Prognostic Value of Resting Energy Expenditure Measured by Indirect Calorimetry in Patients with Cirrhosis Referred for Liver Transplantation †
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
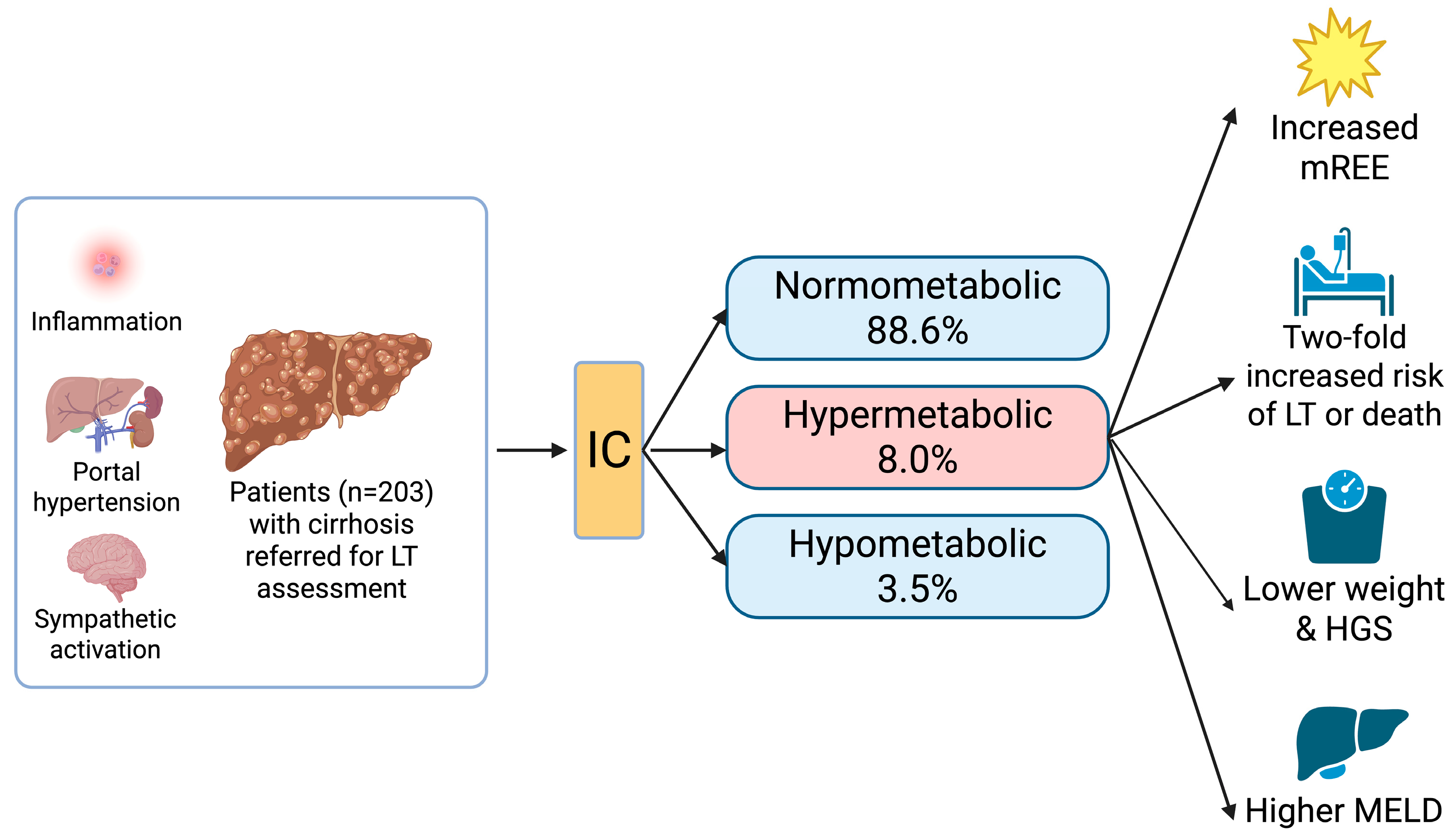
3. Results
3.1. Patient Characteristics
3.2. Calorimetry
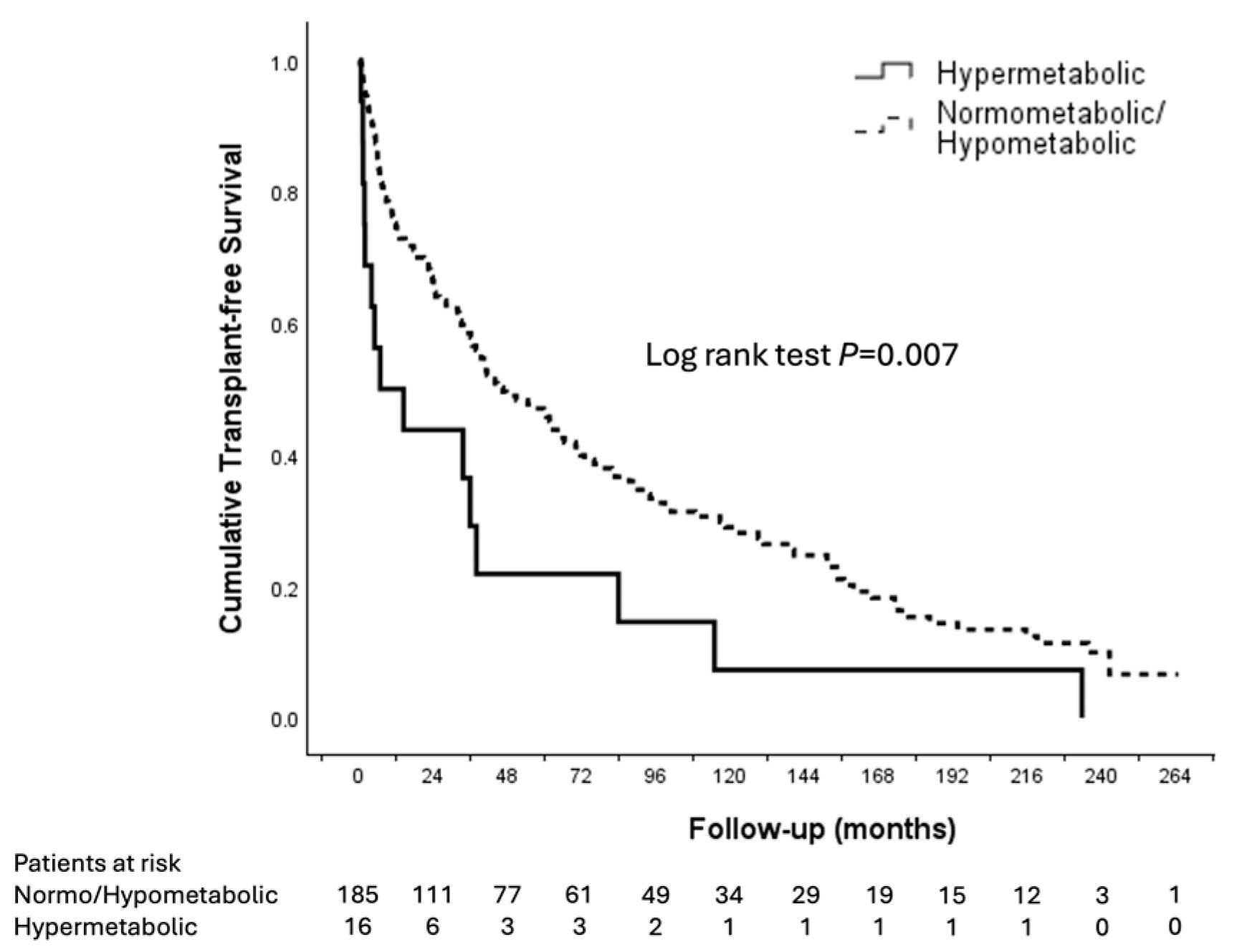
3.3. Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| HCC | Hepatocellular carcinoma |
| HGS | Handgrip strength |
| HR | Hazard ratio |
| IC | Indirect calorimetry |
| IQR | Interquartile range |
| LT | Liver transplantation |
| MAC | Mid-arm circumference |
| MAMC | Mid-arm muscle circumference |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MELD | Model for End-Stage Liver Disease |
| mREE | Measured resting energy expenditure |
| pREE | Predicted resting energy expenditure |
| REE | Resting energy expenditure |
| SD | Standard deviation |
| SGA | Subjective Global Assessment |
| TSFT | Tricep skin fold thickness |
| VIF | Variance inflation factor |
| VCO2 | Coefficient variation of carbon dioxide production |
| VO2 | Coefficient variation of oxygen production |
References
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125. [Google Scholar] [CrossRef]
- Chapman, B.; Goh, S.K.; Parker, F.; Romero, S.; Sinclair, M.; Gow, P.; Ma, R.; Angus, P.; Jones, R.; Luke, J.; et al. Malnutrition and low muscle strength are independent predictors of clinical outcomes and healthcare costs after liver transplant. Clin. Nutr. ESPEN 2022, 48, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Duong, N.; Sadowski, B.; Rangnekar, A.S. The Impact of Frailty, Sarcopenia, and Malnutrition on Liver Transplant Outcomes. Clin. Liver Dis. 2021, 17, 271–276. [Google Scholar] [CrossRef]
- Kalafateli, M.; Mantzoukis, K.; Choi Yau, Y.; Mohammad, A.O.; Arora, S.; Rodrigues, S.; de Vos, M.; Papadimitriou, K.; Thorburn, D.; O’Beirne, J.; et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J. Cachexia Sarcopenia Muscle 2017, 8, 113–121. [Google Scholar] [CrossRef]
- Sanchez, A.J.; Aranda-Michel, J. Nutrition for the liver transplant patient. Liver Transpl. 2006, 12, 1310–1316. [Google Scholar] [CrossRef]
- Madden, A.M.; Morgan, M.Y. Resting energy expenditure should be measured in patients with cirrhosis, not predicted. Hepatology 1999, 30, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.R.; Amaral, T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty—A systematic review. BMC Geriatr. 2017, 17, 238. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Anastacio, L.R.; Lima, A.S.; Correia, M.I. Malnutrition and inadequate food intake of patients in the waiting list for liver transplant. Rev. Assoc. Med. Bras. 2009, 55, 389–393. [Google Scholar] [CrossRef]
- Laish, I.; Braun, M.; Mor, E.; Sulkes, J.; Harif, Y.; Ben Ari, Z. Metabolic syndrome in liver transplant recipients: Prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011, 17, 15–22. [Google Scholar] [CrossRef]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef]
- Chapman, B.; Testro, A.; Gow, P.; Whitcher, B.; Sinclair, M. Determining Energy Requirements in Cirrhosis: An Update on the Role of Indirect Calorimetry. Curr. Hepatol. Rep. 2021, 20, 85–95. [Google Scholar] [CrossRef]
- Dolz, C.; Raurich, J.M.; Ibáñez, J.; Obrador, A.; Marsé, P.; Gayá, J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology 1991, 100, 738–744. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Z.; Wu, S.; Li, L.; Li, J.; Zhang, Y.; Chen, B.; Sun, X.; Sun, C.; Wu, L. Screening and assessment of malnutrition in patients with liver cirrhosis. Front. Nutr. 2024, 11, 1398690. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect calorimetry: The 6 main issues. Clin. Nutr. 2021, 40, 4–14. [Google Scholar] [CrossRef]
- Merli, M.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.J.; Jung, Y.J.; Han, M.; Lee, S.G.; Hong, S.K. Comparison of Measured Energy Expenditure Using Indirect Calorimetry vs Predictive Equations for Liver Transplant Recipients. JPEN J. Parenter. Enteral Nutr. 2021, 45, 761–767. [Google Scholar] [CrossRef]
- Muller, M.J.; Loyal, S.; Schwarze, M.; Lobers, J.; Selberg, O.; Ringe, B.; Pichlmayr, R. Resting energy expenditure and nutritional state in patients with liver cirrhosis before and after liver transplantation. Clin. Nutr. 1994, 13, 145–152. [Google Scholar] [CrossRef]
- Singhvi, A.; Sadowsky, H.S.; Cohen, A.; Demzik, A.; VanWagner, L.; Rinella, M.; Levitsky, J. Resting and Exercise Energy Metabolism After Liver Transplantation for Nonalcoholic Steatohepatitis. Transplant. Direct 2017, 3, e188. [Google Scholar] [CrossRef]
- Ngu, N. Figure 1. BioRender. 2025. Available online: https://BioRender.com/drh96tp (accessed on 18 November 2025).
- Blond, E.; Maitrepierre, C.; Normand, S.; Sothier, M.; Roth, H.; Goudable, J.; Laville, M. A new indirect calorimeter is accurate and reliable for measuring basal energy expenditure, thermic effect of food and substrate oxidation in obese and healthy subjects. ESPEN J. 2011, 6, e7–e15. [Google Scholar]
- Fadeur, M.; Kaux, J.F.; De Flines, J.; Misset, B.; Paquot, N.; Rousseau, A.F. Indirect calorimetry in canopy mode in healthy subjects: Performances of the Q-NRG device compared to the Deltatrac II. Acta Gastro-Enterol. Belg. 2025, 88, 13–17. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Mathur, S.; Peng, S.; Gane, E.J.; McCall, J.L.; Plank, L.D. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition 2007, 23, 398–403. [Google Scholar] [CrossRef]
- Chapman, B.; Sinclair, M.; Gow, P.J.; Testro, A.G. Malnutrition in cirrhosis: More food for thought. World J. Hepatol. 2020, 12, 883–896. [Google Scholar] [CrossRef]
- Müller, M.J.; Böttcher, J.; Selberg, O.; Weselmann, S.; Böker, K.H.; Schwarze, M.; von zur Mühlen, A.; Manns, M.P. Hypermetabolism in clinically stable patients with liver cirrhosis. Am. J. Clin. Nutr. 1999, 69, 1194–1201. [Google Scholar] [CrossRef]
- Knapp, G.; Ngu, N.; Vidot, H.; Craik, J.; Liu, K. Prognostic value of measuring resting energy expenditure by indirect calorimetry in cirrhosis patients referred for liver transplantation. In Proceedings of the World Congress of Gastroenterology, Melbourne, Australia, 19–22 September 2025. [Google Scholar]
- Ferreira, L.G.; Santos, L.F.; Silva, T.R.; Anastácio, L.R.; Lima, A.S.; Correia, M.I. Hyper- and hypometabolism are not related to nutritional status of patients on the waiting list for liver transplantation. Clin. Nutr. 2014, 33, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Frías, C.; Conchillo, M.; Payeras, M.; Iñarrairaegui, M.; Davola, D.; Frühbeck, G.; Salvador, J.; Rodríguez, M.; Richter, J.; Mugueta, C.; et al. Factors related to increased resting energy expenditure in men with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 139–145. [Google Scholar] [CrossRef]
- Peng, S.; Plank, L.D.; McCall, J.L.; Gillanders, L.K.; McIlroy, K.; Gane, E.J. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: A comprehensive study. Am. J. Clin. Nutr. 2007, 85, 1257–1266. [Google Scholar] [CrossRef]
- Knudsen, A.W.; Krag, A.; Nordgaard-Lassen, I.; Frandsen, E.; Tofteng, F.; Mortensen, C.; Becker, U. Effect of paracentesis on metabolic activity in patients with advanced cirrhosis and ascites. Scand. J. Gastroenterol. 2016, 51, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Prabhu, R.; Thomas, S.; Reddy, J.B.; Pulimood, A.; Balasubramanian, K.A. Intestinal mucosal alterations in experimental cirrhosis in the rat: Role of oxygen free radicals. Hepatology 2002, 35, 622–629. [Google Scholar] [CrossRef]
- Müller, M.J.; Lautz, H.U.; Plogmann, B.; Bürger, M.; Körber, J.; Schmidt, F.W. Energy expenditure and substrate oxidation in patients with cirrhosis: The impact of cause, clinical staging and nutritional state. Hepatology 1992, 15, 782–794. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Santos, L.F.; Anastacio, L.R.; Lima, A.S.; Correia, M.I. Resting energy expenditure, body composition, and dietary intake: A longitudinal study before and after liver transplantation. Transplantation 2013, 96, 579–585. [Google Scholar] [CrossRef]
- Santos, B.C.; Correia, M.I.T.D.; Anastácio, L.R. Energy Expenditure and Liver Transplantation: What We Know and Where We Are. JPEN J. Parenter. Enteral Nutr. 2021, 45, 456–464. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J. Indications and limitations of the use of subjective global assessment in clinical practice: An update. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, Y.; Feng, Y.; Wang, R.; Yao, N.; Zhang, M.; Liu, X.; Liu, H.; Shi, L.; Zhu, L.; et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br. J. Nutr. 2020, 124, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, B.C.; Srivastava, S. Malnutrition in cirrhosis increases morbidity and mortality. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar] [CrossRef]
- Reeves, M.M.; Capra, S. Predicting energy requirements in the clinical setting: Are current methods evidence based? Nutr. Rev. 2003, 61, 143–151. [Google Scholar] [CrossRef]
- Reid, C.L. Poor agreement between continuous measurements of energy expenditure and routinely used prediction equations in intensive care unit patients. Clin. Nutr. 2007, 26, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Jackson, C.D.; Eslamparast, T.; Yamanaka-Okumura, H.; Plank, L.D.; Henry, C.J.; Madden, A.M.; Ferreira, L.G.; Kalaitzakis, E.; Prieto de Frías, C.; et al. Predicted estimates of resting energy expenditure have limited clinical utility in patients with cirrhosis. J. Hepatol. 2022, 77, 98–107. [Google Scholar] [CrossRef]
- Eslamparast, T.; Vandermeer, B.; Raman, M.; Gramlich, L.; Den Heyer, V.; Belland, D.; Ma, M.; Tandon, P. Are Predictive Energy Expenditure Equations Accurate in Cirrhosis? Nutrients 2019, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.; Hipskind, P.; Cole, D.; Lopez, R.; Dasarathy, S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: A prospective study. Nutr. Clin. Pract. 2012, 27, 677–688. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Result n (%) or Median (IQR) |
|---|---|
| Male sex | 150 (73.9) |
| Median age (years) | 55 (49–60) |
| Median height (m) | 1.72 (1.67–1.78) |
| Median weight (kg) | 79 (69–93) |
| Median BMI (kg/m2) | 27.0 (23.7–31.1) |
| Cirrhosis cause | |
| 82 (40.4) |
| 62 (30.5) |
| 33 (16.3) |
| 18 (8.9) |
| 8 (3.9) |
| Concomitant HCC | 39 (19.2) |
| Median MELD score | 14 (11–17) |
| Ascites | 90 (44.3) |
| Encephalopathy | 73 (36.0) |
| Subjective Global Assessment | |
| 55 (28.4) |
| 103 (53.1) |
| 36 (18.6) |
| Median mid-arm circumference (cm) | 29.5 (26.5–34.6) |
| Median mid-arm muscle circumference (cm) | 25.5 (22.7–28.1) |
| Grip strength (kg/mmHg) | |
| 27.0 (20.7–33.3) |
| 29.2 (22.5–36.7) |
| Tricep skin fold thickness (mm) | 12.8 (8.6–22.1) |
| Predicted resting energy expenditure (kcal/day) | 1652 (1459–1873) |
| Measured resting energy expenditure (kcal/day) | 1708 (1490–1907) |
| Metabolism | |
| 178 (88.6) |
| 7 (3.5) |
| 16 (8.0) |
| Variable | Spearman Rho | p |
|---|---|---|
| Age | −0.209 | 0.003 |
| Height | 0.574 | <0.001 |
| Weight | 0.645 | <0.001 |
| BMI | 0.431 | <0.001 |
| MAC | 0.458 | <0.001 |
| MAMC | 0.500 | 0.002 |
| Grip strength L | 0.498 | <0.001 |
| Grip strength R | 0.509 | <0.001 |
| TSFT | 0.172 | 0.015 |
| Characteristic | Normometabolic n = 178 | Hypometabolic n = 7 | Hypermetabolic n = 16 | p * |
|---|---|---|---|---|
| Male sex (%) | 138 (77.5) | 6 (85.7) | 6 (37.5) | 0.002 a |
| Age (year) | 55 (48–60) | 55 (53–57) | 56 (52–66) | 0.311 |
| Height (m) | 1.74 (1.67–1.79) | 1.73 (1.65–1.75) | 1.67 (1.60–1.75) | 0.078 |
| Weight (kg) | 80.0 (69.4–92.5) | 101.7 (70.0–107.4) | 74.5 (60.0–78.5) | 0.036 |
| BMI (kg/m2) | 26.9 (23.8–31.0) | 32.5 (25.7–36.3) | 26.3 (23.0–28.9) | 0.112 |
| MELD | 14 (11–17) | 13 (12–15) | 20 (13–24) | 0.051 b |
| Concomitant HCC (%) | 37 (20.6) | 0 (0) | 2 (12.5) | 0.311 |
| Ascites (%) | 97 (54.2) | 3 (42.9) | 10 (62.5) | 0.896 |
| Encephalopathy (%) | 63 (35.2) | 5 (71.4) | 4 (28.6) | 0.123 |
| MAC (cm) | 29.8 (26.6–35.0) | 35.0 (27.9–38.5) | 27.0 (25.0–29.3) | 0.008 c |
| MAMC (cm) | 25.6 (22.7–28.2) | 25.2 (23.6–30.2) | 23.5 (21.4–25.6) | 0.059 |
| TSFT (mm) | 12.8 (8.7–22.9) | 21.5 (13.6–29.3) | 9.9 (7.7–15.0) | 0.033 |
| Grip strength L | 28.0 (22.0–38.5) | 25.7 (19.3–32.0) | 19.7 (16.9–24.9) | 0.010 d |
| Grip strength R | 30.3 (23.3–36.7) | 26.3 (13.6–29.3) | 24.9 (17.1–27.0) | 0.021 e |
| SGA A B C | 52 (29.9) 91 (52.3) 31 (17.8) | 2 (28.6) 5 (71.4) 0 (0) | 2 (13.3) 8 (53.3) 5 (33.3) | 0.294 |
| Predicted REE (kcal/day) | 1662 (1471–1888) | 1967 (1503–2100) | 1418 (1342–1652) | 0.004 f |
| Measured REE (kcal/day) | 1708 (1500–1903) | 1404 (1183–1540) | 1822 (1658–2062) | 0.001 g |
| Difference between predicted and measured REE (kcal/day) | 21 (−128–146) | −496 (−562–−320) | 352 (320–457) | <0.001 h |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | aHR | 95% CI | p-Value | |
| Male sex (vs. female) | 1.349 | 0.932–1.952 | 0.112 | 1.072 | 0.614–1.873 | 0.806 |
| HCC (yes vs. no) | 1.341 | 0.92–1.938 | 0.118 | 1.685 | 1.125–2.523 | 0.011 |
| MELD (per point increase) | 1.065 | 1.039–1.091 | <0.001 | 1.068 | 1.040–1.098 | <0.001 |
| MAC (per cm increase) | 0.998 | 0.995–1.001 | 0.181 | 1.004 | 0.996–1.013 | 0.329 |
| MAMC (per cm increase) | 0.996 | 0.992–0.999 | 0.020 | 0.995 | 0.991–0.998 | 0.003 |
| TSFT (per mm increase) | 0.985 | 0.967–1.003 | 0.097 | 0.991 | 0.960–1.024 | 0.598 |
| SGA | ||||||
| A (Ref) | 1 | 1 | ||||
| B | 1.403 | 0.962–2.045 | 0.079 | 1.356 | 0.866–2.125 | 0.183 |
| C | 1.663 | 1.010–2.738 | 0.045 | 1.394 | 0.765–2.539 | 0.278 |
| REE measured (per 100 kcal/day increase) | 1.053 | 0.999–1.109 | 0.055 | 1.081 | 1.017–1.148 | 0.013 |
| Metabolism | ||||||
| Normometabolic (Ref) | 1 | 1 | ||||
| Hypometabolic | 1.258 | 0.553–2.860 | 0.584 | 2.204 | 0.917–5.302 | 0.078 |
| Hypermetabolic | 2.070 | 1.211–3.537 | 0.008 | 2.113 | 1.161–3.845 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngu, N.L.Y.; Knapp, G.; Vidot, H.; Craik, J.; Jacob, R.; Strasser, S.I.; McCaughan, G.W.; Liu, K. Prognostic Value of Resting Energy Expenditure Measured by Indirect Calorimetry in Patients with Cirrhosis Referred for Liver Transplantation. Nutrients 2025, 17, 3709. https://doi.org/10.3390/nu17233709
Ngu NLY, Knapp G, Vidot H, Craik J, Jacob R, Strasser SI, McCaughan GW, Liu K. Prognostic Value of Resting Energy Expenditure Measured by Indirect Calorimetry in Patients with Cirrhosis Referred for Liver Transplantation. Nutrients. 2025; 17(23):3709. https://doi.org/10.3390/nu17233709
Chicago/Turabian StyleNgu, Natalie L. Y., Georgia Knapp, Helen Vidot, Joanne Craik, Rachael Jacob, Simone I. Strasser, Geoffrey W. McCaughan, and Ken Liu. 2025. "Prognostic Value of Resting Energy Expenditure Measured by Indirect Calorimetry in Patients with Cirrhosis Referred for Liver Transplantation" Nutrients 17, no. 23: 3709. https://doi.org/10.3390/nu17233709
APA StyleNgu, N. L. Y., Knapp, G., Vidot, H., Craik, J., Jacob, R., Strasser, S. I., McCaughan, G. W., & Liu, K. (2025). Prognostic Value of Resting Energy Expenditure Measured by Indirect Calorimetry in Patients with Cirrhosis Referred for Liver Transplantation. Nutrients, 17(23), 3709. https://doi.org/10.3390/nu17233709






