In Vitro Evaluation of Bioavailability of Mg from Daily Food Rations, Dietary Supplements and Medicinal Products from the Polish Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.2.1. Dietary Supplements and Medicinal Products
2.2.2. Reconstructed Diet Duplicates
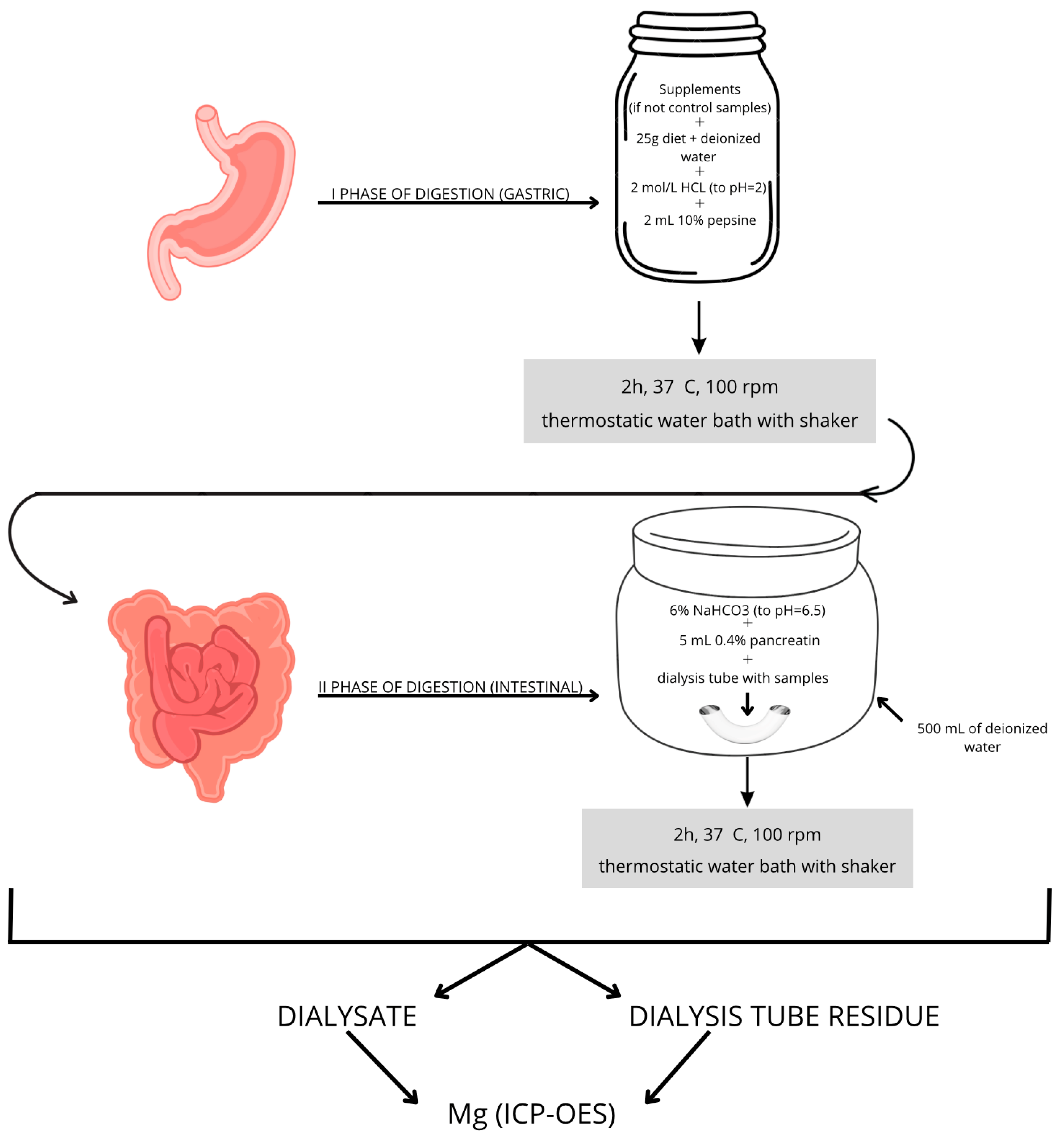
2.3. Two-Phase Enzymatic Model of In Vitro Digestion
2.4. Analytical Determination of Mg
2.5. Calculation of the Bioavailability Value
2.6. Statistical Analysis
3. Results
3.1. Bioavailability of Mg Under the Influence of Various Diets
3.2. Influence of Diet and Chemical Form on the Bioavailability of Mg
3.3. Influence of the Pharmaceutical Form on the Bioavailability of Mg
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of Magnesium Status for Diagnosis and Therapy. Magnes. Res. 2010, 23, S194–S198. [Google Scholar]
- Fawcett, W.J.; Haxby, E.J.; Male, D.A. Magnesium: Physiology and Pharmacology. Br. J. Anaesth. 1999, 83, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.G.; Quinzi, F.; Folino, K.; Greco, F.; Oranges, F.P.; Cerulli, C.; Emerenziani, G.P. Effects of Magnesium Supplementation on Muscle Soreness in Different Type of Physical Activities: A Systematic Review. J. Transl. Med. 2024, 22, 629. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Pardo, M.R.; Garicano Vilar, E.; San Mauro Martín, I.; Camina Martín, M.A. Bioavailability of Magnesium Food Supplements: A Systematic Review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Moscoso-Pérez, C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Bermejo-Barrera, P.; Prada-Rodríguez, D. In-Vivo and in-Vitro Testing to Assess the Bioaccessibility and the Bioavailability of Arsenic, Selenium and Mercury Species in Food Samples. Trends Anal. Chem. 2011, 2, 324–345. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W. Advances in Evaluating the Oral Bioavailability of Inorganics in Soil for Use in Human Health Risk Assessment. Environ. Sci. Technol. 1999, 33. [Google Scholar] [CrossRef]
- Koch, W.; Karim, M.R.; Marzec, Z.; Miyataka, H.; Himeno, S.; Asakawa, Y. Dietary Intake of Metals by the Young Adult Population of Eastern Poland: Results from a Market Basket Study. J. Trace Elem. Med. Biol. 2016, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, L.; Nashalian, O.; Naja, F.; Itani, L.; Parent-Massin, D.; Nabhani-Zeidan, M.; Hwalla, N. Dietary Exposure to Essential and Toxic Trace Elements from a Total Diet Study in an Adult Lebanese Urban Population. Food Chem. Toxicol. 2010, 48, 1262–1269. [Google Scholar] [CrossRef]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.a.M. Development of an in Vitro Digestion Model for Estimating the Bioaccessibility of Soil Contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Goderska, K.; Grajek, K.; Grajek, W. Modele Przewodu Pokarmowego in Vitro Do Badań Nad Biodostepnościa Skladników Odzywczych. Zywnosd. Nauka. Technologia. Jakość 2016, 13, 30–45. [Google Scholar]
- Yun, S.; Habicht, J.-P.; Miller, D.D.; Glahn, R.P. An In Vitro Digestion/Caco-2 Cell Culture System Accurately Predicts the Effects of Ascorbic Acid and Polyphenolic Compounds on Iron Bioavailability in Humans. J. Nutr. 2004, 134, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Marze, S. Bioavailability of Nutrients and Micronutrients: Advances in Modeling and In Vitro Approaches. Annu. Rev. Food Sci. Technol. 2017, 8, 35–55. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Herbello-Hermelo, P.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Bioavailability Assessment of Essential and Toxic Metals in Edible Nuts and Seeds. Food Chem. 2016, 205, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shiowatana, J.; Kitthikhun, W.; Sottimai, U.; Promchan, J.; Kunajiraporn, K. Dynamic Continuous-Flow Dialysis Method to Simulate Intestinal Digestion for in Vitro Estimation of Mineral Bioavailability of Food. Talanta 2006, 68, 549–557. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Domínguez-González, R.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. Trace Metals in Marine Foodstuff: Bioavailability Estimation and Effect of Major Food Constituents. Food Chem. 2012, 134, 339–345. [Google Scholar] [CrossRef]
- Eastwood, M. Principles of Human Nutrition; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Gertig, H.; Przysławski, J. Bromatologia. Zarys Nauki o Żywności i Żywieniu; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2015; ISBN 978-83-200-3603-9. (In Polish) [Google Scholar]
- Ciborowska, H.; Ciborowski, A. Dietetyka Żywienie Zdrowego i Chorego Człowieka; Royal College of Nursing (UK): Warszawa, Poland, 2021. (In Polish) [Google Scholar]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Helon, P.; Pietrzak, K.; Koch, W. In Vitro Evaluation of Bioavailability of Cr from Daily Food Rations and Dietary Supplements from the Polish Market. Nutrients 2024, 16, 1022. [Google Scholar] [CrossRef]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Iłowiecka, K.; Koch, W. In Vitro Evaluation of Bioavailability of Se from Daily Food Rations and Dietary Supplements. Nutrients 2023, 15, 1511. [Google Scholar] [CrossRef]
- Kuchanowicz, H.; Przygoda, B.; Nadolna, I. Tabele Składu i Wartości Odżywczej Żywności; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2005. (In Polish) [Google Scholar]
- Koch, W.; Czop, M.; Nawrocka, A.; Wiącek, D. Contribution of Major Groups of Food Products to the Daily Intake of Selected Elements—Results from Analytical Determinations Supported by Chemometric Analysis. Nutrients 2020, 12, 3412. [Google Scholar] [CrossRef]
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiącek, D. Dietary Intake of Toxic Heavy Metals with Major Groups of Food Products—Results of Analytical Determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Schricker, B.R.; Rasmussen, R.R.; Van Campen, D. An in Vitro Method for Estimation of Iron Availability from Meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, J.; Pietrzak, K.; Kukula-Koch, W.; Czop, M.; Laszuk, J.; Koch, W. Influence of Diet on the Bioavailability of Active Components from Zingiber Officinale Using an In Vitro Digestion Model. Foods 2023, 12, 3897. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber Officinale) Rhizomes from Ecological Plantations. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar] [CrossRef]
- Gibson, R.S. The Role of Diet- and Host-Related Factors in Nutrient Bioavailability and Thus in Nutrient-Based Dietary Requirement Estimates. Food Nutr. Bull. 2007, 28, S77–S100. [Google Scholar] [CrossRef]
- Jabłecka, A.; Korzeniowska, K.; Skołuda, A.; Cieślewicz, A. Preparations of magnesium. Farm. Współczesna 2011, 4, 29–32. [Google Scholar]
- Firoz, M.; Graber, M. Bioavailability of US Commercial Magnesium Preparations. Magnes. Res. 2001, 14, 257–262. [Google Scholar]
- Rylander, R. Bioavailability of Magnesium Salts – A Review. J. Pharm. Nutr. Sci. 2014, 4, 57–59. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg Citrate Found More Bioavailable than Other Mg Preparations in a Randomised, Double-Blind Study. Magnes. Res. 2003, 16, 183–191. [Google Scholar] [PubMed]
- Werner, T.; Kolisek, M.; Vormann, J.; Pilchova, I.; Grendar, M.; Struharnanska, E.; Cibulka, M. Assessment of Bioavailability of Mg from Mg Citrate and Mg Oxide by Measuring Urinary Excretion in Mg-Saturated Subjects. Magnes. Res. 2019, 32, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, N.D.; Radosavljevic, B.; Zekovic, M.; Korcok, D.; Ignjatovic, S.; Djordjevic, B.; Milinkovic, N. Effects of Short-Term Magnesium Supplementation on Ionized, Total Magnesium and Other Relevant Electrolytes Levels. Biometals 2022, 35, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Karmańska, A.; Stańczak, A.; Karwowski, B. Magnez aktualny stan wiedzy. Bromatol. I Chem. Toksykol. 2015, 48, 677–689. [Google Scholar]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. The evaluation of pharmaceutical magnesium availability from unmodified release tablets. Ann. Acad. Medicae Silesiensis 2015, 166–171. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Bartosiewicz, N.; Socha, K. Is the Magnesium Content in Food Supplements Consistent with the Manufacturers’ Declarations? Nutrients 2021, 13, 3416. [Google Scholar] [CrossRef]
- Marzec, Z.; Szalast-Pietrzak, A.; Wyszogrodzka-Koma, L.; Kasperek, R.; Frąk, A. Wpływ produktów spożywczych na biodostępność wapnia i magnezu zawartych w suplementach diety w warunkach trawienia in vitro. Bromatol. I Chem. Toksykol. 2015, 3, 452–456. (In Polish) [Google Scholar]
- Siener, R.; Jahnen, A.; Hesse, A. Bioavailability of Magnesium from Different Pharmaceutical Formulations. Urol. Res. 2011, 39, 123–127. [Google Scholar] [CrossRef]
- Coudray, C.; Demigné, C.; Rayssiguier, Y. Effects of Dietary Fibers on Magnesium Absorption in Animals and Humans. J. Nutr. 2003, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used in Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. A Physiological Approach for Preparing and Conducting Intestinal Bioavailability Studies Using Experimental Systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The Effect of in Vitro Digestion, Food Matrix, and Hydrothermal Treatment on the Potential Bioaccessibility of Selected Phenolic Compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef] [PubMed]
| Mg | ||||
|---|---|---|---|---|
| Product No. | Chemical Form | Supplement Type | Pharmaceutical Form | Registration Form |
| 1 | magnesium hydroxide | vitamin–mineral | coated tablets | dietary supplement |
| 2 | magnesium oxide | vitamin–mineral | coated tablets | dietary supplement |
| 3 | magnesium oxide | vitamin-mineral | capsules | medicinal product |
| 4 | magnesium oxide | single-mineral | capsules | dietary supplement |
| 5 | magnesium oxide | vitamin-mineral | tablets | dietary supplement |
| 6 | magnesium oxide | single-mineral | capsules | dietary supplement |
| 7 | magnesium oxide | vitamin-mineral | tablets | dietary supplement |
| 8 | magnesium bisglycinate (chelate ALBION®-Balchem Corporation, Montvale, NJ, USA) amino acid magnesium) | single-mineral | capsules | dietary supplement |
| 9 | magnesium lactate dihydrate | single-mineral | coated tablets | medicinal product |
| 10 | magnesium citrate | single-mineral | coated tablets | dietary supplement |
| 11 | magnesium chloride hexahydrate | single-mineral | coated tablets | medicinal product |
| 12 | magnesium L-pidolate | single-mineral | sachets for dissolving | dietary supplement |
| Parameter | Mg (ICP-OES) |
|---|---|
| Reference value (mg/kg) | 752.3 |
| Determined value (mg/kg) | 816.4 |
| 734.2 | |
| 808.1 | |
| 794.5 | |
| 783.7 | |
| 771.9 | |
| Average | 784.8 |
| SD | 29.54 |
| RSD (%) | 3.76 |
| Recovery (%) | 104.3 |
| LOD (μg/kg) | 37.0 |
| LOQ (μg/kg) | 135.7 |
| Dietary Supplement No. | Chemical Form | Diet | M | Me | Min | Max | IQR | SD | One-Way ANOVA | Tukey’s Post Hoc Test Results | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | Group 1 | Group 2 | p | |||||||||
| Without (%) | - | Standard | 52.51 | 52.23 | 44.99 | 60.22 | 5.23 | 4.50 | 3.21 | >0.05 | Standard | Basic | >0.05 |
| Basic | 52.23 | 51.77 | 46.71 | 57.75 | 2.14 | 3.00 | Standard | High-residue | >0.05 | ||||
| High-residue | 48.74 | 48.65 | 43.21 | 52.59 | 2.89 | 2.79 | Basic | High-residue | >0.05 | ||||
| 1 | magnesium hydroxide | Standard | 46.95 | 48.51 | 39.52 | 54.38 | 9.41 | 5.43 | 209.68 | <0.001 | Standard | Basic | <0.001 |
| Basic | 74.19 | 74.15 | 71.11 | 75.82 | 0.78 | 1.36 | Standard | High-residue | <0.001 | ||||
| High-residue | 74.20 | 74.23 | 72.98 | 75.03 | 1.30 | 0.72 | Basic | High-residue | >0.05 | ||||
| 2 | magnesium oxide | Standard | 55.95 | 55.39 | 50.56 | 61.05 | 5.20 | 3.43 | 48.55 | <0.001 | Standard | Basic | <0.001 |
| Basic | 64.01 | 63.59 | 61.32 | 66.70 | 2.22 | 1.80 | Standard | High-residue | <0.001 | ||||
| High-residue | 66.01 | 65.82 | 64.78 | 67.23 | 1.36 | 0.89 | Basic | High-residue | >0.05 | ||||
| 3 | magnesium oxide | Standard | 69.63 | 68.97 | 58.15 | 83.84 | 8.22 | 7.49 | 143.17 | <0.001 | Standard | Basic | <0.001 |
| Basic | 40.68 | 40.76 | 39.51 | 41.52 | 0.78 | 0.66 | Standard | High-residue | <0.001 | ||||
| High-residue | 35.85 | 35.44 | 31.06 | 40.65 | 1.58 | 2.54 | Basic | High-residue | >0.05 | ||||
| 4 | magnesium oxide | Standard | 69.29 | 69.88 | 67.09 | 71.23 | 3.30 | 1.77 | 0.44 | >0.05 | Standard | Basic | >0.05 |
| Basic | 70.51 | 70.93 | 67.09 | 71.55 | 0.94 | 1.38 | Standard | High-residue | >0.05 | ||||
| High-residue | 69.63 | 69.91 | 60.95 | 77.51 | 2.95 | 4.40 | Basic | High-residue | >0.05 | ||||
| 5 | magnesium oxide | Standard | 36.54 | 36.19 | 35.14 | 38.56 | 1.92 | 1.26 | 1263.55 | <0.001 | Standard | Basic | <0.001 |
| Basic | 64.79 | 64.79 | 63.85 | 65.91 | 0.92 | 0.67 | Standard | High-residue | <0.001 | ||||
| High-residue | 49.20 | 49.18 | 47.29 | 51.03 | 2.91 | 1.50 | Basic | High-residue | <0.001 | ||||
| 6 | magnesium oxide | Standard | 76.55 | 75.91 | 73.52 | 79.32 | 3.35 | 2.03 | 304.86 | <0.001 | Standard | Basic | <0.001 |
| Basic | 61.54 | 62.03 | 59.12 | 63.11 | 1.88 | 1.45 | Standard | High-residue | <0.001 | ||||
| High-residue | 59.60 | 60.19 | 57.56 | 61.04 | 1.65 | 1.18 | Basic | High-residue | <0.05 | ||||
| 7 | magnesium oxide | Standard | 42.06 | 41.05 | 38.11 | 46.01 | 4.45 | 2.75 | 489.50 | <0.001 | Standard | Basic | <0.001 |
| Basic | 73.03 | 74.09 | 69.92 | 75.79 | 4.06 | 2.24 | Standard | High-residue | <0.001 | ||||
| High-residue | 70.89 | 72.13 | 67.99 | 72.88 | 2.87 | 1.97 | Basic | High-residue | >0.05 | ||||
| 8 | magnesium bisglycinate | Standard | 65.15 | 65.72 | 58.71 | 72.93 | 6.49 | 4.69 | 7.49 | <0.01 | Standard | Basic | <0.01 |
| Basic | 70.08 | 70.48 | 65.42 | 73.73 | 2.88 | 2.56 | Standard | High-residue | >0.05 | ||||
| High-residue | 64.76 | 64.81 | 62.28 | 66.48 | 0.52 | 1.12 | Basic | High-residue | <0.01 | ||||
| 9 | magnesium lactate dihydrate | Standard | 53.50 | 51.54 | 46.74 | 64.62 | 5.36 | 5.70 | 11.02 | <0.001 | Standard | Basic | >0.05 |
| Basic | 56.43 | 56.93 | 54.11 | 58.54 | 2.62 | 1.59 | Standard | High-residue | <0.001 | ||||
| High-residue | 61.42 | 61.42 | 58.14 | 63.94 | 3.20 | 2.05 | Basic | High-residue | <0.05 | ||||
| 10 | magnesium citrate | Standard | 52.86 | 52.39 | 49.71 | 58.42 | 1.37 | 2.57 | 43.84 | <0.001 | Standard | Basic | <0.001 |
| Basic | 57.92 | 58.49 | 55.59 | 59.61 | 2.02 | 1.46 | Standard | High-residue | <0.001 | ||||
| High-residue | 61.80 | 62.23 | 58.78 | 64.09 | 2.89 | 1.91 | Basic | High-residue | <0.01 | ||||
| 11 | magnesium chloride hexahydrate | Standard | 60.65 | 60.89 | 58.77 | 62.14 | 1.82 | 1.12 | 250.98 | <0.001 | Standard | Basic | <0.001 |
| Basic | 75.27 | 74.95 | 73.55 | 77.05 | 1.88 | 1.33 | Standard | High-residue | <0.001 | ||||
| High-residue | 69.19 | 69.44 | 66.67 | 71.70 | 1.72 | 1.67 | Basic | High-residue | <0.001 | ||||
| 12 | magnesium L-pidolate | Standard | 52.95 | 54.75 59.79 48.63 | 45.77 55.03 42.29 | 57.27 61.33 57.89 | 7.24 3.23 4.49 | 4.31 2.37 4.64 | 10.90 | <0.001 | Standard | Basic | <0.05 |
| Basic | 58.54 | Standard | High-residue | >0.05 | |||||||||
| High-residue | 50.09 | Basic | High-residue | <0.001 | |||||||||
| Chemical Form | Diet | M | Me | Min | Max | IQR | SD | One-Way ANOVA | Tukey’s Post Hoc Test Results | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | Group 1 | Group 2 | p | ||||||||
| Magnesium hydroxide | Standard | 46.95 | 48.51 | 39.52 | 54.38 | 9.41 | 5.43 | 209.68 | >0.001 | Standard | Basic | <0.001 |
| Basic | 74.19 | 74.15 | 71.11 | 75.82 | 0.78 | 1.36 | Standard | High-residue | <0.001 | |||
| High-residue | 74.20 | 74.23 | 72.98 | 75.03 | 1.30 | 0.72 | Basic | High-residue | >0.05 | |||
| Magnesium oxide | Standard | 58.34 | 60.38 | 35.14 | 83.84 | 30.10 | 15.42 | 1.68 | >0.05 | Standard | Basic | >0.05 |
| Basic | 62.43 | 64.79 | 39.51 | 75.79 | 8.570 | 10.68 | Standard | High-residue | >0.05 | |||
| High-residue | 58.53 | 60.99 | 31.06 | 77.51 | 19.11 | 12.80 | Basic | High-residue | >0.05 | |||
| Magnesium bisglycinate | Standard | 65.15 | 65.72 | 58.71 | 72.93 | 6.49 | 4.69 | 7.49 | <0.01 | Standard | Basic | <0.01 |
| Basic | 70.08 | 70.48 | 65.42 | 73.73 | 2.88 | 2.56 | Standard | High-residue | >0.05 | |||
| High-residue | 64.76 | 64.81 | 62.28 | 66.48 | 0.52 | 1.12 | Basic | High-residue | <0.01 | |||
| Magnesium lactate dihydrate | Standard | 53.50 | 51.54 | 46.74 | 64.62 | 5.36 | 5.70 | 11.02 | <0.001 | Standard | Basic | >0.05 |
| Basic | 56.43 | 56.93 | 54.11 | 58.54 | 2.62 | 1.59 | Standard | High-residue | <0.001 | |||
| High-residue | 61.42 | 61.42 | 58.14 | 63.94 | 3.20 | 2.05 | Basic | High-residue | <0.05 | |||
| Magnesium Citrate | Standard | 52.86 | 52.39 | 49.71 | 58.42 | 1.37 | 2.57 | 43.84 | <0.001 | Standard | Basic | <0.001 |
| Basic | 57.92 | 58.49 | 55.59 | 59.61 | 2.02 | 1.46 | Standard | High-residue | <0.001 | |||
| High-residue | 61.80 | 62.23 | 58.78 | 64.09 | 2.89 | 1.91 | Basic | High-residue | <0.01 | |||
| Magnesium chloride hexahydrate | Standard | 60.65 | 60.89 | 58.77 | 62.14 | 1.82 | 1.12 | 250.98 | <0.001 | Standard | Basic | <0.001 |
| Basic | 75.27 | 74.95 | 73.55 | 77.05 | 1.88 | 1.33 | Standard | High-residue | <0.001 | |||
| High-residue | 69.19 | 69.44 | 66.67 | 71.70 | 1.72 | 1.67 | Basic | High-residue | <0.001 | |||
| Magnesium L-pidolate | Standard | 52.95 | 54.75 | 45.77 | 57.27 | 7.24 | 4.31 | 10.90 | <0.001 | Standard | Basic | <0.05 |
| Basic | 58.54 | 59.79 | 55.03 | 61.33 | 3.23 | 2.37 | Standard | High-residue | >0.05 | |||
| High-residue | 50.09 | 48.63 | 42.29 | 57.89 | 4.49 | 4.64 | Basic | High-residue | <0.001 | |||
| Chemical Form | M | Me | Min | Max | IQR | SD | Kruskal–Wallis ANOVA | |
|---|---|---|---|---|---|---|---|---|
| H | p | |||||||
| Magnesium hydroxide (%) | 65.11 | 73.62 | 39.52 | 75.82 | 23.55 | 13.46 | 81.30 | <0.001 |
| Magnesium oxide (%) | 59.76 | 63.94 | 31.06 | 83.84 | 20.98 | 13.16 | ||
| Magnesium bisglycinate (%) | 66.66 | 65.72 | 58.71 | 73.73 | 5.70 | 3.91 | ||
| Magnesium lactate dihydrate (%) | 57.12 | 57.59 | 46.74 | 64.62 | 6.04 | 4.82 | ||
| Magnesium Citrate (%) | 57.53 | 58.49 | 49.71 | 64.09 | 7.27 | 4.21 | ||
| Magnesium chloride hexahydrate (%) | 68.37 | 69.44 | 58.77 | 77.05 | 12.88 | 6.26 | ||
| Magnesium L-pidolate (%) | 53.86 | 55.41 | 42.29 | 61.33 | 8.66 | 5.18 | ||
| Diet (%) | 51.16 | 51.13 | 43.21 | 60.22 | 4.54 | 3.80 | ||
| Pharmaceutical Form | M | Me | Min | Max | IQR | SD | Kruskal–Wallis ANOVA | |
|---|---|---|---|---|---|---|---|---|
| H | p | |||||||
| Diet without preparation (%) | 51.16 | 51.13 | 43.21 | 60.22 | 4.54 | 3.80 | 53.99 | <0.001 |
| Coated tablets (%) | 62.02 | 61.42 | 39.52 | 77.05 | 11.81 | 8.62 | ||
| Capsules (%) | 62.77 | 65.83 | 31.06 | 83.84 | 10.91 | 12.25 | ||
| Tablets (%) | 56.08 | 57.44 | 35.14 | 75.79 | 28.88 | 14.43 | ||
| Sachets (%) | 53.86 | 55.41 | 42.29 | 61.33 | 8.66 | 5.18 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bawiec, P.; Jaworowska, A.; Sawicki, J.; Czop, M.; Szalak, R.; Koch, W. In Vitro Evaluation of Bioavailability of Mg from Daily Food Rations, Dietary Supplements and Medicinal Products from the Polish Market. Nutrients 2025, 17, 748. https://doi.org/10.3390/nu17050748
Bawiec P, Jaworowska A, Sawicki J, Czop M, Szalak R, Koch W. In Vitro Evaluation of Bioavailability of Mg from Daily Food Rations, Dietary Supplements and Medicinal Products from the Polish Market. Nutrients. 2025; 17(5):748. https://doi.org/10.3390/nu17050748
Chicago/Turabian StyleBawiec, Piotr, Agnieszka Jaworowska, Jan Sawicki, Marcin Czop, Radosław Szalak, and Wojciech Koch. 2025. "In Vitro Evaluation of Bioavailability of Mg from Daily Food Rations, Dietary Supplements and Medicinal Products from the Polish Market" Nutrients 17, no. 5: 748. https://doi.org/10.3390/nu17050748
APA StyleBawiec, P., Jaworowska, A., Sawicki, J., Czop, M., Szalak, R., & Koch, W. (2025). In Vitro Evaluation of Bioavailability of Mg from Daily Food Rations, Dietary Supplements and Medicinal Products from the Polish Market. Nutrients, 17(5), 748. https://doi.org/10.3390/nu17050748








