascular oxidative stress. Vasc. Pharmacol. 2015, 74, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Awata, W.M.; Alves, J.V.; Costa, R.M.; Bruder-Nascimento, A.; Singh, S.; Barbosa, G.S.; Tirapelli, C.R.; Bruder-Nascimento, T. Vascular injury associated with ethanol intake is driven by AT1 receptor and mitochondrial dysfunction. Biomed. Pharmacother. 2023, 169, 115845. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Chen, R.; Zheng, Q.; Yao, M.; Li, K.; Cao, Y.; Jiang, L. Oxidative stress disrupts vascular microenvironmental homeostasis affecting the development of atherosclerosis. Cell Biol. Int. 2024, 48, 1781–1801. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.A.; Osborn, K.; Hwang, C.-L.; Sabbahi, A.; Piano, M.R. Ethanol Induced Oxidative Stress in the Vasculature: Friend or Foe. Curr. Hypertens. Rev. 2019, 16, 181–191. [Google Scholar] [CrossRef]
- Padovan, J.C.; Dourado, T.M.H.; Pimenta, G.F.; Bruder-Nascimento, T.; Tirapelli, C.R. Reactive oxygen species are central mediators of vascular dysfunction and hypertension induced by ethanol consumption. Antioxidants 2023, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Lexis, L.; Lewandowski, P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr. J. 2007, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúnez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP -1 in Atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Badía, E.; Sacanella, E.; Fernández-Solá, J.; Nicolás, J.M.; Antúnez, E.; Rotilio, D.; de Gaetano, G.; Urbano-Márquez, A.; Estruch, R. Decreased tumor necrosis factor-induced adhesion of human monocytes to endothelial cells after moderate alcohol consumption. Am. J. Clin. Nutr. 2004, 80, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.; Guruge, B.L.; Miller, D.D.; Chaitman, B.R.; Bora, P.S. Moderate alcohol feeding attenuates postinjury vascular cell proliferation in rabbit angioplasty model. J. Cardiovasc. Pharmacol. 1997, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.P.; Sayeed, S.; Jin, Y.; Theodorakis, N.G.; Sitzmann, J.V.; Cahill, P.A.; Redmond, E.M. Ethanol inhibits monocyte chemotactic protein-1 expression in interleukin-1{beta}-activated human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1669–H1675. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15908470 (accessed on 21 February 2025).
- Saeed, R.W.; Varma, S.; Peng, T.; Tracey, K.J.; Sherry, B.; Metz, C.N. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J. Immunol. 2004, 173, 6376–6383. [Google Scholar] [CrossRef] [PubMed]
- Redmond, E.M.; Morrow, D.; Kundimi, S.; Miller-Graziano, C.L.; Cullen, J.P. Acetaldehyde stimulates monocyte adhesion in a p-selectin- and tnfalpha-dependent manner. Atherosclerosis 2009, 204, 372–380. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19036374 (accessed on 21 February 2025). [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.P. Endothelial-Leukocyte Adhesion Molecules. Annu. Rev. Immunol. 1993, 11, 767–804. [Google Scholar] [CrossRef]
- Sacanella, E.; Estruch, R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict. Biol. 2003, 8, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Shirpoor, A.; Salami, S.; Khadem-Ansari, M.H.; Heshmatian, B.; Ilkhanizadeh, B. Long-term ethanol consumption initiates atherosclerosis in rat aorta through inflammatory stress and endothelial dysfunction. Vasc. Pharmacol. 2012, 57, 72–77. [Google Scholar] [CrossRef]
- Sacanella, E.; Estruch, R.; Badía, E.; Fernández-Sola, J.; Nicolás, J.M.; Urbano-Márquez, A. Chronic alcohol consumption increases serum levels of circulating endothelial cell/leucocyte adhesion molecules e-selectin and icam-1. Alcohol. Alcohol. 1999, 34, 678–684. [Google Scholar] [CrossRef][Green Version]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic. Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- Biros, E.; Reznik, J.E.; Moran, C.S. Role of inflammatory cytokines in genesis and treatment of atherosclerosis. Trends Cardiovasc. Med. 2021, 32, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Bourcier, T.; Bonnefoy, J.-Y.; Pober, J.S.; Libby, P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40–CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Santolaria-Fernández, F.; Martín-González, M.C.; Fernández-Rodríguez, C.M.; Quintero-Platt, G. Alcoholism: A systemic proinflammatory condition. World J. Gastroenterol. 2014, 20, 14660–14671. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Pahor, M.; Ferrucci, L.; Simonsick, E.M.; Guralnik, J.M.; Kritchevsky, S.B.; Fellin, R.; Harris, T.B. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitior-1 in well-functioning older adults. Circulation 2004, 109, 607–612. [Google Scholar] [CrossRef]
- Blake, G.; Ridker, P. High sensitivity C-reactive protein for predicting cardiovascular disease: An inflammatory hypothesis. Eur. Heart J. 2001, 22, 349–352. [Google Scholar] [CrossRef]
- Imhof, A.; Koenig, W. Alcohol inflammation and coronary heart disease. Addict. Biol. 2003, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Bochud, M.; Bastardot, F.; von Känel, R.; Ferrero, F.; Gaspoz, J.-M.; Paccaud, F.; Urwyler, A.; Lüscher, T.; Hock, C.; et al. Associations between alcohol consumption and selected cytokines in a Swiss population-based sample (CoLaus study). Atherosclerosis 2012, 222, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.D.; Elmadhun, N.Y.; Liu, Y.; Feng, J.; Burgess, T.A.; Karlson, N.W.; Laham, R.J.; Sellke, F.W.; Bito, V.; van der Velden, J.; et al. Ethanol promotes arteriogenesis and restores perfusion to chronically ischemic myocardium. Circulation 2013, 128, S136–S143. [Google Scholar] [CrossRef][Green Version]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.R.; Tsiang, M.; E Sadler, J. Regulation of thrombomodulin by tumor necrosis factor-alpha: Comparison of transcriptional and posttranscriptional mechanisms. Blood 1991, 77, 542–550. [Google Scholar]
- Huang, Y.; Li, Y.; Zheng, S.; Yang, X.; Wang, T.; Zeng, J. Moderate alcohol consumption and atherosclerosis. Wien. Klin. Wochenschr. 2017, 129, 835–843. [Google Scholar] [CrossRef]
- McCarter, K.D.; Li, C.; Jiang, Z.; Lu, W.; Smith, H.A.; Xu, G.; Mayhan, W.G.; Sun, H. Effect of low-dose alcohol consumption on inflammation following transient focal cerebral ischemia in rats. Sci. Rep. 2017, 7, 12547. [Google Scholar] [CrossRef] [PubMed]
- Kanthi, Y.M.; Sutton, N.R.; Pinsky, D.J. CD39: Interface Between Vascular Thrombosis and Inflammation. Curr. Atheroscler. Rep. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Pinsky, D.J. Cd39 as a critical ectonucleotidase defense against pathological vascular remodeling. Trans. Am. Clin. Climatol. Assoc. 2018, 129, 132–139. [Google Scholar]
- Caiazzo, E.; Tedesco, I.; Spagnuolo, C.; Russo, G.L.; Ialenti, A.; Cicala, C. Red wine inhibits aggregation and increases atp-diphosphohydrolase (cd39) activity of rat platelets in vitro. Nat. Prod. Commun. 2016, 11, 771–774. [Google Scholar] [CrossRef]
- Schmatz, R.; Mann, T.R.; Spanevello, R.; Machado, M.M.; Zanini, D.; Pimentel, V.C.; Stefanello, N.; Martins, C.C.; Cardoso, A.M.; Bagatini, M.; et al. Moderate red wine and grape juice consumption modulates the hydrolysis of the adenine nucleotides and decreases platelet aggregation in streptozotocin-induced diabetic rats. Cell Biochem. Biophys. 2012, 65, 129–143. [Google Scholar] [CrossRef]
- Xia, G.-Q.; Cai, J.-N.; Wu, X.; Fang, Q.; Zhao, N.; Lv, X.-W. The mechanism by which ATP regulates alcoholic steatohepatitis through P2X4 and CD39. Eur. J. Pharmacol. 2022, 916, 174729. [Google Scholar] [CrossRef]
- Fuhrman, B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis 2012, 222, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y. The upa/upar system orchestrates the inflammatory response, vascular homeostasis, and immune system in fibrosis progression. Int. J. Mol. Sci. 2023, 24, 1796. [Google Scholar] [CrossRef]
- Hajjar, K.A.; Acharya, S.S. Annexin ii and regulation of cell surface fibrinolysis. Ann. N. Y. Acad. Sci. 2000, 902, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Aikens, M.L.; E Grenett, H.; Benza, R.L.; Tabengwa, E.M.; Davis, G.C.; Booyse, F.M. Alcohol-induced upregulation of plasminogen activators and fibrinolytic activity in cultured human endothelial cells. Alcohol. Clin. Exp. Res. 1998, 22, 375–381. [Google Scholar] [PubMed]
- Tabengwa, E.M.; Abou-Agag, L.H.; Benza, R.L.; Torres, J.A.; Aikens, M.L.; Booyse, F.M. Ethanol-induced up-regulation of candidate plasminogen receptor annexin ii in cultured human endothelial cells. Alcohol. Clin. Exp. Res. 2000, 24, 754–761. Available online: https://www.ncbi.nlm.nih.gov/pubmed/10888061 (accessed on 21 February 2025). [PubMed]
- Booyse, F.M.; Aikens, M.L.; Grenett, H.E. Endothelial cell fibrinolysis: Transcriptional regulation of fibrinolytic protein gene expression (t-pa, u-pa, and pai-1) by low alcohol. Alcohol. Clin. Exp. Res. 1999, 23, 1119–1124. [Google Scholar] [CrossRef]
- E Grenett, H.; Aikens, M.L.; Tabengwa, E.M.; Davis, G.C.; Booyse, F.M. Ethanol downregulates transcription of the pai-1 gene in cultured human endothelial cells. Thromb. Res. 2000, 97, 247–255. [Google Scholar] [CrossRef]
- Van De Wiel, A.; Van Golde, P.M.; Kraaijenhagen, R.J.; Borne, P.A.K.V.D.; Bouma, B.N.; Hart, H.C. Acute inhibitory effect of alcohol on fibrinolysis. Eur. J. Clin. Investig. 2001, 31, 164–170. [Google Scholar] [CrossRef]
- Kiviniemi, T.O.; Saraste, A.; Lehtimäki, T.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Pärkkä, J.P.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. High dose of red wine elicits enhanced inhibition of fibrinolysis. Eur. J. Prev. Cardiol. 2009, 16, 161–163. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Mehta, A.; Dhindsa, D.S.; Bonora, B.M.; Sreejit, G.; Nagareddy, P.; Quyyumi, A.A. Circulating stem cells and cardiovascular outcomes: From basic science to the clinic. Eur. Heart J. 2019, 41, 4271–4282. [Google Scholar] [CrossRef]
- Pelliccia, F.; Zimarino, M.; De Luca, G.; Viceconte, N.; Tanzilli, G.; De Caterina, R. Endothelial progenitor cells in coronary artery disease: From bench to bedside. Stem Cells Transl. Med. 2022, 11, 451–460. [Google Scholar] [CrossRef]
- Kong, J.; Wang, F.; Zhang, J.; Cui, Y.; Pan, L.; Zhang, W.; Wen, J.; Liu, P. Exosomes of endothelial progenitor cells inhibit neointima formation after carotid artery injury. J. Surg. Res. 2018, 232, 398–407. [Google Scholar] [CrossRef]
- Chen, D.-X.; Lu, C.-H.; Na, N.; Yin, R.-X.; Huang, F. Endothelial progenitor cell-derived extracellular vesicles: The world of potential prospects for the treatment of cardiovascular diseases. Cell Biosci. 2024, 14, 1–21. [Google Scholar] [CrossRef]
- Premer, C.; Schulman, I.H. Predictive value of circulating progenitor cells in acute coronary syndrome. Circ. Res. 2018, 122, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowska-Ciastek, B.; Sokup, A.; Leszcz, M.; Drela, E.; Stankowska, K.; Boinska, J.; Haor, B.; Ślusarz, R.; Lisewska, B.; Gadomska, G.; et al. The number of circulating endothelial progenitor cells in healthy individuals—Effect of some anthropometric and environmental factors (a pilot study). Adv. Med. Sci. 2015, 60, 58–63. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Condines, X.; Magraner, E.; Roth, I.; Valderas-Martínez, P.; Arranz, S.; Casas, R.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Quifer-Rada, P.; et al. The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: A randomized clinical trial. Atherosclerosis 2014, 233, 518–524. [Google Scholar] [CrossRef]
- Xiao, Q.; Kiechl, S.; Patel, S.; Oberhollenzer, F.; Weger, S.; Mayr, A.; Metzler, B.; Reindl, M.; Hu, Y.; Willeit, J.; et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—results from a large population-based study. PLoS ONE 2007, 2, e975. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chen, Y.-H.; Tsai, H.-Y.; Chen, J.-S.; Wu, T.-C.; Lin, F.-Y.; Sata, M.; Chen, J.-W.; Lin, S.-J. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, L.; Schröder-Heurich, B.; Kipke, B.; Schmidt, C.; von Kaisenberg, C.S.; von Versen-Höynck, F. Low ethanol concentrations promote endothelial progenitor cell capacity and reparative function. Cardiovasc. Ther. 2020, 2020, 4018478. [Google Scholar] [CrossRef]
- Gil-Bernabe, P.; Boveda-Ruiz, D.; D’Alessandro-Gabazza, C.; Toda, M.; Miyake, Y.; Mifuji-Moroka, R.; Iwasa, M.; Morser, J.; Gabazza, E.C.; Takei, Y. Atherosclerosis amelioration by moderate alcohol consumption is associated with increased circulating levels of stromal cell-derived factor-1. Circ. J. 2011, 75, 2269–2279. [Google Scholar] [CrossRef]
- Vergori, L.; Lauret, E.; Soleti, R.; Martinez, M.C.; Andriantsitohaina, R. Low concentration of ethanol favors progenitor cell differentiation and neovascularization in high-fat diet-fed mice model. Int. J. Biochem. Cell Biol. 2016, 78, 43–51. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Sega, F.V.D.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch signaling regulates immune responses in atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef]
- Mack, J.J.; Iruela-Arispe, M.L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352. [Google Scholar] [CrossRef]
- Morrow, D.; Cullen, J.P.; Liu, W.; Cahill, P.A.; Redmond, E.M. Alcohol Inhibits Smooth Muscle Cell Proliferation via Regulation of the Notch Signaling Pathway. Arter. Thromb. Vasc. Biol. 2010, 30, 2597–2603. [Google Scholar] [CrossRef]
- Morrow, D.; Cullen, J.P.; Cahill, P.A.; Redmond, E.M. Ethanol stimulates endothelial cell angiogenic activity via a Notch- and angiopoietin-1-dependent pathway. Cardiovasc. Res. 2008, 79, 313–321. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18448572 (accessed on 21 February 2025). [CrossRef]
- Morrow, D.; Hatch, E.; Hamm, K.; Cahill, P.A.; Redmond, E.M. Flk-1/KDR Mediates Ethanol-Stimulated Endothelial Cell Notch Signaling and Angiogenic Activity. J. Vasc. Res. 2014, 51, 315–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusti, Y.; Liu, W.; Athar, F.; Cahill, P.A.; Redmond, E.M. Endothelial Homeostasis Under the Influence of Alcohol—Relevance to Atherosclerotic Cardiovascular Disease. Nutrients 2025, 17, 802. https://doi.org/10.3390/nu17050802
Gusti Y, Liu W, Athar F, Cahill PA, Redmond EM. Endothelial Homeostasis Under the Influence of Alcohol—Relevance to Atherosclerotic Cardiovascular Disease. Nutrients. 2025; 17(5):802. https://doi.org/10.3390/nu17050802
Chicago/Turabian StyleGusti, Yusof, Weimin Liu, Fathima Athar, Paul A. Cahill, and Eileen M. Redmond. 2025. "Endothelial Homeostasis Under the Influence of Alcohol—Relevance to Atherosclerotic Cardiovascular Disease" Nutrients 17, no. 5: 802. https://doi.org/10.3390/nu17050802
APA StyleGusti, Y., Liu, W., Athar, F., Cahill, P. A., & Redmond, E. M. (2025). Endothelial Homeostasis Under the Influence of Alcohol—Relevance to Atherosclerotic Cardiovascular Disease. Nutrients, 17(5), 802. https://doi.org/10.3390/nu17050802




