Consumption of Unprocessed and Ultraprocessed Foods in Adolescents with Obesity: Associations with Neuroendocrine Mediators of Appetite Regulation and Binge Eating Symptoms
Abstract
1. Introduction
2. Materials and Methods
3. Results
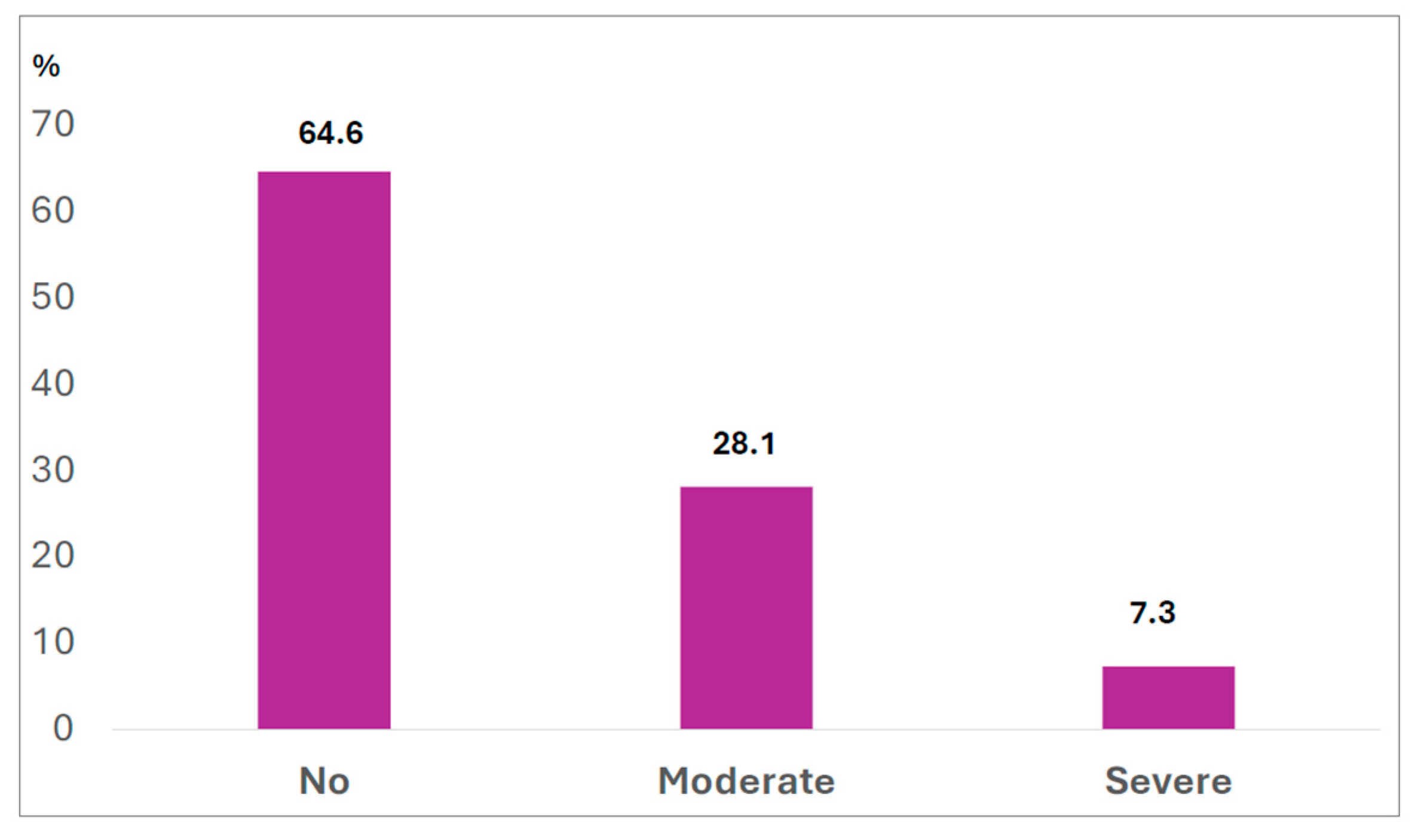
3.1. Binge Eating Symptoms
3.2. Comparison of Adolescents According to Tertiles of Unprocessed and Ultraprocessed Food
3.3. Predictors of Ultraprocessed and Unprocessed/Minimally Consumption Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgRP | Agouti-related peptide |
| α-MSH | Alpha-melanocyte-stimulating hormone |
| MCH | Melanin-concentrating hormone |
| NPY | Neuropeptide Y |
| BES | Binge eating scale |
| BMI | Body mass index |
| FFQ | Food frequency questionary |
References
- World Health Organization. Obesity and Overweight. WHO: Geneva, Switzerland, 2024. Available online: https://www.who.int/health-topics/obesity (accessed on 29 August 2025).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A Pooled Analysis of 3663 Population-Representative Studies with 222 Million Children, Adolescents, and Adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Matos, R.A.; Adams, M.; Sabaté, J. Review: The Consumption of Ultra-Processed Foods and Non-Communicable Diseases in Latin America. Front. Nutr. 2021, 8, 622714. [Google Scholar] [CrossRef]
- Carvalho-Ferreira, J.P.; Finlayson, G.; da Cunha, D.T.; Caldas, G.; Bandoni, D.; de Rosso, V.V. Adiposity and Binge Eating Are Related to Liking and Wanting for Food in Brazil: A Cultural Adaptation of the Leeds Food Preference Questionnaire. Appetite 2019, 133, 174–183. [Google Scholar] [CrossRef]
- Rathi, N.; Worsley, A.; Bruening, M. Perceived Influences of Fruit and Vegetable Consumption among Indian Adolescents—A Qualitative Inquiry. BMC Public Health 2025, 25, 271. [Google Scholar] [CrossRef]
- Chavez-Ugalde, I.Y.; de Vocht, F.; Jago, R.; Adams, J.; Ong, K.K.; Forouhi, N.G.; Colombet, Z.; Ricardo, L.I.C.; van Sluijs, E.; Toumpakari, Z. Ultra-Processed Food Consumption in UK Adolescents: Distribution, Trends, and Sociodemographic Correlates. Eur. J. Nutr. 2024, 63, 2709–2723. [Google Scholar] [CrossRef]
- Williams, A.M.; Couch, C.A.; Emmerich, S.E.; Ogburn, D.F. Ultra-Processed Food Consumption in Youth and Adults: United States, August 2021–August 2023. NCHS Data Brief 2025, 536, 1–11. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The Physiological Control of Eating: Signals, Neurons, and Networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef] [PubMed]
- Dâmaso, A.R.; Masquio, D.C.L.; Campos, R.M.D.S.; Corgosinho, F.C.; Cercato, C. Effects of Multidisciplinary Therapy on Energy Balance, Inflammation, and Metabolic Diseases in Adolescents with Obesity: A Narrative Review. Ann. N. Y. Acad. Sci. 2024, 1542, 25–50. [Google Scholar] [CrossRef]
- van Galen, K.A.; ter Horst, K.W.; Serlie, M.J. Serotonin, Food Intake, and Obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef] [PubMed]
- Via, E.; Contreras-Rodríguez, O. Binge-Eating Precursors in Children and Adolescents: Neurodevelopment, and the Potential Contribution of Ultraprocessed Foods. Nutrients 2023, 15, 2994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, L.; Liu, Z.; Cao, B. Emotion Regulation Difficulties and Disordered Eating in Adolescents and Young Adults: A Meta-Analysis. J. Eat. Disord. 2025, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Ayton, A.; Ibrahim, A.; Dugan, J.; Galvin, E.; Wright, O.W. Ultra-Processed Foods and Binge Eating: A Retrospective Observational Study. Nutrition 2021, 84, 111023. [Google Scholar] [CrossRef]
- Formisano, A.; Dello Russo, M.; Lissner, L.; Russo, P.; Ahrens, W.; De Henauw, S.; Hebestreit, A.; Intemann, T.; Hunsberger, M.; Molnár, D.; et al. Ultra-Processed Foods Consumption and Metabolic Syndrome in European Children, Adolescents, and Adults: Results from the I.Family Study. Nutrients 2025, 17, 2252. [Google Scholar] [CrossRef]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-Processed Foods and Obesity and Adiposity Parameters among Children and Adolescents: A Systematic Review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultraprocessed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 11 2002, 246, 1–203. Available online: https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf (accessed on 10 August 2025).
- Tanner, J.M. Growth at Adolescence, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1962; Available online: https://archive.org/details/growthatadolesce0000jmta/page/n5/mode/2up (accessed on 10 August 2025).
- Brazilian Society of Pediatrics. Avaliação Nutricional da Criança e do Adolescente: Manual de Orientação, 2nd ed.; Scientific Department of Nutrology, Brazilian Society of Pediatrics: São Paulo, Brazil, 2021. [Google Scholar]
- Bijlsma, A.; van Beijsterveldt, I.A.L.P.; Vermeulen, M.J.; Beunders, V.A.A.; Dorrepaal, D.J.; Boeters, S.C.M.; van den Akker, E.L.T.; Vlug, L.E.; de Koning, B.A.E.; Bracké, K.F.M.; et al. Challenges in Body Composition Assessment Using Air-Displacement Plethysmography by BOD POD in Pediatric and Young Adult Patients. Clin. Nutr. 2023, 42, 1588–1594. [Google Scholar] [CrossRef]
- Zieff, G.; Cornwall, J.; Blue, M.N.; Smith-Ryan, A.E.; Stoner, L. Ultrasound-Based Measurement of Central Adiposity: Key Considerations and Guidelines. Obes. Rev. 2024, 25, e13716. [Google Scholar] [CrossRef]
- Gutin, B.; Ramsey, L.; Barbeau, P.; Cannady, W.; Ferguson, M.; Litaker, M.; Owens, S. Plasma Leptin Concentrations in Obese Children: Changes during 4-mo Periods With and Without Physical Training. Am. J. Clin. Nutr. 1999, 69, 388–394. [Google Scholar] [CrossRef]
- Freitas, S.; Lopes, C.; Coutinho, W.; Appolinario, J.C. Tradução e adaptação para o português da Escala de Compulsão Alimentar Periódica. Braz. J. Psychiatry 2001, 23, 215–220. [Google Scholar] [CrossRef]
- Slater, B.; Philippi, S.T.; Marchioni, D.M.L.; Fisberg, R.M. Validação de Questionários de Freqüência Alimentar—QFA: Considerações metodológicas. Rev. Bras. Epidemiol. 2003, 6, 200–208. [Google Scholar] [CrossRef]
- Marchioni, D.M.L.; Voci, S.M.; de Lima, F.E.L.; Fisberg, R.M.; Slater, B. Reproducibility of a food frequency questionnaire for adolescents. Cad Saude Publica 2007, 23, 2187–2196. [Google Scholar] [CrossRef]
- Voci, S.M.; Enes, C.C.; Slater, B. Validação do Questionário de Frequência Alimentar para Adolescentes (QFAA) por grupos de alimentos em uma população de escolares. Rev. Bras. Epidemiol. 2008, 11, 561–572. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef]
- Martinez-Steele, E.; Khandpur, N.; Batis, C.; Bes-Rastrollo, M.; Bonaccio, M.; Cediel, G.; Huybrechts, I.; Juul, F.; Levy, R.B.; da Costa Louzada, M.L.; et al. Best Practices for Applying the NOVA Food Classification System. Nat. Food 2023, 4, 445–448. [Google Scholar] [CrossRef]
- Fornes, N.S.; Martins, I.S.; Velasquez-Melendez, G.; Latorre Mdo, R. Escores de consumo alimentar e níveis lipêmicos em população de São Paulo, Brasil. Rev. Saude Publica 2002, 36, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Barbosa, L.B.; Vasconcelos, S.M.L. Studies assessing food consumption by the scores method: A systematic review. Cienc. Saude Colet. 2019, 24, 1777–1792. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Button, A.M.; Tate, C.M.; Kracht, C.L.; Champagne, C.M.; Staiano, A.E. Adolescent Diet Quality, Cardiometabolic Risk, and Adiposity: A Prospective Cohort. J. Nutr. Educ. Behav. 2023, 55, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Kowalkowska, J.; Hamulka, J.; Wadolowska, L.; Górnicka, M.; Czarniecka-Skubina, E.; Gutkowska, K. Restrained Eating and Disinhibited Eating: Association with Diet Quality and Body Weight Status among Adolescents. Nutrients 2024, 16, 3601. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, E.M.; Botticelli, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Cifani, C.; Di Bonaventura, M.V.M. Assessing the role of ghrelin and the enzyme ghrelin O-acyltransferase (GOAT) system in food reward, food motivation, and binge eating behavior. Pharmacol. Res. 2021, 172, 105847. [Google Scholar] [CrossRef] [PubMed]
- De Ruyter, T.; Martens, D.S.; Bijnens, E.M.; De Henauw, S.; Nawrot, T.S.; Michels, N. Exploring the Impact of Lifestyle and Environmental Exposures on Appetite Hormone Levels in Children and Adolescents: An Observational Study. Environ. Res. 2024, 252, 118846. [Google Scholar] [CrossRef] [PubMed]
- Galdino-Silva, M.B.; Almeida, K.M.M.; Oliveira, A.D.S.; Santos, J.V.L.; Macena, M.L.; Silva, D.R.; Pereira, M.R.; Silva-Júnior, A.E.; Ferro, D.C.; Paula, D.T.D.C.; et al. A Meal with Ultra-Processed Foods Leads to a Faster Rate of Intake and to a Lesser Decrease in the Capacity to Eat When Compared to a Similar, Matched Meal without Ultra-Processed Foods. Nutrients 2024, 16, 4398. [Google Scholar] [CrossRef]
- Moreno-Altamirano, L.; Robles-Rivera, K.; Castelán-Sánchez, H.G.; Vaca-Paniagua, F.; Iñarritu Pérez, M.D.C.; Hernández-Valencia, S.E.; Cruz-Casarrubias, C.; García-García, J.J.; Ruíz de la Cruz, M.; Martínez-Gregorio, H.; et al. Gut Microbiota: Association with Fiber Intake, Ultra-Processed Food Consumption, Sex, Body Mass Index, and Socioeconomic Status in Medical Students. Nutrients 2024, 16, 4241. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Solanas, M.; Escorihuela, R.M. Dissecting ultra-processed foods and drinks: Do they have a potential to impact the brain? Rev. Endocr. Metab. Disord. 2022, 23, 697–717. [Google Scholar] [CrossRef]
- Reichenbach, A.; Clarke, R.E.; Stark, R.; Lockie, S.H.; Mequinion, M.; Dempsey, H.; Rawlinson, S.; Reed, F.; Sepehrizadeh, T.; DeVeer, M.; et al. Metabolic Sensing in AgRP Neurons Integrates Homeostatic State with Dopamine Signalling in the Striatum. eLife 2022, 11, e72668. [Google Scholar] [CrossRef]
- Mottis, G.; Kandasamey, P.; Peleg-Raibstein, D. The Consequences of Ultra-Processed Foods on Brain Development during Prenatal, Adolescent and Adult Stages. Front. Public Health 2025, 13, 1590083. [Google Scholar] [CrossRef]
- Larruy-García, A.; Mahmood, L.; Miguel-Berges, M.L.; Masip, G.; Seral-Cortés, M.; De Miguel-Etayo, P.; Moreno, L.A. Diet Quality Scores, Obesity and Metabolic Syndrome in Children and Adolescents: A Systematic Review and Meta-Analysis. Curr. Obes. Rep. 2024, 13, 755–788. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Louzada, M.L.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.; Canella, D.S.; Moubarac, J.C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. 2015, 81, 9–15. [Google Scholar] [CrossRef]
- Masquio, D.C.; de Piano, A.; Campos, R.M.; Sanches, P.L.; Carnier, J.; Corgosinho, F.C.; Netto, B.D.; Carvalho-Ferreira, J.P.; Oyama, L.M.; Nascimento, C.M.; et al. The role of multicomponent therapy in the metabolic syndrome, inflammation and cardiovascular risk in obese adolescents. Br. J. Nutr. 2015, 113, 1920–1930. [Google Scholar] [CrossRef]
- Kravchychyn, A.C.P.; Campos, R.M.D.S.; Oliveira E Silva, L.; Ferreira, Y.A.M.; Corgosinho, F.C.; Masquio, D.C.L.; Vicente, S.E.C.F.; Oyama, L.M.; Tock, L.; de Mello, M.T.; et al. Adipocytokine and appetite-regulating hormone response to weight loss in adolescents with obesity: Impact of weight loss magnitude. Nutrition 2021, 87–88, 111188. [Google Scholar] [CrossRef]

| NOVA Classification | Items from Food Frequency Questionary |
|---|---|
| Unprocessed food | Whole milk, skimmed milk, natural yogurt, lettuce, kale/cabbage, watercress/arugula, cauliflower, beetroot, spinach/collard greens, peas, tomato, carrot, coffee, green corn, potato, boiled cassava, orange/tangerine, banana, pineapple, apple/pear, papaya, strawberry, avocado, melon/watermelon, grape, mango, cooked rice, cooked beans, chicken, beef, fish, pork, coffee, mate tea (chimarrão) |
| Ultraprocessed and minimally processed food | Potato chips or savory snacks, chocolate/brigadeiro, plain or packaged cake, ice cream (tub or popsicle), powdered chocolate drink, candies, cheeseburger (beef or chicken), cheese bread, hot dog, diet yogurt, cream cheese, mayonnaise, margarine, plain biscuits, filled biscuits, breakfast cereals, processed meats, sausage, frankfurter, soft drink (regular), diet soft drink, flavored mate tea, artificial juices, sweetener, mousse-type desserts, chocolate croissant, ham and cheese croissant, fermented milk drink |
| Consumption Frequency | Conversion Factor Used to Calculate Annual Consumption Score (0–1) |
|---|---|
| Never | 0.00 |
| Less than once a month | 0.02 |
| 1–3 times per month | 0.07 |
| Once per week | 0.14 |
| 2–4 times per week | 0.43 |
| Once per day | 1.00 |
| Two or more times per day | 1.00 |
| Ultraprocessed Food Consumption Score | ||||
|---|---|---|---|---|
| Tertile 1 (n = 33) Median 2.91 (Range 0.48–4.25) | Tertile 2 (n = 31) Median 5.34 Range (4.29–6.68) | Tertile 3 (n = 32) Median 8.20 Range (6.71–17.6) | p 1 | |
| Age (years) | 16.51 ± 1.85 | 17.25 ± 1.97 | 16.90 ± 1.46 | 0.349 |
| BES score 2 | 16 (1–46) | 12 (3–32) | 16.5 (0–30) | 0.376 |
| Annual consumption score | ||||
| Unprocessed/minimally processed food score | 6.06 (1.35–14.9) b | 6.40 (1.66–14.7) | 8.17 (4.00–23.4) | 0.006 |
| Ingredients score | 0.45 (0.07–3.00) b | 1.07 (0.02–3.00) c | 1.65 (0.02–3.00) | <0.001 |
| Processed food score | 1.79 (0.23–4.08) a,b | 2.50 (0.64–5.32) c | 3.03 (1.27–9.59) | <0.001 |
| Anthropometry | ||||
| Body weight (kg) | 101.00 (71.60–162.90) | 98.40 (77.50–155.80) | 96.70 (76.80–145.70) | 0.867 |
| Height (m) | 1.68 ± 0.10 | 1.69 ± 0.09 | 1.68 ± 0.09 | 0.857 |
| BMI (kg/m2) | 34.50 (28.30–48.50) | 34.80 (29.40–45.50) | 34.40 (28.20–48.10) | 0.969 |
| BMI percentile | 98.8 (96.5–99.7) | 98.7 (95.9–99.9) | 98.7 (96.0–99.9) | 0.453 |
| BMI z score | 2.27 ± 0.27 | 2.19 ± 0.33 | 2.23 ± 0.32 | 0.603 |
| Body fat (%) | 42.93 ± 5.95 | 44.38 ± 6.08 | 44.13 ± 6.34 | 0.594 |
| Lean mass (%) | 57.07 ± 5.95 | 55.62 ± 6.09 | 55.87 ± 6.34 | 0.594 |
| Body fat (kg) | 44.25 ± 11.63 | 44.86 ± 9.67 | 44.55 ± 12.16 | 0.975 |
| Lean mass (kg) | 58.20 ± 10.95 | 56.16 ± 11.11 | 55.37 ± 8.77 | 0.517 |
| Visceral fat (cm) | 4.38 ± 1.11 | 4.35 ± 1.45 | 4.68 ± 1.28 | 0.785 |
| Subcutaneous fat (cm) | 4.12 ± 1.05 | 4.23 ± 3.99 | 3.99 ± 0.73 | 0.535 |
| Waist circumference (cm) | 99.83 ± 10.86 | 98.57 ± 10.34 | 98.05 ± 9.51 | 0.785 |
| Unprocessed/Minimally Food Consumption Score | ||||
| Tertile 1 (n = 32) Median 3.94 Range (1.35–5.55) | Tertile 2 (n = 32) Median 6.80 Range (5.65–8.39) | Tertile 3 (n = 32) Median Range 10.9 (8.46–23.4) | p 1 | |
| Age (years) | 16.80 ± 2.17 | 16.78 ± 1.40 | 17.04 ± 1.72 | 0.646 |
| BES score 2 | 13 (0–33) | 15 (3–43) | 15 (2–37) | 0.908 |
| Annual consumption score | ||||
| Ultraprocessed food score | 4.93 (0.48–8.24) b | 4.38 (1.15–12.4) | 6.87 (0.62–17.6) | 0.004 |
| Ingredient score | 0.28 (0.02–2.39) b | 1.13 (0.07–3.00) c | 1.89 (0.02–3.00) | <0.001 |
| Processed food score | 1.62 (0.33–3.51) b | 2.25 (0.23–6.01) c | 3.49 (1.27–6.01) | <0.001 |
| Anthropometry | ||||
| Body weight (kg) | 101.00 (71.60–162.90) | 98.40 (77.50–155.80) | 96.70 (76.90–145.70) | 0.967 |
| Height (m) | 1.67 ± 0.09 | 1.70 ± 0.09 | 1.67 ± 0.09 | 0.262 |
| BMI (kg/m2) | 34.50 (28.30–48.50) | 34.80 (29.40–45.50) | 34.40 (28.20–48.10) | 0.307 |
| BMI percentile | 98.8 (95.9–99.9) | 98.7 (96.0–99.9) | 98.4 (95.9–99.8) | 0.608 |
| BMI z score | 2.28 ± 0.32 | 2.22 ± 0.29 | 2.20 ± 0.31 | 0.591 |
| Body fat (%) | 45.22 ± 5.02a | 41.55 ± 6.67 | 44.63 ± 6.01 | 0.046 |
| Lean mass (%) | 54.78 ± 5.02a | 58.45 ± 6.67 | 55.38 ± 6.01 | 0.047 |
| Body fat (kg) | 45.40 ± 9.57 | 42.40 ± 11.23 | 45.84 ± 12.31 | 0.422 |
| Lean mass (kg) | 54.71 ± 9.71 | 59.03 ± 10.49 | 56.07 ± 10.49 | 0.234 |
| Visceral fat (cm) | 4.79 ± 1.46 | 4.22 ± 0.98 | 4.39 ± 1.31 | 0.199 |
| Subcutaneous fat (cm) | 4.26 ± 0.87 | 3.84 ± 0.93 | 4.24 ± 0.88 | 0.125 |
| Waist circumference (cm) | 101.45 ± 10.20 | 96.91 ± 9.85 | 98.12 ± 10.19 | 0.193 |
| Ultraprocessed Food Consumption Score | ||||
|---|---|---|---|---|
| Tertile 1 (n = 33) | Tertile 2 (n = 31) | Tertile 3 (n = 32) | p 1 | |
| AgRP (ng/mL) | 0.34 (0.12–5.24) | 0.96 (0.13–2.71) | 0.82 (0.12–14.50) | 0.434 |
| NPY (ng/mL) | 1.23 (0.50–30.90) | 2.10 (0.48–8.12) | 2.08 (0.54–12.90) | 0.249 |
| NPY/AGRP ratio | 3.17 (0.47–11.70) | 2.54 (10.70–6.99) | 2.80(0.41–11.68) | 0.674 |
| Leptin (ng/mL) | 35.25 (7.78–61.74) | 33.45 (49.56–124.38) | 34.31 (85.61–80.26) | 0.945 |
| Ghrelin (ng/mL) | 1.17 (0.20–1.53) | 1.05 (0.88–1.44) | 1.00 (0.31–1.44) | 0.221 |
| MCH (ng/mL) | 4.77 (1.68–10.84) | 6.40 (1.45–1.93) | 5.93 (1.36–21.10) | 0.638 |
| α-MSH (ng/mL) | 0.76 (0.07–3.63) | 1.84 (0.17–9.14) | 1.63 (0.25–6.44) | 0.428 |
| Unprocessed/Minimally Food Consumption Score | ||||
| Tertile 1 (n = 32) | Tertile 2 (n = 32) | Tertile 3 (n = 32) | p 1 | |
| AgRP (ng/mL) | 0.45 (0.12–3.23) | 0.67 (0.12–10.66) | 0.38 (0.13–14.48) | 0.911 |
| NPY (ng/mL) | 1.56 (0.55–8.12) | 1.69 (0.60–9.61) | 1.54 (0.48–30.89) | 0.916 |
| NPY/AGRP ratio | 2.86 (0.47–11.68) | 1.94 (0.41–11.70) | 3.11 (0.67–9.83) | 0.165 |
| Leptin (ng/mL) | 34.24 (1.67–61.19) | 34.11 (4.96–62.59) | 35.24 (14.75–124.38) | 0.344 |
| Ghrelin (ng/mL) | 1.18 (0.31–1.44) a | 0.96 (0.20–1.49) | 1.11 (0.53–1.44) | 0.038 |
| MCH (ng/mL) | 7.21 (1.36–10.24) | 4.88 (1.50–11.60) | 5.50 (1.88–21.10) | 0.713 |
| α-MSH (ng/mL) | 0.85 (0.30–6.44) | 1.53 (0.24–3.31) | 1.16 (0.07–9.14) | 0.955 |
| Ultraprocessed Food Consumption Score | |||||||
|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | |||||||
| Estimate | R2 | Standard Error | Lower Limit | Upper Limit | t | p | |
| AgRP | 0.262 | 0.03 | 0.147 | −0.03 | 0.555 | 1.78 | 0.079 |
| NPY | 0.011 | 1.79 | 0.087 | −0.161 | 0.184 | 0.130 | 0.897 |
| Adiponectin | 0.026 | 0.02 | 0.029 | −0.015 | 0.067 | 1.240 | 0.217 |
| Leptin | 6.48 | 1.66 | 0.018 | −0.036 | 0.038 | 0.034 | 0.973 |
| Ghrelin | −2.10 | 0.05 | 1.40 | −4.920 | 0.711 | −1.510 | 0.139 |
| MCH | 0.069 | 0.001 | 0.111 | −0.151 | 0.291 | 0.632 | 0.530 |
| α-MSH | 0.342 | 0.026 | 0.217 | −0.088 | 0.772 | 1.580 | 0.118 |
| Unprocessed/Minimally Processed Food Consumption Score | |||||||
| 95% Confidence Interval | |||||||
| Estimate | R2 | Standard Error | Lower Limit | Upper Limit | t | p | |
| AgRP | 0.123 | 0.000 | 0.183 | −0.240 | 0.486 | 0.675 | 0.502 |
| NPY | 0.166 | 0.025 | 0.105 | −0.043 | 0.376 | 1.580 | 0.118 |
| Adiponectin | 0.024 | 0.000 | 0.025 | −0.027 | 0.075 | 0.920 | 0.360 |
| Leptin | 0.023 | 0.014 | 0.023 | −0.022 | 0.069 | 1.02 | 0.313 |
| Ghrelin | −1.200 | 0.010 | 1.750 | −4.730 | 2.32 | −0.687 | 0.496 |
| MCH | 0.053 | 0.002 | 0.137 | −0.221 | 0.326 | 0.382 | 0.703 |
| α-MSH | 0.224 | 0.001 | 0.268 | −0.308 | 0.757 | 0.836 | 0.405 |
| Ultraprocessed Food Consumption Score | Unprocessed/Minimally Processed Food Consumption Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | ||||||||||||
| Predictors | Estimate | Standard Error | Lower Limit | Upper Limit | t | p | Predictors | Estimate | Standard Error | Lower Limit | Upper Limit | t | p |
| R2 = 0.05 | R2 = 0.01 | ||||||||||||
| Intercept | 0.84 | 2.18 | 0.024 | 8.68 | 1.99 | 0.04 | Intercept | 7.58 | 4.55 | 16.63 | 16.63 | 1.66 | 0.10 |
| Age | 0.19 | 0.18 | −0.17 | 0.56 | 1.05 | 0.29 | Age | 0.09 | 0.23 | −0.36 | 0.55 | 0.41 | 0.68 |
| Body fat (%) | 0.02 | 0.05 | −0.08 | 0.13 | 0.51 | 0.61 | Body fat (%) | −0.04 | 0.06 | −0.18 | 0.08 | −0.71 | 0.47 |
| AgRP | 0.30 | 0.15 | 0.001 | 0.61 | 1.99 | 0.04 | AgRP | 0.10 | 0.19 | −0.27 | 0.48 | 0.56 | 0.57 |
| BES (Yes-No) | 0.29 | 0.67 | −1.03 | 1.63 | 0.44 | 0.66 | BES (Yes-No) | 0.53 | 0.83 | −1.12 | 2.20 | 0.64 | 0.52 |
| R2 = 0.07 | R2 = 0.03 | ||||||||||||
| Intercept | 2.89 | 3.61 | −4.23 | 10.07 | 0.80 | 0.42 | Intercept | 7.28 | 4.37 | −1.40 | 15.96 | 1.66 | 0.09 |
| Age | 0.14 | 0.18 | −0.22 | 0.52 | 0.79 | 0.43 | Age | 0.12 | 0.22 | −0.32 | 0.57 | 0.55 | 0.58 |
| Body fat (%) | 0.01 | 0.05 | −0.10 | 0.11 | 0.12 | 0.89 | Body fat (%) | −0.05 | 0.06 | −0.18 | 0.07 | −0.89 | 0.37 |
| NPY | 0.01 | 0.08 | −0.15 | 0.19 | 0.20 | 0.83 | NPY | 0.16 | 0.10 | −0.04 | 0.38 | 1.54 | 0.12 |
| BES (Yes-No) | 0.30 | 0.68 | −1.05 | 1.66 | 0.44 | 0.65 | BES (Yes-No) | 0.37 | 0.82 | −1.26 | 2.02 | 0.45 | 0.64 |
| R2 = 0.11 | R2 = 0.07 | ||||||||||||
| Intercept | −2.72 | 4.48 | −11.77 | 6.33 | −0.60 | 0.54 | Intercept | 5.98 | 5.73 | −5.59 | 17.55 | 1.04 | 0.30 |
| Age | 0.03 | 0.19 | −0.35 | 0.42 | 0.18 | 0.85 | Age | 0.24 | 0.24 | −0.25 | 0.74 | 0.99 | 0.32 |
| Body fat (%) | 0.16 | 0.08 | −0.01 | 0.33 | 1.98 | 0.05 | Body fat (%) | −0.04 | 0.10 | −0.26 | 0.17 | −0.99 | 0.66 |
| Ghrelin | −0.26 | 0.50 | −1.27 | 0.75 | −0.52 | 0.60 | Ghrelin | 0.25 | 0.64 | −2.10 | 0.48 | −1.26 | 0.21 |
| BES (Yes-No) | 0.67 | 0.84 | −1.02 | 2.36 | 0.79 | 0.43 | BES (Yes-No) | −0.81 | 1.07 | −1.92 | 2.42 | 0.23 | 0.81 |
| R2 = 0.01 | R2 = 0.01 | ||||||||||||
| Intercept | 3.04 | 4.56 | −6.07 | 12.15 | 0.66 | 0.50 | Intercept | 11.42 | 5.63 | 0.17 | 22.67 | 2.02 | 0.04 |
| Age | 0.10 | 0.23 | −0.36 | 0.57 | 0.44 | 0.65 | Age | −0.06 | 0.28 | −0.63 | 0.51 | −0.21 | 0.83 |
| Body fat (%) | 0.01 | 0.06 | −0.10 | 0.14 | 0.30 | 0.76 | Body fat (%) | −0.07 | 0.07 | −0.22 | 0.08 | −0.90 | 0.37 |
| MCH | 0.08 | 0.11 | −0.14 | 0.31 | 0.74 | 0.45 | MCH | 0.04 | 0.14 | −0.24 | 0.32 | 0.29 | 0.77 |
| BES (Yes-No) | 0.29 | 0.85 | −1.41 | 2.01 | 0.34 | 0.73 | BES (Yes-No) | 0.58 | 1.05 | −1.53 | 2.69 | 0.55 | 0.58 |
| R2 = 0.04 | R2 = 0.01 | ||||||||||||
| Intercept | 1.11 | 3.64 | −6.13 | 8.36 | 0.30 | 0.76 | Intercept | 7.63 | 4.53 | −1.36 | 16.64 | 1.68 | 0.09 |
| Age | 0.17 | 0.18 | −0.19 | 0.53 | 0.93 | 0.35 | Age | 0.09 | 0.22 | −0.36 | 0.54 | 0.39 | 0.69 |
| Body fat (%) | 0.02 | 0.05 | −0.08 | 0.13 | 0.46 | 0.64 | Body fat (%) | −0.05 | 0.06 | −0.18 | 0.08 | −0.76 | 0.44 |
| α-MSH | 0.39 | 0.22 | −0.04 | 0.84 | 1.76 | 0.59 | α-MSH | 0.52 | 0.83 | −1.13 | 0.75 | 0.72 | 0.46 |
| BES (Yes-No) | 0.36 | 0.66 | −0.96 | 1.69 | 0.54 | 0.08 | BES (Yes-No) | 0.20 | 0.27 | −0.35 | 2.17 | 0.63 | 0.53 |
| R2 = 0.04 | R2 = 0.01 | ||||||||||||
| Intercept | 1.87 | 4.38 | −6.87 | 10.63 | 0.42 | 0.67 | Intercept | 7.02 | 5.45 | −3.86 | 17.97 | 1.28 | 0.20 |
| Age | 0.31 | 0.26 | −0.20 | 0.83 | 1.19 | 0.23 | Age | −0.01 | 0.32 | −0.65 | 0.64 | −0.02 | 0.98 |
| Visceral fat | −0.27 | −0.27 | 0.29 | 0.31 | −0.93 | 0.35 | Visceral fat | −0.11 | 0.36 | −0.85 | 0.62 | −0.31 | 0.75 |
| Leptin | −0.01 | 0.01 | 0.01 | 0.03 | −0.28 | 0.77 | Leptin | 0.02 | −0.02 | −0.02 | 0.94 | 0.94 | 0.34 |
| BES (Yes-No) | 0.79 | 0.81 | −0.82 | 2.42 | 0.98 | 0.32 | BES (Yes-No) | 0.20 | 1.01 | −1.81 | 2.22 | 0.20 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neres, P.S.; Ganen, A.d.P.; Campos, R.M.d.S.; Carvalho Ferreira, J.P.d.; Oyama, L.M.; Dâmaso, A.R.; Masquio, D.C.L. Consumption of Unprocessed and Ultraprocessed Foods in Adolescents with Obesity: Associations with Neuroendocrine Mediators of Appetite Regulation and Binge Eating Symptoms. Nutrients 2025, 17, 3711. https://doi.org/10.3390/nu17233711
Neres PS, Ganen AdP, Campos RMdS, Carvalho Ferreira JPd, Oyama LM, Dâmaso AR, Masquio DCL. Consumption of Unprocessed and Ultraprocessed Foods in Adolescents with Obesity: Associations with Neuroendocrine Mediators of Appetite Regulation and Binge Eating Symptoms. Nutrients. 2025; 17(23):3711. https://doi.org/10.3390/nu17233711
Chicago/Turabian StyleNeres, Patrícia Sousa, Aline de Piano Ganen, Raquel Munhoz da Silveira Campos, Joana Pereira de Carvalho Ferreira, Lila Missae Oyama, Ana Raimunda Dâmaso, and Deborah Cristina Landi Masquio. 2025. "Consumption of Unprocessed and Ultraprocessed Foods in Adolescents with Obesity: Associations with Neuroendocrine Mediators of Appetite Regulation and Binge Eating Symptoms" Nutrients 17, no. 23: 3711. https://doi.org/10.3390/nu17233711
APA StyleNeres, P. S., Ganen, A. d. P., Campos, R. M. d. S., Carvalho Ferreira, J. P. d., Oyama, L. M., Dâmaso, A. R., & Masquio, D. C. L. (2025). Consumption of Unprocessed and Ultraprocessed Foods in Adolescents with Obesity: Associations with Neuroendocrine Mediators of Appetite Regulation and Binge Eating Symptoms. Nutrients, 17(23), 3711. https://doi.org/10.3390/nu17233711







