The Cholesterol Paradox in Long-Livers from a Sardinia Longevity Hot Spot (Blue Zone)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
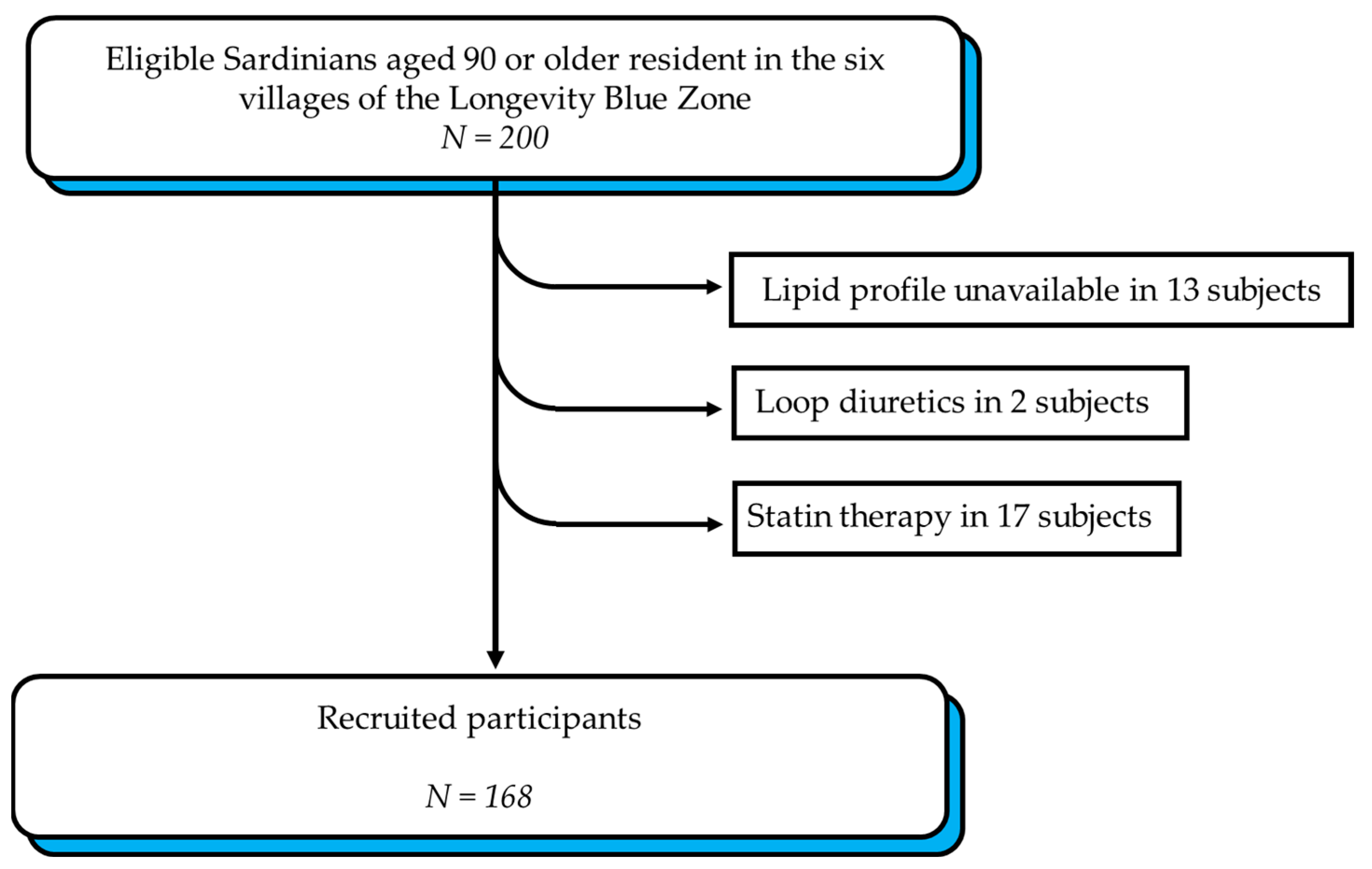
2.2. Study Participants
2.3. Data Collection
2.4. Lipid Profile
2.5. Dietary Assessment
2.6. Statistical Analysis
3. Results
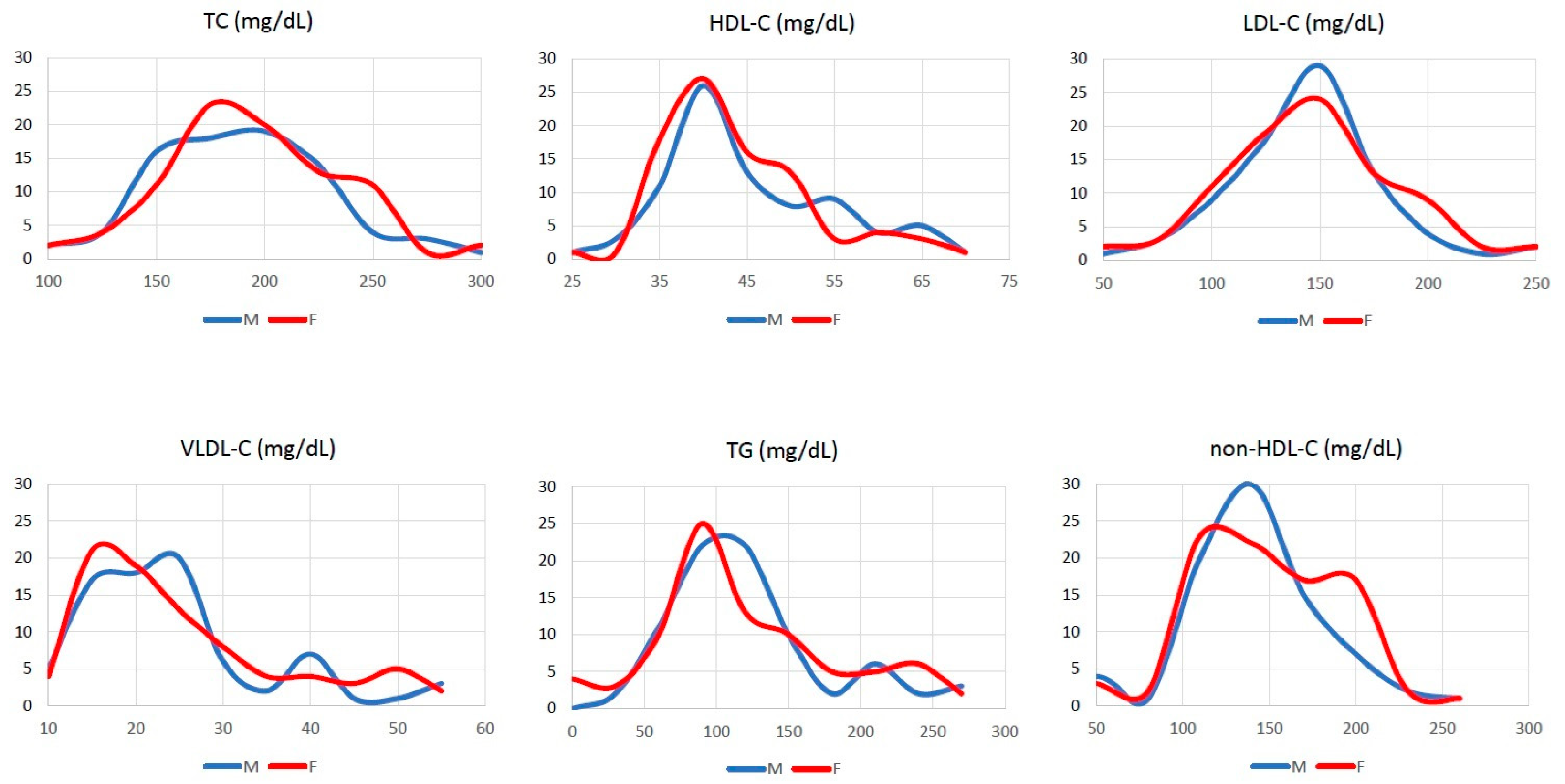
3.1. Descriptive Statistics
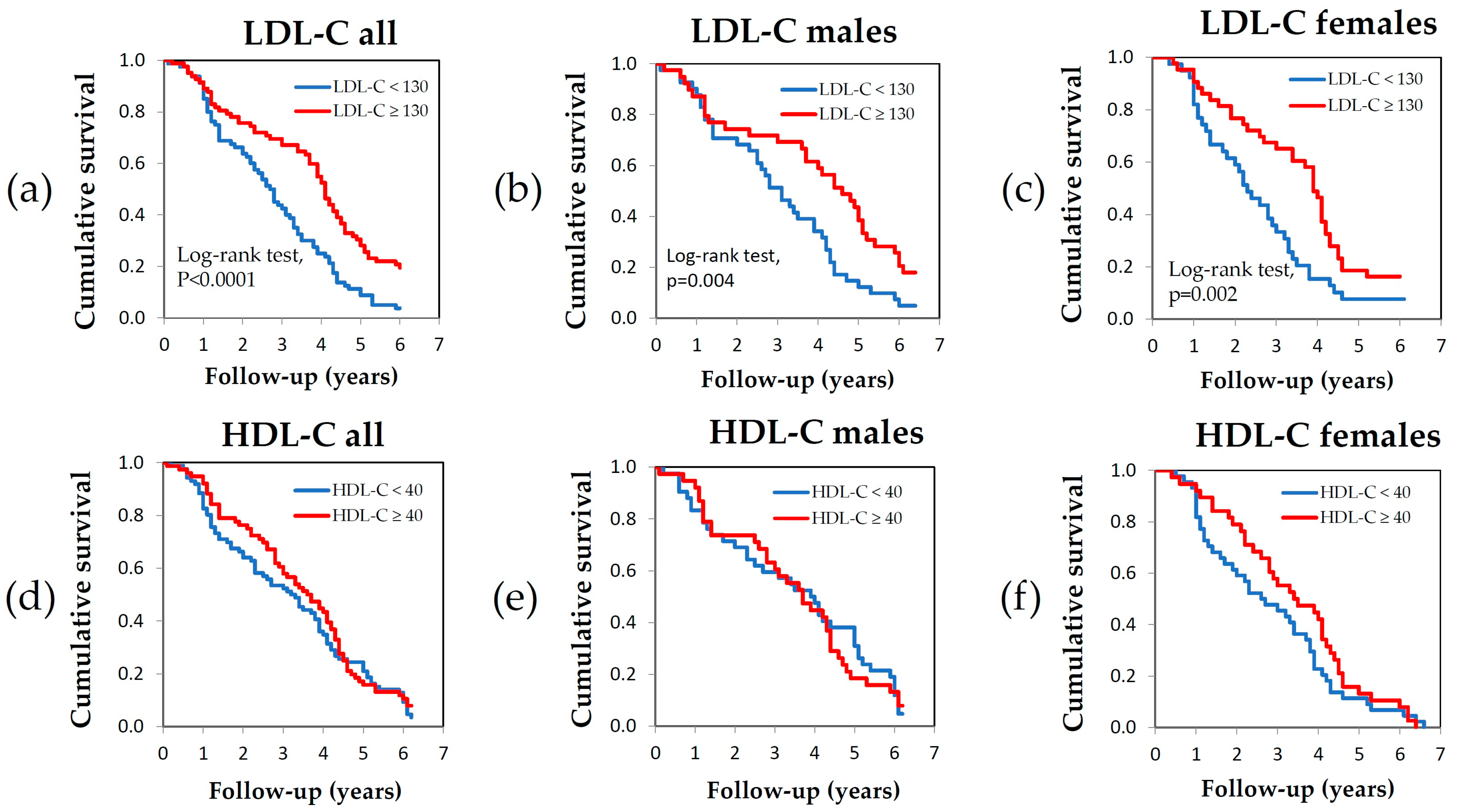
3.2. Survival Analysis
3.3. Correlation Between Cholesterol Levels and Anthropometric Parameters
3.4. Correlation Between Cholesterol Levels and Comorbidity
3.5. Correlation Between Cholesterol Levels and Diet
4. Discussion
4.1. Reverse Causality
4.2. The Cholesterol Paradox as a Real Phenomenon
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT2 | Acetyl-CoA acetyltransferase 2 |
| BMI | Body mass index |
| CI | Confidence interval |
| CIRS | Cumulative Illness Rating Scale |
| HDL | High-density cholesterol |
| HR | Hazard ratio |
| HUNT | Trøndelag Health Study |
| KM | Kaplan–Meier |
| LBZ | Longevity Blue Zone |
| LDL | Low-density cholesterol |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| VLDL | Very low-density cholesterol |
| TG | Triglycerides |
References
- Patel, S.A.; Winkel, M.; Ali, M.K.; Narayan, K.M.; Mehta, N.K. Cardiovascular mortality associated with 5 leading risk factors: National and state preventable fractions estimated from survey data. Ann. Intern. Med. 2015, 163, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Anichkow, N.; Chalatow, S. Uber experimentelle cholesterin-steatose und ihre bedeuntung fur die entstehung einiger pathologischer prozess. Zentralbl Allg. Pathol. Anat. 1913, 24, 1–9. [Google Scholar]
- Windaus, A. Ueber der Gehalt normaler und atheromatoser Aorten an Cholesterol und Cholesterinester. Z. Physiol. Chem. 1910, 67, 174. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Krauss, R.M. The early years of lipoprotein research: From discovery to clinical application. J. Lipid Res. 2016, 57, 1771–1777. [Google Scholar] [CrossRef]
- Higgins, M.W. The Framingham Heart Study: Review of epidemiological design and data, limitations and prospects. Prog. Clin. Biol. Res. 1984, 147, 51–64. [Google Scholar]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. The seven countries study: 2,289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Dawber, T.R.; Kannel, W.B. Atherosclerosis and you: Pathogentic implications from epidemiologic observations. J. Am. Geriatr. Soc. 1962, 10, 805–821. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Cheung, B.M.; Lauder, I.J.; Lau, C.P.; Kumana, C.R. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br. J. Clin. Pharmacol. 2004, 57, 640–651. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Daghlas, I.; Gill, D. Low-density lipoprotein cholesterol and lifespan: A Mendelian randomization study. Br. J. Clin. Pharmacol. 2021, 87, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Silaeva, Y.Y.; Orekhov, A.N.; Deykin, A.V. Animal models of human atherosclerosis: Current progress. Braz. J. Med. Biol. Res. 2020, 53, e9557. [Google Scholar] [CrossRef] [PubMed]
- Sari, G.; Meester, E.J.; van der Zee, L.C.; Wouters, K.; van Lennep, J.R.; Peppelenbosch, M.; Boonstra, A.; Van der Heiden, K.; Mulder, M.M.T.; Vanwolleghem, T. A mouse model of humanized liver shows a human-like lipid profile, but does not form atherosclerotic plaque after western type diet. Biochem. Biophys. Res. Commun. 2020, 524, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, T.; Okuyama, H.; Ogushi, Y.; Hama, R. Towards a Paradigm Shift in Cholesterol Treatment. A Re-examination of the Cholesterol Issue in Japan. Ann. Nutr. Metab. 2015, 66 (Suppl. 4), 1–116. [Google Scholar] [CrossRef]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Ravnskov, U. Is atherosclerosis caused by high cholesterol? QJM 2002, 95, 397–403. [Google Scholar] [CrossRef]
- Ravnskov, U. The Cholesterol Myths: Exposing the Fallacy That Saturated Fat and Cholesterol Cause Heart Disease; New Trends: Washington, DC, USA, 2003. [Google Scholar]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Orkaby, A.R. The Highs and Lows of Cholesterol: A Paradox of Healthy Aging? J. Am. Geriatr. Soc. 2020, 68, 236–237. [Google Scholar] [CrossRef]
- Wang, T.Y.; Chang, W.L.; Wei, C.Y.; Liu, C.H.; Tzeng, R.C.; Chiu, P.Y. Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study. Nutrients 2023, 15, 3270. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, Z.; Li, H.; Zhou, Z.; Lu, H.; Ying, M.; Mai, Z.; Yu, Y.; Yang, Y.; Deng, J.; et al. Non-HDL cholesterol paradox and effect of underlying malnutrition in patients with coronary artery disease: A 41,182 cohort study. Clin. Nutr. 2022, 41, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, J.; Tojek, K.; Wustrau, B.; Czerniak, B.; Winiarski, P.; Korzycka-Wilinska, W.; Banaszkiewicz, Z. The “cholesterol paradox” among inpatients—Retrospective analysis of medical documentation. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, e46–e57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T.; Ho, Y.L.; Li, Y.; Song, R.J.; Leung, K.H.; Rahman, S.U.; Orkaby, A.R.; Vassy, J.L.; Gagnon, D.R.; Cho, K.; et al. Serum Cholesterol and Impact of Age on Coronary Heart Disease Death in More Than 4 Million Veterans. J. Am. Heart Assoc. 2023, 12, e030496. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ponce, E.; Santolaria, F.; Aleman-Valls, M.R.; Gonzalez-Reimers, E.; Martinez-Riera, A.; Rodriguez-Gaspar, M.; Rodriguez-Rodriguez, E. Factors involved in the paradox of reverse epidemiology. Clin. Nutr. 2010, 29, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kishore, B.K. Reverse epidemiology of obesity paradox: Fact or fiction? Physiol. Rep. 2024, 12, e70107. [Google Scholar] [CrossRef]
- Frohlich, H.; Raman, N.; Tager, T.; Schellberg, D.; Goode, K.M.; Kazmi, S.; Grundtvig, M.; Hole, T.; Cleland, J.G.F.; Katus, H.A.; et al. Statins attenuate but do not eliminate the reverse epidemiology of total serum cholesterol in patients with non-ischemic chronic heart failure. Int. J. Cardiol. 2017, 238, 97–104. [Google Scholar] [CrossRef]
- Ahmadi, S.F.; Streja, E.; Zahmatkesh, G.; Streja, D.; Kashyap, M.; Moradi, H.; Molnar, M.Z.; Reddy, U.; Amin, A.N.; Kovesdy, C.P.; et al. Reverse Epidemiology of Traditional Cardiovascular Risk Factors in the Geriatric Population. J. Am. Med. Dir. Assoc. 2015, 16, 933–939. [Google Scholar] [CrossRef]
- Nilsson, G.; Leppert, J.; Ohrvik, J. Enigma of the cholesterol paradox in acute myocardial infarction: Lessons from an 8-year follow-up of all-cause mortality in an age-matched and sex-matched case-control study with controls from the patients’ recruitment area. BMJ Open 2022, 12, e057562. [Google Scholar] [CrossRef]
- Poulain, M.; Pes, G.M.; Grasland, C.; Carru, C.; Ferrucci, L.; Baggio, G.; Franceschi, C.; Deiana, L. Identification of a geographic area characterized by extreme longevity in the Sardinia island: The AKEA study. Exp. Gerontol. 2004, 39, 1423–1429. [Google Scholar] [CrossRef]
- Pes, G.M.; Tolu, F.; Dore, M.P.; Sechi, G.P.; Errigo, A.; Canelada, A.; Poulain, M. Male longevity in Sardinia, a review of historical sources supporting a causal link with dietary factors. Eur. J. Clin. Nutr. 2015, 69, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Pes, G.M.; Poulain, M.; Errigo, A.; Dore, M.P. Evolution of the Dietary Patterns Across Nutrition Transition in the Sardinian Longevity Blue Zone and Association with Health Indicators in the Oldest Old. Nutrients 2021, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- Pes, G.M.; Errigo, A.; Dore, M.P. Association between Mild Overweight and Survival: A Study of an Exceptionally Long-Lived Population in the Sardinian Blue Zone. J. Clin. Med. 2024, 13, 5322. [Google Scholar] [CrossRef] [PubMed]
- Pes, G.M.; Errigo, A.; Tedde, P.; Dore, M.P. Sociodemographic, Clinical and Functional Profile of Nonagenarians from Two Areas of Sardinia Characterized by Distinct Longevity Levels. Rejuvenation Res. 2020, 23, 341–348. [Google Scholar] [CrossRef]
- Marche, C.; Poulain, M.; Nieddu, A.; Errigo, A.; Dore, M.P.; Pes, G.M. Is a plant-based diet effective to maintain a good psycho-affective status in old age? Results of a survey of a long-lived population from Sardinia. Nutr. Neurosci. 2024, 27, 382–391. [Google Scholar] [CrossRef]
- Idler, E.L.; Benyamini, Y. Self-rated health and mortality: A review of twenty-seven community studies. J. Health Soc. Behav. 1997, 38, 21–37. [Google Scholar] [CrossRef]
- Norton, K.; Whittingham, N.; Carter, L.; Kerr, D.; Gore, C.; Marfell-Jones, M. Measurement Techniques in Anthropometry; University of New South Wales Press: Sydney, Australia, 1996. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Nesto, R.W. Beyond low-density lipoprotein: Addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am. J. Cardiovasc. Drugs 2005, 5, 379–387. [Google Scholar] [CrossRef]
- Di Giorgi, N.; Michelucci, E.; Smit, J.M.; Scholte, A.; El Mahdiui, M.; Knuuti, J.; Buechel, R.R.; Teresinska, A.; Pizzi, M.N.; Roque, A.; et al. A specific plasma lipid signature associated with high triglycerides and low HDL cholesterol identifies residual CAD risk in patients with chronic coronary syndrome. Atherosclerosis 2021, 339, 1–11. [Google Scholar] [CrossRef]
- Raja, V.; Aguiar, C.; Alsayed, N.; Chibber, Y.S.; ElBadawi, H.; Ezhov, M.; Hermans, M.P.; Pandey, R.C.; Ray, K.K.; Tokgozoglu, L.; et al. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis 2023, 383, 117312. [Google Scholar] [CrossRef] [PubMed]
- Nieddu, A.; Vindas, L.; Errigo, A.; Vindas, J.; Pes, G.M.; Dore, M.P. Dietary Habits, Anthropometric Features and Daily Performance in Two Independent Long-Lived Populations from Nicoya peninsula (Costa Rica) and Ogliastra (Sardinia). Nutrients 2020, 12, 1621. [Google Scholar] [CrossRef]
- Tessier, S.; Gerber, M. Factors determining the nutrition transition in two Mediterranean islands: Sardinia and Malta. Public. Health Nutr. 2005, 8, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.; Martin, S.S.; Michos, E.D.; Lamba, I.; Blumenthal, R.S.; Saeed, A.; Lima, J.; Puri, R.; Nomura, S.; Tsai, M.; et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: A primary prevention study. Eur. Heart J. 2021, 42, 4324–4332. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef]
- Scuteri, A.; Najjar, S.S.; Orru, M.; Albai, G.; Strait, J.; Tarasov, K.V.; Piras, M.G.; Cao, A.; Schlessinger, D.; Uda, M.; et al. Age- and gender-specific awareness, treatment, and control of cardiovascular risk factors and subclinical vascular lesions in a founder population: The SardiNIA Study. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 532–541. [Google Scholar] [CrossRef][Green Version]
- Manca, C.; Carta, G.; Murru, E.; Abolghasemi, A.; Ansar, H.; Errigo, A.; Cani, P.D.; Banni, S.; Pes, G.M. Circulating fatty acids and endocannabinoidome-related mediator profiles associated to human longevity. Geroscience 2021, 43, 1783–1798. [Google Scholar] [CrossRef]
- Caselli, G.; Lipsi, R. Survival differences among the oldest old in Sardinia: Who, what, where, and why? Demogr. Res. 2006, 14, 267–294. [Google Scholar] [CrossRef]
- Pes, G.M.; Dore, M.P.; Errigo, A.; Poulain, M. Analysis of Physical Activity Among Free-Living Nonagenarians from a Sardinian Longevous Population. J. Aging Phys. Act. 2018, 26, 254–258. [Google Scholar] [CrossRef]
- Pau, M.; Brandas, B.; Fastame, M.C. Women have the power: When motor efficiency makes the difference in older individuals of the sardinian blue zone. Physiol. Behav. 2025, 291, 114811. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.M.; Boardley, D.; Lambert, C.P.; Flynn, M.G. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B54–B60. [Google Scholar] [CrossRef]
- Varady, K.A.; Jones, P.J. Combination diet and exercise interventions for the treatment of dyslipidemia: An effective preliminary strategy to lower cholesterol levels? J. Nutr. 2005, 135, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.S.; Sanchez, O.A. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc. 2001, 33, S502–S515, discussion S528-509. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Roberts, S.; Haskell, W. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. Clin. Nutr. 2012, 31, 156–167. [Google Scholar] [CrossRef]
- Albarrati, A.M.; Alghamdi, M.S.M.; Nazer, R.I.; Alkorashy, M.M.; Alshowier, N.; Gale, N. Effectiveness of Low to Moderate Physical Exercise Training on the Level of Low-Density Lipoproteins: A Systematic Review. Biomed. Res. Int. 2018, 2018, 5982980. [Google Scholar] [CrossRef]
- Petursson, H.; Sigurdsson, J.A.; Bengtsson, C.; Nilsen, T.I.; Getz, L. Is the use of cholesterol in mortality risk algorithms in clinical guidelines valid? Ten years prospective data from the Norwegian HUNT 2 study. J. Eval. Clin. Pract. 2012, 18, 927–928. [Google Scholar] [CrossRef]
- Song, Y.M.; Sung, J.; Kim, J.S. Which cholesterol level is related to the lowest mortality in a population with low mean cholesterol level: A 6.4-year follow-up study of 482,472 Korean men. Am. J. Epidemiol. 2000, 151, 739–747. [Google Scholar] [CrossRef][Green Version]
- Stamler, J.; Daviglus, M.L.; Garside, D.B.; Dyer, A.R.; Greenland, P.; Neaton, J.D. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000, 284, 311–318. [Google Scholar] [CrossRef]
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- Petersen, L.K.; Christensen, K.; Kragstrup, J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010, 39, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Spolaore, P.; Ginocchio, G.; Colangeli, G.; Di Menza, G.; Marchioro, M.; Mazza, A.; Ambrosio, G.B. Predictors of mortality in very old subjects aged 80 years or over. Eur. J. Epidemiol. 1993, 9, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Wilcox, B.J.; Wilcox, C.D. Implications from and for food cultures for cardiovascular disease: Longevity. Asia Pac. J. Clin. Nutr. 2001, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, M.; Sawayama, Y.; Maeda, N.; Okada, K.; Furusyo, N.; Kashiwagi, S.; Hayashi, J. Carotid atherosclerosis and cardiovascular risk factors: A comparison of residents of a rural area of Okinawa with residents of a typical suburban area of Fukuoka, Japan. Atherosclerosis 2004, 172, 337–343. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hasegawa, Y.; Higa, M.; Amano, R.; Yamada, H.; Mizushima, S.; Masuzaki, H.; Sata, M. Subclinical Carotid Atherosclerosis Burden in the Japanese: Comparison between Okinawa and Nagano Residents. J. Atheroscler. Thromb. 2015, 22, 854–868. [Google Scholar] [CrossRef]
- Rosero-Bixby, L.; Dow, W.H.; Rehkopf, D.H. The Nicoya region of Costa Rica: A high longevity island for elderly males. Vienna Yearb. Popul. Res. 2013, 11, 109–136. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Pitsavos, C.; Lazaros, G.; Skoumas, J.; Tousoulis, D.; Stefanadis, C.; Ikaria Study, I. Determinants of All-Cause Mortality and Incidence of Cardiovascular Disease (2009 to 2013) in Older Adults: The Ikaria Study of the Blue Zones. Angiology 2016, 67, 541–548. [Google Scholar] [CrossRef]
- Taylor, R.; Zhang, C.; George, D.; Kotecha, S.; Abdelghaffar, M.; Forster, T.; Santos Rodrigues, P.D.; Reisinger, A.C.; White, D.; Hamilton, F.; et al. Low circulatory levels of total cholesterol, HDL-C and LDL-C are associated with death of patients with sepsis and critical illness: Systematic review, meta-analysis, and perspective of observational studies. eBioMedicine 2024, 100, 104981. [Google Scholar] [CrossRef]
- Mc Auley, M.T. Effects of obesity on cholesterol metabolism and its implications for healthy ageing. Nutr. Res. Rev. 2020, 33, 121–133. [Google Scholar] [CrossRef]
- Bathum, L.; Depont Christensen, R.; Engers Pedersen, L.; Lyngsie Pedersen, P.; Larsen, J.; Nexoe, J. Association of lipoprotein levels with mortality in subjects aged 50 + without previous diabetes or cardiovascular disease: A population-based register study. Scand. J. Prim. Health Care 2013, 31, 172–180. [Google Scholar] [CrossRef]
- Wang, J.; Shi, L.; Zou, Y.; Tang, J.; Cai, J.; Wei, Y.; Qin, J.; Zhang, Z. Positive association of familial longevity with the moderate-high HDL-C concentration in Bama Aging Study. Aging 2018, 10, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, J.; Rozycki, B.; Ji, J.; Song, F. Interplay of receptor-ligand binding and lipid domain formation during cell adhesion. Front. Mol. Biosci. 2022, 9, 1019477. [Google Scholar] [CrossRef] [PubMed]
- Stamerra, C.A.; Di Giosia, P.; Ferri, C.; Giorgini, P.; Reiner, Z.; Johnston, T.P.; Sahebkar, A. Statin therapy and sex hormones. Eur. J. Pharmacol. 2021, 890, 173745. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, X.; Fu, S.; Sun, D.; Zhang, W.; Liu, W.; Miao, X.; Luo, Q.; Li, H.; Zhang, J.; et al. Associations of Serum 25(OH)D, PTH, and beta-CTX Levels with All-Cause Mortality in Chinese Community-Dwelling Centenarians. Nutrients 2022, 15, 94. [Google Scholar] [CrossRef]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinue, A.; Martinez-Hervas, S.; Gonzalez-Navarro, H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef]
- Rudd-Schmidt, J.A.; Hodel, A.W.; Noori, T.; Lopez, J.A.; Cho, H.J.; Verschoor, S.; Ciccone, A.; Trapani, J.A.; Hoogenboom, B.W.; Voskoboinik, I. Lipid order and charge protect killer T cells from accidental death. Nat. Commun. 2019, 10, 5396. [Google Scholar] [CrossRef]
- Ravnskov, U.; McCully, K.S. The importance of LDL-cholesterol and infection in the etiology of cardiovascular disease: A meta-analysis of COVID-19 survivors and non-survivors. Med. Res. Arch. 2024, 12, 5. [Google Scholar] [CrossRef]
- Ravnskov, U. High cholesterol may protect against infections and atherosclerosis. QJM 2003, 96, 927–934. [Google Scholar] [CrossRef]
- Mielke, M.M.; Zandi, P.P.; Sjogren, M.; Gustafson, D.; Ostling, S.; Steen, B.; Skoog, I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 2005, 64, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Tolu, F.; Palermo, M.; Dore, M.P.; Errigo, A.; Canelada, A.; Poulain, M.; Pes, G.M. Association of endemic goitre and exceptional longevity in Sardinia: Evidence from an ecological study. Eur. J. Ageing 2019, 16, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Holmes, R.; Arbeev, K.; Bagley, O.; Wu, D.; Yashin, A.; Ukraintseva, S. A Mendelian randomization study supports causal effect of overweight on longevity. Innov. Aging 2023, 7, 904–905. [Google Scholar] [CrossRef]
- Tognotti, E. Program to eradicate malaria in Sardinia, 1946–1950. Emerg. Infect. Dis. 2009, 15, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Onongbu, I.C.; Onyeneke, E.C. Plasma lipid changes in human malaria. Tropenmed Parasitol. 1983, 34, 193–196. [Google Scholar]
| Variables | Males | Females |
|---|---|---|
| Number of participants at baseline | 81 | 87 |
| Mean age at recruitment (years) | 92.7 ± 4.8 | 93.7 ± 4.4 |
| Age range at recruitment (years) | 90–107 | 90–106 |
| Education (years) | 4.1 ± 1.4 | 3.3 ± 1.4 |
| Marital status, n (%) | ||
| Single | 11 (13.6) | 16 (18.4) |
| Married | 45 (55.6) | 12 (13.8) |
| Widowed | 25 (30.9) | 59 (67.8) |
| Divorced | 0 (0.0) | 0 (0.0) |
| Household composition, n (%) | ||
| Alone | 5 (6.2) | 10 (11.5) |
| Married without children | 6 (7.4) | 2 (2.3) |
| Married with children | 39 (48.1) | 10 (11.5) |
| Widowed with children | 24 (29.6) | 59 (67.8) |
| Other | 7 (8.6) | 6 (6.9) |
| Smoking habits, n (%) | ||
| Never smokers | 54 (66.7) | 83 (95.4) |
| Former smokers | 27 (33.3) | 4 (4.6) |
| Current smokers | 0 (0.0) | 0 (0.0) |
| Body mass index, n (%) | ||
| <24.9 | 52 (64.2) | 60 (69.0) |
| 25.0–29.9 | 16 (19.8) | 19 (21.8) |
| ≥30 | 13 (16.0) | 8 (9.2) |
| Physical activity, n (%) | ||
| <3 times/week | 12 (14.8) | 27 (31.0) |
| ≥3 times/week | 69 (85.2) | 60 (69.0) |
| Number of participants alive at the end of follow-up | 12 | 8 |
| Mean survival time for 148 participants who died during the follow-up (years) | 3.17 ± 1.81 | 2.86 ± 1.45 |
| Survival time for all participants (years) | 3.51 ± 1.93 | 3.08 ± 1.65 |
| Lipid Parameters (Median, Range) | Males | Females | p Value |
|---|---|---|---|
| Total cholesterol, mg/dL | 199.5 [89, 314] | 202.5 [89, 324] | 0.552 |
| HDL * cholesterol, mg/dL | 38 [23, 68] | 39 [23, 74] | 0.883 |
| TG †, mg/dL | 127 [52, 304] | 130.5 [52, 304] | 0.761 |
| LDL # cholesterol, mg/dL | 129.9 [48, 245] | 132.8 [31, 254] | 0.474 |
| VLDL ¶ cholesterol, mg/dL | 26 [10.4, 60.8] | 26.1 [10.4, 60.8] | 0.761 |
| Non-HDL * cholesterol, mg/dL | 160 [57, 274] | 161 [48, 285] | 0.496 |
| TG/HDL | 3.21 [1.09, 8.44] | 3.12 [1.13, 8.44] | 0.751 |
| LDL/HDL | 3.32 [0.6, 7.2] | 3.39 [0.8, 6.5] | 0.381 |
| Lipid Levels | Males | Females | ||||
|---|---|---|---|---|---|---|
| No. of Participants | Survival (yrs) | p Value | No. of Participants | Survival (yrs) | p Value | |
| Total cholesterol (mg/dL) | ||||||
| <200 | 40 | 3.37 ± 1.94 | Ref. | 34 | 2.54 ± 1.49 | Ref. |
| 200–249 | 31 | 3.69 ± 1.92 | 0.525 | 37 | 3.93 ± 1.51 | <0.0001 * |
| ≥250 | 10 | 3.66 ± 2.12 | 0.747 | 16 | 2.31 ± 1.48 | 0.574 |
| HDL cholesterol (mg/dL) | ||||||
| <40 | 43 | 3.54 ± 2.04 | 0.870 | 47 | 2.82 ± 1.66 | 0.077 |
| ≥40 | 38 | 3.62 ± 1.82 | 40 | 3.40 ± 1.61 | ||
| Triglycerides (mg/dL) | ||||||
| <150 | 58 | 3.62 ± 1.96 | 0.434 | 55 | 3.12 ± 1.60 | 0.860 |
| ≥150 | 23 | 3.30 ± 1.88 | 32 | 3.03 ± 1.77 | ||
| LDL cholesterol (mg/dL) | ||||||
| <130 | 41 | 3.06 ± 1.69 | 0.015 | 42 | 2.50 ± 1.37 | 0.003 |
| ≥130 | 40 | 4.04 ± 2.05 | 45 | 3.62 ± 1.72 | ||
| VLDL cholesterol (mg/dL) | ||||||
| <24 | 42 | 3.54 ± 1.97 | 0.688 | 41 | 2.95 ± 1.63 | 0.415 |
| ≥24 | 39 | 3.65 ± 1.91 | 46 | 3.15 ± 1.67 | ||
| Non-HDL cholesterol (mg/dL) | ||||||
| <158 | 51 | 3.24 ± 1.84 | 0.114 | 43 | 2.51 ± 1.31 | 0.010 |
| ≥158 | 30 | 3.94 ± 1.97 | 44 | 3.48 ± 1.76 | ||
| TG/HDL | ||||||
| <3.5 | 45 | 3.56 ± 2.02 | 0.834 | 44 | 3.02 ± 1.52 | 0.861 |
| ≥3.5 | 36 | 3.67 ± 1.77 | 43 | 3.10 ± 1.78 | ||
| LDL/HDL | ||||||
| <3.3 | 45 | 3.38 ± 1.80 | 0.285 | 42 | 2.80 ± 1.41 | 0.290 |
| ≥3.3 | 36 | 3.84 ± 2.03 | 45 | 3.30 ± 1.81 | ||
| Covariates | Full Model (HR # and 95% CI) |
|---|---|
| Male sex | 1.320 (0.817–2.132) |
| Age at recruitment | 1.270 (1.200–1.344) ** |
| Body mass index (kg/m2) | |
| <24 | Ref. |
| 24–28 | 0.616 (0.378–0.988) * |
| ≥28 | 2.643 (1.539–4.537) ** |
| Smoking | |
| Never smoker | Ref. |
| Former smokers † | 1.653 (0.964–2.835) |
| Self-rated health | 1.020 (0.842–1.237) |
| CIRS | 1.009 (0.843–1.207) |
| LDL cholesterol range (mg/dL) | |
| <130 | Ref. |
| ≥130 | 0.606 (0.403–0.912) * |
| Foods | Lipid Profile | |||||||
|---|---|---|---|---|---|---|---|---|
| TC | HDL-C | LDL-C | Non-HDL-C | VLDL-C | TG | TG/HDL | LDL/HDL | |
| Meat | 0.243 * | 0.249 | 0.115 | 0.076 | −0.078 | −0.024 | −0.163 | −0.089 |
| Fish | 0.121 | 0.095 | 0.134 | 0.106 | −0.137 | −0.140 | −0.183 * | 0.005 |
| Legumes | 0.012 | 0.087 | 0.069 | 0.135 | 0.133 | 0.117 | 0.059 | −0.012 |
| Greens | 0.107 | 0.076 | 0.167 | 0.193 | 0.127 | 0.126 | 0.088 | 0.090 |
| Cereals | 0.194 * | −0.115 | 0.199 * | 0.241 * | 0.184 * | 0.118 | 0.161 | 0.147 |
| Potato | −0.123 | 0.001 | 0.025 | 0.018 | 0.044 | 0.036 | 0.058 | −0.010 |
| Fruit | 0.234 | 0.141 | 0.214 | 0.205 | 0.022 | 0.016 | −0.041 | 0.083 |
| Sweets | −0.070 | 0.081 | −0.077 | −0.041 | 0.119 | 0.114 | 0.016 | −0.097 |
| Olive oil | −0.187 | −0.015 | −0.098 | −0.156 * | −0.017 | −0.007 | −0.025 | −0.054 |
| Dairy food | −0.072 | −0.023 | −0.034 | −0.029 | 0.104 | 0.110 | 0.069 | −0.022 |
| Coffee | −0.011 | 0.204 * | −0.116 | 0.025 | −0.113 | −0.099 | 0.064 | −0.163 |
| Wine | −0.157 | 0.036 | −0.094 | −0.107 | −0.111 | −0.101 | −0.078 | −0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errigo, A.; Dore, M.P.; Portoghese, M.; Pes, G.M. The Cholesterol Paradox in Long-Livers from a Sardinia Longevity Hot Spot (Blue Zone). Nutrients 2025, 17, 765. https://doi.org/10.3390/nu17050765
Errigo A, Dore MP, Portoghese M, Pes GM. The Cholesterol Paradox in Long-Livers from a Sardinia Longevity Hot Spot (Blue Zone). Nutrients. 2025; 17(5):765. https://doi.org/10.3390/nu17050765
Chicago/Turabian StyleErrigo, Alessandra, Maria Pina Dore, Michele Portoghese, and Giovanni Mario Pes. 2025. "The Cholesterol Paradox in Long-Livers from a Sardinia Longevity Hot Spot (Blue Zone)" Nutrients 17, no. 5: 765. https://doi.org/10.3390/nu17050765
APA StyleErrigo, A., Dore, M. P., Portoghese, M., & Pes, G. M. (2025). The Cholesterol Paradox in Long-Livers from a Sardinia Longevity Hot Spot (Blue Zone). Nutrients, 17(5), 765. https://doi.org/10.3390/nu17050765









