Association Between Vitamin D and Cortisol Concentrations Among Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Study Design
2.3. Salivary Collection and Cortisol Analysis
2.4. Perceived Stress Questionnaire
2.5. Vitamin D Analysis (25(OH)D)
2.6. Sociodemographic and Substance Use Data
2.7. U.S Household Food Security Survey Module (HFSSM)
2.8. Automated Self-Administered 24-h (ASA-24) Recall
2.9. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association Between Vitamin Concentration and Cortisol Concentration Among Pregnant Women
3.3. Odds Ratios Associated with Vitamin D Concentration and Cortisol Concentratoion Among Pregnant Women
3.4. Pearson’s Correlation Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| AUCg | Area Under the Curve with Respect to the Ground |
| BMI | Body Mass Index |
| CAR | Cortisol Awakening Response |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PHAN | Prenatal Health and Nutrition Study |
| VDD | Vitamin D Deficiency |
| WIC | Women Infants and Children |
Appendix A
Prenatal Supplementation and Vitamin D Status During Pregnancy
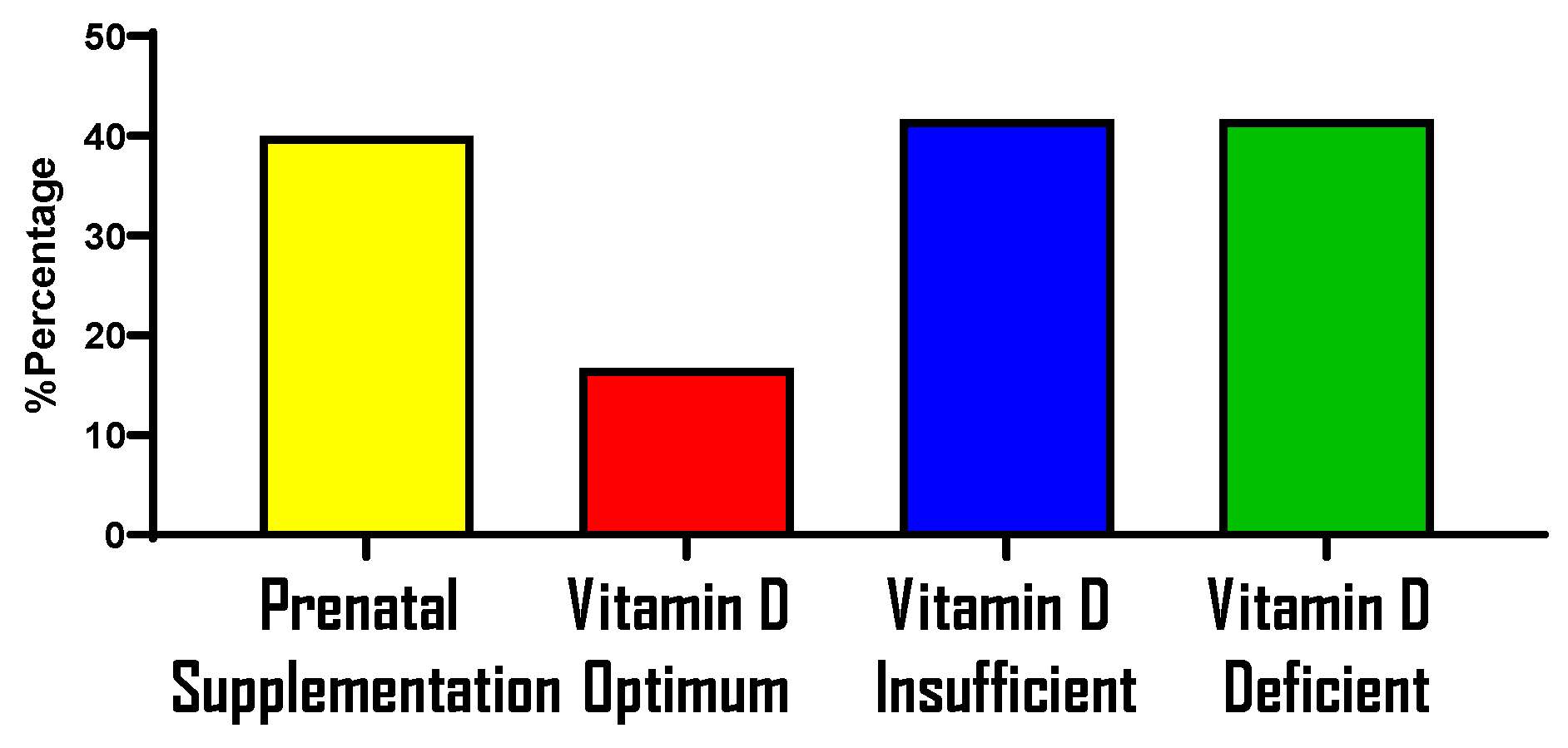
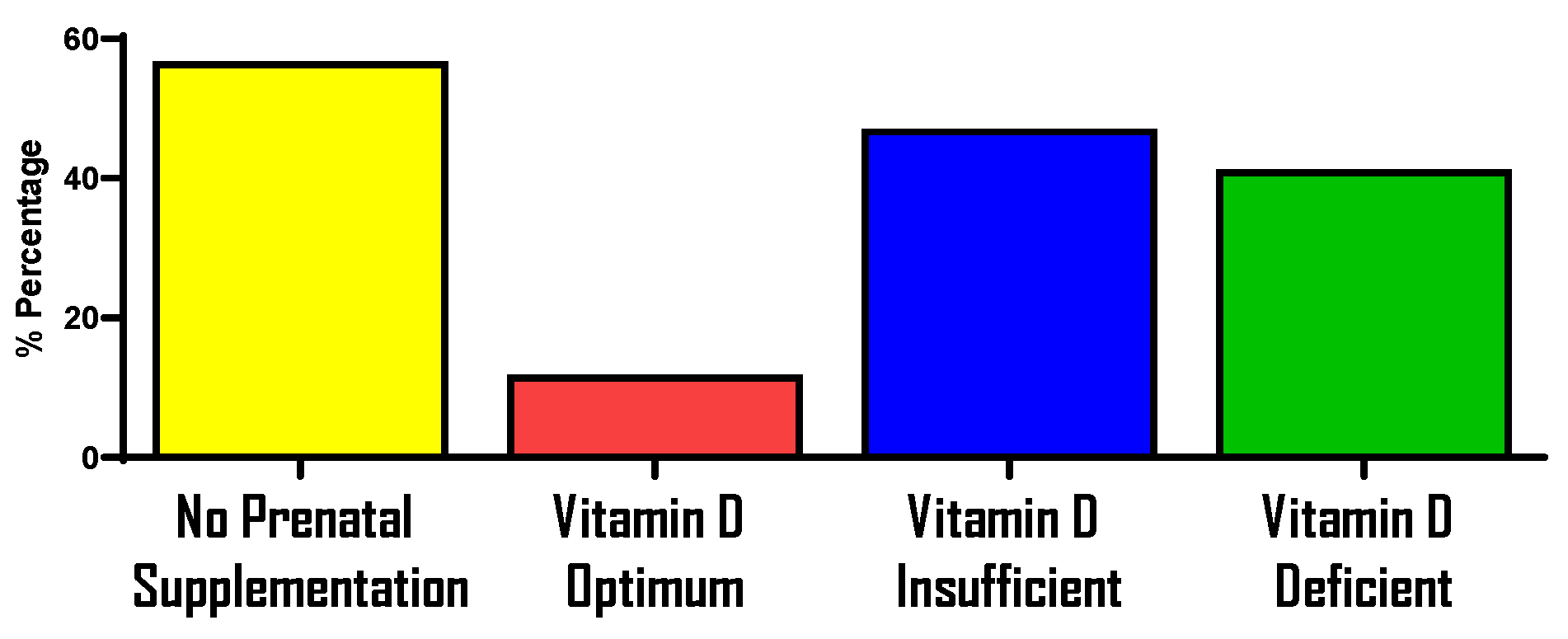
References
- Bleker, L.S.; Roseboom, T.J.; Vrijkotte, T.G.; Reynolds, R.M.; de Rooij, S.R. Determinants of cortisol during pregnancy—The ABCD cohort. Psychoneuroendocrinology 2017, 83, 172–181. [Google Scholar] [CrossRef]
- Giurgescu, C. Are maternal cortisol levels related to preterm birth? JOGNN—J. Obstet. Gynecol. Neonatal Nurs. 2009, 38, 377–390. [Google Scholar] [CrossRef]
- World-Health-Statistics-2014, n.d. Available online: www.who.int/docs/default-source/gho-documents/world-health-statistic-reports/world-health-statistics-2014.pdf (accessed on 20 October 2024).
- Nath, A.; Murthy, G.V.S.; Babu, G.R.; Renzo, G.C. Effect of prenatal exposure to maternal cortisol and psychological distress on infant development in Bengaluru, southern India: A prospective cohort study. BMC Psychiatry 2017, 17, 255. [Google Scholar] [CrossRef]
- Graham, A.M.; Rasmussen, J.M.; Entringer, S.; Ben Ward, E.; Rudolph, M.D.; Gilmore, J.H.; Styner, M.; Wadhwa, P.D.; Fair, D.A.; Buss, C. Maternal Cortisol Concentrations During Pregnancy and Sex-Specific Associations With Neonatal Amygdala Connectivity and Emerging Internalizing Behaviors. Biol. Psychiatry 2019, 85, 172–181. [Google Scholar] [CrossRef]
- Welberg, L.A.M.; Thrivikraman, K.V.; Plotsky, P.M. Chronic maternal stress inhibits the capacity to up-regulate placental 11β-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 2005, 186, 7–12. [Google Scholar] [CrossRef]
- Lesage, J.; Blondeau, B.; Grino, M.; Bréant, B.; Dupouy, J.P. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001, 142, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Al Refaie, A.; Baldassini, L.; De Vita, M.; Gonnelli, S.; Caffarelli, C. Vitamin D and adrenal gland: Myth or reality? A systematic review. Front. Endocrinol. 2022, 13, 1001065. [Google Scholar] [CrossRef]
- Reynolds, R.M. Impact des stéroïdes maternels pendant la grossesse. Ann. Endocrinol. 2016, 77, 677–679. [Google Scholar] [CrossRef]
- McLean, M.; Smith, R. Corticotropin-releasing Hormone in Human Pregnancy and Parturition. Trends Endocrinol. Metab. 1999, 10, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Howland, M.A.; Sandman, C.A.; Glynn, L.M. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev. Endocrinol. Metab. 2017, 12, 321–339. [Google Scholar] [CrossRef]
- Al-Dujaili, E.A.S.; Munir, N.; Iniesta, R.R. Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: A randomized placebo-controlled preliminary study. Ther. Adv. Endocrinol. Metab. 2016, 7, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Woźniak, J.; Włodarek, D. The combination of a diversified intake of carbohydrates and fats and supplementation of vitamin d in a diet does not affect the levels of hormones (Testosterone, estradiol, and cortisol) in men practicing strength training for the duration of 12 weeks. Int. J. Environ. Res. Public Health 2020, 17, 8057. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Mcaree, T.; Jacobs, B.; Manickavasagar, T.; Sivalokanathan, S.; Brennan, L.; Bassett, P.; Rainbow, S.; Blair, M. Vitamin D deficiency in pregnancy—Still a public health issue. Matern. Child. Nutr. 2013, 9, 23–30. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: A systematic review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Moan, J.; Porojnicu, A.C.; Dahlback, A.; Setlow, R.B. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc. Natl. Acad. Sci. USA 2008, 105, 668–673. [Google Scholar] [CrossRef]
- Wilkes, N.C. Vitamin D Deficiency. In Essence of Anesthesia Practice E-Book; Elsevier: Amsterdam, The Netherlands, 2010; pp. 382–383. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Sullivan, S.S.; Rosen, C.J.; Halteman, W.A.; Chen, T.C.; Holick, M.F. Adolescent girls in maine are at risk for vitamin D insufficiency. J. Am. Diet. Assoc. 2005, 105, 971–974. [Google Scholar] [CrossRef]
- Gordon, C.M.; DePeter, K.C.; Feldman, H.A.; Grace, E.; Emans, S.J. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 531–537. [Google Scholar] [CrossRef]
- Bärebring, L.; O’Connell, M.; Winkvist, A.; Johannsson, G.; Augustin, H. Serum cortisol and vitamin D status are independently associated with blood pressure in pregnancy. J. Steroid Biochem. Mol. Biol. 2019, 189, 259–264. [Google Scholar] [CrossRef]
- Nelson, A.R.; Jackson, L.; Clarke, J.; Stellingwerff, T.; Broadbent, S.; Rowlands, D.S. Effect of post-exercise protein-leucine feeding on neutrophil function, immunomodulatory plasma metabolites and cortisol during a 6-day block of intense cycling. Eur. J. Appl. Physiol. 2013, 113, 2211–2222. [Google Scholar] [CrossRef]
- Fitzgerald, J.S.; Orysiak, J.; Wilson, P.B.; Mazur-Różycka, J.; Obminski, Z. Association between Vitamin D status and testosterone and cortisol in ice hockey players. Biol. Sport 2018, 35, 207–213. [Google Scholar] [CrossRef]
- Guarnotta, V.; Di Gaudio, F.; Giordano, C. Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation. Nutrients 2022, 14, 973. [Google Scholar] [CrossRef]
- Tesic, D.; Hawes, J.E.; Zosky, G.R.; Wyrwoll, C.S. Vitamin D deficiency in BALB/c mouse pregnancy increases placental transfer of glucocorticoids. Endocrinology 2015, 156, 3673–3679. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. Maternal Vitamin D supplementation during pregnancy. Br. Med. Bull. 2018, 126, 57–77. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Guenther, P.M.; Durward, C.; Douglass, D.; Zimmerman, T.P.; Kahle, L.L.; Atoloye, A.T.; Marcinow, M.L.; Savoie-Roskos, M.R.; Herrick, K.A.; et al. The Accuracy of Portion Size Reporting on Self-Administered Online 24-Hour Dietary Recalls Among Women With Low Incomes. J. Acad. Nutr. Diet. 2022, 122, 2243–2256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef]
- Food, C.; Data, S. Household Food Security Survey Module Quick Guide What is the Household Food Security Survey Module? n.d. pp. 1–3. Available online: www.nutritionincentivehub.org/media/u0hbzjr2/food-security-quick-guide_final.pdf (accessed on 20 October 2024).
- Gulliford, M.C.; Mahabir, D.; Rocke, B. Reliability and validity of a short form household food security scale in a Caribbean community. BMC Public Health 2004, 4, 22. [Google Scholar] [CrossRef]
- 26621_hh2012. n.d. Available online: https://share.google/WBmMLpbAajIHVcACb (accessed on 20 October 2024).
- Flannery, J.E.; Gabard-Durnam, L.J.; Shapiro, M.; Goff, B.; Caldera, C.; Louie, J.; Gee, D.G.; Telzer, E.H.; Humphreys, K.L.; Lumian, D.S.; et al. Diurnal cortisol after early institutional care—Age matters. Dev. Cogn. Neurosci. 2017, 25, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, M.; Neubauer, A.B.; Ditzen, B. How to assess and interpret everyday life salivary cortisol measures: A tutorial on practical and statistical considerations. Psychoneuroendocrinology 2021, 133, 105391. [Google Scholar] [CrossRef]
- Obel, C.; Hedegaard, M.; Henriksen, T.B.; Secher, N.J.; Olsen, J.; Levine, S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology 2005, 30, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Buss, C.; Shirtcliff, E.A.; Cammack, A.L.; Yim, I.S.; Chicz-Demet, A.; Sandman, C.A.; Wadhwa, P.D. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress 2010, 13, 258–268. [Google Scholar] [CrossRef]
- Maldonado, G. Simulation Study of Confounder-Selection Strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef]
- Pruessner, M.; Hellhammer, D.H.; Pruessner, J.C.; Lupien, S.J. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom. Med. 2003, 65, 92–99. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Hellgren, C.; Åkerud, H.; Skalkidou, A.; Sundström-Poromaa, I. Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology 2013, 38, 3150–3154. [Google Scholar] [CrossRef]
- Tiedje, L.B. Vitamin D deficiency. MCN Am. J. Matern. /Child Nurs. 2008, 33, 394. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Appendix A—Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. n.d. Available online: https://nces.ed.gov/programs/handbook/data/pdf/appendix_a.pdf (accessed on 20 October 2024).
- Khoury, J.E.; Gonzalez, A.; Levitan, R.D.; Pruessner, J.C.; Chopra, K.; Basile, V.S.; Masellis, M.; Goodwill, A.; Atkinson, L. Summary cortisol reactivity indicators: Interrelations and meaning. Neurobiol. Stress 2015, 2, 34–43. [Google Scholar] [CrossRef]
- Specker, B. Vitamin D requirements during pregnancy. Am. J. Clin. Nutr. 2004, 80, 1740S–1747S. [Google Scholar] [CrossRef]
- Flood-Nichols, S.K.; Tinnemore, D.; Huang, R.R.; Napolitano, P.G.; Ippolito, D.L. Vitamin D deficiency in early pregnancy. PLoS ONE 2015, 10, e0123763. [Google Scholar] [CrossRef]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women—Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Gynecol. Scand. 2021, 100, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Akinbi, H. Vitamin D nutrition in pregnancy: Current opinion. Int. J. Womens Health 2013, 5, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Y.; Xu, Y. Vitamin D deficiency in pregnant women: Influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine 2021, 100, e27505. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. New insights into the Vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Simhan, H.N.; Powers, R.W.; Frank, M.P.; Cooperstein, E.; Roberts, J.M. High Prevalence of Vitamin D Insufficiency in Black and White Pregnant Women Residing in the Northern United States and Their Neonates. J. Nutr. 2007, 137, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Hemmingway, A.; O’Callaghan, K.M. Vitamin D in pregnancy: Current perspectives and future directions. Ther. Adv. Musculoskelet. Dis. 2017, 9, 145–154. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Orta, O.; Tworoger, S.; Terry, K.; Coull, B.; Gelaye, B.; Kirschbaum, C.; Sanchez, S.E.; Williams, M.A. Stress and hair cortisol concentrations from preconception to the third trimester. Stress 2018, 22, 60–69. [Google Scholar] [CrossRef]
- Wakefield, C.; Janoschek, B.; Frank, Y.; Karp, F.; Reyes, N.; Schulkin, J.; Frasch, M.G. Chronic stress may disrupt covariant fluctuations of vitamin D and cortisol plasma levels in pregnant sheep during the last trimester: A preliminary report. arXiv 2019, arXiv:1904.06377. [Google Scholar] [CrossRef]


| Participant’s Characteristics | Frequency (%)/Mean ± SD |
|---|---|
| Age (yr) | 28 ± 6 |
| Pre-pregnancy weight (kg) | 71.3 ± 16.6 |
| Pre-pregnancy BMI (kg/m2) | 27.9 ± 6.2 |
| Underweight (BMI < 18.5 kg/m2) | 2 (6.7) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 10 (33.3) |
| Overweight (BMI 25–29.9 kg/m2) | 10 (33.3) |
| Obese (BMI 30 kg/m2) | 8 (26.7) |
| Annual household income a | |
| Less than USD 60,000 | 27 (90) |
| Over USD 60,000 | 3 (10) |
| Highest education attained | |
| Completed high school and or associate degree | 17 (56.7) |
| Bachelor’s degree or graduate degree | 13 (43.3) |
| Race b | |
| Asian | 1 (3.3) |
| Black or African American | 7 (23.3) |
| Native Hawaiian or other Pacific Islander | 2 (6.7) |
| White | 17 (56.7) |
| Other race | 3 (10) |
| Ethnicity b | |
| Hispanic, Latinx, or of Spanish origin | 14 (46.7) |
| Not Hispanic, Latinx, or of Spanish origin | 16 (53.3) |
| Marital status % | |
| Single | 16 (53.3) |
| Married or in a domestic partnership | 13 (43.3) |
| Divorce/separated | 1 (3.3) |
| Number of children given birth to | |
| None | 16 (53.7) |
| 1–2 | 10 (33.3) |
| 3–4 | 4 (13.3) |
| Cortisol AUCg (nmol/L) | 650.5 ± 212.1 |
| Cortisol average at wake time * (AM) | 8:20 |
| Cortisol average at bedtime * (PM) | 10:22 |
| Weekday cortisol * | 19 (74) |
| Weekend cortisol * | 6 (26) |
| Pregnancy trimester | |
| First trimester | 3 (10.0) |
| Second trimester | 16 (53.3) |
| Third trimester | 11 (36.7) |
| Mean gestational age (wk) | 22.2 ± 7.1 |
| PSS c | |
| High PSS | 5 (16.7) |
| Moderate PSS | 20 (66.7) |
| Low PSS | 5 (16.7) |
| Variables | Frequency (%)/Mean ± SD |
|---|---|
| Alcohol intake during pregnancy | |
| Less than or equal to 3 drinks per week | 1 (13.3) |
| >3 drinks per week | 1 (3.3) |
| Do not drink at all | 28 (93.3) |
| Alcohol intake 3 months before pregnancy | |
| 1 to 3 drinks a week | 8 (26.7) |
| 14 drinks or more a week | 1 (3.3) |
| 4 to 7 drinks a week | 2 (6.7) |
| Less than 1 drink a week | 10 (33.3) |
| No drink at all | 9 (30.0) |
| Use of cigarettes 3 months before pregnancy per day | |
| 1 to 5 cigarettes | 2 (6.7) |
| 6 to 10 cigarettes | 2 (6.7) |
| Not all | 26 (86.7) |
| Smoking cigarettes during pregnancy | |
| Do not smoke | 30 (100) |
| Vaping of nicotine 3 months before pregnancy | |
| 1 day a week or less | 1 (3.3) |
| 2–6 days a week | 4 (13.3) |
| More than once a day | 2 (6.7) |
| I didn’t vape nicotine then | 23 (76.7) |
| Vaping of nicotine now (during pregnancy) | |
| I don’t vape | 30 (100) |
| Vaping of marijuana/cannabis now | |
| I don’t vape | 30 (100) |
| Cortisol Assessments | Vitamin D (25(OH)D) | ||
|---|---|---|---|
| β | (95% CI) | p-Value | |
| Waking cortisol | |||
| Unadjusted | −0.002 | (−0.012, 0.008) | 0.69 |
| Adjusted a | 0.014 | (−0.014, 0.016) | 0.72 |
| Cortisol 30 min after waking | |||
| Unadjusted | −0.004 | (−0.013, 0.006) | 0.40 |
| Adjusted a | −0.003 | (−0.015, 0.009) | 0.96 |
| Cortisol 45 min after waking | |||
| Unadjusted | 0.002 | (−0.007, 0.010) | 0.70 |
| Adjusted a | 0.003 | (−0.007, 0.014) | 0.91 |
| Cortisol 60 min after waking | |||
| Unadjusted | 0.002 | (−0.007, 0.012) | 0.62 |
| Adjusted a | 0.004 | (−0.008, 0.015) | 0.91 |
| Bedtime cortisol | |||
| Unadjusted | −0.004 | (−0.019, 0.011) | 0.61 |
| Adjusted a | 0.001 | (−0.016, 0.020) | 0.62 |
| Cortisol AUCg | |||
| Unadjusted | 0.093 | (−0.054, 0.241) | 0.21 |
| Adjusted a | 2.987 | (−7.269, 13.244) | 0.57 |
| Cortisol awakening response | |||
| Unadjusted | −0.008 | (−0.012, 0.008) | 0.53 |
| Adjusted a | 0.002 | (0.001, 0.006) | 0.71 |
| Risk of Having Elevated Cortisol (Ref. = Optimum Vitamin D) | OR (95% CI) | p-Value |
|---|---|---|
| Ref: Optimum Vitamin D | 1 | |
| Waking Cortisol | ||
| Unadjusted | 0.79 (0.09, 5.61) | 0.81 |
| Adjusted * | 0.07 (0.04, 0.09) | 0.83 |
| Cortisol +30 min | ||
| Unadjusted | 0.21 (0.01, 1.83) | 0.21 |
| Adjusted * | 0.15 (0.001, 2.12) | 0.23 |
| Cortisol + 45 min | ||
| Unadjusted | 1.09 (0.15, 9.79) | 0.92 |
| Adjusted | 0.001 (0.001, 19.81) | 0.75 |
| Cortisol + 60 min | ||
| Unadjusted | 1.25 (0.17, 10.96) | 0.82 |
| Adjusted * | 0.11 (0.10, 15.22) | 0.88 |
| Bedtime Cortisol | ||
| Unadjusted | 0.25 (0.012, 2.04) | 0.27 |
| Adjusted * | 0.047 (0.010, 6.963) | 0.60 |
| Cortisol AUCg | ||
| Unadjusted | 1.63 (0.23, 2.04) | 0.63 |
| Adjusted * | 1.11 (0.09, 14.86) | 0.93 |
| Cortisol Awakening Response (CAR) | ||
| Unadjusted | 0.62 (0.06, 3.70) | 0.41 |
| Adjusted * | 0.01 (0.003, 0.005) | 0.78 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. PSS | 1.00 | 0.13 | 0.44 | 0.15 |
| 2. Cortisol * | 0.13 | 1.00 | 0.40 | 0.23 |
| 3. HFSSM | 0.44 | 0.399 | 1.00 | 0.28 |
| 4. Vitamin D | 0.15 | 0.24 | 0.23 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addae, K.S.; Agbemafle, I.; Zhu, G.; Abreu, A.; Jacques, Z.; Owens, B.; Vatral, C.; Oaks, B.M. Association Between Vitamin D and Cortisol Concentrations Among Pregnant Women. Nutrients 2025, 17, 3055. https://doi.org/10.3390/nu17193055
Addae KS, Agbemafle I, Zhu G, Abreu A, Jacques Z, Owens B, Vatral C, Oaks BM. Association Between Vitamin D and Cortisol Concentrations Among Pregnant Women. Nutrients. 2025; 17(19):3055. https://doi.org/10.3390/nu17193055
Chicago/Turabian StyleAddae, Kenneth S., Isaac Agbemafle, Guangyu Zhu, Alyssa Abreu, Zachary Jacques, Bridget Owens, Christopher Vatral, and Brie M. Oaks. 2025. "Association Between Vitamin D and Cortisol Concentrations Among Pregnant Women" Nutrients 17, no. 19: 3055. https://doi.org/10.3390/nu17193055
APA StyleAddae, K. S., Agbemafle, I., Zhu, G., Abreu, A., Jacques, Z., Owens, B., Vatral, C., & Oaks, B. M. (2025). Association Between Vitamin D and Cortisol Concentrations Among Pregnant Women. Nutrients, 17(19), 3055. https://doi.org/10.3390/nu17193055






