Camellia Tea Saponin Ameliorates 5–Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Crude Tea Saponin
2.3. Keratinocyte Culture
2.4. Cytotoxicity Test
2.5. Measurement of Cellular ROS
2.6. Assessment of Cellular LPOs Content
2.6.1. Membrane LPOs
2.6.2. Mitochondrial LPOs
2.7. Detection of Cellular LIP
2.7.1. Cytosolic LIP
2.7.2. Mitochondrial LIP
2.8. Determination of GSH Content
2.9. Assay of GPX–4 Activity
2.10. Measurement of Protein Content
2.11. Assessment of Apoptotic Cells
2.12. Quantification of Inflammatory Cytokines
2.13. Scratch Assay
2.14. Statistical Analysis
3. Results
3.1. Protective Effects of TS Against 5–FU-Induced Cytotoxicity
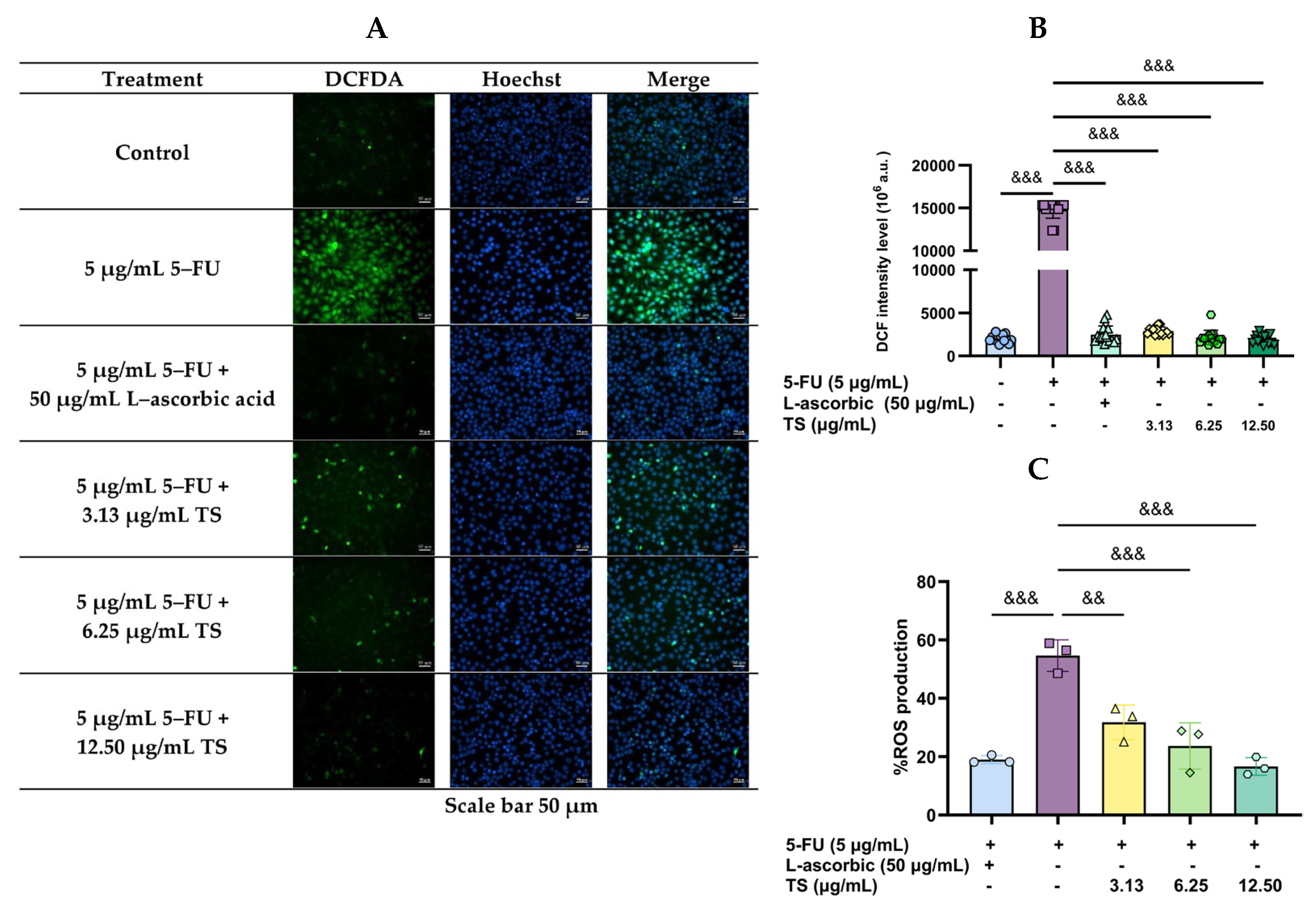
3.2. Reduction of 5–FU-Induced Oxidative Stress by TS
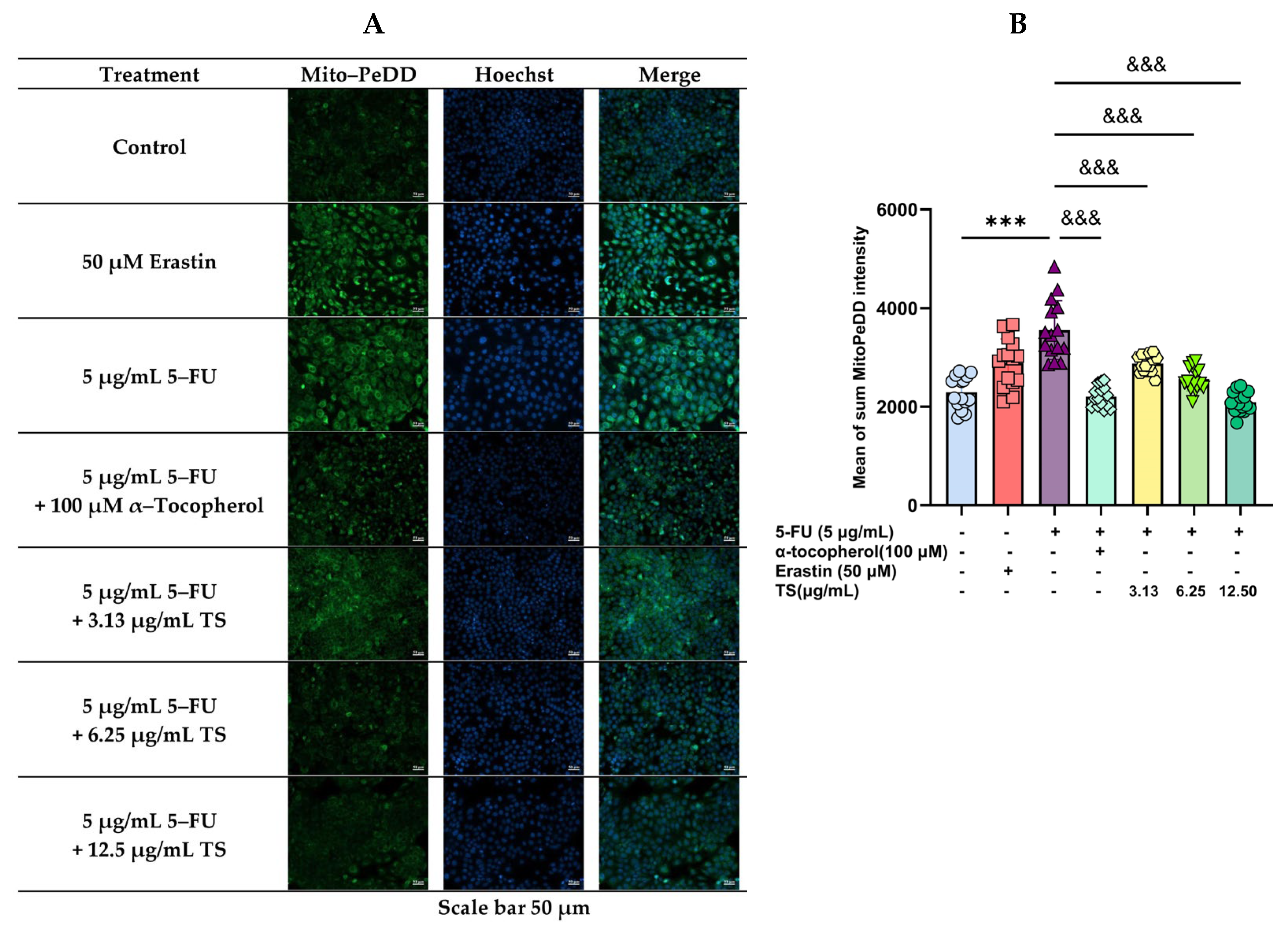
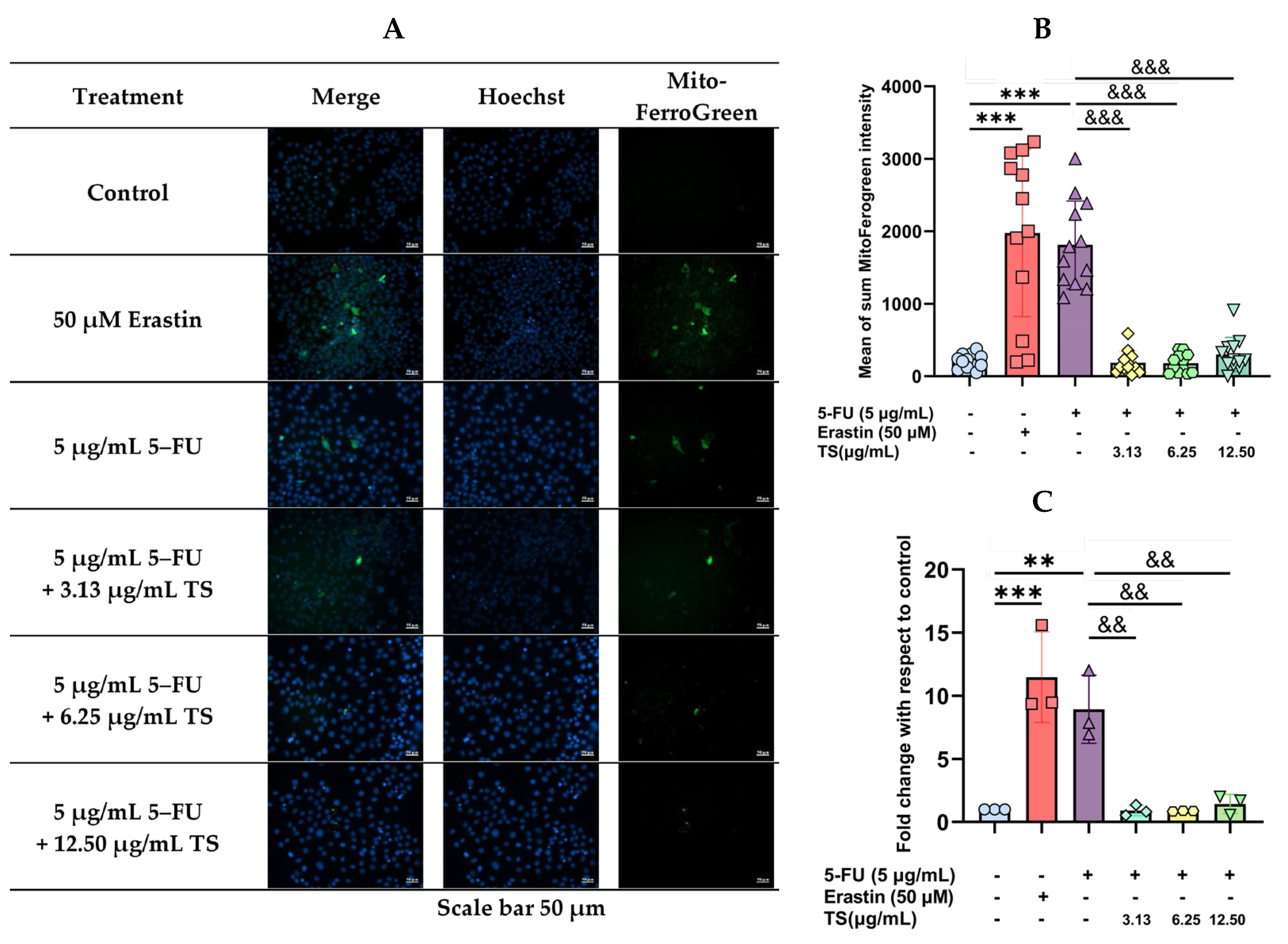
3.3. Inhibition of Lipid Peroxidation by TS
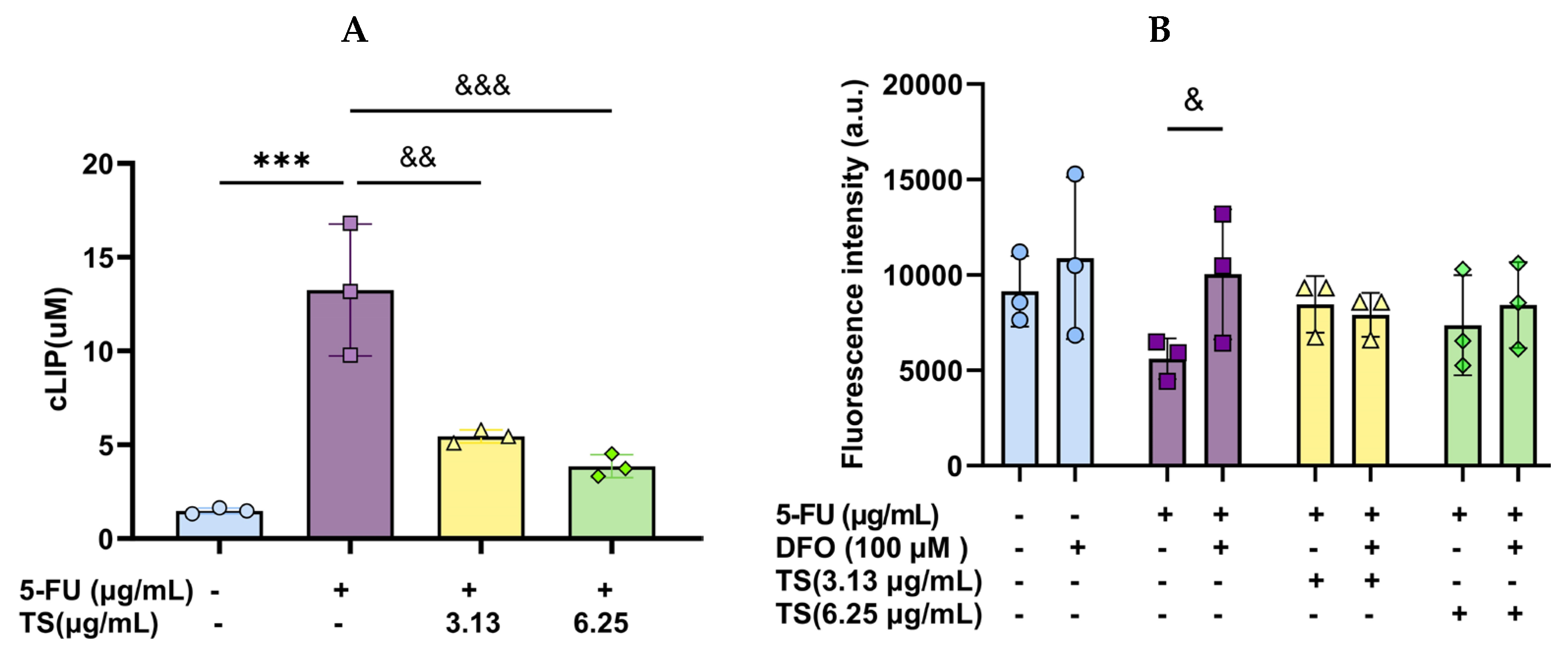
3.4. Effect of TS Treatment on Cellular LIP
3.5. Restoration of Cellular GSH and GPX–4 Levels by TS
3.6. Anti-Ferroptosis Effects of TS Against Erastin- or 5–FU-Induced HaCaT Cells
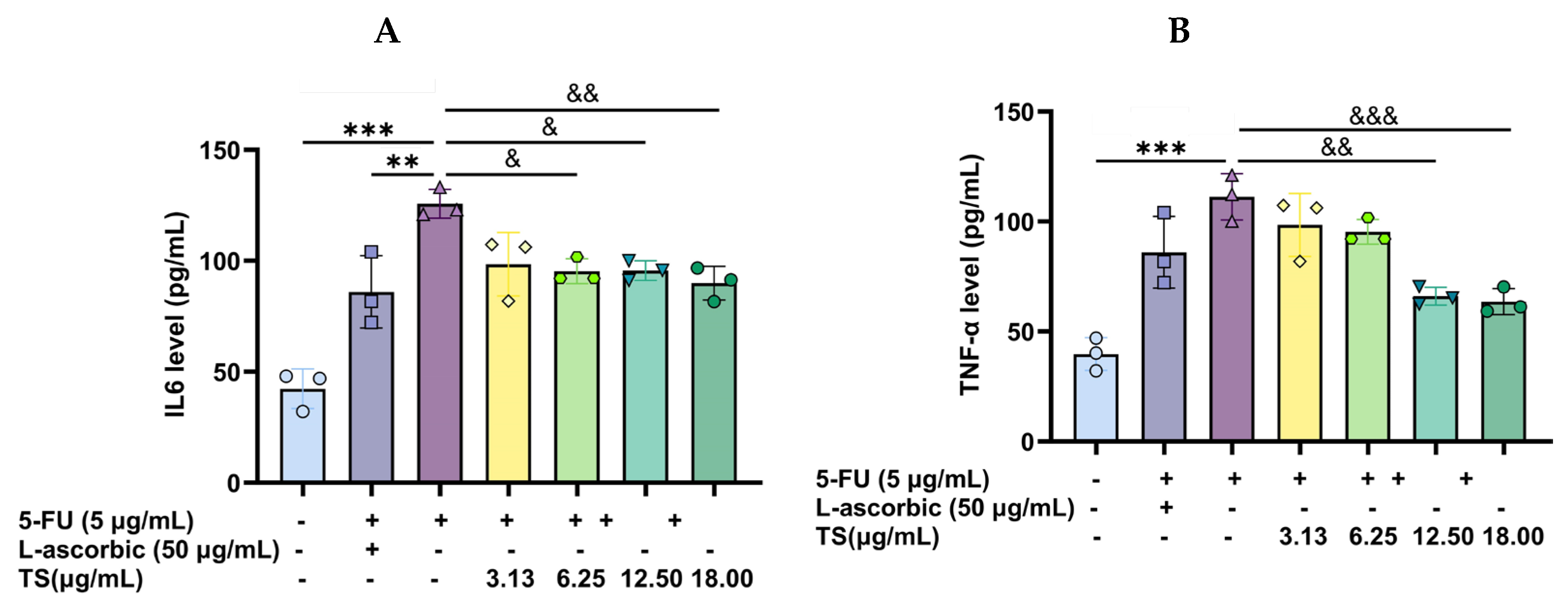
3.7. Anti-Inflammatory Activity of TS
3.8. Wound-Healing Effects of TS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| CAT | Catalase |
| H2DCFDA | 2′,7′–Dichlorodihydrofluorescein diacetate reduced form |
| DAPI | Diamino propidium iodide |
| DFO | Desferrioxamine mesylate |
| DMSO | Dimethyl sulfoxide |
| DNCB | 2,4–Dinitrochlorobenzene |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ELISA | Enzyme-linked immune absorbent assay |
| FAS | Ferrous ammonium sulfate |
| FBS | Fetal bovine serum |
| FI | Fluorescence intensity |
| 5–FU | 5–Fluorouracil |
| GSH | Glutathione |
| GPX–4 | Glutathione peroxidase 4 |
| GR | Glutathione reductase |
| HBS | Hanks’ balanced salt |
| HEPES | 4–(2–Hydroxyethyl)–1–piperazine ethanesulfonic acid |
| IC50 | Half-maximal inhibitory concentration |
| IL–6 | Interleukin 6 |
| IFN–γ | Interferon–gamma |
| LPO | Lipid–peroxidation |
| LPS | Lipopolysaccharide |
| MAPK | Macrophage-associated protein kinase |
| mTOR | Mammalian target of rapamycin |
| MTT | 3–(4,5–Dimethylthiazol–2–yl) 2,5–diphenyl tetrazolium bromide |
| NF–κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OD | Optical density |
| PBS | Phosphate-buffered saline pH 7.4 |
| ROS | Reactive oxygen species |
| RIPA | Radioimmunoprecipitation assay |
| SD | Mean ± standard deviation |
| SOD | Superoxide dismutase |
| TNF–α | Tumor–necrotic factor alpha |
| Trypsin–EDTA | Trypsin/disodium ethylenediamine tetraacetate |
| TS | Tea saponin extract |
| UVA | Ultraviolet A |
| UVB | Ultraviolet B |
| VEGF | Vascular endothelial growth factor |
References
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, A.; Torres-Lagares, D.; Robles-Garcia, M.; Pachon-Ibanez, J.; Gonzalez-Padilla, D.; Gutierrez-Perez, J.L. Cancer treatment-induced oral mucositis: A critical review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.D.; Borges, A.D.; Trindade, L.M.; Andrade, M.E.R.; Cavalcante, G.G.; Leocádio, P.C.L.; Alvarez-Leite, J.I.; Cassali, G.D.; Costa, B.G.; Martins, F.d.S.; et al. Mitigation of chemotherapy-induced experimental intestinal mucositis through postbiotic lactate. Lett. Appl. Microbiol. 2024, 77, 103. [Google Scholar] [CrossRef]
- Deng, S.; Wu, D.; Li, L.; Li, J.; Xu, Y. TBHQ attenuates ferroptosis against 5-fluorouracil-induced intestinal epithelial cell injury and intestinal mucositis via activation of Nrf2. Cell Mol. Biol. Lett. 2021, 26, 48. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Resveratrol alleviated 5-FU-induced cardiotoxicity by attenuating GPX4 dependent ferroptosis. J. Nutr. Biochem. 2023, 112, 109241. [Google Scholar] [CrossRef]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer. 2013, 21, 327–332. [Google Scholar] [CrossRef]
- Ono, K.; Ueno, T.; Kido, M.A.; Hitomi, S.; Naniwa, M.; Nakatomi, C.; Yoshimoto, R.U.; Sawada, T.; Kato, T. Recent advances in the treatment of oral ulcerative mucositis from clinical and basic perspectives. J. Oral Biosci. 2024, 66, 504–510. [Google Scholar] [CrossRef]
- Ouyang, M.; Wu, J.; Hu, X.; Liu, C.; Zhou, D. Decoding the power of saponins in ferroptosis regulation and disease intervention: A review. J. Pharm. Pharmacol. 2024, 10, 144. [Google Scholar] [CrossRef]
- Guo, C.; Yue, Y.; Wang, B.; Chen, S.; Li, D.; Zhen, F.; Liu, L.; Zhu, H.; Xie, M. Anemoside B4 alleviates arthritis pain via suppressing ferroptosis-mediated inflammation. J. Cell. Mol. Med. 2024, 28, e18136. [Google Scholar] [CrossRef]
- Guo, J.; Le, Y.; Yuan, A.; Liu, J.; Chen, H.; Qiu, J.; Wang, C.; Dou, X.; Yuan, X.; Lu, D. Astragaloside IV ameliorates cisplatin-induced liver injury by modulating ferroptosis-dependent pathways. J. Ethnopharmacol. 2024, 328, 118080. [Google Scholar] [CrossRef] [PubMed]
- Sagesaka, Y.M.; Uemura, T.; Watanabe, N.; Sakata, K.; Uzawa, J. A new glucuronide saponin from tea leaves (Camellia sinensis var. sinensis. Biosci. Biotechnol. Biochem. 1994, 58, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Umeda, T.; Yagi, A.; Sakata, K.; Chaudhuri, T.; Ganguly, D.K.; Sarma, S. Triterpenoid saponins from the roots of tea plant (Camellia sinensis var. assamica). Phytochemistry 2000, 53, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.F.; Hong, Z.B.; Hu, Z.H.; Hou, R.Y. Two new triterpenoid saponins with antifungal activity from Camellia sinensis flowers. Int. J. Mol. Sci. 2025, 26, 1147. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Kim, H.R.; Ko, J.; Kong, C.S. Camellioside A, isolated from Camellia japonica flowers, attenuates UVA-induced production of MMP-1 in HaCaT keratinocytes via suppression of MAPK activation. Exp. Ther. Med. 2021, 21, 16. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.; Ren, N.; Tu, Y.; Li, B. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhang, X.; Deng, M.; Xie, L.; Zhang, L.; Li, X. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int. J. Pharm. 2020, 583, 119406. [Google Scholar] [CrossRef]
- Akagi, M.; Fukuishi, N.; Kan, T.; Sagesaka, Y.M.; Akagi, R. Anti-allergic effect of tea-leaf saponin (TLS) from tea leaves (Camellia sinensis var. sinensis). Biol. Pharm. Bull. 1997, 20, 565–567. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Besra, S.E.; Gomes, A.; Das, M.; Sur, P.; Mitra, S.; Vedasiromoni, J.R. Anti-inflammatory activity of tea (Camellia sinensis) root extract. Life Sci. 2004, 74, 1839–1849. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I.A.-O. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol.Sci. 2021, 21, 12827. [Google Scholar] [CrossRef]
- Hartinger, J.; Vesely, P.; Sima, M.; Netikova, I.; Matouskova, E.; Petruzelka, L. 5-Fluorouracil toxicity mechanism determination in human keratinocytes: In vitro study on HaCaT cell line. Prague Med. Rep. 2017, 118, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Magliocca, G.; Pepe, G.; Amodio, G.; Autore, G.; Campiglia, P.; Marzocco, S. Protective effect of pomegranate on oxidative stress and inflammatory response induced by 5-fluorouracil in human keratinocytes. Antioxidants 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, W.; Zhou, L.; Huang, J. Probing lipid peroxidation in ferroptosis: Emphasizing the utilization of C11-BODIPY-based protocols. Methods Mol. Biol. 2023, 2712, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.Y.; Luo, Y.B.; Ju, X.D.; Zhang, B.; Cui, Y.J.; Pan, Y.B.; Tian, J.H.; Teng, W.J.; Wu, J.; Li, Y. Solasonine causes redox imbalance and mitochondrial oxidative stress of ferroptosis in lung adenocarcinoma. Front. Oncol. 2022, 12, 874900. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Pourzand, C.; Ma, Y.; Cilibrizzi, A. Optical imaging opportunities to inspect the nature of cytosolic iron pools. Molecules 2023, 28, 6467. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, D.; Zhao, L.; Li, W.; Han, J.; Hu, B.; Nie, G.; He, Y. Liproxstatin-1 protects hair cell-like HEI-OC1 cells and cochlear hair cells against neomycin ototoxicity. Oxid. Med. Cell Longev. 2020, 2020, 1782659. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the bradford protein assay. J. Vis. Exp. 2010, 38, e1918. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Chen, D.; Pi, Y.; Zhou, W.; Xiong, X.; Ren, Y.; Lai, Y.; Hua, Z. Quantitative analysis of annexin V-membrane interaction by flow cytometry. Eur. Biophys. J. 2015, 44, 325–336. [Google Scholar] [CrossRef]
- Cory, G. Scratch-wound assay. Method Mol. Bol. 2011, 769, 25–30. [Google Scholar] [CrossRef]
- Koonyosying, P.; Paradee, N.; Srichairatanakool, S. Chapter 7—Hormetic effects of plant bioactives on healthy aging and longevity. In Plant Bioactives as Natural Panacea Against Age-Induced Diseases; Pandey, K.B., Suttajit, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 129–146. [Google Scholar] [CrossRef]
- Katz, K.A. Problematic end points in a trial of topical fluorouracil, 5%, for chemoprevention of keratinocyte carcinoma. JAMA Dermatol. 2018, 154, 850–851. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, D.; Christoforou, M.; Christou, K.; Christou, M.; Ververis, A.; Andreou, M.; Christodoulou, K.; Koutsoulidou, A.; Papachrysostomou, C.; Pantelidou, M. Saponin and phenolic composition and assessment of biological activities of Saponaria officinalis L. root extracts. Plants 2024, 13, 1982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, S.; Wan, F.; Zhou, Y.; Wang, Z.; Fan, G.; Wang, P.; Luo, H.; Liao, S.; He, L.; et al. Quantitative analysis of Camellia oleifera seed saponins and aqueous two-phase extraction and separation. Molecules 2023, 28, 2132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Wu, J.P.; Gao, B.B.; Ren, H.T.; Liu, Y.L.; Li, X.R.; Li, K.P.; Xu, Q.M.; Yang, S.L. Two new 28-nor-oleanane-type triterpene saponins from roots of Camellia oleifera and their cytotoxic activity. J. Asian Nat. Prod. Res. 2016, 18, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; He, M.; Huang, Q.R.; Liu, D.; Huang, M. Sasanquasaponin protects rat cardiomyocytes against oxidative stress induced by anoxia-reoxygenation injury. Eur. J. Pharmacol. 2007, 575, 21–27. [Google Scholar] [CrossRef]
- Zong, J.F.; Peng, Y.R.; Bao, G.H.; Hou, R.Y.; Wan, X.C. Two new oleanane-type saponins with anti-proliferative activity from Camellia oleifera Abel. seed cake. Molecules 2016, 21, 188. [Google Scholar] [CrossRef]
- Iliescu, I.A.; Peter, S.; Albert, I.; Skalicka-Wozniak, K.; Miron, A.; Luca, S.V.; Wolfram, E. Verbascum nigrum: Cytotoxicity evaluation in A431 epidermoid carcinoma cells and untargeted LC-HR-MS/MS metabolite profiling. Chem. Biodivers. 2020, 17, e2000644. [Google Scholar] [CrossRef]
- Basar, N.; Oridupa, O.A.; Ritchie, K.J.; Nahar, L.; Osman, N.M.; Stafford, A.; Kushiev, H.; Kan, A.; Sarker, S.D. Comparative cytotoxicity of Glycyrrhiza glabra roots from different geographical origins against immortal human keratinocyte (HaCaT), lung adenocarcinoma (A549) and liver carcinoma (HepG2) cells. Phytother. Res. 2015, 29, 944–948. [Google Scholar] [CrossRef]
- Ko, K.; Wahyudi, L.D.; Kwon, Y.S.; Kim, J.H.; Yang, H. Nuclear factor erythroid 2-related factor 2 activating triterpenoid saponins from Camellia japonica roots. J. Nat. Prod. 2018, 81, 2399–2409. [Google Scholar] [CrossRef]
- Ahn, H.; Han, B.C.; Hong, E.J.; An, B.S.; Lee, E.; Lee, S.H.; Lee, G.S. Korean Red Ginseng attenuates ultraviolet-mediated inflammasome activation in keratinocytes. J. Ginseng Res. 2021, 45, 456–463. [Google Scholar] [CrossRef]
- Ahn, H.; Yu, S.; Han, B.C.; Ro, Y.; Kim, Y.H.; Kizaki, K.; Lee, E.; Lee, S.H.; Lee, G.S. Maltol, a compound in Korean Red Ginseng, attenuates the Staphylococcus aureus-induced inflammasome activation in the skin. J. Ginseng Res. 2024, 48, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ren, H.; Chai, J.; Zhai, C.; Li, T.; Zhou, X.; Lee, J.; Li, X.; Zhao, Y. Protective effect of ginseng berry saponin conversion products on skin photodamage caused by UVB in vitro and in vivo. Food Res. Int. 2024, 198, 115379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Li, T.; Wang, K.; Huang, X.; Shi, J.; Wang, Y. Mechanisms and inhibitors of ferroptosis in psoriasis. Front. Mol. Biosci. 2022, 9, 1019447. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Jimenez Perez, Z.E.; Kim, Y.J.; Yang, D.C. Ginsenoside Rg5:Rk1 attenuates TNF-alpha/IFN-gamma-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-kappaB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef]
- Ahn, S.S.; Lee, Y.H.; Yeo, H.; Jung, E.; Lim, Y.; Shin, S.Y. Saikosaponin A and Saikosaponin C reduce TNF-alpha-induced TSLP expression through inhibition of MAPK-mediated EGR1 expression in HaCaT keratinocytes. Int. J. Mol. Sci. 2022, 23, 4857. [Google Scholar] [CrossRef]
- Ahn, S.S.; Yeo, H.; Jung, E.; Kim, T.Y.; Han, J.; Lee, Y.H.; Shin, S.Y. Saikosaponin A recovers impaired filaggrin levels in inflamed skin by downregulating the expression of FRA1 and c-Jun. Molecules 2024, 29, 4064. [Google Scholar] [CrossRef]
- Ahn, K.S.; Hahn, B.S.; Kwack, K.; Lee, E.B.; Kim, Y.S. Platycodin D-induced apoptosis through nuclear factor-kappaB activation in immortalized keratinocytes. Eur. J. Pharmacol. 2006, 537, 1–11. [Google Scholar] [CrossRef]
- Niu, J.; Jia, X.; Yang, N.; Ran, Y.; Wu, X.; Ding, F.; Tang, D.; Tian, M. Phytochemical analysis and anticancer effect of Camellia oleifera bud ethanol extract in non-small cell lung cancer A549 cells. Front. Pharmacol. 2024, 15, 1359632. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006, 148, 860–870. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, W.L.; Li, Z.X.; Kirkby, N.S.; Tsai, W.C.; Huang, S.F.; Ou, C.H.; Chang, T.C. Astragaloside VI and cycloastragenol-6-O-beta-D-glucoside promote wound healing in vitro and in vivo. Phytomedicine 2018, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ai, R.; Liu, B.Z.; He, L. Tea polyphenol nano-crosslinked dynamical hyaluronic acid-based hydrogel for diabetic wound healing. Int. J. Biol. Macromol. 2024, 282, 136856. [Google Scholar] [CrossRef]
- Huang, C.C.; Wu, W.B.; Fang, J.Y.; Chiang, H.S.; Chen, S.K.; Chen, B.H.; Chen, Y.T.; Hung, C.F. (-)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules 2007, 12, 1845–1858. [Google Scholar] [CrossRef]
- Huang, C.C.; Fang, J.Y.; Wu, W.B.; Chiang, H.S.; Wei, Y.J.; Hung, C.F. Protective effects of (-)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch. Dermatol. Res. 2005, 296, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.U.; Park, Y.M. The effect of green tea polyphenols on macrophage migration inhibitory factor-associated steroid resistance. Br. J. Dermatol. 2012, 166, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef]
- Cui, Y.; Kim, D.S.; Park, S.H.; Yoon, J.A.; Kim, S.K.; Kwon, S.B.; Park, K.C. Involvement of ERK AND p38 MAP kinase in AAPH-induced COX-2 expression in HaCaT cells. Chem. Phys. Lipids 2004, 129, 43–52. [Google Scholar] [CrossRef]
- Cho, M.; Yoon, H.; Park, M.; Kim, Y.H.; Lim, Y. Flavonoids promoting HaCaT migration: I. Hologram quantitative structure-activity relationships. Phytomedicine 2014, 21, 560–569. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likitsatian, T.; Koonyosying, P.; Paradee, N.; Roytrakul, S.; Ge, H.; Pourzand, C.; Srichairatanakool, S. Camellia Tea Saponin Ameliorates 5–Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation. Nutrients 2025, 17, 764. https://doi.org/10.3390/nu17050764
Likitsatian T, Koonyosying P, Paradee N, Roytrakul S, Ge H, Pourzand C, Srichairatanakool S. Camellia Tea Saponin Ameliorates 5–Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation. Nutrients. 2025; 17(5):764. https://doi.org/10.3390/nu17050764
Chicago/Turabian StyleLikitsatian, Tanrada, Pimpisid Koonyosying, Narisara Paradee, Sittiruk Roytrakul, Haobo Ge, Charareh Pourzand, and Somdet Srichairatanakool. 2025. "Camellia Tea Saponin Ameliorates 5–Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation" Nutrients 17, no. 5: 764. https://doi.org/10.3390/nu17050764
APA StyleLikitsatian, T., Koonyosying, P., Paradee, N., Roytrakul, S., Ge, H., Pourzand, C., & Srichairatanakool, S. (2025). Camellia Tea Saponin Ameliorates 5–Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation. Nutrients, 17(5), 764. https://doi.org/10.3390/nu17050764





