Association Between Maternal Egg Consumption During Pregnancy and Breastfeeding Initiation and Duration †
Abstract
1. Introduction
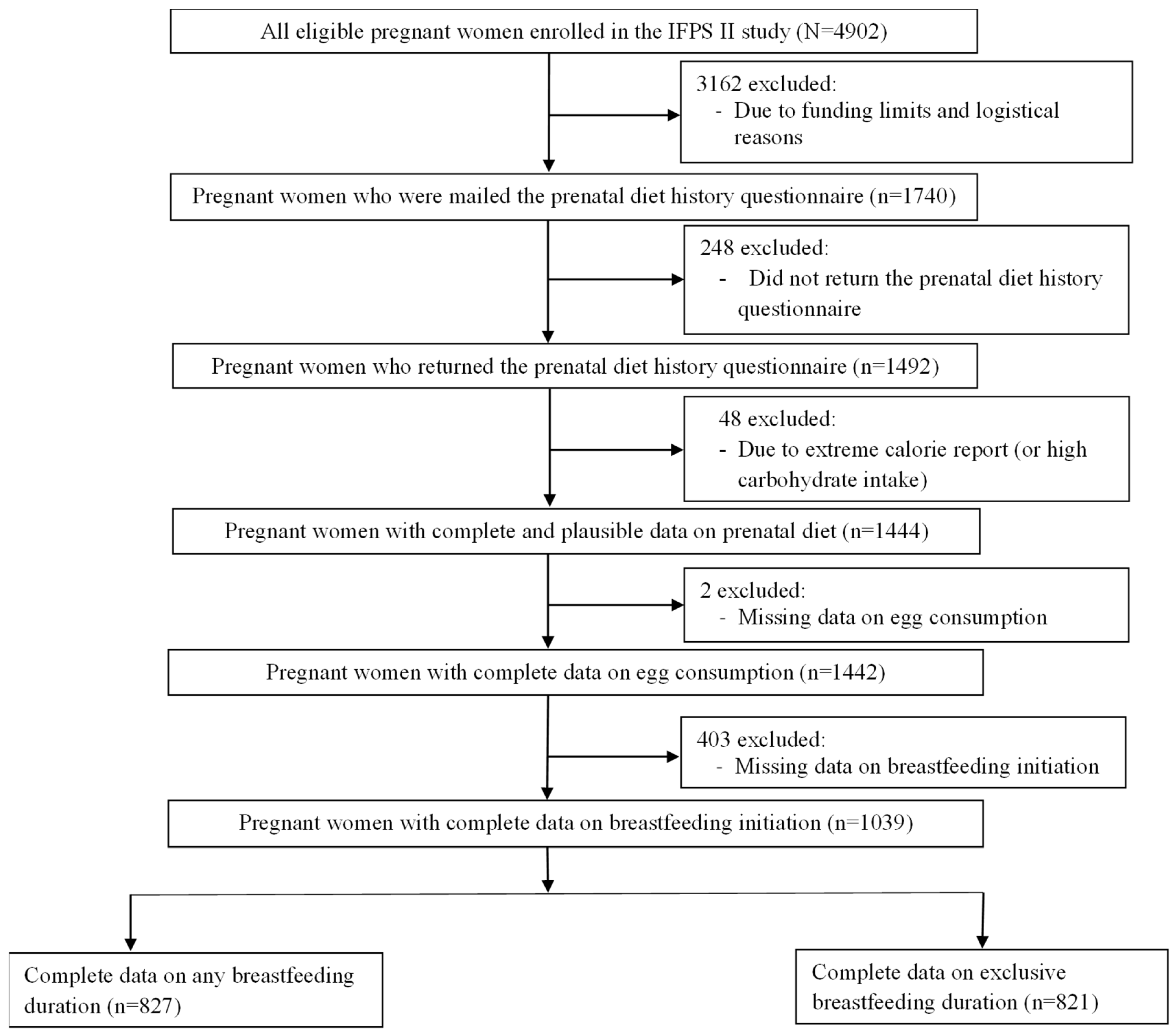
2. Materials and Methods
2.1. Participants and Setting
2.2. Measures for Maternal Egg Consumption During Pregnancy
2.3. Breastfeeding Outcome Measures
2.4. Confounders
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Correlates of Egg Consumption During Pregnancy
3.3. Distribution and Correlates of Breastfeeding Outcomes
3.4. Association of Egg Consumption During Pregnancy with Breastfeeding Initiation
3.5. Association of Egg Consumption During Pregnancy with Any Breastfeeding Duration
3.6. Association of Egg Consumption During Pregnancy with Exclusive Breastfeeding Duration
4. Discussion
4.1. Distribution and Correlates of Maternal Egg Consumption
4.2. Associations Between Maternal Egg Consumption and Breastfeeding Outcomes
4.3. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIDS | sudden infant death syndrome |
| AAP | American Academy of Pediatrics |
| ACOG | American College of Obstetricians and Gynecologists |
| IFPS II | Infant Feeding Practices Study II |
| FDA | U.S. Food and Drug Administration |
| CDC | Centers for Disease Control and Prevention |
| Y6FU | Year 6 Follow-Up |
| WIC | Special Supplemental Nutrition Program for Women, Infants, and Children |
| BMI | body mass index |
| DHQ | Diet History Questionnaire |
| HEI | Healthy Eating Index |
| USDA | U.S. Department of Agriculture |
| SD | standard deviation |
| ANOVA | analysis of variance |
| LSD | least significant difference |
| OR | odds ratio |
| CI | confidence interval |
| RDA | Recommended Dietary Allowance |
| LCPUFA | long-chain polyunsaturated fatty acids |
Appendix A
| Analytic Sample (N = 1039) | Excluded Sample (N = 3863) | ||||
|---|---|---|---|---|---|
| Characteristics * | n (%) | Mean ± SD | n (%) | Mean ± SD | p-Value ** |
| Maternal age, years | 29.02 ± 5.34 | 27.97 ± 5.80 | <0.001 | ||
| % of Federal poverty level | 262.10 ± 189.30 | 243.90 ± 199.00 | 0.007 | ||
| Pre-pregnancy BMI, kg/m2 | 26.28 ± 6.45 | 26.47 ± 6.97 | 0.396 | ||
| Maternal race/ethnicity | |||||
| Non-Hispanic White | 880 (85.2) | 2983 (80.2) | 0.001 | ||
| Non-Hispanic Black | 40 (3.9) | 260 (7.0) | |||
| Hispanic | 66 (6.4) | 269 (7.2) | |||
| Non-Hispanic Asian/Pacific Islander/Other | 47 (4.6) | 209 (5.6) | |||
| Maternal highest education level | <0.001 | ||||
| High school or lower | 187 (18.9) | 869 (26.4) | |||
| 1–3 years of college | 388 (39.2) | 1369 (41.6) | |||
| College graduate | 309 (31.2) | 781 (23.8) | |||
| Postgraduate | 105 (10.6) | 270 (8.2) | |||
| Maternal employment status | |||||
| Unemployed | 364 (35.2) | 1271 (33.1) | 0.207 | ||
| Employed | 671 (64.8) | 2571 (66.9) | |||
| Household size | |||||
| 1–2 people | 260 (25.0) | 1051 (27.2) | 0.008 | ||
| 3 people | 400 (38.5) | 1275 (33.0) | |||
| 4 people | 217 (20.9) | 839 (21.7) | |||
| ≥5 people | 162 (15.6) | 698 (18.1) | |||
| Annual household income level | |||||
| <$25,000 | 220 (21.2) | 985 (25.5) | 0.001 | ||
| $25,000 ≤ $39,999 | 228 (21.9) | 929 (24.1) | |||
| $40,000 ≤ $59,999 | 243 (23.4) | 858 (22.2) | |||
| ≥$60,000 | 348 (33.5) | 1091 (28.2) | |||
| Maternal WIC recipient status | |||||
| Non-recipient | 619 (59.6) | 2042 (52.9) | <0.001 | ||
| Recipient | 420 (40.4) | 1821 (47.1) | |||
| Region of residency | |||||
| New England | 52 (5.0) | 154 (4.0) | 0.052 | ||
| Middle Atlantic | 122 (11.7) | 497 (12.9) | |||
| East North Central | 225 (21.7) | 758 (19.6) | |||
| West North Central | 96 (9.2) | 332 (8.6) | |||
| South Atlantic | 153 (14.7) | 698 (18.1) | |||
| East South Central | 56 (5.4) | 245 (6.3) | |||
| West South Central | 117 (11.3) | 435 (11.3) | |||
| Mountain | 107 (10.3) | 319 (8.3) | |||
| Pacific | 111 (10.7) | 425 (11.0) | |||
| Maternal cigarette smoking during pregnancy | |||||
| No | 940 (91.0) | 3341 (87.1) | 0.001 | ||
| Yes | 93 (9.0) | 496 (12.9) | |||
| Potential Confounder | Type of Variable | Unit or Categories |
|---|---|---|
| Age | Continuous | Years |
| Healthy Eating Index | Continuous | Scoring unit |
| Pre-pregnancy body mass index | Continuous | Kg/m2 |
| Race/ethnicity | Categorical | Non-Hispanic White, non-Hispanic Black, Hispanic, and Non-Hispanic Asian/Pacific Islander/other |
| The highest education level | Categorical | High school or lower, 1–3 years college, college graduate, and postgraduate |
| Employment status | Categorical | Unemployed and employed |
| Household size | Categorical | 1–2, 3, 4, and ≥5 people |
| Annual household income | Categorical | <$25,000, $25,000 to $39,999, $40,000 to $59,999, and ≥$60,000 |
| WIC recipient status | Categorical | Non-recipient and recipient |
| Region of residency | Categorical | New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, and Pacific |
| Smoking status during pregnancy | Categorical | Yes and no |
| Any Breastfeeding Duration, Weeks | Exclusive Breastfeeding Duration, Weeks | |||
|---|---|---|---|---|
| Characteristics * | Mean ± SD | p-Value ** | Mean ± SD | p-Value ** |
| Overall | 26.43 ± 20.69 | 6.31 ± 9.37 | ||
| Maternal age, years (mean difference ± SE) *** | 1.09 ± 0.09 | <0.001 | 0.14 ± 0.03 | <0.001 |
| 100% of Federal poverty level (mean difference ± SE) *** | 1.10 ± 0.26 | <0.001 | 0.20 ± 0.08 | 0.017 |
| Pre-pregnancy BMI, kg/m2 *** | −0.28 ± 0.08 | <0.001 | −0.13 ± 0.02 | <0.001 |
| Maternal race/ethnicity | <0.001 | <0.001 | ||
| Non-Hispanic White | 25.42 ± 26.49 | 5.79 ± 8.91 | ||
| Non-Hispanic Black | 16.82 ± 21.87 | 1.59 ± 4.27 | ||
| Hispanic | 20.91 ± 23.83 | 3.53 ± 6.63 | ||
| Non-Hispanic Asian/Pacific Islander/Other | 27.11 ± 24.45 | 4.37 ± 8.18 | ||
| Maternal highest education level | <0.001 | <0.001 | ||
| High school or lower | 15.76 ± 20.22 | 3.32 ± 6.51 | ||
| 1–3 years of college | 23.17 ± 24.86 | 5.02 ± 8.29 | ||
| College graduate | 31.97 ± 26.35 | 7.06 ± 9.59 | ||
| Postgraduate | 36.78 ± 30.81 | 8.09 ± 10.48 | ||
| Maternal employment status | <0.001 | <0.001 | ||
| Unemployed | 27.17 ± 28.26 | 6.24 ± 9.44 | ||
| Employed | 23.30 ± 24.70 | 4.87 ± 8.11 | ||
| Household size | 0.599 | 0.723 | ||
| 1–2 people | 24.30 ± 27.69 | 5.07 ± 8.44 | ||
| 3 people | 25.39 ± 24.42 | 5.54 ± 8.62 | ||
| 4 people | 23.58 ± 25.52 | 5.28 ± 8.59 | ||
| ≥5 people | 25.02 ± 28.06 | 5.26 ± 8.80 | ||
| Annual household income level | <0.001 | <0.001 | ||
| <$25,000 | 17.65 ± 22.96 | 4.01 ± 7.63 | ||
| $25,000 ≤ $39,999 | 23.56 ± 25.42 | 5.33 ± 8.51 | ||
| $40,000 ≤ $59,999 | 28.14 ± 27.71 | 6.09 ± 9.21 | ||
| ≥$60,000 | 27.49 ± 26.53 | 5.59 ± 8.69 | ||
| Maternal WIC recipient status | <0.001 | 0.008 | ||
| Non-recipient | 27.23 ± 26.70 | 5.68 ± 8.85 | ||
| Recipient | 21.03 ± 24.90 | 4.80 ± 8.19 | ||
| Region of residency | <0.001 | <0.001 | ||
| New England | 24.64 ± 26.80 | 5.07 ± 8.27 | ||
| Middle Atlantic | 23.85 ± 25.97 | 4.19 ± 7.97 | ||
| East North Central | 23.66 ± 27.91 | 5.01 ± 8.38 | ||
| West North Central | 24.28 ± 22.60 | 5.88 ± 8.72 | ||
| South Atlantic | 22.48 ± 26.15 | 4.59 ± 7.97 | ||
| East South Central | 18.67 ± 22.84 | 4.63 ± 8.30 | ||
| West South Central | 22.44 ± 25.17 | 4.81 ± 8.26 | ||
| Mountain | 29.29 ± 20.96 | 6.72 ± 9.20 | ||
| Pacific | 32.50 ± 29.82 | 7.56 ± 9.92 | ||
| Maternal cigarette smoking during pregnancy | <0.001 | <0.001 | ||
| No | 26.32 ± 26.37 | 5.71 ± 8.85 | ||
| Yes | 10.29 ± 18.68 | 1.92 ± 4.62 | ||
| Child sex | 0.402 | 0.128 | ||
| Male | 25.10 ± 26.97 | 5.07 ± 8.41 | ||
| Female | 24.24 ± 25.32 | 5.56 ± 8.76 | ||
References
- Chen, Y.; Jiang, P.; Geng, Y. The role of breastfeeding in breast cancer prevention: A literature review. Front. Oncol. 2023, 13, 1257804. [Google Scholar] [CrossRef]
- Babic, A.; Sasamoto, N.; Rosner, B.A.; Tworoger, S.S.; Jordan, S.J.; Risch, H.A.; Harris, H.R.; Rossing, M.A.; Doherty, J.A.; Fortner, R.T.; et al. Association Between Breastfeeding and Ovarian Cancer Risk. JAMA Oncol. 2020, 6, e200421. [Google Scholar] [CrossRef]
- Jiang, M.; Gao, H.; Vinyes-Pares, G.; Yu, K.; Ma, D.; Qin, X.; Wang, P. Association between breastfeeding duration and postpartum weight retention of lactating mothers: A meta-analysis of cohort studies. Clin. Nutr. 2018, 37, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Rich-Edwards, J.W.; Willett, W.C.; Manson, J.E.; Michels, K.B. Duration of lactation and incidence of type 2 diabetes. JAMA 2005, 294, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin. Perinatol. 2015, 39, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Krol, K.M.; Grossmann, T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61, 977–985. [Google Scholar] [CrossRef]
- Muro-Valdez, J.C.; Meza-Rios, A.; Aguilar-Uscanga, B.R.; Lopez-Roa, R.I.; Medina-Diaz, E.; Franco-Torres, E.M.; Zepeda-Morales, A.S.M. Breastfeeding-Related Health Benefits in Children and Mothers: Vital Organs Perspective. Medicina 2023, 59, 1535. [Google Scholar] [CrossRef]
- Stuebe, A. The risks of not breastfeeding for mothers and infants. Rev. Obstet. Gynecol. 2009, 2, 222–231. [Google Scholar]
- American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- American College of Obstetricians Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet. Gynecol. 2016, 127, e86–e92. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Breastfeeding Report Card United States, 2022; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Moret-Tatay, A.; Perez-Bermejo, M.; Asins-Cubells, A.; Moret-Tatay, C.; Murillo-Llorente, M.T. A Systematic Review of Multifactorial Barriers Related to Breastfeeding. Healthcare 2025, 13, 1225. [Google Scholar] [CrossRef]
- Carretero-Krug, A.; Montero-Bravo, A.; Morais-Moreno, C.; Puga, A.M.; Samaniego-Vaesken, M.L.; Partearroyo, T.; Varela-Moreiras, G. Nutritional Status of Breastfeeding Mothers and Impact of Diet and Dietary Supplementation: A Narrative Review. Nutrients 2024, 16, 301. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. The Health Benefits of Egg Protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef] [PubMed]
- Lutter, C.K.; Iannotti, L.L.; Stewart, C.P. The potential of a simple egg to improve maternal and child nutrition. Matern. Child Nutr. 2018, 14 (Suppl. S3), e12678. [Google Scholar] [CrossRef] [PubMed]
- Buntuchai, G.; Pavadhgul, P.; Kittipichai, W.; Satheannoppakao, W. Traditional Galactagogue Foods and Their Connection to Human Milk Volume in Thai Breastfeeding Mothers. J. Hum. Lact. 2017, 33, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Huang, C.R.; Du, W.W.; Li, J.Y.; Redding, S.R.; Ouyang, Y.Q. Galactagogue Food Consumption, Perception of Insufficient Milk Supply, and Exclusive Breastfeeding in Chinese Postpartum Women: An Analysis of Repeated Measures. J. Transcult. Nurs. 2023, 34, 365–374. [Google Scholar] [CrossRef]
- Santos, H.O.; Gomes, G.K.; Schoenfeld, B.J.; de Oliveira, E.P. The Effect of Whole Egg Intake on Muscle Mass: Are the Yolk and Its Nutrients Important? Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 514–521. [Google Scholar] [CrossRef]
- Pin, S.S. Effects of preparation methods on protein and amino acid contents of various eggs available in Malaysian local markets. ACTA Sci. Pol. Technol. Aliment. 2013, 12, 21–32. [Google Scholar]
- Xue, H.; Han, T.; Xu, M.; Yao, Y.; Wu, N.; Chen, S.; Zhang, G.; Wang, W.; Zhao, Y.; Tu, Y. Processing technology, principle, and nutritional characteristics of preserved eggs: A review. Trends Food Sci. Technol. 2022, 128, 265–277. [Google Scholar] [CrossRef]
- Moradi, F.; Tabarrai, M.; Nejatbakhsh, F. Methods of preparation of egg and therapeutic uses: A review perspective of Persian Medicine. Food Ther. Health Care 2020, 2, 32–39. [Google Scholar] [CrossRef]
- Wen, X.; Mohammed, F.; Giancarlo, E.M.; Botchway, A.; Rideout, T.C. Maternal Egg Consumption during Pregnancy and Breastfeeding Practices. In Proceedings of the Nutrition 2024 (The American Society for Nutrition [ASN] Annual Scientific Meeting), Chicago, IL, USA, 29 June–2 July 2024. [Google Scholar]
- Fein, S.B.; Labiner-Wolfe, J.; Shealy, K.R.; Li, R.; Chen, J.; Grummer-Strawn, L.M. Infant Feeding Practices Study II: Study methods. Pediatrics 2008, 122 (Suppl. S2), S28–S35. [Google Scholar] [CrossRef]
- Fein, S.B.; Li, R.; Chen, J.; Scanlon, K.S.; Grummer-Strawn, L.M. Methods for the year 6 follow-up study of children in the Infant Feeding Practices Study II. Pediatrics 2014, 134 (Suppl. S1), S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Eini-Zinab, H.; Shoaibinobarian, N.; Ranjbar, G.; Norouzian Ostad, A.; Sobhani, S.R. Association between the socio-economic status of households and a more sustainable diet. Public Health Nutr. 2021, 24, 6566–6574. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Kogan, M.D.; Dee, D.L. Nativity/immigrant status, race/ethnicity, and socioeconomic determinants of breastfeeding initiation and duration in the United States, 2003. Pediatrics 2007, 119 (Suppl. S1), S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Hjartaker, A.; Lund, E. Relationship between dietary habits, age, lifestyle, and socio-economic status among adult Norwegian women. The Norwegian Women and Cancer Study. Eur. J. Clin. Nutr. 1998, 52, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D. Disparities in the usage of maternity leave according to occupation, race/ethnicity, and education. Am. J. Ind. Med. 2020, 63, 1134–1144. [Google Scholar] [CrossRef]
- Bennett, G.; Bardon, L.A.; Gibney, E.R. A Comparison of Dietary Patterns and Factors Influencing Food Choice among Ethnic Groups Living in One Locality: A Systematic Review. Nutrients 2022, 14, 941. [Google Scholar] [CrossRef]
- Biesbroek, S.; Kneepkens, M.C.; van den Berg, S.W.; Fransen, H.P.; Beulens, J.W.; Peeters, P.H.M.; Boer, J.M.A. Dietary patterns within educational groups and their association with CHD and stroke in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Br. J. Nutr. 2018, 119, 949–956. [Google Scholar] [CrossRef]
- Yourkavitch, J.; Kane, J.B.; Miles, G. Neighborhood Disadvantage and Neighborhood Affluence: Associations with Breastfeeding Practices in Urban Areas. Matern. Child Health J. 2018, 22, 546–555. [Google Scholar] [CrossRef]
- Chau, C.A.; Pan, W.H.; Chen, H.J. Employment status and temporal patterns of energy intake: Nutrition and Health Survey in Taiwan, 2005–2008. Public Health Nutr. 2017, 20, 3295–3303. [Google Scholar] [CrossRef]
- Ryan, A.S.; Zhou, W.; Arensberg, M.B. The effect of employment status on breastfeeding in the United States. Women’s Health Issues 2006, 16, 243–251. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E. Association between Healthy Eating Index and Mental Health in Middle-Aged Adults Based on Household Size in Korea. Int. J. Environ. Res. Public Health 2022, 19, 4692. [Google Scholar] [CrossRef]
- Manyeh, A.K.; Amu, A.; Akpakli, D.E.; Williams, J.E.; Gyapong, M. Estimating the rate and determinants of exclusive breastfeeding practices among rural mothers in Southern Ghana. Int. Breastfeed. J. 2020, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Backholer, K.; Spencer, E.; Gearon, E.; Magliano, D.J.; McNaughton, S.A.; Shaw, J.E.; Peeters, A. The association between socio-economic position and diet quality in Australian adults. Public Health Nutr. 2016, 19, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Shepherd-Banigan, M.; Bell, J.F. Paid leave benefits among a national sample of working mothers with infants in the United States. Matern. Child Health J. 2014, 18, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Melo Herrera, Y.; Tovar, A.; Oaks, B.M.; Quashie, N.T.; Vadiveloo, M. Associations between Participation in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and Maternal Diet Quality. J. Nutr. 2023, 153, 3317–3326. [Google Scholar] [CrossRef]
- Heck, K.E.; Braveman, P.; Cubbin, C.; Chavez, G.F.; Kiely, J.L. Socioeconomic status and breastfeeding initiation among California mothers. Public Health Rep. 2006, 121, 51–59. [Google Scholar] [CrossRef]
- Gray, M.S.; Lakkur, S.; Howard, V.J.; Pearson, K.; Shikany, J.M.; Safford, M.; Gutierrez, O.M.; Colabianchi, N.; Judd, S.E. The Association between Residence in a Food Desert Census Tract and Adherence to Dietary Patterns in the REGARDS Cohort. Food Public Health 2018, 8, 79–85. [Google Scholar]
- Nuttens, M.C.; Romon, M.; Ruidavets, J.B.; Arveiler, D.; Ducimetiere, P.; Lecerf, J.M.; Richard, J.L.; Cambou, J.P.; Simon, C.; Salomez, J.L. Relationship between smoking and diet: The MONICA-France project. J. Intern. Med. 1992, 231, 349–356. [Google Scholar] [CrossRef]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- Pan, X.F.; Yang, J.J.; Lipworth, L.P.; Shu, X.O.; Cai, H.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Yu, D. Cholesterol and Egg Intakes with Cardiometabolic and All-Cause Mortality among Chinese and Low-Income Black and White Americans. Nutrients 2021, 13, 2094. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. The cost of US foods as related to their nutritive value. Am. J. Clin. Nutr. 2010, 92, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.; Cui, M.; Liu, J.; Yang, C.; Li, X.; Liu, P.; Wang, L. Impact of maternal nutrition during early pregnancy and diet during lactation on lactoferrin in mature breast milk. Nutrition 2022, 93, 111500. [Google Scholar] [CrossRef] [PubMed]
- USDA Agricultural Research Service. Eggs, Grade A, Large, Egg Yolk. Available online: https://fdc.nal.usda.gov/food-details/748236/nutrients (accessed on 13 May 2025).
- USDA Agricultural Research Service. Eggs, Grade A, Large, Egg White. Available online: https://fdc.nal.usda.gov/food-details/747997/nutrients (accessed on 13 May 2025).
- Ward, E.; Yang, N.; Muhlhausler, B.S.; Leghi, G.E.; Netting, M.J.; Elmes, M.J.; Langley-Evans, S.C. Acute changes to breast milk composition following consumption of high-fat and high-sugar meals. Matern. Child Nutr. 2021, 17, e13168. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, L. The investigation of fatty acid composition of breast milk and its relationship with dietary fatty acid intake in 5 regions of China. Medicine 2019, 98, e15855. [Google Scholar] [CrossRef]
- Mandel, D.; Lubetzky, R.; Dollberg, S.; Barak, S.; Mimouni, F.B. Fat and energy contents of expressed human breast milk in prolonged lactation. Pediatrics 2005, 116, e432–e435. [Google Scholar] [CrossRef]
- Nysenbaum, A.N.; Smart, J.L. Sucking behaviour and milk intake of neonates in relation to milk fat content. Early Hum. Dev. 1982, 6, 205–213. [Google Scholar] [CrossRef]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef]
- Ollivier-Bousquet, M.; Guesnet, P.; Seddiki, T.; Durand, G. Deficiency of (n-6) but not (n-3) polyunsaturated fatty acids inhibits the secretagogue effect of prolactin in lactating rat mammary epithelial cells. J. Nutr. 1993, 123, 2090–2100. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutrition in pregnancy: The argument for including a source of choline. Int. J. Women’s Health 2013, 5, 193–199. [Google Scholar] [CrossRef]
- Radzyminski, S. Neurobehavioral functioning and breastfeeding behavior in the newborn. J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 335–341. [Google Scholar] [CrossRef]
- Hernandez-Luengo, M.; Alvarez-Bueno, C.; Martinez-Hortelano, J.A.; Cavero-Redondo, I.; Martinez-Vizcaino, V.; Notario-Pacheco, B. The relationship between breastfeeding and motor development in children: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 1827–1835. [Google Scholar] [CrossRef]
| Analytic Sample (N = 1039) | ||
|---|---|---|
| Characteristics * | n (%) | Mean ± SD |
| Maternal age, years | 29.02 ± 5.34 | |
| % of Federal poverty level | 262.09 ± 189.28 | |
| Pre-pregnancy BMI, kg/m2 | 26.28 ± 6.45 | |
| Maternal race/ethnicity | ||
| Non-Hispanic White | 880 (85.2) | |
| Non-Hispanic Black | 40 (3.9) | |
| Hispanic | 66 (6.4) | |
| Non-Hispanic Asian/Pacific Islander/Other | 47 (4.6) | |
| Maternal highest education level | ||
| High school or lower | 187 (18.9) | |
| 1–3 years of college | 388 (39.2) | |
| College graduate | 309 (31.2) | |
| Postgraduate | 105 (10.6) | |
| Maternal employment status | ||
| Unemployed | 364 (35.2) | |
| Employed | 671 (64.8) | |
| Household size | ||
| 1–2 people | 260 (25.0) | |
| 3 people | 400 (38.5) | |
| 4 people | 217 (20.9) | |
| ≥5 people | 162 (15.6) | |
| Annual household income level | ||
| <$25,000 | 220 (21.2) | |
| $25,000 ≤ $39,999 | 228 (21.9) | |
| $40,000 ≤ $59,999 | 243 (23.4) | |
| ≥$60,000 | 348 (33.5) | |
| Maternal WIC recipient status | ||
| Non-recipient | 619 (59.6) | |
| Recipient | 420 (40.4) | |
| Region of residency | ||
| New England | 52 (5.0) | |
| Middle Atlantic | 122 (11.7) | |
| East North Central | 225 (21.7) | |
| West North Central | 96 (9.2) | |
| South Atlantic | 153 (14.7) | |
| East South Central | 56 (5.4) | |
| West South Central | 117 (11.3) | |
| Mountain | 107 (10.3) | |
| Pacific | 111 (10.7) | |
| Maternal cigarette smoking during pregnancy | ||
| No | 940 (91.0) | |
| Yes | 93 (9.0) | |
| Total Egg | Whole Egg | Egg White | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** |
| Overall | 1038 | 2.79 ± 3.39 | 1037 | 2.51 ± 3.22 | 1037 | 0.19 ± 1.05 | ||||||
| Maternal race/ethnicity | 0.013 | 0.019 | 0.310 | |||||||||
| Non-Hispanic White | 879 | 2.66 ± 3.25 | a | 878 | 2.38 ± 3.10 | a | 878 | 0.16 ± 0.96 | ||||
| Non-Hispanic Black | 40 | 3.95 ± 5.22 | b | 40 | 3.29 ± 4.72 | a,b | 40 | 0.46 ± 1.71 | ||||
| Hispanic | 66 | 3.10 ± 3.40 | a,b | 66 | 3.01 ± 3.38 | a,b | 66 | 0.24 ± 1.33 | ||||
| Non-Hispanic Asian/Pacific Islander/Other | 47 | 3.79 ± 3.53 | b | 47 | 3.53 ± 3.51 | b | 47 | 0.24 ± 0.75 | ||||
| Maternal education level | 0.500 | 0.330 | 0.603 | |||||||||
| High school or lower | 187 | 2.86 ± 3.99 | 187 | 2.59 ± 3.98 | 187 | 0.11 ± 0.56 | ||||||
| 1–3 years of college | 387 | 2.94 ± 3.46 | 387 | 2.71 ± 3.36 | 387 | 0.19 ± 1.05 | ||||||
| College graduate | 309 | 2.55 ± 2.98 | 308 | 2.29 ± 2.69 | 309 | 0.19 ± 1.16 | ||||||
| Postgraduate | 105 | 2.77 ± 3.11 | 105 | 2.31 ± 2.79 | 104 | 0.27 ± 1.26 | ||||||
| Maternal employment status | 0.180 | 0.260 | 0.632 | |||||||||
| Unemployed | 364 | 2.99 ± 3.62 | 364 | 2.67 ± 3.50 | 364 | 0.21 ± 1.16 | ||||||
| Employed | 670 | 2.69 ± 3.25 | 669 | 2.43 ± 3.07 | 669 | 0.18 ± 0.99 | ||||||
| Household size | 0.840 | 0.664 | 0.999 | |||||||||
| 1–2 people | 259 | 2.81 ± 3.34 | 259 | 2.52 ± 3.18 | 259 | 0.19 ± 1.05 | ||||||
| 3 people | 400 | 2.69 ± 3.33 | 400 | 2.38 ± 3.10 | 399 | 0.19 ± 1.12 | ||||||
| 4 people | 217 | 2.95 ± 3.42 | 216 | 2.72 ± 3.37 | 217 | 0.20 ± 0.96 | ||||||
| ≥5 people | 162 | 2.81 ± 3.59 | 162 | 2.56 ± 3.43 | 162 | 0.19 ± 0.97 | ||||||
| Annual household income level | 0.514 | 0.279 | 0.426 | |||||||||
| <$25,000 | 220 | 3.00 ± 3.99 | 220 | 2.78 ± 3.89 | 220 | 0.27 ± 1.37 | ||||||
| $25,000 ≤ $39,999 | 228 | 2.87 ± 3.09 | 227 | 2.64 ± 2.97 | 228 | 0.13 ± 0.73 | ||||||
| $40,000 ≤ $59,999 | 242 | 2.83 ± 3.61 | 242 | 2.50 ± 3.43 | 242 | 0.22 ± 1.21 | ||||||
| ≥$60,000 | 348 | 2.59 ± 2.97 | 348 | 2.27 ± 2.73 | 347 | 0.16 ± 0.84 | ||||||
| Maternal WIC recipient status | 0.116 | 0.063 | 0.446 | |||||||||
| Non-recipient | 618 | 2.66 ± 3.08 | 618 | 2.36 ± 2.88 | 617 | 0.17 ± 0.98 | ||||||
| Recipient | 420 | 2.99 ± 3.79 | 419 | 2.74 ± 3.67 | 420 | 0.22 ± 1.14 | ||||||
| Region of residency | 0.390 | 0.469 | 0.237 | |||||||||
| New England | 52 | 3.01 ± 3.93 | a,b | 52 | 2.54 ± 3.25 | a,b | 52 | 0.44 ± 2.34 | a | |||
| Middle Atlantic | 122 | 3.00 ± 3.36 | a.b | 122 | 2.56 ± 3.06 | a,b | 122 | 0.33 ± 1.37 | a,c | |||
| East North Central | 225 | 2.61 ± 2.94 | a | 225 | 2.33 ± 2.85 | a,b | 225 | 0.16 ± 0.81 | a,b | |||
| West North Central | 96 | 2.22 ± 2.93 | a | 96 | 2.06 ± 2.83 | a | 96 | 0.03 ± 0.13 | b | |||
| South Atlantic | 153 | 2.69 ± 3.32 | a,b | 152 | 2.34 ± 3.08 | a,b | 153 | 0.27 ± 1.27 | a,b | |||
| East South Central | 56 | 2.54 ± 2.98 | a,b | 56 | 2.26 ± 2.95 | a,b | 56 | 0.16 ± 0.97 | a,b | |||
| West South Central | 116 | 2.94 ± 3.67 | a,b | 116 | 2.80 ± 3.65 | a,b | 116 | 0.10 ± 0.57 | b,c | |||
| Mountain | 107 | 2.86 ± 3.99 | a,b | 107 | 2.81 ± 4.00 | a,b | 107 | 0.09 ± 0.70 | b,c | |||
| Pacific | 111 | 3.41 ± 3.68 | b | 111 | 2.99 ± 3.43 | b | 110 | 0.23 ± 0.87 | a,b | |||
| Maternal cigarette smoking during pregnancy | 0.164 | 0.275 | 0.127 | |||||||||
| No | 939 | 2.85 ± 3.42 | 938 | 2.55 ± 3.25 | 938 | 0.21 ± 1.10 | ||||||
| Yes | 93 | 2.33 ± 3.07 | 93 | 2.17 ± 3.08 | 93 | 0.03 ± 0.31 | ||||||
| Egg Substitute | Egg with Fat | Egg Salad | ||||||||||
| Characteristics | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** | n | Mean ± SD (Eggs/Week) | Overall p-Value * | Pairwise Comparisons ** |
| Overall | 1038 | 0.07 ± 0.45 | 1038 | 1.65 ± 2.84 | 1036 | 0.23 ± 0.74 | ||||||
| Maternal race/ethnicity | 0.737 | <0.001 | 0.043 | |||||||||
| Non-Hispanic White | 879 | 0.08 ± 0.48 | 879 | 1.50 ± 2.65 | a | 877 | 0.20 ± 0.62 | a | ||||
| Non-Hispanic Black | 40 | 0.03 ± 0.16 | 40 | 3.30 ± 4.99 | b | 40 | 0.47 ± 1.51 | b | ||||
| Hispanic | 66 | 0.04 ± 0.21 | 66 | 1.96 ± 2.82 | a,c | 66 | 0.22 ± 0.69 | a,b | ||||
| Non-Hispanic Asian/Pacific Islander/Other | 47 | 0.04 ± 0.26 | 47 | 2.48 ± 3.33 | b,c | 47 | 0.41 ± 1.43 | a,b | ||||
| Maternal education level | 0.275 | 0.002 | 0.927 | |||||||||
| High school or lower | 187 | 0.03 ± 0.16 | 187 | 2.07 ± 3.45 | a | 187 | 0.25 ± 0.82 | |||||
| 1–3 years of college | 387 | 0.08 ± 0.55 | 387 | 1.85 ± 3.23 | a | 386 | 0.21 ± 0.76 | |||||
| College graduate | 309 | 0.06 ± 0.40 | 309 | 1.20 ± 1.94 | b | 308 | 0.23 ± 0.74 | |||||
| Postgraduate | 105 | 0.13 ± 0.50 | 105 | 1.44 ± 2.19 | a,b | 105 | 0.20 ± 0.59 | |||||
| Maternal employment status | 0.314 | 0.322 | 0.424 | |||||||||
| Unemployed | 364 | 0.09 ± 0.59 | 364 | 1.77 ± 2.89 | 364 | 0.20 ± 0.66 | ||||||
| Employed | 670 | 0.06 ± 0.35 | 670 | 1.59 ± 2.81 | 668 | 0.24 ± 0.77 | ||||||
| Household size | 0.091 | 0.309 | 0.764 | |||||||||
| 1-2 people | 259 | 0.12 ± 0.64 | a | 259 | 1.74 ± 2.84 | 259 | 0.23 ± 0.63 | |||||
| 3 people | 400 | 0.08 ± 0.46 | a,b | 400 | 1.45 ± 2.71 | 399 | 0.20 ± 0.77 | |||||
| 4 people | 217 | 0.03 ± 0.18 | b | 217 | 1.87 ± 3.27 | 217 | 0.24 ± 0.73 | |||||
| ≥5 people | 162 | 0.04 ± 0.27 | a,b | 162 | 1.72 ± 2.50 | 161 | 0.26 ± 0.82 | |||||
| Annual household income level | 0.306 | 0.002 | 0.770 | |||||||||
| <$25,000 | 220 | 0.06 ± 0.50 | 220 | 2.25 ± 3.91 | a | 220 | 0.21 ± 0.88 | |||||
| $25,000 ≤ $39,999 | 228 | 0.03 ± 0.29 | 228 | 1.58 ± 2.45 | b | 227 | 0.20 ± 0.50 | |||||
| $40,000 ≤ $59,999 | 242 | 0.08 ± 0.53 | 242 | 1.64 ± 2.76 | b | 241 | 0.26 ± 0.88 | |||||
| ≥$60,000 | 348 | 0.10 ± 0.44 | 348 | 1.33 ± 2.21 | b | 348 | 0.23 ± 0.65 | |||||
| Maternal WIC recipient status | 0.080 | <0.001 | 0.703 | |||||||||
| Non-recipient | 618 | 0.09 ± 0.48 | 618 | 1.39 ± 2.36 | a | 617 | 0.23 ± 0.73 | |||||
| Recipient | 420 | 0.04 ± 0.39 | 420 | 2.03 ± 3.39 | b | 419 | 0.21 ± 0.74 | |||||
| Region of residency | 0.350 | 0.705 | 0.379 | |||||||||
| New England | 52 | 0.04 ± 0.24 | a,b | 52 | 1.18 ± 2.79 | 51 | 0.39 ± 0.70 | a | ||||
| Middle Atlantic | 122 | 0.07 ± 0.33 | a,b | 122 | 1.79 ± 2.80 | 122 | 0.24 ± 0.56 | a,b | ||||
| East North Central | 225 | 0.06 ± 0.29 | a,b | 225 | 1.61 ± 2.55 | 225 | 0.21 ± 0.76 | a,b | ||||
| West North Central | 96 | 0.05 ± 0.37 | a,b | 96 | 1.44 ± 2.55 | 96 | 0.10 ± 0.38 | b | ||||
| South Atlantic | 153 | 0.13 ± 0.66 | a,b | 153 | 1.56 ± 2.89 | 153 | 0.23 ± 0.73 | a,b | ||||
| East South Central | 56 | 0.03 ± 0.16 | a,b | 56 | 1.61 ± 2.79 | 56 | 0.19 ± 0.48 | a,b | ||||
| West South Central | 116 | 0.06 ± 0.50 | a,b | 116 | 1.76 ± 3.02 | 115 | 0.25 ± 0.77 | a,b | ||||
| Mountain | 107 | 0.03 ± 0.20 | b | 107 | 1.59 ± 2.86 | 107 | 0.15 ± 0.52 | a,b | ||||
| Pacific | 111 | 0.16 ± 0.73 | a | 111 | 2.10 ± 3.42 | 111 | 0.32 ± 1.20 | a | ||||
| Maternal cigarette smoking during pregnancy | 0.095 | 0.850 | 0.328 | |||||||||
| No | 939 | 0.08 ± 0.47 | 939 | 1.65 ± 2.86 | 937 | 0.23 ± 0.76 | ||||||
| Yes | 93 | 0.00 ± 0.00 | 93 | 1.71 ± 2.75 | 93 | 0.15 ± 0.36 | ||||||
| Probability of Breastfeeding Initiation | |||
|---|---|---|---|
| Characteristics * | n (%) | Mean Difference ± SE ** | p-Value *** |
| Overall | 905 (87.1) | ||
| Maternal age, years | 0.27 ± 0.49 | 0.581 | |
| % of Federal poverty level | 34.35 ± 17.50 | 0.050 | |
| Pre-pregnancy BMI, kg/m2 | −1.64 ± 0.60 | 0.018 | |
| Maternal race/ethnicity | 0.088 | ||
| Non-Hispanic White | 762 (86.6) | ||
| Non-Hispanic Black | 32 (80.0) | ||
| Hispanic | 62 (93.9) | ||
| Non-Hispanic Asian/Pacific Islander/Other | 44 (93.6) | ||
| Maternal highest education level | <0.001 | ||
| High school or lower | 145 (77.5) | ||
| 1–3 years of college | 335 (86.3) | ||
| College graduate | 286 (92.6) | ||
| Postgraduate | 96 (91.4) | ||
| Maternal employment status | 0.183 | ||
| Unemployed | 310 (85.2) | ||
| Employed | 591 (88.1) | ||
| Household size | 0.122 | ||
| 1–2 people | 236 (90.8) | ||
| 3 people | 347 (86.8) | ||
| 4 people | 181 (83.4) | ||
| ≥5 people | 141 (87.0) | ||
| Annual household income level | 0.164 | ||
| <$25,000 | 182 (82.7) | ||
| $25,000 ≤ $39,999 | 200 (87.7) | ||
| $40,000 ≤ $59,999 | 213 (87.7) | ||
| ≥$60,000 | 310 (89.1) | ||
| Maternal WIC recipient status | 0.003 | ||
| Non-recipient | 555 (89.7) | ||
| Recipient | 350 (83.3) | ||
| Region of residency | 0.005 | ||
| New England | 44 (84.6) | ||
| Middle Atlantic | 99 (81.2) | ||
| East North Central | 192 (85.3) | ||
| West North Central | 84 (87.5) | ||
| South Atlantic | 139 (90.9) | ||
| East South Central | 46 (82.1) | ||
| West South Central | 97 (82.9) | ||
| Mountain | 95 (88.8) | ||
| Pacific | 109 (98.2) | ||
| Maternal cigarette smoking during pregnancy | 0.003 | ||
| No | 830 (88.3) | ||
| Yes | 72 (77.4) | ||
| Maternal Egg Consumption During Pregnancy | Probability of Breastfeeding Initiation | ||||
|---|---|---|---|---|---|
| n (%) | Crude OR (95% CI) | Crude p-Value * | Adjusted OR (95% CI) ** | Adjusted p-Value * | |
| Frequency of total egg consumption | |||||
| Never | 103 (81.1) | Reference | Reference | ||
| 1–3 times per month | 319 (86.5) | 1.49 (0.87–2.54) | 0.146 | 1.47 (0.82–2.62) | 0.195 |
| 1–2 times per week | 302 (86.3) | 1.47 (0.86–2.51) | 0.164 | 1.34 (0.75–2.39) | 0.327 |
| 3 times per week or more | 181 (93.8) | 3.51 (1.69–7.32) | <0.001 | 3.34 (1.51–7.39) | 0.003 |
| Amount of egg consumption, 1 egg/week increment | 1.11 (1.03–1.19) | 0.006 | 1.11 (1.03–1.20) | 0.005 | |
| Frequency of whole egg consumption | |||||
| Never | 141 (83.4) | Reference | Reference | ||
| 1–3 times per month | 327 (85.6) | 1.18 (0.72–1.94) | 0.512 | 1.25 (0.73–2.13) | 0.421 |
| 1 time per week | 135 (85.4) | 1.17 (0.64–2.12) | 0.617 | 0.99 (0.52–1.87) | 0.972 |
| 2 times per week or more | 301 (91.5) | 2.13 (1.22–3.74) | 0.008 | 2.21 (1.20–4.04) | 0.010 |
| Amount of whole egg consumption, 1 egg/week increment | 1.12 (1.03–1.21) | 0.006 | 1.13 (1.04–1.23) | 0.004 | |
| Frequency of egg white consumption | |||||
| Never | 836 (87.3) | Reference | Reference | ||
| 1–3 times per month | 39 (84.8) | 0.81 (0.36–1.86) | 0.624 | 0.42 (0.17–1.03) | 0.058 |
| 1 time per week or more | 29 (85.3) | 0.85 (0.32–2.23) | 0.736 | 0.49 (0.17–1.43) | 0.193 |
| Amount of egg white consumption, 1 egg/week increment | 1.01 (0.84–1.21) | 0.892 | 0.95 (0.79–1.13) | 0.550 | |
| Frequency of egg substitute consumption | |||||
| Never | 856 (87.1) | Reference | Reference | ||
| Ever consumption | 49 (87.5) | 1.04 (0.46–2.34) | 0.927 | 0.61 (0.25–1.46) | 0.266 |
| Amount of egg substitute consumption, 1 egg/week increment | 1.50 (0.72–3.14) | 0.283 | 1.09 (0.54–2.22) | 0.804 | |
| Frequency of eggs with fat consumption | |||||
| Never | 341 (86.8) | Reference | Reference | ||
| 1–3 times per month | 293 (86.4) | 0.97 (0.63–1.49) | 0.894 | 1.12 (0.70–1.77) | 0.643 |
| 1 time per week | 81 (81.8) | 0.69 (0.38–1.24) | 0.210 | 0.74 (0.39–1.39) | 0.347 |
| 2 times per week or more | 190 (91.4) | 1.61 (0.92–2.83) | 0.098 | 2.19 (1.16–4.13) | 0.015 |
| Amount of eggs with fat consumption, 1 egg/week increment | 1.04 (0.97–1.12) | 0.259 | 1.09 (1.00–1.19) | 0.041 | |
| Frequency of egg salad consumption | |||||
| Never | 708 (87.2) | Reference | Reference | ||
| 1–3 times per month | 165 (86.4) | 0.93 (0.59–1.48) | 0.766 | 1.04 (0.63–1.72) | 0.878 |
| 1 time per week or more | 30 (88.2) | 1.10 (0.38–3.19) | 0.858 | 1.07 (0.35–3.34) | 0.901 |
| Amount of egg salad consumption, 1 egg/week increment | 1.02 (0.79–1.32) | 0.865 | 1.03 (0.77–1.38) | 0.834 | |
| Maternal Egg Consumption During Pregnancy | Any Breastfeeding Duration, Weeks | |||||
|---|---|---|---|---|---|---|
| Sample Size | Mean ± SD | Crude Mean Difference (95% CI) | Crude p-Value * | Adjusted Mean Difference (95% CI) ** | Adjusted p-Value * | |
| Frequency of total egg consumption | ||||||
| Never | 102 | 19.35 ± 19.91 | Reference | Reference | ||
| 1–3 times per month | 301 | 26.93 ± 20.60 | 7.58 (2.98–12.18) | 0.001 | 5.80 (1.55–10.06) | 0.008 |
| 1–2 times per week | 270 | 27.76 ± 20.57 | 8.41 (3.74–13.08) | <0.001 | 6.48 (2.18–10.78) | 0.003 |
| 3 times per week or more | 154 | 27.82 ± 20.82 | 8.47 (3.34–13.60) | 0.001 | 5.16 (0.41–9.92) | 0.033 |
| Amount of total egg consumption, 1 egg/week increment | 0.18 (−0.23–0.58) | 0.396 | 0.13 (−0.25–0.51) | 0.497 | ||
| Frequency of whole egg consumption | ||||||
| Never | 136 | 21.35 ± 20.56 | Reference | Reference | ||
| 1–3 times per month | 310 | 26.84 ± 20.67 | 5.48 (1.34–9.62) | 0.009 | 4.80 (0.98–8.61) | 0.014 |
| 1 time per week | 121 | 29.18 ± 21.23 | 7.82 (2.79–12.86) | 0.002 | 6.41 (1.81–11.00) | 0.006 |
| 2 times per week or more | 260 | 27.33 ± 20.20 | 5.97 (1.71–10.23) | 0.006 | 4.64 (0.71–8.56) | 0.021 |
| Amount of whole egg consumption, 1 egg/week increment | 0.15 (−0.28–0.57) | 0.495 | 0.16 (−0.23–0.55) | 0.413 | ||
| Frequency of egg white consumption | ||||||
| Never | 761 | 26.37 ± 20.64 | Reference | Reference | ||
| 1–3 times per month | 39 | 29.32 ± 20.20 | 2.96 (−3.69–9.60) | 0.383 | −0.65 (−6.83–5.52) | 0.836 |
| 1 time per week or more | 26 | 22.97 ± 22.79 | −3.39 (−11.46–4.68) | 0.410 | −5.93 (−13.46–1.61) | 0.123 |
| Amount of egg white consumption, 1 egg/week increment | −0.35 (−1.91–1.22) | 0.666 | −0.90 (−2.41–0.60) | 0.240 | ||
| Frequency of egg substitute consumption | ||||||
| Never | 780 | 26.34 ± 20.59 | Reference | Reference | ||
| Ever consumption | 47 | 27.99 ± 22.41 | 1.66 (−4.43–7.74) | 0.593 | −2.74 (−8.50–3.02) | 0.351 |
| Amount of egg substitute consumption, 1 egg/week increment | 2.72 (−0.21–5.65) | 0.069 | −0.05 (−2.85–2.74) | 0.971 | ||
| Frequency of eggs with fat consumption | ||||||
| Never | 314 | 26.00 ± 20.92 | Reference | Reference | ||
| 1–3 times per month | 272 | 27.77 ± 21.15 | 1.77 (−1.59–5.12) | 0.302 | 3.51 (0.37–6.66) | 0.029 |
| 1 time per week | 78 | 25.59 ± 20.04 | −0.42 (−5.54–4.71) | 0.874 | 1.70 (−3.01–6.41) | 0.479 |
| 2 times per week or more | 163 | 25.42 ± 19.82 | −0.58 (−4.49–3.33) | 0.770 | 1.51 (−2.19–5.21) | 0.423 |
| Amount of eggs with fat consumption, 1 egg/week increment | −0.27 (−0.76–0.21) | 0.274 | 0.02 (−0.44–0.48) | 0.938 | ||
| Frequency of egg salad consumption | ||||||
| Never | 660 | 26.28 ± 20.84 | Reference | Reference | ||
| 1–3 times per month | 139 | 26.19 ± 20.21 | −0.10 (−3.88–3.68) | 0.959 | 0.89 (−2.62–4.39) | 0.620 |
| 1 time per week or more | 26 | 31.28 ± 19.85 | 4.99 (−3.11–13.09) | 0.227 | 5.98 (−1.55–13.50) | 0.120 |
| Amount of egg salad consumption, 1 egg/week increment | 0.95 (−1.09–2.99) | 0.363 | 1.18 (−0.70–3.07) | 0.219 | ||
| Maternal Egg Consumption During Pregnancy | Exclusive Breastfeeding Duration, Weeks | |||||
|---|---|---|---|---|---|---|
| Sample Size | Mean ± SD | Crude Mean Difference (95% CI) | Crude p-Value * | Adjusted Mean Difference (95% CI) ** | Adjusted p-Value * | |
| Frequency of total egg consumption | ||||||
| Never | 102 | 3.64 ± 7.29 | Reference | Reference | ||
| 1–3 times per month | 301 | 5.69 ± 8.99 | 2.06 (−0.03–4.14) | 0.053 | 1.51 (−0.48–3.51) | 0.137 |
| 1–2 times per week | 265 | 7.25 ± 9.83 | 3.61 (1.50–5.73) | 0.001 | 2.91 (0.89–4.93) | 0.005 |
| 3 times per week or more | 153 | 7.69 ± 10.10 | 4.05 (1.73–6.38) | 0.001 | 2.84 (0.61–5.07) | 0.013 |
| Amount of total egg consumption, 1 egg/week increment | 0.13 (−0.05–0.32) | 0.162 | 0.12 (−0.06–0.30) | 0.193 | ||
| Frequency of whole egg consumption | ||||||
| Never | 136 | 4.26 ± 7.93 | Reference | Reference | ||
| 1–3 times per month | 310 | 5.94 ± 9.08 | 1.69 (−0.19–3.56) | 0.078 | 1.65 (−0.14–3.44) | 0.070 |
| 1 time per week | 119 | 7.88 ± 9.99 | 3.63 (1.34–5.91) | 0.002 | 3.21 (1.05–5.38) | 0.004 |
| 2 times per week or more | 256 | 7.13 ± 9.93 | 2.87 (0.94–4.81) | 0.004 | 2.66 (0.82–4.51) | 0.005 |
| Amount of whole egg consumption, 1 egg/week increment | 0.10 (−0.09–0.30) | 0.288 | 0.12 (−0.06–0.31) | 0.194 | ||
| Frequency of egg white consumption | ||||||
| Never | 755 | 6.32 ± 9.38 | Reference | Reference | ||
| 1–3 times per month | 39 | 5.97 ± 8.74 | −0.35 (−3.36–2.67) | 0.822 | −2.26 (−5.16–0.64) | 0.126 |
| 1 time per week or more | 26 | 6.18 ± 10.15 | −0.14 (−3.80–3.52) | 0.940 | −1.15 (−4.69–2.39) | 0.524 |
| Amount of egg white consumption, 1 egg/week increment | 0.01 (−0.70–0.72) | 0.983 | −0.24 (−0.95–0.46) | 0.500 | ||
| Frequency of egg substitute consumption | ||||||
| Never | 774 | 6.27 ± 9.28 | Reference | Reference | ||
| Ever consumption | 47 | 7.09 ± 10.81 | 0.83 (−1.93–3.58) | 0.557 | −0.93 (−3.64–1.77) | 0.499 |
| Amount of egg substitute consumption, 1 egg/week increment | 1.81 (0.49–3.14) | 0.007 | 0.72 (−0.60–2.03) | 0.285 | ||
| Frequency of eggs with fat consumption | ||||||
| Never | 311 | 5.95 ± 9.09 | Reference | Reference | ||
| 1–3 times per month | 272 | 6.49 ± 9.49 | 0.54 (−0.99–2.06) | 0.490 | 1.31 (−0.17–2.79) | 0.082 |
| 1 time per week | 78 | 5.34 ± 8.52 | −0.61 (−2.93–1.71) | 0.605 | −0.11 (−2.32–2.10) | 0.922 |
| 2 times per week or more | 160 | 7.21 ± 10.07 | 1.26 (−0.52–3.04) | 0.166 | 2.06 (0.31–3.81) | 0.021 |
| Amount of eggs with fat consumption, 1 egg/week increment | −0.05 (−0.27–0.17) | 0.635 | 0.04 (−0.17–0.26) | 0.708 | ||
| Frequency of egg salad consumption | ||||||
| Never | 657 | 6.27 ± 9.42 | Reference | Reference | ||
| 1–3 times per month | 136 | 6.24 ± 9.13 | −0.02 (−1.75–1.71) | 0.979 | 0.20 (−1.46–1.86) | 0.813 |
| 1 time per week or more | 26 | 7.88 ± 9.75 | 1.61 (−2.06–5.28) | 0.390 | 2.76 (−0.77–6.30) | 0.125 |
| Amount of egg salad consumption, 1 egg/week increment | 0.38 (−0.55–1.30) | 0.426 | 0.61 (−0.28–1.49) | 0.180 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Mohammed, F.; Giancarlo, E.M.; Botchway, A.; Albert-Ducasse, D.; Ritchie, I.; Rideout, T.C. Association Between Maternal Egg Consumption During Pregnancy and Breastfeeding Initiation and Duration. Nutrients 2025, 17, 3710. https://doi.org/10.3390/nu17233710
Wen X, Mohammed F, Giancarlo EM, Botchway A, Albert-Ducasse D, Ritchie I, Rideout TC. Association Between Maternal Egg Consumption During Pregnancy and Breastfeeding Initiation and Duration. Nutrients. 2025; 17(23):3710. https://doi.org/10.3390/nu17233710
Chicago/Turabian StyleWen, Xiaozhong, Fatima Mohammed, Eve M. Giancarlo, Andrea Botchway, Daphkar Albert-Ducasse, Isabella Ritchie, and Todd C. Rideout. 2025. "Association Between Maternal Egg Consumption During Pregnancy and Breastfeeding Initiation and Duration" Nutrients 17, no. 23: 3710. https://doi.org/10.3390/nu17233710
APA StyleWen, X., Mohammed, F., Giancarlo, E. M., Botchway, A., Albert-Ducasse, D., Ritchie, I., & Rideout, T. C. (2025). Association Between Maternal Egg Consumption During Pregnancy and Breastfeeding Initiation and Duration. Nutrients, 17(23), 3710. https://doi.org/10.3390/nu17233710






