Defining Low Milk Supply: A Data-Driven Diagnostic Framework and Risk Factor Analysis for Breastfeeding Women
Abstract
1. Introduction
2. Materials and Methods
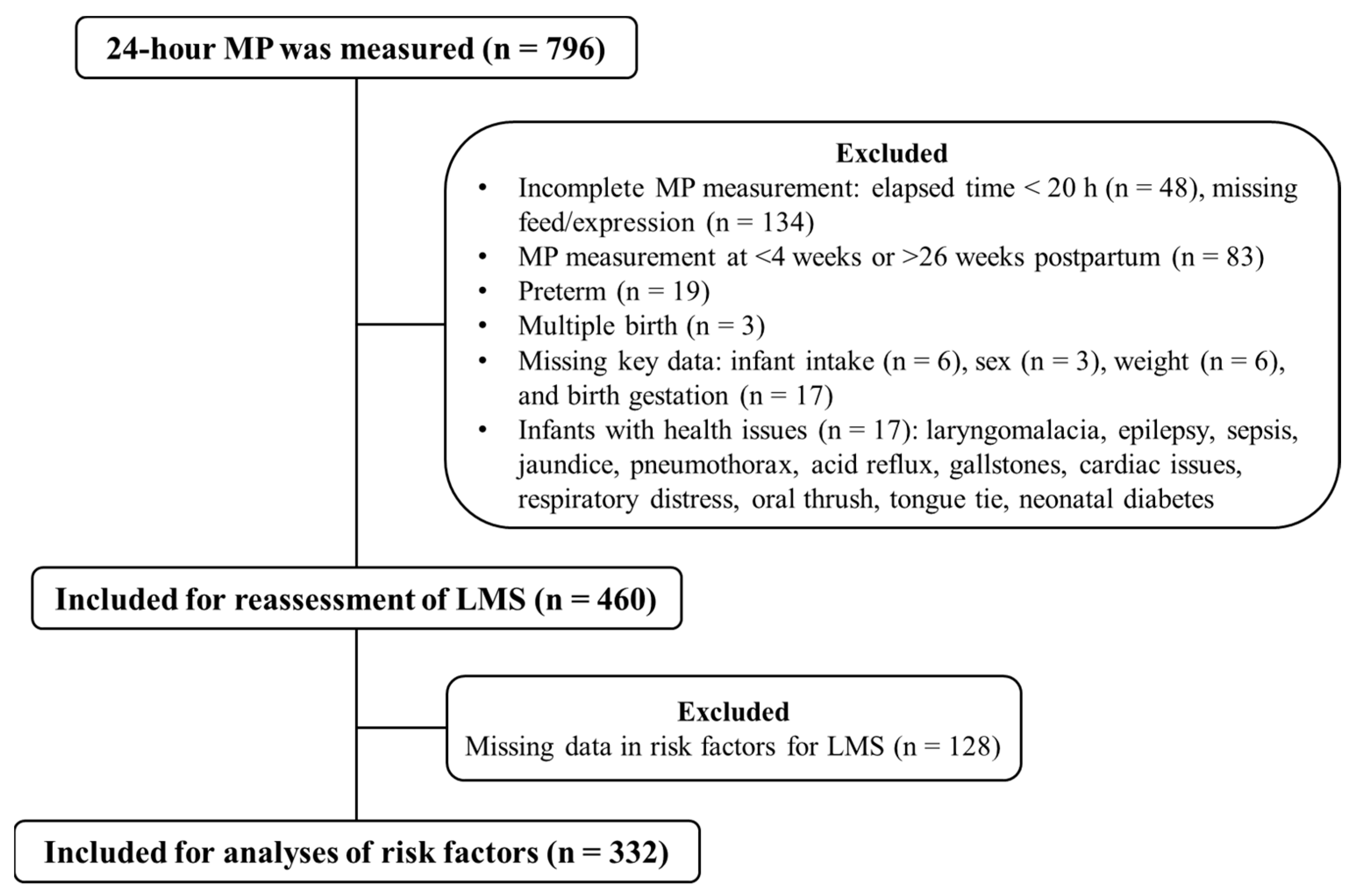
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
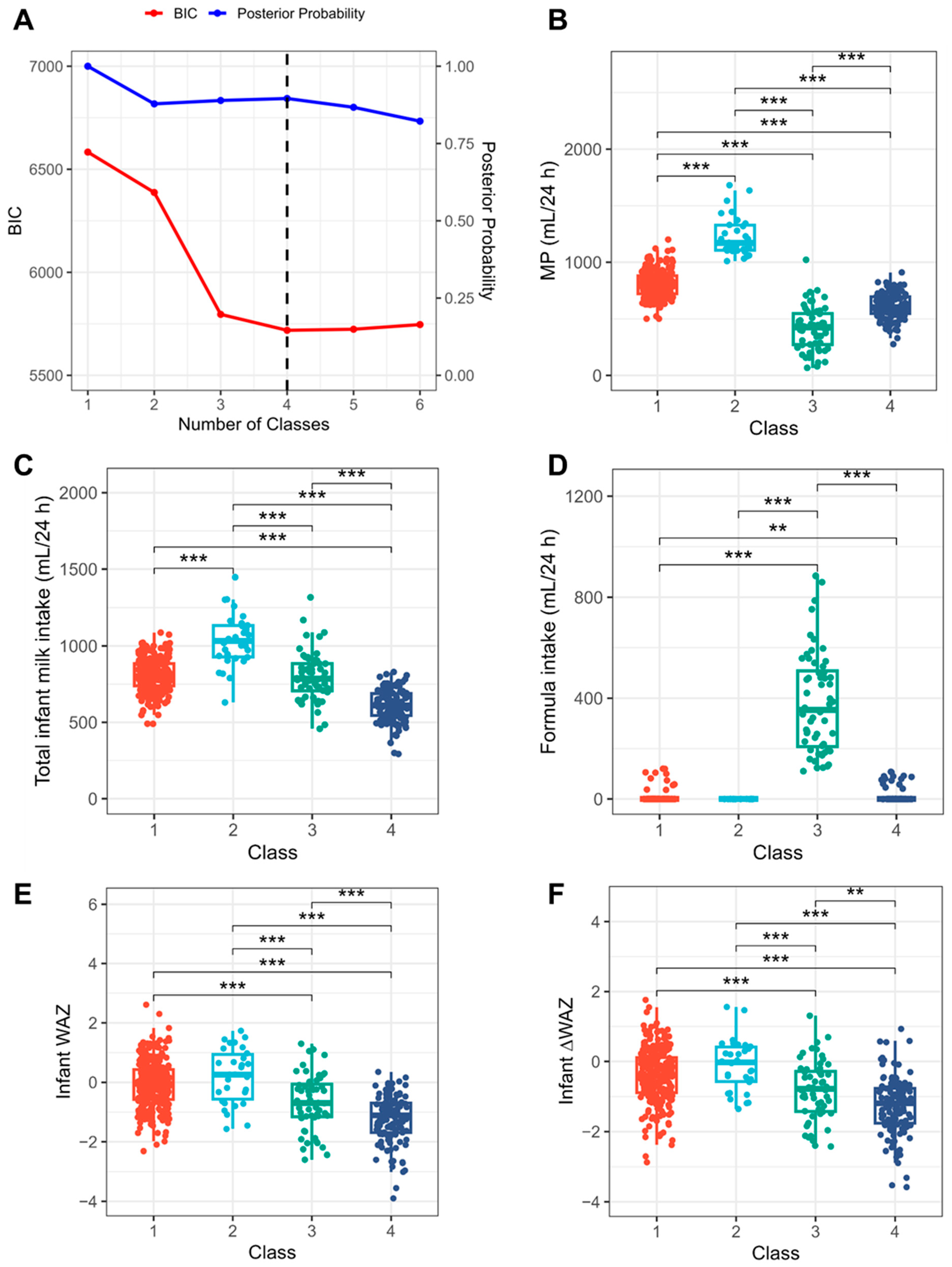
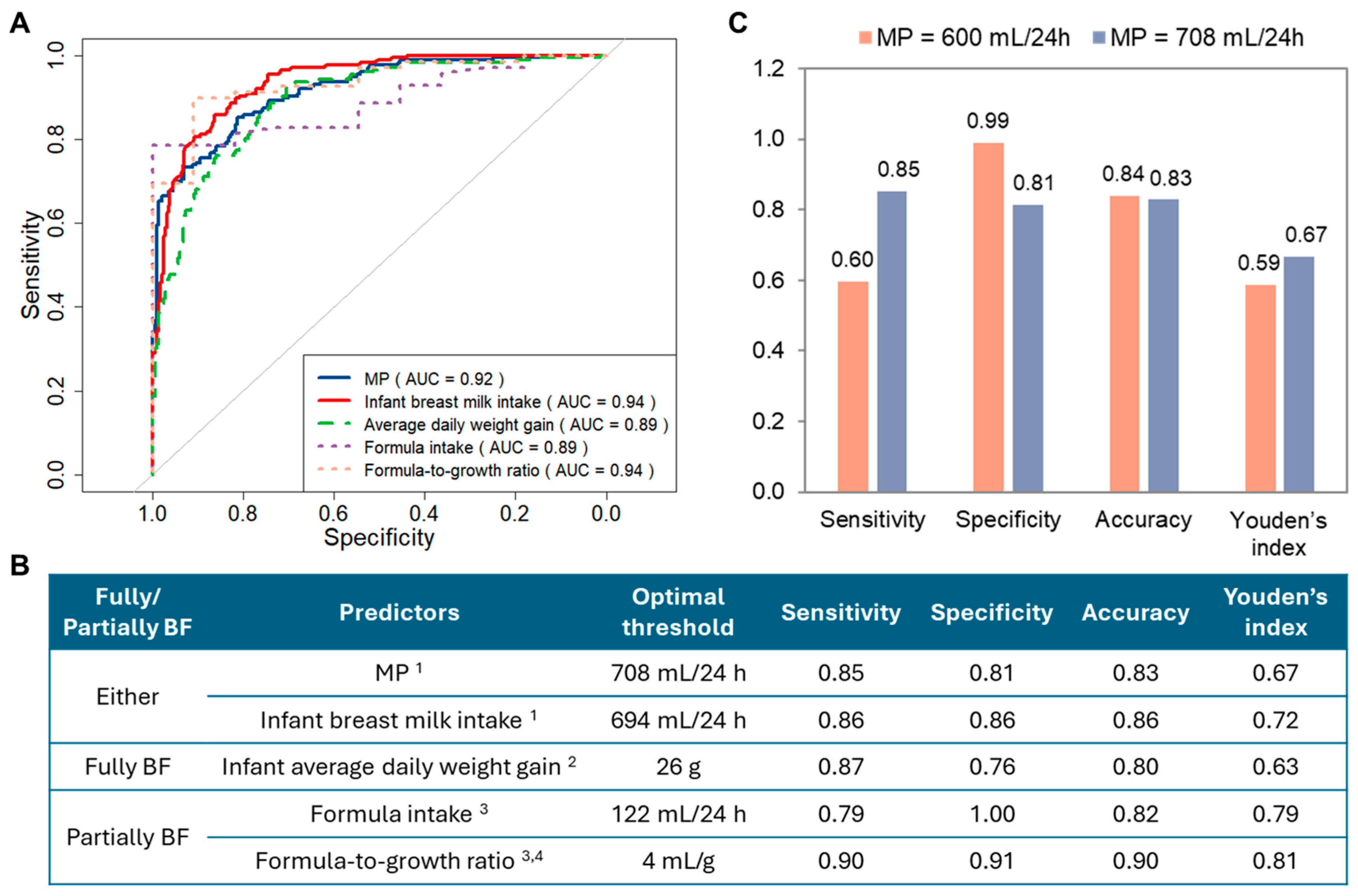
3.2. Reassessment of LMS
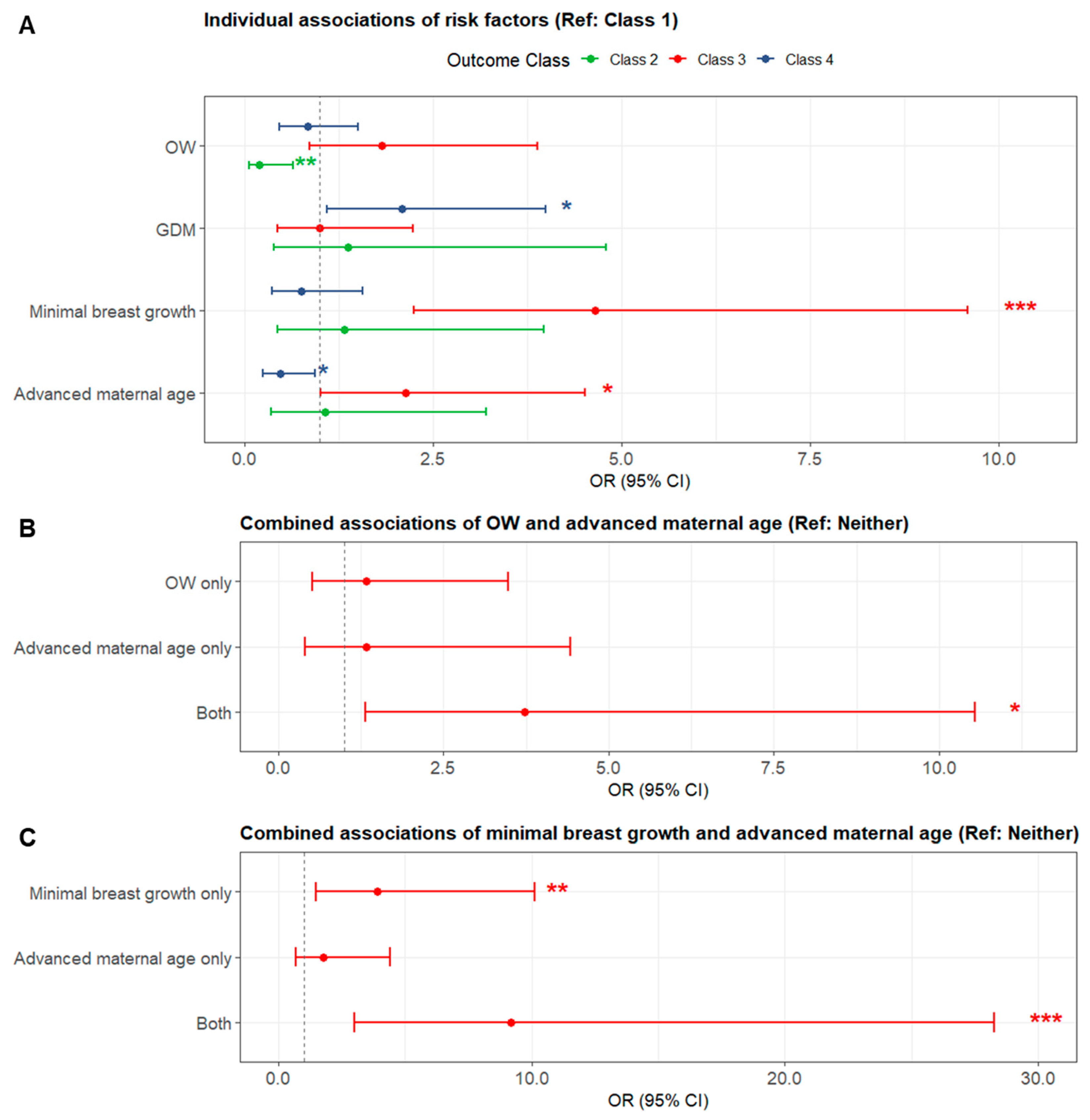
3.3. Risk Factors for LMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIC | Bayesian information criterion |
| BLRT | Bootstrap likelihood ratio test |
| BMI | Body mass index |
| BV | Breast volume |
| CI | Confidence interval |
| GDM | Gestational diabetes mellitus |
| LPA | Latent profile analysis |
| LMS | Low milk supply |
| MP | Milk production |
| NMS | Normal milk supply |
| OW | Overweight/obese weight |
| PCOS | Polycystic ovary syndrome |
| ROC | Receiver operation characteristics |
| OR | Odds ratio |
| WAZ | Weight-for-age z-score |
References
- Riddle, S.W.; Nommsen-Rivers, L.A. Low milk supply and the pediatrician. Curr. Opin. Pediatr. 2017, 29, 249–256. [Google Scholar] [CrossRef]
- Segura-Pérez, S.; Richter, L.; Rhodes, E.C.; Hromi-Fiedler, A.; Vilar-Compte, M.; Adnew, M.; Nyhan, K.; Pérez-Escamilla, R. Risk factors for self-reported insufficient milk during the first 6 months of life: A systematic review. Matern. Child Nutr. 2022, 18, e13353. [Google Scholar] [CrossRef]
- Jin, X.; Lai, C.T.; Perrella, S.L.; Zhou, X.; Hassan, G.M.; McEachran, J.L.; Gridneva, Z.; Taylor, N.L.; Wlodek, M.E.; Geddes, D.T. Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches. Diagnostics 2025, 15, 191. [Google Scholar] [CrossRef]
- Kent, J.C.; Ashton, E.; Hardwick, C.M.; Rea, A.; Murray, K.; Geddes, D.T. Causes of perception of insufficient milk supply in Western Australian mothers. Matern. Child Nutr. 2021, 17, e13080. [Google Scholar] [CrossRef]
- Gridneva, Z.; Warden, A.H.; McEachran, J.L.; Perrella, S.L.; Lai, C.T.; Geddes, D.T. Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants. Nutrients 2025, 17, 366. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, R.; Dumas, L.; Lepage, M. Perception of Not Having Enough Milk and Actual Milk Production of First-Time Breastfeeding Mothers: Is There a Difference? Breastfeed. Med. 2017, 12, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hannan, F.M.; Elajnaf, T.; Vandenberg, L.N.; Kennedy, S.H.; Thakker, R.V. Hormonal regulation of mammary gland development and lactation. Nat. Rev. Endocrinol. 2023, 19, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lai, C.T.; Perrella, S.L.; Zhou, X.; Hassan, G.M.; McEachran, J.L.; Gridneva, Z.; Taylor, N.L.; Wlodek, M.E.; Geddes, D.T. Causes of Low Milk Supply: The Roles of Estrogens, Progesterone, and Related External Factors. Adv. Nutr. 2024, 15, 100129. [Google Scholar] [CrossRef]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L. Breastfeeding after gestational diabetes mellitus: Maternal, milk and infant outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 257–262. [Google Scholar] [CrossRef]
- Turcksin, R.; Bel, S.; Galjaard, S.; Devlieger, R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: A systematic review. Matern. Child Nutr. 2014, 10, 166–183. [Google Scholar] [CrossRef]
- Muktamath, V.U.; Sunitha, N.; Ravi, Y.; Hegde, P.R.; Biradar, S.; Saptagiri, T.; Ilager, S. Understanding Insufficient Maternal Milk Production: Clinical Causes and Remedies. In Clinical Guidance in Breastfeeding—Physiology, Success, and Challenges; IntechOpen: London, UK, 2025. [Google Scholar]
- Perrella, S.L.; Prosser, S.A.; Vlaskovsky, P.; Geddes, D.T. Prevalence of Antenatally Identified Lactation Risk Factors and Risk of Not Fully Breastfeeding at 6 to 8 Weeks Postpartum. J. Midwifery Women’s Health, 2025; ahead of print. [Google Scholar] [CrossRef]
- Sadovnikova, A.; Wysolmerski, J.J.; Hovey, R.C. The Onset and Maintenance of Human Lactation and its Endocrine Regulation, in Maternal-Fetal and Neonatal Endocrinology. In Maternal-Fetal and Neonatal Endocrinology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–205. [Google Scholar]
- Kent, J.C.; Hepworth, A.R.; Langton, D.B.; Hartmann, P.E. Impact of measuring milk production by test weighing on breastfeeding confidence in mothers of term infants. Breastfeed. Med. 2015, 10, 318–325. [Google Scholar] [CrossRef]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef]
- Jin, X.; Lai, C.T.; Perrella, S.L.; McEachran, J.L.; Gridneva, Z.; Geddes, D.T. Maternal Breast Growth and Body Mass Index Are Associated with Low Milk Production in Women. Nutrients 2024, 16, 2854. [Google Scholar] [CrossRef]
- World Health Organization. Child Growth Standards. 2024. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 1 June 2024).
- Macdonald, P.D.; Ross, S.R.; Grant, L.; Young, D. Neonatal weight loss in breast and formula fed infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, F472–F476. [Google Scholar] [CrossRef]
- Spurk, D.; Hirschi, A.; Wang, M.; Valero, D.; Kauffeld, S. Latent profile analysis: A review and “how to” guide of its application within vocational behavior research. J. Vocat. Behav. 2020, 120, 103445. [Google Scholar] [CrossRef]
- Sinha, P.M.; Calfee, C.S.; Delucchi, K.L. Practitioner’s Guide to Latent Class Analysis: Methodological Considerations and Common Pitfalls. Crit. Care Med. 2021, 49, e63–e79. [Google Scholar] [CrossRef]
- Rios-Leyvraz, M.; Yao, Q. The Volume of Breast Milk Intake in Infants and Young Children: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2023, 18, 188–197. [Google Scholar] [CrossRef]
- Shafer, E.F.; Hawkins, S.S. The Impact of Sex of Child on Breastfeeding in the United States. Matern. Child Health J. 2017, 21, 2114–2121. [Google Scholar] [CrossRef]
- Huang, S.-K.; Chih, M.-H. Increased Breastfeeding Frequency Enhances Milk Production and Infant Weight Gain: Correlation with the Basal Maternal Prolactin Level. Breastfeed. Med. 2020, 15, 639–645. [Google Scholar] [CrossRef]
- Li, L.; Wan, W.; Zhu, C. Breastfeeding after a cesarean section: A literature review. Midwifery 2021, 103, 103117. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Weight, D.; McEachran, J.L.; Lai, C.T.; Perrella, S.L.; Geddes, D.T. Maternal Factors, Breast Anatomy, and Milk Production During Established Lactation—An Ultrasound Investigation. J. Imaging 2025, 11, 313. [Google Scholar] [CrossRef]
- Lubke, G.H.; Campbell, I.; McArtor, D.; Miller, P.; Luningham, J.; Berg, S.M.v.D. Assessing Model Selection Uncertainty Using a Bootstrap Approach: An update. Struct. Equ. Model. 2017, 24, 230–245. [Google Scholar] [CrossRef]
- Bookhart, L.H.; Anstey, E.H.; Kramer, M.R.; Perrine, C.G.; Reis-Reilly, H.; Ramakrishnan, U.; Young, M.F. A nation-wide study on the common reasons for infant formula supplementation among healthy, term, breastfed infants in US hospitals. Matern. Child Nutr. 2022, 18, e13294. [Google Scholar] [CrossRef]
- Sandhi, A.; Lee, G.T.; Chipojola, R.; Huda, M.H.; Kuo, S.-Y. The relationship between perceived milk supply and exclusive breastfeeding during the first six months postpartum: A cross-sectional study. Int. Breastfeed. J. 2020, 15, 65. [Google Scholar] [CrossRef]
- Mampane, T.; Wolvaardt, J.E. The acceptability of a donor human milk bank and donated human milk among mothers in Limpopo Province, South Africa. Matern. Child Nutr. 2024, 20, e13651. [Google Scholar] [CrossRef] [PubMed]
- Anders, L.A.; Robinson, K.; Ohlendorf, J.M.; Hanson, L. Unseen, unheard: A qualitative analysis of women’s experiences of exclusively expressing breast milk. BMC Pregnancy Childbirth 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Trimeloni, L.; Spencer, J. Diagnosis and Management of Breast Milk Oversupply. J. Am. Board Fam. Med. 2016, 29, 139–142. [Google Scholar] [CrossRef]
- Hill, R.R.; Lyons, K.S.; Kelly-Weeder, S.; Pados, B.F. Effect of Frenotomy on Maternal Breastfeeding Symptoms and the Relationship Between Maternal Symptoms and Problematic Infant Feeding. Glob. Pediatr. Health 2022, 9, 2333794x211072835. [Google Scholar] [CrossRef]
- Su, L.-L.; Chong, Y.-S.; Chan, Y.-H.; Chan, Y.-S.; Fok, D.; Tun, K.-T.; Ng, F.S.P.; Rauff, M. Antenatal education and postnatal support strategies for improving rates of exclusive breast feeding: Randomised controlled trial. BMJ 2007, 335, 596. [Google Scholar] [CrossRef]
- Walker, R.E.; Harvatine, K.J.; Ross, A.C.; Wagner, E.A.; Riddle, S.W.; Gernand, A.D.; Nommsen-Rivers, L.A. Fatty Acid Transfer from Blood to Milk Is Disrupted in Mothers with Low Milk Production, Obesity, and Inflammation. J. Nutr. 2022, 152, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Hawke, K.; Bowman, A.; Cameron, C.; Peterson, K.L.; Middleton, P.; Leane, C.; Deverix, J.; Collins-Clinch, A.; Rumbold, A.; Glover, K. Breastfeeding experiences and infant feeding decisions for women birthing Aboriginal children in Adelaide, South Australia: A qualitative study. Int. Breastfeed. J. 2025, 20, 48. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Wlodek, M.E.; Lai, C.T.; Prosser, S.A.; Geddes, D.T.; Perrella, S.L. Delayed secretory activation and low milk production in women with gestational diabetes: A case series. BMC Pregnancy Childbirth 2022, 22, 350. [Google Scholar] [CrossRef]
- Otter, G.; Davis, D.; Kurz, E.; Hooper, M.-E.; Shield, A.; Samarawickrema, I.; Spiller, S.; Atchan, M. Promoting breastfeeding in women with gestational diabetes mellitus in high-income settings: An integrative review. Int. Breastfeed. J. 2024, 19, 4. [Google Scholar] [CrossRef]
- Riddle, S.W.; Nommsen-Rivers, L.A. A Case Control Study of Diabetes During Pregnancy and Low Milk Supply. Breastfeed. Med. 2016, 11, 80–85. [Google Scholar] [CrossRef]
- Kam, R.L.; Amir, L.H.; Cullinane, M.; Ingram, J.; Li, X.; Nommsen-Rivers, L.A. Breast hypoplasia markers among women who report insufficient milk production: A retrospective online survey. PLoS ONE 2024, 19, e0299642. [Google Scholar] [CrossRef]
- Kam, R.L.; Amir, L.H.; Cullinane, M. Is There an Association Between Breast Hypoplasia and Breastfeeding Outcomes? A Systematic Review. Breastfeed. Med. 2021, 16, 594–602. [Google Scholar] [CrossRef]
- Luke, B.; Brown, M.B. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum. Reprod. 2007, 22, 1264–1272. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired lactation: Review of delayed lactogenesis and insufficient lactation. J. Midwifery Women’s Health 2021, 66, 631–640. [Google Scholar] [CrossRef]
- Basu, R.; Breda, E.; Oberg, A.L.; Powell, C.C.; Dalla Man, C.; Basu, A.; Vittone, J.L.; Klee, G.G.; Arora, P.; Jensen, M.D.; et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003, 52, 1738–1748. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv. Nutr. 2016, 7, 407–414. [Google Scholar] [CrossRef]
- Arbour, M.W.; Kessler, J.L. Mammary hypoplasia: Not every breast can produce sufficient milk. J. Midwifery Women’s Health 2013, 58, 457–461. [Google Scholar] [CrossRef]
- Katulski, K.; Katulski, A.; Nykowska, A.; Beutler, K.; Kozielek, K.; Antczak, S.; Katulska, K. Physiological changes in the mammary glands during a female’s life. Pol. J. Radiol. 2024, 89, e386–e390. [Google Scholar] [CrossRef]
- Kam, R.L.; Cullinane, M.; Amir, L.H. Breast Hypoplasia and Polycystic Ovary Syndrome: Is There a Link? Clin. Lact. 2021, 12, 159–167. [Google Scholar]
- Suwaydi, M.A. Circadian Variation and Intake Estimation of Human Milk Composition: Exploring the Impact of Gestational Diabetes Mellitus. Ph.D. Thesis, The University of Western Australia, Crawley, Australia, 2024. [Google Scholar]
- Schellscheidt, J.; Oyen, N.; Jorch, G. Interactions between maternal smoking and other prenatal risk factors for sudden infant death syndrome (SIDS). Acta Paediatr. 1997, 86, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Chen, Y.; Zhang, Y.-J. Risk factors combine in a complex manner in assessment for macrosomia. BMC Public Health 2023, 23, 271. [Google Scholar] [CrossRef] [PubMed]
| Value 1 | Missing 2 | |
|---|---|---|
| Maternal characteristics | ||
| Age (years) | 33.1 ± 4.3 (22.7–45.9) | 4 (0.9%) |
| Change in BV during pregnancy (cm3) | 185 ± 134 (−230–660) | 74 (16.1%) |
| BMI (kg/m2) | 26.2 ± 5.7 (17.2–62.5) | 51 (11.1%) |
| Education: bachelor or above | 331 (72.1%) | 1 (0.2%) |
| Marital status: married or de facto | 438 (95.4%) | 1 (0.2%) |
| Parity: primiparous | 273 (59.3%) | 0 |
| Birth mode: vaginal | 267 (60.3%) | 17 (3.7%) |
| Infant characteristics | ||
| Sex: male | 207 (45%) | 0 |
| Birth gestation (weeks) | 39.3 ± 1.1 (37.0–42.4) | 0 |
| Birth weight (g) | 3.4 ± 0.4 (2.0–5.0) | 0 |
| Birth WAZ | 0.2 ± 0.9 (−3.2–3.5) | 0 |
| WAZ at MP measurement | −0.4 ± 1.0 (−3.9–2.6) | 0 |
| ∆WAZ | −0.7 ± 0.9 (−3.6–1.8) | 0 |
| Average daily weight gain (g) | 29.0 ± 9.0 (−1.0–63.1) | 0 |
| Age at MP measurement (weeks) | 12.8 ± 5.1 (4.2–25.9) | 0 |
| Feeding characteristics | ||
| Fully breastfeeding | 379 (82.4%) | 0 |
| MP (mL/24 h) | 734 ± 228 (67–1682) | 0 |
| Total infant milk intake (g/24 h) 3 | 771 ± 162 (293–1448) | 0 |
| Infant breast milk intake (g/24 h) | 721 ± 202 (39–1448) | 0 |
| Infant formula intake (g/24 h) | 51 ± 142 (0–884) | 0 |
| Breastfeeding duration (min/feed) | 13.2 ± 7.0 (0–45) | 0 |
| Total milk removal frequency (times/24 h) 4 | 13.6 ± 4.4 (6–31) | 0 |
| Breastfeeding frequency (times/24 h) 5 | 11.0 ± 4.6 (0–29) | 0 |
| Breast expression frequency (times/24 h) 5 | 2.6 ± 4.0 (0–20) | 0 |
| Risk factors for LMS | ||
| OW (BMI ≥ 25 kg/m2) | 202 (49.4%) | 51 (11.1%) |
| Advanced maternal age (≥35 years) | 141 (30.9%) | 4 (0.9%) |
| GDM | 103 (22.4%) | 0 |
| Minimal breast growth during pregnancy (<100 cm3) | 92 (23.8%) | 74 (16.1%) |
| Fertility issues | 51 (11.1%) | 0 |
| Thyroid disorders | 41 (8.9%) | 0 |
| PCOS | 40 (8.7%) | 0 |
| Nipple piercing or breast surgery | 35 (7.6%) | 0 |
| Postpartum haemorrhage | 29 (6.3%) | 0 |
| Hypertensive disorders in pregnancy | 23 (5.0%) | 0 |
| Risk Factors for LMS | Class 1 (n = 187) | Class 2 (n = 22) | Class 3 (n = 47) | Class 4 (n = 76) |
|---|---|---|---|---|
| OW (BMI ≥ 25 kg/m2) | 101 (54.0%) a | 4 (18.2%) b | 31 (66.0%) ac | 37 (48.7%) ac |
| Advanced maternal age (≥35 years) | 60 (32.1%) a | 6 (27.3%) ab | 24 (51.1%) b | 19 (25.0%) a |
| GDM | 37 (19.8%) a | 4 (18.2%) ab | 14 (29.8%) ab | 25 (32.9%) b |
| Minimal breast growth during pregnancy (<100 cm3) | 36 (19.3%) a | 6 (27.3%) ab | 24 (51.1%) b | 13 (17.1%) a |
| Fertility issues | 20 (10.7%) | 3 (13.6%) | 7 (14.9%) | 11 (14.5%) |
| Thyroid disorders | 17 (9.1%) | 3 (13.6%) | 4 (8.5%) | 6 (7.9%) |
| PCOS | 17 (9.1%) | 3 (13.6%) | 4 (8.5%) | 7 (9.2%) |
| Nipple piercing or breast surgery | 15 (8.0%) | 0 (0%) | 2 (4.3%) | 5 (6.6%) |
| Postpartum haemorrhage | 16 (8.6%) | 2 (9.1%) | 5 (10.6%) | 4 (5.3%) |
| Hypertensive disorders in pregnancy | 16 (8.6%) | 0 (0%) | 1 (2.1%) | 2 (2.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Lai, C.T.; Perrella, S.L.; Gridneva, Z.; McEachran, J.L.; Hassan, G.M.; Taylor, N.L.; Geddes, D.T. Defining Low Milk Supply: A Data-Driven Diagnostic Framework and Risk Factor Analysis for Breastfeeding Women. Nutrients 2025, 17, 3524. https://doi.org/10.3390/nu17223524
Jin X, Lai CT, Perrella SL, Gridneva Z, McEachran JL, Hassan GM, Taylor NL, Geddes DT. Defining Low Milk Supply: A Data-Driven Diagnostic Framework and Risk Factor Analysis for Breastfeeding Women. Nutrients. 2025; 17(22):3524. https://doi.org/10.3390/nu17223524
Chicago/Turabian StyleJin, Xuehua, Ching Tat Lai, Sharon L. Perrella, Zoya Gridneva, Jacki L. McEachran, Ghulam Mubashar Hassan, Nicolas L. Taylor, and Donna T. Geddes. 2025. "Defining Low Milk Supply: A Data-Driven Diagnostic Framework and Risk Factor Analysis for Breastfeeding Women" Nutrients 17, no. 22: 3524. https://doi.org/10.3390/nu17223524
APA StyleJin, X., Lai, C. T., Perrella, S. L., Gridneva, Z., McEachran, J. L., Hassan, G. M., Taylor, N. L., & Geddes, D. T. (2025). Defining Low Milk Supply: A Data-Driven Diagnostic Framework and Risk Factor Analysis for Breastfeeding Women. Nutrients, 17(22), 3524. https://doi.org/10.3390/nu17223524





