Complex Probiotics Ameliorate Fecal Microbiota Transplantation-Induced IBS in Mice via Gut Microbiota and Metabolite Modulation
Abstract
1. Introduction
2. Materials and Methods
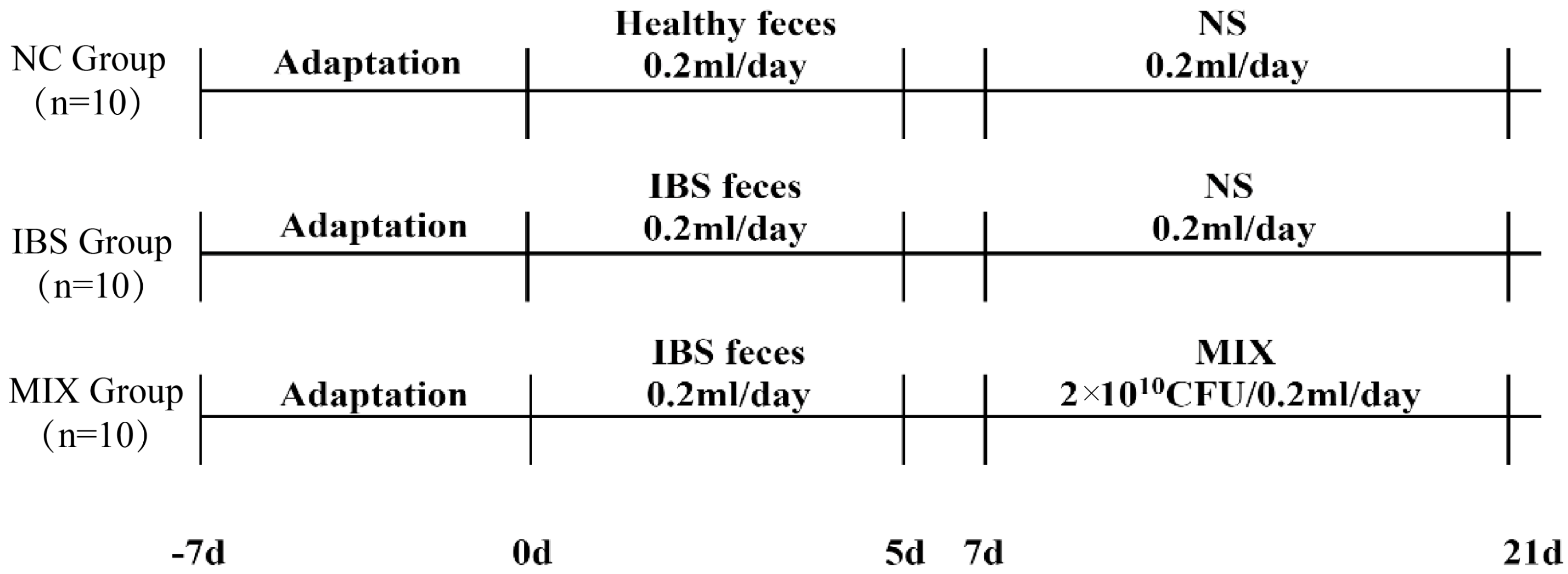
2.1. IBS Animal Experimental Design
2.2. Number of Fecal Pellets and Water Content
2.3. Intestinal Permeability
2.4. Gastrointestinal Transit Time
2.5. Histological Analysis
2.6. ELISA
2.7. Fecal DNA Extraction, Sequencing and Analysis
2.8. Determination of Fecal Short-Chain Fatty Acids and Tryptophan
2.9. Statistical Analysis
3. Results
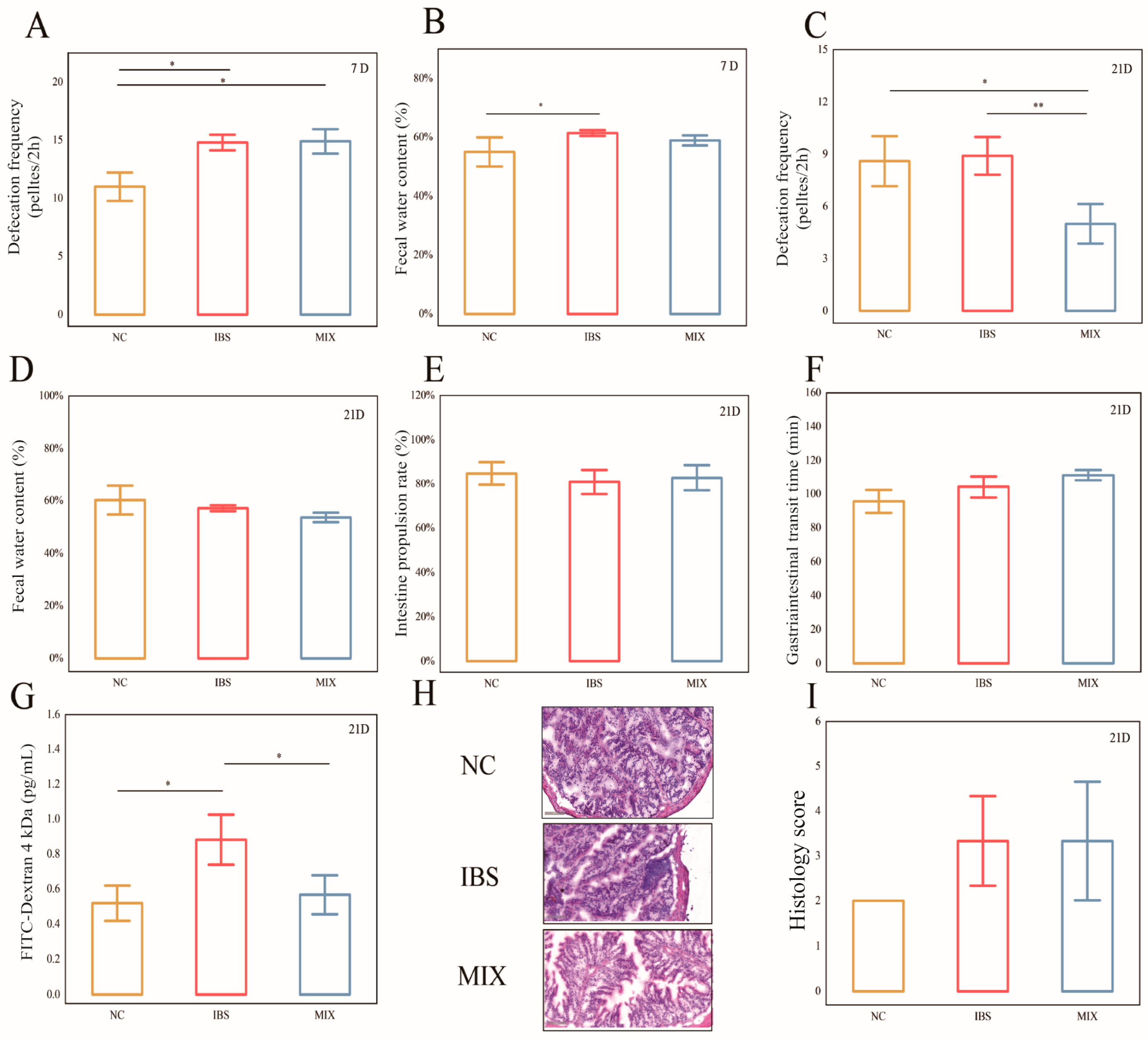
3.1. Effects of Complex Probiotic on the Pathological Indicators of IBS
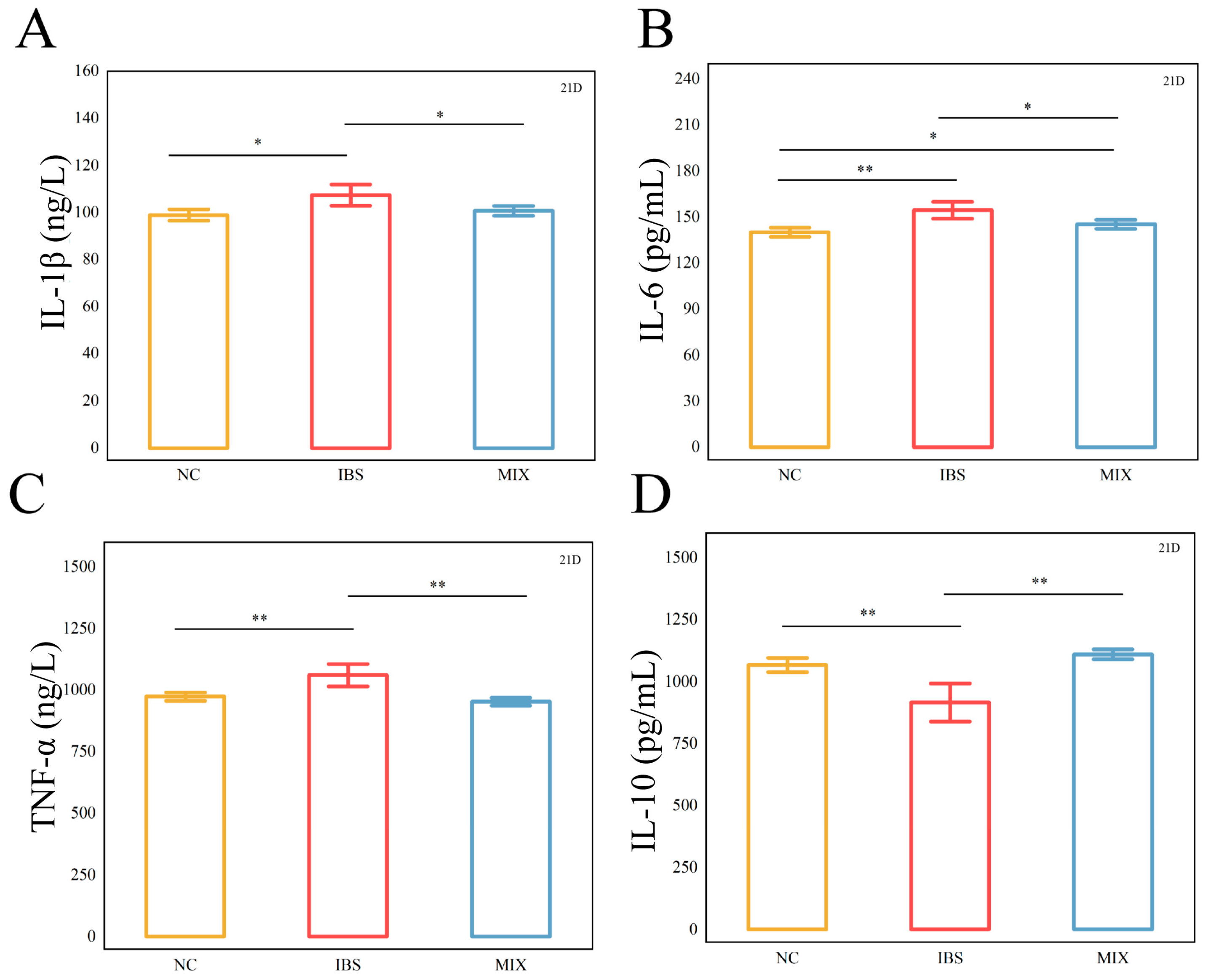
3.2. Effects of Complex Probiotic on the Inflammatory Factors in IBS
3.3. Effects of Complex Probiotic on IBS 5-Hydroxytryptamine and Serotonin Transporter
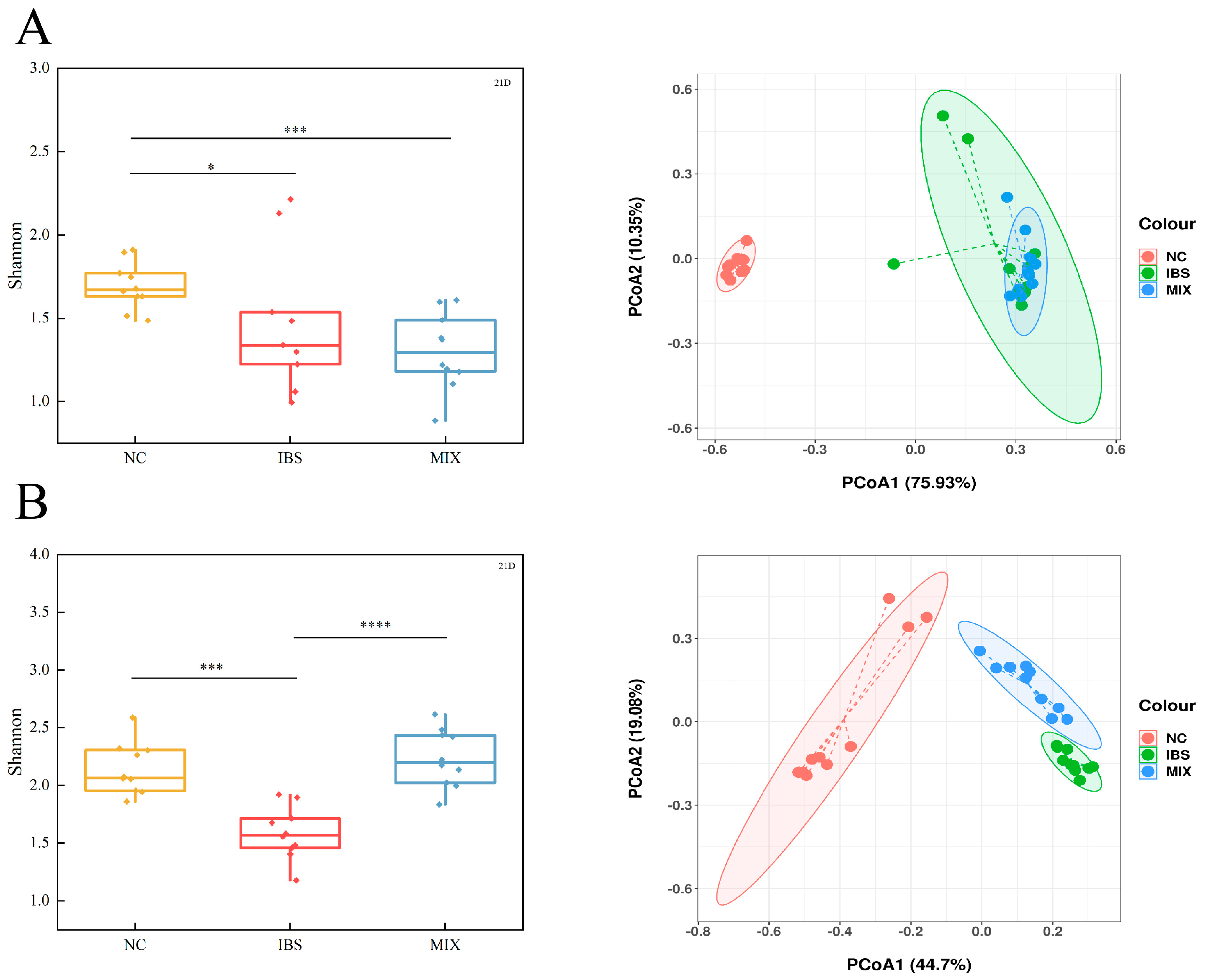
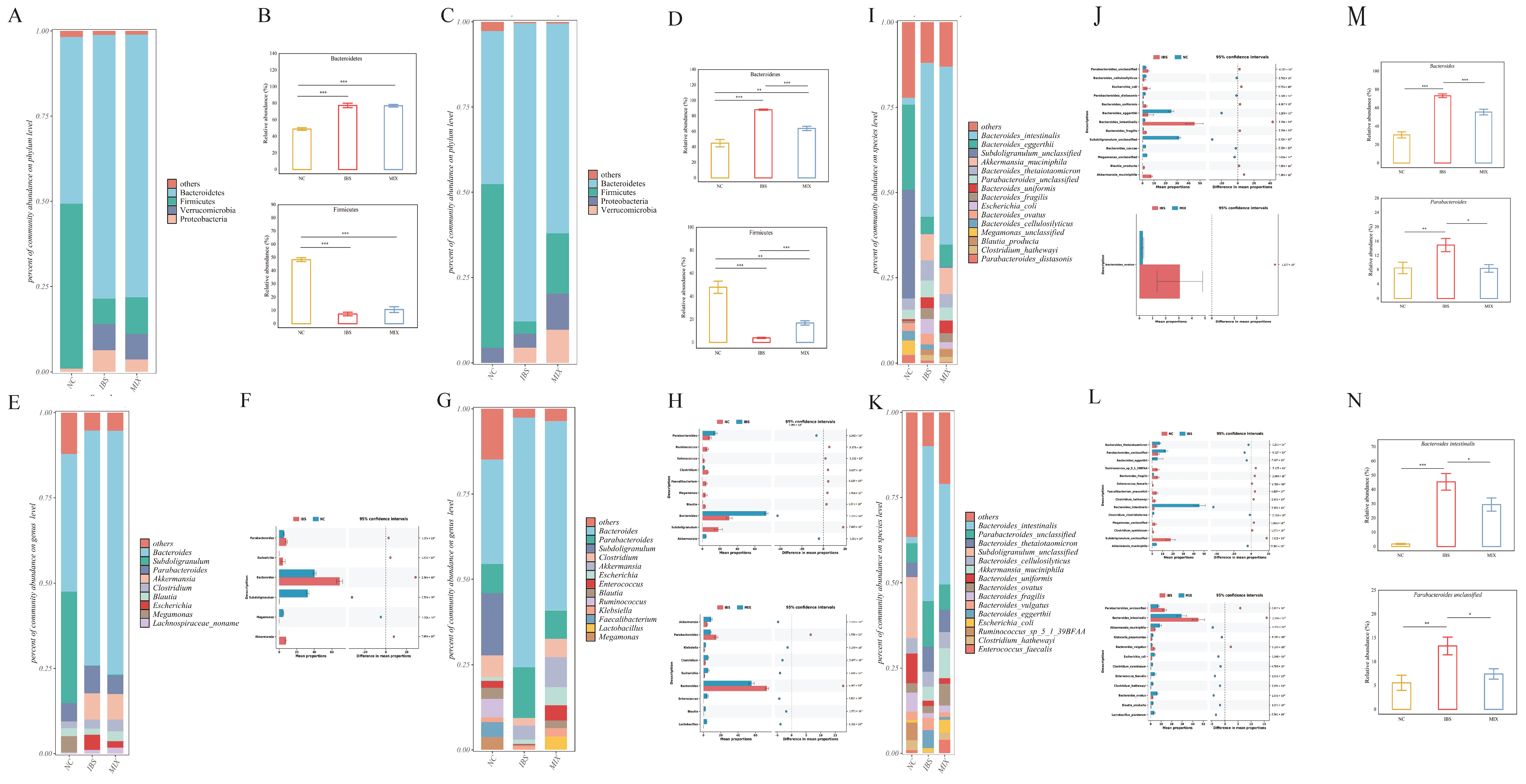
3.4. Effects of Complex Probiotic on Gut Microbiota in IBS
3.5. Effects of the Complex Probiotic on Fecal Metabolites in IBS
3.6. Correlation Analysis Between Gut Microbiota and Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef]
- Spiller, R.; Major, G. IBS and IBD—Separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; A Paine, P.; Black, C.J.; A Houghton, L.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines for the Management of Irritable Bowel Syndrome. Gut 2022, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut dysbiosis in irritable bowel syndrome: A narrative review on correlation with disease subtypes and novel therapeutic implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Chey, W.D.; Jackson, K.; Pillai, S.; Chey, S.W.; Han-Markey, T. A diet low in fermentable oligo-, di-, and monosaccharides and polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin. Gastroenterol. Hepatol. 2017, 15, 1890–1899.e3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Eswaran, S.; Photenhauer, A.L.; Merchant, J.L.; Chey, W.D.; D’amato, M. Reduced efficacy of low FODMAPs diet in patients with IBS-D carrying sucrase-isomaltase (SI) hypomorphic variants. Gut 2020, 69, 397–398. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion properties and competitive pathogen exclusion ability of bifidobacteria with acquired acid resistance. J. Food Prot. 2006, 69, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.S. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Tian, Z.; Li, L.; Zeng, Z.; Chen, M.; Xiong, L. Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front. Microbiol. 2018, 9, 1600. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2011, 61, 997–1006. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Li, M.; Li, Y.-Y.; Li, L.-X.; Zhai, W.-Z.; Wang, P.; Yang, X.-X.; Gu, X.; Song, L.-J.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.-L.; Xiao, J.-Y. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int. J. Surg. 2020, 75, 116–127. [Google Scholar] [CrossRef]
- Preston, K.; Krumian, R.; Hattner, J.; de Montigny, D.; Stewart, M.; Gaddam, S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef. Microbes 2018, 9, 697–706. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xie, J.; Zhang, Y.; Wang, J.; Sun, X.; Zhang, H. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int. Immunopharmacol. 2013, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Chen, M.; Ya, T.; Huang, W.; Gao, P.; Zhang, H. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 2015, 29, 901–907. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Peng, C.; Yu, Z.; Li, B.; Zhang, W.; Sun, Z.; Zhang, H. Fermented milk containing Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis V9 alleviated constipation symptoms through regulation of intestinal microbiota, inflammation, and metabolic pathways. J. Dairy Sci. 2020, 103, 11025–11038. [Google Scholar] [CrossRef] [PubMed]
- Kwok, L.; Wang, L.; Zhang, J.; Guo, Z.; Zhang, H. A pilot study on the effect of Lactobacillus casei Zhang on intestinal microbiota parameters in Chinese subjects of different age. Benef. Microbes 2014, 5, 295–304. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783.e1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.-Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021, 60, 2553–2565. [Google Scholar] [CrossRef]
- Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.; Diolintzi, A.; Zacharisen, S.M.; Lau, Y.H.; Quadro, L.; Storch, J. Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice. Nutrients 2025, 17, 753. [Google Scholar] [CrossRef]
- Tan, S.; Gu, J.; Yang, J.; Dang, X.; Liu, K.; Gong, Z.; Xiao, W.J. L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice. Nutrients 2025, 17, 720. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, M.-H.; Huang, H.-L.; Zhou, A.-C.; Chu, Z.-D.; Huang, X.-D.; Li, C.-W. Protective effects of citrulline supplementation in ulcerative colitis rats. PLoS ONE 2020, 15, e0240883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhan, J.; Sun, C.; Zhu, S.; Zhai, Y.; Dai, Y.; Wang, X.; Gao, X. Sishen Wan enhances intestinal barrier function via regulating endoplasmic reticulum stress to improve mice with diarrheal irritable bowel syndrome. Phytomedicine 2024, 129, 155541. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Hao, Z.; Jing, X.; Zhao, Y.; Cheng, X.; Ma, H.; Wang, J.; Wang, J. Mitigation effects of selenium nanoparticles on depression-like behavior induced by fluoride in mice via the JAK2-STAT3 pathway. ACS Appl. Mater. Interfaces 2022, 14, 3685–3700. [Google Scholar] [CrossRef] [PubMed]
- He, D.-F.; Ren, Y.-P.; Liu, M.-Y. Effects of ginseng fruit saponins on serotonin system in Sprague-Dawley rats with myocardial infarction, depression, and myocardial infarction complicated with depression. Chin. Med. J. 2016, 129, 2913–2919. [Google Scholar] [CrossRef]
- Li, B.; Hui, F.; Yuan, Z.; Shang, Q.; Shuai, G.; Bao, Y.; Chen, Y. Untargeted fecal metabolomics revealed biochemical mechanisms of the blood lipid-lowering effect of koumiss treatment in patients with hyperlipidemia. J. Funct. Foods 2021, 78, 104355. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, G. Gut microbiome, surgical complications and probiotics. Ann. Gastroenterol. 2016, 30, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kanarek, E.; Sowińska, A.; Cukrowska, B. The effectiveness and safety of multi-strain probiotic preparation in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled study. Nutrients 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Chimienti, G.; Notarnicola, M.; Russo, F. The ketogenic diet improves gut–brain axis in a rat model of irritable bowel syndrome: Impact on 5-HT and BDNF systems. Int. J. Mol. Sci. 2022, 23, 1098. [Google Scholar] [CrossRef]
- Abdellah, S.A.; Gal, C.; Laterza, L.; Velenza, V.; Settanni, C.R.; Napoli, M.; Schiavoni, E.; Mora, V.; Petito, V.; Gasbarrini, A. Effect of a multistrain probiotic on leaky gut in patients with diarrhea-predominant irritable bowel syndrome: A pilot study. Dig. Dis. 2022, 41, 489–499. [Google Scholar] [CrossRef]
- Friedman, G. A Multi-Strain Probiotic Reduces the Frequency of Diarrhea in IBS-D Patients. Am. J. Gastroenterol. 2008, 103, S455. [Google Scholar] [CrossRef]
- Wang, H.; Gong, J.; Wang, W.; Long, Y.; Fu, X.; Fu, Y.; Qian, W.; Hou, X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE 2014, 9, e90153. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Scarpa, M.; Marchiori, C.; Sarasin, G.; Caputi, V.; Porzionato, A.; Giron, M.C.; Palù, G.; Castagliuolo, I. Saccharomyces boulardii CNCM I-745 supplementation reduces gastrointestinal dysfunction in an animal model of IBS. PLoS ONE 2017, 12, e0181863. [Google Scholar] [CrossRef]
- Si, J.-M.; Yu, Y.-C.; Fan, Y.-J.; Chen, S.-J. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J. Gastroenterol. 2004, 10, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Rajilić–Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; de Vos, W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2013, 63, 1737–1745. [Google Scholar] [CrossRef]
- Gryaznova, M.; Smirnova, Y.; Burakova, I.; Morozova, P.; Lagutina, S.; Chizhkov, P.; Korneeva, O.; Syromyatnikov, M. Fecal Microbiota Characteristics in Constipation-Predominant and Mixed-Type Irritable Bowel Syndrome. Microorganisms 2024, 12, 1414. [Google Scholar] [CrossRef]
- Ryan, F.J.; Ahern, A.M.; Fitzgerald, R.S.; Laserna-Mendieta, E.J.; Power, E.M.; Clooney, A.G.; O’donoghue, K.W.; McMurdie, P.J.; Iwai, S.; Crits-Christoph, A.; et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Olyaiee, A.; Sadeghi, A.; Yadegar, A.; Mirsamadi, E.S.; Mirjalali, H. Gut Microbiota Shifting in Irritable Bowel Syndrome: The Mysterious Role of Blastocystis sp. Front. Med. 2022, 9, 890127. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the gut microbiome in hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- El Mouzan, M.; Assiri, A.; Al Sarkhy, A. Gut microbiota predicts the diagnosis of celiac disease in Saudi children. World J. Gastroenterol. 2023, 29, 1994–2000. [Google Scholar] [CrossRef]
- Yasuma, T.; Toda, M.; Abdel-Hamid, A.M.; D’alessandro-Gabazza, C.; Kobayashi, T.; Nishihama, K.; D’alessandro, V.F.; Pereira, G.V.; Mackie, R.I.; Gabazza, E.C.; et al. Degradation products of complex arabinoxylans by Bacteroides intestinalis enhance the host immune response. Microorganisms 2021, 9, 1126. [Google Scholar] [CrossRef]
- Costa, L.M.; Mendes, M.M.; Oliveira, A.C.; Magalhães, K.G.; Shivappa, N.; Hebert, J.R.; da Costa, T.H.M.; Botelho, P.B. Dietary inflammatory index and its relationship with gut microbiota in individuals with intestinal constipation: A cross-sectional study. Eur. J. Nutr. 2022, 61, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Camilleri, M. Serotonin: A mediator of the brain–gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How serotonin level fluctuation affects the effectiveness of treatment in irritable bowel syndrome. Cureus 2020, 12, 9871. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Han, Y.; Wang, Z.; Qin, Z.; Yang, C.; Cao, J.; Chen, Y. Role of serotonin on the intestinal mucosal immune response to stress-induced diarrhea in weaning mice. BMC Gastroenterol. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bearcroft, C.P.; Perrett, D.; Farthing, M.J.G. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: A pilot study. Gut 1998, 42, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Feng, Y.; Hang, L.; Zhou, Y.; Wang, E.; Yuan, J. No synergistic effect of fecal microbiota transplantation and shugan decoction in water avoidance stress-induced IBS-D rat model. Front. Microbiol. 2022, 13, 995567. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, J.; Zhu, S.; Xin, L.; Yu, C.; Shen, Z. The role of short chain fatty acids in irritable bowel syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic. Int. J. Oncol. 2024, 64, 5632. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Shaidullov, I.F.; Sorokina, D.M.; Sitdikov, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter dysfunction in irritable bowel syndrome: Emerging approaches for management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Chojnacki, C.; Medrek-Socha, M.; Blonska, A.; Zajdel, R.; Chojnacki, J.; Poplawski, T. A Reduced Tryptophan Diet in Patients with Diarrhoea-Predominant Irritable Bowel Syndrome Improves Their Abdominal Symptoms and Their Quality of Life through Reduction of Serotonin Levels and Its Urinary Metabolites. Int. J. Mol. Sci. 2022, 23, 15314. [Google Scholar] [CrossRef]
- Bai, T.; Xu, Z.; Xia, P.; Feng, Y.; Liu, B.; Liu, H.; Chen, Y.; Yan, G.; Lv, B.; Yan, Z.; et al. The short-term efficacy of bifidobacterium quadruple viable tablet in patients with diarrhea-predominant irritable bowel syndrome: Potentially mediated by metabolism rather than diversity regulation. Am. J. Gastroenterol. 2022, 118, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, F.; Mao, L.; Feng, T.; Wang, K.; Xu, M.; Lv, B.; Wang, X. Bifico relieves irritable bowel syndrome by regulating gut microbiota dysbiosis and inflammatory cytokines. Eur. J. Nutr. 2023, 62, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, Y.; Wang, G.; Xiong, Z.; Wei, G.; Liao, Z.; Qian, Y.; Cai, Z.; Ai, L. Lactobacillus plantarum AR495 improves colonic transport hyperactivity in irritable bowel syndrome through tryptophan metabolism. Food Funct. 2024, 15, 7416–7429. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, R.; Li, X.; Gao, C.; Hu, X.; Hussain, S.; Zhang, L.; Wang, M.; Ma, X.; Pan, Q.; et al. Long-term simulated microgravity alters gut microbiota and metabolome in mice. Front. Microbiol. 2023, 14, 747. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Borjihan, Q.; Zhang, W.; Li, L.; Wang, D.; Bai, L.; Zhu, S.; Chen, Y. Complex Probiotics Ameliorate Fecal Microbiota Transplantation-Induced IBS in Mice via Gut Microbiota and Metabolite Modulation. Nutrients 2025, 17, 801. https://doi.org/10.3390/nu17050801
Gao Y, Borjihan Q, Zhang W, Li L, Wang D, Bai L, Zhu S, Chen Y. Complex Probiotics Ameliorate Fecal Microbiota Transplantation-Induced IBS in Mice via Gut Microbiota and Metabolite Modulation. Nutrients. 2025; 17(5):801. https://doi.org/10.3390/nu17050801
Chicago/Turabian StyleGao, Yuan, Qinggele Borjihan, Weiqin Zhang, Lu Li, Dandan Wang, Lu Bai, Shiming Zhu, and Yongfu Chen. 2025. "Complex Probiotics Ameliorate Fecal Microbiota Transplantation-Induced IBS in Mice via Gut Microbiota and Metabolite Modulation" Nutrients 17, no. 5: 801. https://doi.org/10.3390/nu17050801
APA StyleGao, Y., Borjihan, Q., Zhang, W., Li, L., Wang, D., Bai, L., Zhu, S., & Chen, Y. (2025). Complex Probiotics Ameliorate Fecal Microbiota Transplantation-Induced IBS in Mice via Gut Microbiota and Metabolite Modulation. Nutrients, 17(5), 801. https://doi.org/10.3390/nu17050801





