Nutritional and Supplemental Interventions for Prevention and Treatment of Oral Mucositis in Pediatric Oncology
Highlights
- Topical, nutrition-based agents—particularly honey and vitamin E—consistently reduced the severity, pain, and duration of oral mucositis (OM) in paediatric cancer patients.
- Across studies, structured oral hygiene protocols (ADD), including regular toothbrushing, bland rinses, and caregiver/child education, emerged as the most effective foundational measure for OM prevention, enhancing the efficacy of topical adjuncts.
- Topical natural agents can serve as low-cost, accessible adjuncts to standard oral care, offering a feasible alternative for pediatric centers where high-cost interventions such as photobiomodulation or palifermin are unavailable.
Abstract
1. Introduction
- Children more often receive leukemia/lymphoma regimens and HSCT, while adults dominate head-and-neck RT/CRT—so the dominant mucositis causal agents differ by age. This can also be seen through differences in prevalence rates between the two populations regarding type of therapy involved [29,30,31,32].
2. Materials and Methods
2.1. Guideline and PICO
- Population/Participants (P): Studies including children and adolescents (≤18 years at treatment initiation) with any type of cancer or undergoing hematopoietic stem cell transplantation treated with chemotherapy, radiotherapy, or chemoradiotherapy, with follow-up data permitted up to 25 years of age.
- Intervention/Index (I): Nutritional supplementation, dietary modification, or widely available natural products (e.g., honey, omega-3 fatty acids, Aloe vera, olive oil, probiotics, glutamine, herbal preparations, or similar agents) administered orally/systemically, with the aim of preventing or treating oral mucositis and/or mucositis-associated pain.
- Comparator/Comparison (C): Standard of care, placebo, or alternative active interventions.
- Outcomes (O): Incidence, severity, and duration of oral mucositis; mucositis-associated pain; and, where reported, secondary outcomes such as treatment tolerability, nutritional status, or quality of life.
2.2. Search Strategy
2.3. Selection of Articles
2.4. Inclusion Criteria
- (1)
- Studies including children and adolescents (≤18 years, up to 25 years of age for follow-ups) with any type of cancer or undergoing hematopoietic stem cell transplantation treated with chemotherapy, radiotherapy, or chemoradiotherapy.
- (2)
- Studies evaluating nutritional supplementation, dietary modification, or widely available natural products (e.g., honey, omega-3 fatty acids, Aloe vera, olive oil, probiotics, glutamine, herbal preparations, or similar agents) administered for the prevention or treatment of oral mucositis and/or mucositis-associated pain.
- (3)
- Studies comparing the intervention against standard of care, placebo, or alternative active interventions.
- (4)
- Studies reporting clinical outcomes related to oral mucositis, including incidence, severity, duration, and associated pain.
- (5)
- Studies that assessed digestive tract mucositis, in which oral mucositis outcomes were clearly reported.
- (6)
- Studies involving human participants, specifically randomized controlled trials (RCTs). RCTs were chosen exclusively for inclusion because they provide the highest level of evidence, allow for proper comparison between interventions and controls, and ensure greater reliability and reproducibility of results.
2.5. Exclusion Criteria
- (1)
- Studies based on adult study populations or pediatric populations not receiving cancer therapy.
- (2)
- Non-RCT designs, including quasi-experimental studies, controlled clinical trials without randomization, observational cohorts, case–control studies, cross-sectional studies, case reports, case series, and preclinical (animal or in vitro) studies.
- (3)
- Articles published in languages other than English, without an available translation.
- (4)
- Publications not appearing in peer-reviewed journals.
- (5)
- Studies not yet published or lacking accessible full texts (abstract-only).
- (6)
- Publications with unsuitable formats, such as letters, case reports, editorials, conference abstracts, systematic reviews, or meta-analyses.
2.6. Quality and Risk of Bias Assessment of the Studies
3. Results
3.1. Systemically Administered Nutrient Supplementation
3.2. Topically Applied Nutritional Agents
3.3. Nutrient-Containing Oral Rinses
4. Discussion
4.1. General Considerations
4.2. Most Impactful Nutritional Agents on Prevention and Evolution of Oral Mucositis
4.3. Importance of Oral Hygiene
4.4. Implications for Clinical Practice
- Adjunctive use of honey and other accessible natural products may be recommended in centers where PBM and palifermin are unavailable, particularly in resource-limited settings.
- Oral care remains foundational, and natural topical agents should be considered as adjuncts rather than replacements.
- Routine use of systemic supplementation or Caphosol rinses is not supported in pediatrics and may divert resources without clinical benefit.
4.5. Limitations
4.6. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, G.; Logan, R.; Revesz, T.; Keefe, D.; Gue, S. The Prevalence and Investigation of Risk Factors of Oral Mucositis in a Pediatric Oncology Inpatient Population; a Prospective Study. J. Pediatr. Hematol./Oncol. 2018, 40, 15–21. [Google Scholar] [CrossRef]
- Hurrell, L.; Burgoyne, L.L.; Logan, R.M.; Revesz, T.; Gue, S. Factors Associated with Oral Mucositis Severity in Children Who Have Received Chemotherapy. J. Pediatr. Hematol./Oncol. 2022, 44, e1016–e1022. [Google Scholar] [CrossRef]
- Curra, M.; Gabriel, A.F.; Ferreira, M.B.C.; Martins, M.A.T.; Brunetto, A.T.; Gregianin, L.J.; Martins, M.D. Incidence and Risk Factors for Oral Mucositis in Pediatric Patients Receiving Chemotherapy. Support. Care Cancer 2021, 29, 6243–6251. [Google Scholar] [CrossRef]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Rivière, G.; Treister, N.; et al. Clinical Practice Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients: 2021 Update. Eur. J. Cancer 2021, 154, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Soga, Y.; Fujiwara, H.; Miura, R.; Matsuoka, K.-I.; Maeda, Y.; Kuboki, T. Characteristics of Oral Mucositis in Patients Undergoing Haploidentical Stem Cell Transplantation with Posttransplant Cyclophosphamide: Marked Difference between Busulfan and Melphalan Regimens. Support. Care Cancer 2025, 33, 252. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Choquette, L.E.; Curley, K.F.; Dowsett, R.J.; Feinn, R.S.; Hegde, U.P.; Pilbeam, C.C.; Salner, A.L.; Sonis, S.T.; Peterson, D.E. Randomized Double-Blind Placebo-Controlled Trial of Celecoxib for Oral Mucositis in Patients Receiving Radiation Therapy for Head and Neck Cancer. Oral Oncol. 2014, 50, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.F.; Lee, V.; Li, C.H.; Yuen, H.L.; Epstein, J.B. Oral Mucositis in Pediatric and Adolescent Patients Undergoing Chemotherapy: The Impact of Symptoms on Quality of Life. Support. Care Cancer 2012, 20, 2335–2342. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear Factor-κB (NF-κB) and Cyclooxygenase-2 (COX-2) Expression in the Oral Mucosa Following Cancer Chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.-J.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. The Role of Pro-Inflammatory Cytokines in Cancer Treatment-Induced Alimentary Tract Mucositis: Pathobiology, Animal Models and Cytotoxic Drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Serum Levels of NF-κB and pro-Inflammatory Cytokines Following Administration of Mucotoxic Drugs. Cancer Biol. Ther. 2008, 7, 1139–1145. [Google Scholar] [CrossRef]
- Bamba, S.; Andoh, A.; Yasui, H.; Araki, Y.; Bamba, T.; Fujiyama, Y. Matrix Metalloproteinase-3 Secretion from Human Colonic Subepithelial Myofibroblasts: Role of Interleukin-17. J. Gastroenterol. 2003, 38, 548–554. [Google Scholar] [CrossRef]
- Alikhani, M.; Alikhani, Z.; He, H.; Liu, R.; Popek, B.I.; Graves, D.T. Lipopolysaccharides Indirectly Stimulate Apoptosis and Global Induction of Apoptotic Genes in Fibroblasts. J. Biol. Chem. 2003, 278, 52901–52908. [Google Scholar] [CrossRef]
- Huang, C.-J.; Huang, M.-Y.; Fang, P.-T.; Chen, F.; Wang, Y.-T.; Chen, C.-H.; Yuan, S.-S.; Huang, C.-M.; Luo, K.-H.; Chuang, H.-Y.; et al. Randomized Double-Blind, Placebo-Controlled Trial Evaluating Oral Glutamine on Radiation-Induced Oral Mucositis and Dermatitis in Head and Neck Cancer Patients. Am. J. Clin. Nutr. 2019, 109, 606–614. [Google Scholar] [CrossRef]
- Xia, J.; Tao, X.; Hu, Q.; Luo, W.; Tong, X.; Zhou, G.; Zhou, H.; Hua, H.; Tang, G.; Wu, T.; et al. Expert Consensus on the Prevention and Treatment of Radiochemotherapy-Induced Oral Mucositis. Int. J. Oral Sci. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Coppini, M.; Caponio, V.C.A.; Mauceri, R.; Bizzoca, M.E.; Laino, L.; Lorenzo-Pouso, A.I.; Russo, D.; Troiano, G.; Silva, F.F.V.E.; Lo Muzio, L.; et al. Efficacy of Topical Agents in Oral Mucositis Prevention: Systematic Review and Network Meta-analysis. Oral Dis. 2024, 30, 4126–4144. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abu Eid, R.; Sawair, F.; Landini, G.; Saku, T. Age and the Architecture of Oral Mucosa. Age 2012, 34, 651–658. [Google Scholar] [CrossRef]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Hajishengallis, G.; Divaris, K.; et al. Human Oral Mucosa Cell Atlas Reveals a Stromal-Neutrophil Axis Regulating Tissue Immunity. Cell 2021, 184, 4090–4104.e15. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the Immune System in Humans from Infancy to Old Age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Estimates, from Salivary Analyses, of the Turnover Time of the Oral Mucosal Epithelium in Humans and the Number of Bacteria in an Edentulous Mouth. Arch. Oral Biol. 2003, 48, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Zubeidat, K.; Saba, Y.; Horev, Y.; Barel, O.; Wilharm, A.; Heyman, O.; Wald, S.; Eli-berchoer, L.; Shapiro, H.; et al. Maturation of the Neonatal Oral Mucosa Involves Unique Epithelium-Microbiota Interactions. Cell Host Microbe 2021, 29, 197–209.e5. [Google Scholar] [CrossRef]
- Fiwek, P.; Irga-Jaworska, N.; Wojtylak, S.; Biernat, W.; Emerich, K.; Pomiecko, D. Assessment of Cytological Changes in the Oral Mucosa in Young Hematological Patients Treated with Systemic Chemotherapy. J. Clin. Med. 2023, 12, 2665. [Google Scholar] [CrossRef]
- Kennedy, D.D.; Tucker, K.L.; Ladas, E.D.; Rheingold, S.R.; Blumberg, J.; Kelly, K.M. Low Antioxidant Vitamin Intakes Are Associated with Increases in Adverse Effects of Chemotherapy in Children with Acute Lymphoblastic Leukemia. Am. J. Clin. Nutr. 2004, 79, 1029–1036. [Google Scholar] [CrossRef]
- Mosby, T.T.; Cosgrove, M.; Sarkardei, S.; Platt, K.L.; Kaina, B. Nutrition in Adult and Childhood Cancer: Role of Carcinogens and Anti-Carcinogens. Anticancer. Res. 2012, 32, 4171–4192. [Google Scholar]
- Alexandru, A.; Ivan, C.-S.; Tanasescu, S.; Oprisoni, L.A.; Dragomir, T.-L.; Varga, N.-I.; Mateescu, D.; Diaconu, M.; Margan, M.-M.; Boeriu, E. Are Pediatric Cancer Patients a Risk Group for Vitamin D Deficiency? A Systematic Review. Cancers 2024, 16, 4201. [Google Scholar] [CrossRef] [PubMed]
- Margan, M.-M.; Alexandru, A.; Ivan, C.-S.; Boeriu, E.; Tanasescu, S.; Cârstea, A.M.; Varga, N.-I.; Margan, R.; Cindrea, A.C.; Negrean, R.A. Vitamin D Status in Children: Romania’s National Vitamin D Screening Programme in Context of the COVID-19 Pandemic. Med. Sci. 2025, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Faizan, U.; Rouster, A.S. Nutrition and Hydration Requirements in Children and Adults. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cheng, K.K.F.; Goggins, W.B.; Lee, V.W.S.; Thompson, D.R. Risk Factors for Oral Mucositis in Children Undergoing Chemotherapy: A Matched Case-Control Study. Oral Oncol. 2008, 44, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- He, X.; Rong, X.; Wang, W.; Liang, L.; Liao, X.; Huang, J.; Zhang, J.; Zhu, W.; Liu, W.; Shi, L. Prevalence and Risk Factors of Chemotherapy-Induced Oral Mucositis in 470 Children with Acute Lymphoblastic Leukemia. Int. J. Paediatr. Dent. 2025, 35, 888–897. [Google Scholar] [CrossRef]
- Padure, A.; Horhat, R.; Talpos-Niculescu, I.C.; Scheusan, R.; Anghel, M.D.; Rusu, L.-C.; Lungeanu, D. Oral Mucositis in Adult Cancer Patients Undergoing Chemotherapy: Six-Month On-Treatment Follow-Up. J. Clin. Med. 2024, 13, 5723. [Google Scholar] [CrossRef]
- Bell, A.; Kasi, A. Oral Mucositis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhou, S.; He, T.; Zhang, Y.; Zhang, H. Comparison of the Main Pathogenic Microorganisms of Various Common Oral Diseases in Children and Adults. Pediatr. Discov. 2023, 1, e35. [Google Scholar] [CrossRef]
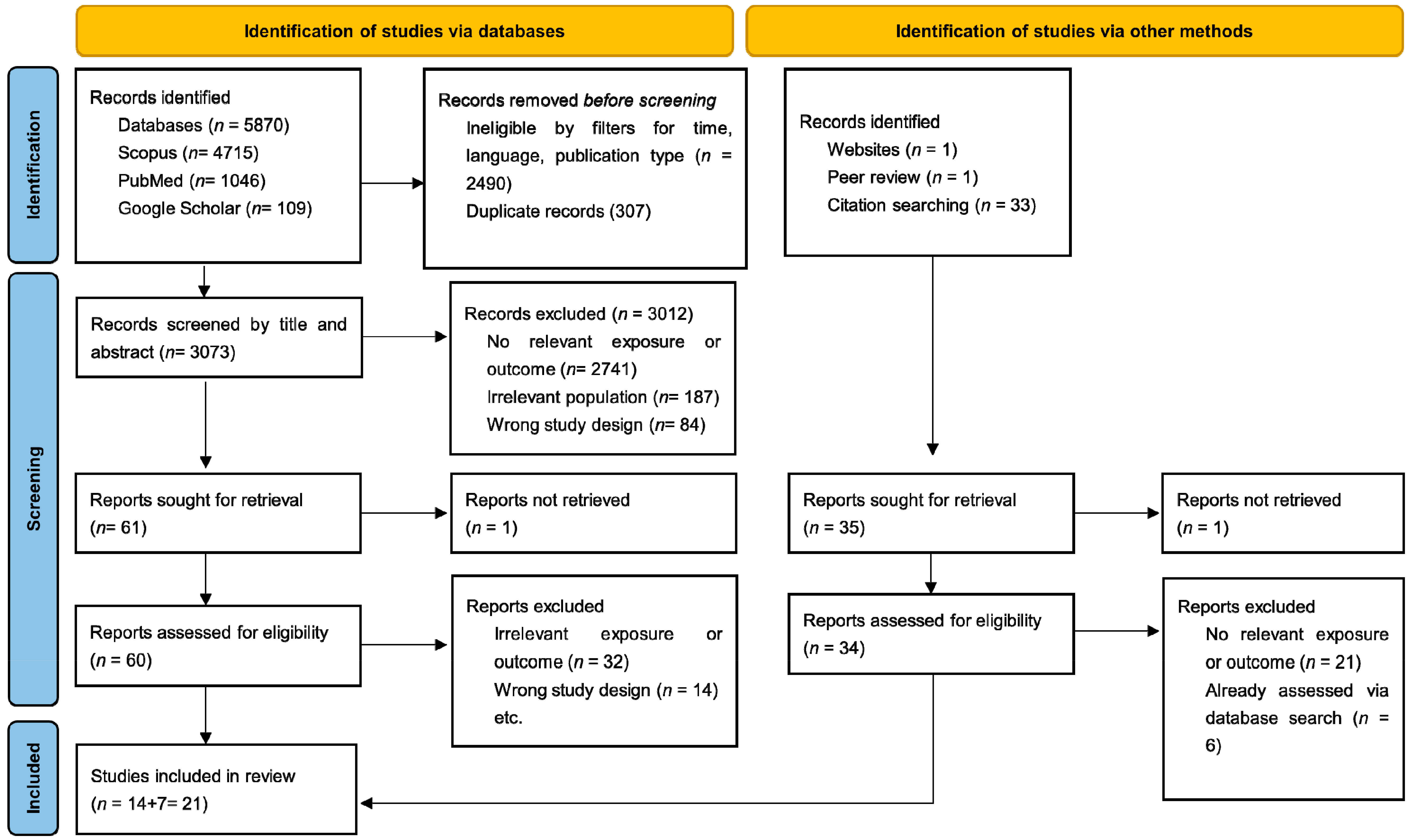
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Uderzo, C.; Rebora, P.; Marrocco, E.; Varotto, S.; Cichello, F.; Bonetti, M.; Maximova, N.; Zanon, D.; Fagioli, F.; Nesi, F.; et al. Glutamine-Enriched Nutrition Does Not Reduce Mucosal Morbidity or Complications After Stem-Cell Transplantation for Childhood Malignancies: A Prospective Randomized Study. Transplantation 2011, 91, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Nieder, M.; Baggott, C.; Olson, E.; Chen, L.; Dang, H.; Krailo, M.; August, A.; Sung, L. Caphosol for Prevention of Oral Mucositis in Pediatric Myeloablative Haematopoietic Cell Transplantation. Br. J. Cancer 2017, 116, 21–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Oberbaum, M.; Yaniv, I.; Ben-Gal, Y.; Stein, J.; Ben-Zvi, N.; Freedman, L.S.; Branski, D. A Randomized, Controlled Clinical Trial of the Homeopathic Medication TRAUMEEL S® in the Treatment of Chemotherapy-induced Stomatitis in Children Undergoing Stem Cell Transplantation. Cancer 2001, 92, 684–690. [Google Scholar] [CrossRef]
- Aquino, V.M.; Harvey, A.R.; Garvin, J.H.; Godder, K.T.; Nieder, M.L.; Adams, R.H.; Jackson, G.B.; Sandler, E.S. A Double-Blind Randomized Placebo-Controlled Study of Oral Glutamine in the Prevention of Mucositis in Children Undergoing Hematopoietic Stem Cell Transplantation: A Pediatric Blood and Marrow Transplant Consortium Study. Bone Marrow Transplant. 2005, 36, 611–616. [Google Scholar] [CrossRef]
- El-Housseiny, A.; Saleh, S.; El-Masry, A.; Allam, A. The Effectiveness of Vitamin “E” in the Treatment of Oral Mucositis in Children Receiving Chemotherapy. J. Clin. Pediatr. Dent. 2007, 31, 167–170. [Google Scholar] [CrossRef]
- Sung, L.; Tomlinson, G.A.; Greenberg, M.L.; Koren, G.; Judd, P.; Ota, S.; Feldman, B.M. Serial Controlled N-of-1 Trials of Topical Vitamin E as Prophylaxis for Chemotherapy-Induced Oral Mucositis in Paediatric Patients. Eur. J. Cancer 2007, 43, 1269–1275. [Google Scholar] [CrossRef]
- Ward, E.; Smith, M.; Henderson, M.; Reid, U.; Lewis, I.; Kinsey, S.; Allgar, V.; Bowers, D.; Picton, S.V. The Effect of High-Dose Enteral Glutamine on the Incidence and Severity of Mucositis in Paediatric Oncology Patients. Eur. J. Clin. Nutr. 2009, 63, 134–140. [Google Scholar] [CrossRef]
- Abdulrhman, M.; Samir Elbarbary, N.; Ahmed Amin, D.; Saeid Ebrahim, R. Honey and a Mixture of Honey, Beeswax, and Olive Oil–Propolis Extract in Treatment of Chemotherapy-Induced Oral Mucositis: A Randomized Controlled Pilot Study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef]
- Khurana, H.; Pandey, R.; Saksena, A.; Kumar, A. An Evaluation of Vitamin E and Pycnogenol in Children Suffering from Oral Mucositis during Cancer Chemotherapy. Oral Dis. 2013, 19, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sencer, S.F.; Zhou, T.; Freedman, L.S.; Ives, J.A.; Chen, Z.; Wall, D.; Nieder, M.L.; Grupp, S.A.; Yu, L.C.; Sahdev, I.; et al. Traumeel S in Preventing and Treating Mucositis in Young Patients Undergoing SCT: A Report of the Children’s Oncology Group. Bone Marrow Transplant. 2012, 47, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Tomaževič, T.; Jazbec, J. A Double Blind Randomised Placebo Controlled Study of Propolis (Bee Glue) Effectiveness in the Treatment of Severe Oral Mucositis in Chemotherapy Treated Children. Complement. Ther. Med. 2013, 21, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.F.; Den Boer, A.M.; Kollen, W.J.W.; Mekelenkamp, H.; Abbink, F.C.H.; Kaspers, G.J.L.; Zomer-Kooijker, K.; Molmans, B.H.W.; Tissing, W.J.E. Caphosol, a Therapeutic Option in Case of Cancer Therapy-Induced Oral Mucositis in Children?: Results from a Prospective Multicenter Double Blind Randomized Controlled Trial. Support. Care Cancer 2014, 22, 3–6. [Google Scholar] [CrossRef]
- Al Jaouni, S.K.; Al Muhayawi, M.S.; Hussein, A.; Elfiki, I.; Al-Raddadi, R.; Al Muhayawi, S.M.; Almasaudi, S.; Kamal, M.A.; Harakeh, S. Effects of Honey on Oral Mucositis among Pediatric Cancer Patients Undergoing Chemo/Radiotherapy Treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid.-Based Complement. Altern. Med. 2017, 2017, 5861024. [Google Scholar] [CrossRef]
- Pourdeghatkar, F.; Motaghi, M.; Darbandi, B.; Baghersalimi, A. Comparative Effect of Chamomile Mouthwash and Topical Mouth Rinse in Prevention of Chemotherapy-Induced Oral Mucositis in Iranian Pediatric Patients with Acute Lymphoblastic Leukemia. Iran. J. Blood Cancer 2017, 9, 84–88. [Google Scholar]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Evaluation of the Effectiveness of Olive Oil to Prevent Chemotherapy Induced Oral Mucositis: A Randomized Controlled Clinical Trial. Pediatr. Dent. J. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Rathe, M.; De Pietri, S.; Wehner, P.S.; Frandsen, T.L.; Grell, K.; Schmiegelow, K.; Sangild, P.T.; Husby, S.; Müller, K. Bovine Colostrum Against Chemotherapy-Induced Gastrointestinal Toxicity in Children with Acute Lymphoblastic Leukemia: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Parenter. Enteral Nutr. 2020, 44, 337–347. [Google Scholar] [CrossRef]
- Widjaja, N.A.; Pratama, A.; Prihaningtyas, R.; Irawan, R.; Ugrasena, I. Efficacy Oral Glutamine to Prevent Oral Mucositis and Reduce Hospital Costs During Chemotherapy in Children with Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2020, 21, 2117–2121. [Google Scholar] [CrossRef]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Efficacy of Aloe-Vera Use for Prevention of Chemotherapy-Induced Oral Mucositis in Children with Acute Lymphoblastic Leukemia: A Randomized Controlled Clinical Trial. Compr. Child Adolesc. Nurs. 2021, 44, 49–62. [Google Scholar] [CrossRef]
- Alkhouli, M.; Laflouf, M.; Comisi, J.C. Assessing the Topical Application Efficiency of Two Biological Agents in Managing Chemotherapy-Induced Oral Mucositis in Children: A Randomized Clinical Trial. J. Oral Biol. Craniofacial Res. 2021, 11, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Badr, L.K.; El Asmar, R.; Hakim, S.; Saad, R.; Merhi, R.; Zahreddine, A.; Muwakkit, S. The Efficacy of Honey or Olive Oil on the Severity of Oral Mucositis and Pain Compared to Placebo (Standard Care) in Children with Leukemia Receiving Intensive Chemotherapy: A Randomized Controlled Trial (RCT). J. Pediatr. Nurs. 2023, 70, e48–e53. [Google Scholar] [CrossRef]
- Shah, D.; Gupta, A.; Meena, J.P.; Kumar Gupta, A.; Velpandian, T.; Pandey, R.M.; Makkar, H.; Seth, R. Efficacy and Safety of Zinc in the Prevention of Oral Mucositis in Children with Cancer Receiving Intensified Chemotherapy: A Randomized Double-blind Placebo-controlled Trial. Pediatr. Blood Cancer 2023, 70, e30309. [Google Scholar] [CrossRef] [PubMed]
- Megari, K.; Katsarou, D.V.; Mantsos, E.; Miliadi, V.; Kosmidou, E.; Argyriadi, A.; Papadopoulou, S.; Argyriadis, A.; Toki, E.I.; Sofologi, M. Quality of Life and Anxiety of Adolescents with Cancer and Their Parents: Neurodevelopmental Implications in Adolescence. Int. J. Dev. Neurosci. 2025, 85, e70040. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.C.G.D.S.; De Fátima Souto Maior, L.; Gueiros, L.A.M.; Leão, J.C.; Higino, J.S.; Carvalho, A.A.T. Clinical Applicability of Natural Products for Prevention and Treatment of Oral Mucositis: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 4115–4124. [Google Scholar] [CrossRef]
- De Sousa Melo, A.; De Lima Dantas, J.B.; Medrado, A.R.A.P.; Lima, H.R.; Martins, G.B.; Carrera, M. Nutritional Supplements in the Management of Oral Mucositis in Patients with Head and Neck Cancer: Narrative Literary Review. Clin. Nutr. ESPEN 2021, 43, 31–38. [Google Scholar] [CrossRef]
- Nurhidayah, I.; Nurhaeni, N.; Allenidekania, A.; Gayatri, D.; Mediani, H. The Effect of Oral Care Intervention in Mucositis Management Among Pediatric Cancer Patients: An Updated Systematic Review. J. Multidiscip. Healthc. 2024, 17, 3497–3515. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; et al. Systematic Review of Basic Oral Care for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef]
- Miranda-Silva, W.; Da Fonseca, F.P.; Gomes, A.A.; Mafra, A.B.B.; Rocha, V.; Fregnani, E.R. Oral Mucositis in Paediatric Cancer Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation Preventively Treated with Professional Dental Care and Photobiomodulation: Incidence and Risk Factors. Int. J. Paediatr. Dent. 2022, 32, 251–263. [Google Scholar] [CrossRef]
- Kurzrock, D.; Ryan, A. Nutrition Tips for Sore Mouth and Throat; Stanford Health Care: Stanford, CA, USA, 2013; Available online: https://stanfordhealthcare.org/content/dam/SHC/programs-services/cancer-nutrition/docs/sore-mouth-throat-during-cancer-treatment-nutrition-facts.pdf (accessed on 18 September 2025).
- On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); Miranda-Silva, W.; Gomes-Silva, W.; Zadik, Y.; Yarom, N.; Al-Azri, A.R.; Hong, C.H.L.; Ariyawardana, A.; Saunders, D.P.; Correa, M.E.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis: Sub-Analysis of Current Interventions for the Management of Oral Mucositis in Pediatric Cancer Patients. Support. Care Cancer 2021, 29, 3539–3562. [Google Scholar] [CrossRef]
- Sung, L.; Robinson, P.; Treister, N.; Baggott, T.; Gibson, P.; Tissing, W.; Wiernikowski, J.; Brinklow, J.; Dupuis, L.L. Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Children Receiving Treatment for Cancer or Undergoing Haematopoietic Stem Cell Transplantation. BMJ Support. Palliat. Care 2017, 7, 7–16. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Oral Mucositis—Self-Care; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. [Google Scholar]
- The Children’s Hospital of Philadelphia (CHOP). Managing Mucositis in Children; Children’s Hospital of Philadelphia: Philadelphia, PA, USA, 2025. [Google Scholar]
- Shafique, S.; Siddique, H.; Rahim, R.G.; Haroon, H.M. Relationship of Oral Hygiene and Oral Mucositis with Concurrent Chemo-Radiotherapy in Head and Neck Cancers. J. Health Rehabil. Res. 2024, 4, 1209–1213. [Google Scholar] [CrossRef]
- Miller, M.M.; Donald, D.V.; Hagemann, T.M. Prevention and Treatment of Oral Mucositis in Children with Cancer. J. Pediatr. Pharmacol. Ther. 2012, 17, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Y.; Long, J.; Yin, Y.; Fu, Q.; Wang, L.; Patil, S. Enhancing Standardized Practices for Oral Mucositis Prevention in Pediatric Hematopoietic Stem Cell Transplantation: A Best Practice Implementation Project. Risk Manag. Healthc. Policy 2024, 17, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Fregnani, E.R.; Epstein, J.B.; Blijlevens, N.M.A.; Yarom, N.; Villa, A.; Raber-Durlacher, J.E.; Gardner, P.J.; Shem, D.; Beaumont, S.; Bossi, P.; et al. MASCC/ISOO Clinical Practice Statement: Management of Oral Complications of Immunotherapy. Support. Care Cancer 2025, 33, 851. [Google Scholar] [CrossRef]
| Author | Year | Country | Study Design | Number of Patients 1 | Number of Patients in Intervention Group | Age (Median or Mean Age ± SD) | Tumor Type | Assessment Measures | Intervention Group | Control Group | Reference Number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oberbaum | 2001 | USA | Randomized, double-blind, placebo-controlled clinical trial | 30 | 15 | 9.9 ± 6.38 years | NR | WHO | Traumeel S oral rinse (homeopathic complex of 14 herbs/minerals), 5× daily from day + 2 post-SCT for ≥14 days or until OM resolution. | Placebo (saline solution, indistinguishable in appearance and taste) | [40] |
| Aquino | 2005 | USA | Randomized controlled study | 120 | 57 | 9.81 ± 0.81 years | Mixed malignancies | Walsh | Oral glutamine at a dose of 2 g/m2/dose (maximum dose 4 g) administered in a solution of 500 mg/mL twice daily beginning on the day of admission for HSCT. | Oral glycine at a dose of 2 g/m2/dose (maximum dose 4 g) administered in a solution of 500 mg/mL twice daily beginning on the day of admission for HSCT. | [41] |
| El-Housseiny | 2007 | Egypt | Randomized controlled trial (two-arm) | 80 | 80 | 5.75 + 3.38 years | Mixed malignancies | WHO | Group A (topical): 100 mg vitamin E (from 100 IU capsule) emptied into mouth twice daily × 5 days. | Group B (systemic): 100 mg vitamin E capsule swallowed twice daily × 5 days. | [42] |
| Sung | 2007 | Canada | Serial N-of-1 randomized, double-blind, placebo-controlled trials combined with Bayesian meta-analysis | 16 | 16 | 12.7 years (range 6.4–15.1 years) | Pediatric cancers requiring ≥2 identical doxorubicin-containing chemotherapy cycles (Ewing’s sarcoma, lymphoma, osteosarcoma, rhabdomyosarcoma) | Objective mucositis scale, WHO mucositis scale (0–4), VAS pain/swallowing | Topical vitamin E solution (800 mg DL-α-tocopheryl acetate in corn oil, 2 mL once daily × 14 days after each doxorubicin-containing cycle, swish and spit) | Placebo solution (corn oil carrier, identical taste/appearance) | [43] |
| Ward | 2009 | UK | Randomized cross-over study (patients as own controls) | 50 | 50 | 8.7 ± 5.8 years | Mixed malignancies | CTC | Enteral glutamine 0.65 g/kg/day (oral or via NG/gastrostomy) × 7 days during chemo | Identical chemotherapy course without glutamine (self-control design) | [44] |
| Uderzo | 2011 | Italy | Prospective multicenter randomized double-blind controlled trial | 118 | 62 | 8.1 years (range 0.4–18.6) | Mixed malignancies | WHO | GE-TPN containing 0.4 g/kg/day L-alanyl-glutamine dipeptide (≈0.25 g/kg glutamine) | S-TPN without glutamine enrichment | [36] |
| Abdulrham | 2012 | Egypt | Randomized controlled trial | 90 | 60 | 6.9 ± 3.8 years | ALL | NCI-CTC | Group 1: Honey (0.5 g/kg, max 15 g, applied topically TID × 10 days); Group 2: HOPE mixture (honey + olive oil–propolis extract + beeswax, 0.25 g/kg, max 5 g, topically TID × 10 days) | Benzocaine 7.5% gel, TID × 10 days | [45] |
| Khurana | 2013 | India | Randomized single-blind controlled trial (three-arm) | 72 | 48 | Group 1: 8.98 ± 2.58 years Group 2: 9.29 ± 2.58 years Group 3: 9.48 ± 2.53 years | Mixed malignancies | WHO/ChiMES/OMAS | Group II: Vitamin E (200 mg/day topical solution in glycerine, 3× daily × 7 days); Group III: Pycnogenol (pine bark extract, 1 mg/kg/day in glycerine, 3× daily × 7 days) | Group 1: Glycerine solution | [46] |
| Sencer | 2012 | USA and Israel | International multicenter double-blind randomized placebo-controlled trial | 181 | 98 | Intervention group: 12 years (3.24) Control group: 11 years (3.25) | Mixed malignancies | Modified Walsh mucositis scale (daily, days −1 to +20) and WHO scale | Traumeel S oral rinse (complex homeopathic solution with 14 herbal/mineral components), 5× daily from day −1 to day +20 after HSCT | Placebo saline solution (identical ampoules) | [47] |
| Tomaževič | 2013 | Slovenia | Double-blind randomized placebo-controlled trial | 40 | 19 | Intervention group: 6.7 ± 5.3 years Control group: 9.3 ± 6.6 years | Mixed malignancies | OAG, score 3 = severe OM | 70% ethanolic extract of Chinese propolis (0.38 g per application, applied twice daily to vestibular mucosa) | Placebo solution (70% alcohol + caramel dye, matched for color, viscosity, taste) | [48] |
| Raphael | 2014 | The Netherlands | Randomized controlled trial | 29 | 15 | Intervention group: 11.3 ± 3.9 years Control group: 9.9 ± 4.7 years | Mixed malignancies | NCI-CTCAE | Caphosol (supersaturated calcium phosphate rinse), 4× daily during OM episode | Placebo (NaCl 0.9% rinse), identical in appearance and taste | [49] |
| Treister | 2016 | USA, Canada, Australia/NZ | Phase III international multicenter randomized double-blind placebo-controlled trial | 220 | 110 | 13.7 (4.0–21.9) years | Mixed malignancies | WHO Oral Toxicity Scale (grades 0–4); also Mouth Pain Categorical Scale (0–10) and OMDQ | Caphosol oral rinse (supersaturated calcium phosphate electrolyte solution), 4× daily from start of conditioning to day +20 post-HCT | Placebo oral rinse (0.9% saline), same schedule | [37] |
| Al Jaouni | 2017 | India | Randomized controlled trial | 40 | 20 | Intervention group: 7.9 ± 4.1 years Control group: 8.1 ± 4.9 years | Mixed malignancies | WHO | Local Saudi commercial honey, applied topically 4–6× daily to oral mucosa + saline rinse | Standard oral hygiene (lidocaine, Mycostatin, Daktarin gel, mouthwash) without honey | [50] |
| Pourdeghatkar | 2017 | Iran | Randomized double-blind clinical trial | 62 | 31 | Intervention group: 9.9 ± 2.9 years Control group: 9.7 ± 3.01 years | ALL | WHO | Chamomile mouthwash (30 drops diluted in 20 mL water, swish/gargle 1 min, 3× daily for 14 days, starting 1 day before chemotherapy). | Topical mouth rinse (sucralfate, allopurinol, bicarbonate 7.5%, half-saline solution, 20 mL swish 3× daily × 14 days). | [51] |
| Alkhouli | 2019 | Syria | Triple-blind randomized controlled clinical trial | 22 | 11 | Intervention group: 5.4 years Control group: 5.2 years | ALL | WHO | Topical olive oil, swabbed twice daily on oral mucosa (tongue, buccal mucosa, lips, palate) starting 2 days before chemotherapy | Sodium bicarbonate 5% solution | [52] |
| Rathe | 2020 | Denmark | Randomized, double-blind, placebo-controlled trial | 62 | 30 | Intervention group: 4 years (1–15) Control group: 5 years (2–14) | ALL | NCI-CTCAE | Bovine colostrum powder (0.5–1 g/kg/day, reconstituted in water, oral/NG tube, daily × 29 days) | Isocaloric milk protein powder placebo | [53] |
| Widjaja | 2020 | Indonesia | Randomized, double-blind, placebo-controlled trial | 48 | 24 | Intervention group: 6.29 ± 4.42 years Control group: 5.9 ± 2.9 years | ALL | WHO | Oral glutamine at a dose of 400 mg/kg/day started 24 h before methotrexate for 14 days | Placebo not detailed | [54] |
| Alkhouli | 2021 | Syria | Triple-blind randomized controlled clinical trial | 26 | 11 | Intervention group: 4.6 years Control group: 4.8 years | ALL | WHO | 70% Aloe vera solution, applied topically with sponge swab 2× daily starting 3 days before chemo, continued during induction | Sodium bicarbonate 5% (control) | [55] |
| Alkhouli | 2021 | Syria | Randomized controlled three-arm clinical trial (double-blind) | 36 | 22 | Intervention group A: 7.5 years Intervention group B: 8.1 years Placebo group: 6.9 years | ALL | WHO | Group A: Aloe vera 70% solution (swab, 4× daily × 10 days) Group B: Extra virgin olive oil (swab, 4× daily × 10 days) | Sodium bicarbonate 5% solution (swab, 4× daily × 10 days) | [56] |
| Badr | 2023 | Lebanon | Single-blind randomized controlled phase II trial (3 arms) | 46 | 20 | Intervention group 1: 10.89 ± 4.10 years Intervention group 2: 9.63 ± 4.17 years Control group: 9.03 ± 3.98 years | ALL | WHO | Group 1: Manuka honey (2.5 cc, swish 1 min then swallow, TID × 7 days) Group 2: Extra virgin olive oil (2.5 cc, swish 1 min then swallow, TID × 7 days) | Standard care (5 cc 3% sodium bicarbonate + 5 cc Rinsidin, swish and spit, TID × 7 days) | [57] |
| Shah | 2023 | India | Double-blind randomized placebo-controlled trial | 90 | 44 | Intervention group: 6 (4;11) years Control group: 7 (4;10) years | Mixed malignancies | WHO | Oral zinc gluconate syrup, 1 mg/kg/day (max 30 mg), once daily × 14 days. | Placebo syrup (matched for taste, color, and smell). | [58] |
| Author (Year) | Country | Population (n) 1 | Intervention | Comparator | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Aquino (2005) | USA | 120 | Oral glutamine at a dose of 2 g/m2/dose (maximum dose 4 g) administered in a solution of 500 mg/mL twice daily beginning on the day of admission for HSCT. | Oral glycine at a dose of 2 g/m2/dose (maximum dose 4 g) administered in a solution of 500 mg/mL twice daily beginning on the day of admission for HSCT. | A non-significant trend toward reduced average mucositis scores with glutamine (p = 0.07); no difference in maximum mucositis score (p = 0.7). Significant reduction in days of morphine use with glutamine (12.1 vs. 19.3 days, p = 0.01). Significant reduction in days of TPN use with glutamine (17.3 vs. 27.3 days, p = 0.02). No excess toxicity was observed with glutamine compared to glycine. No significant differences in bacteremia episodes (p = 0.9), total hospital days (p = 0.4), or day-100 mortality (p = 0.7). | [41] |
| El-Housseiny (2007) | Egypt | 80 | Group A: 100 mg vitamin E (from 100 IU capsule) emptied into mouth twice daily × 5 days. Group B: 100 mg vitamin E capsule swallowed twice daily × 5 days. | Head-to-head comparison between topical and systemic vitamin E. | Topical group: 80% (24/30) healed completely; significant improvement (p < 0.001). Systemic group: No complete healing, most remained at grade ≥ 1; no significant improvement (p = 0.317). Topical vitamin E was well-tolerated; systemic administration not effective at tested dose. | [42] |
| Ward (2009) | UK | 50 | Enteral glutamine 0.65 g/kg/day (oral or via NG/gastrostomy) × 7 days during chemotherapy | Identical chemotherapy course without glutamine (self-control design) | Fever: Duration significantly shorter with glutamine (5.7 vs. 12.9 days, p = 0.021). Infections: Lower (38% vs. 55%). Severe OM (grade 3–4): Lower with glutamine (29% vs. 55%), but NS (p = 0.118). SOS incidence: Lower in glutamine group (10% vs. 35%), borderline (p = 0.067). Drug-related toxicity: Lower with glutamine (14% vs. 40%, p = 0.085). Engraftment, GVHD, hospital stay, mortality were similar. | [44] |
| Uderzo (2011) | Italy | 118 | GE-TPN containing 0.4 g/kg/day L-alanyl-glutamine dipeptide (≈0.25 g/kg glutamine) | S-TPN without glutamine enrichment | Incidence of OM: 94.8% (S-TPN) vs. 96.7% (GE-TPN), p = 0.68. Severity: No significant differences in OM grade distribution (OR 1.73, 95% CI 0.27–11.27). Analgesic use: Duration and type of opioids/analgesics similar. Engraftment, infections, GVHD, TRM (8.6% vs. 11.7%), relapse (17.2% vs. 8.3%), and hospital stay all comparable. Weight, albumin, prealbumin, cholinesterase values unchanged between groups. No adverse effects or increased relapse risk with glutamine. | [36] |
| Rathe (2020) | Denmark | 62 | Bovine colostrum powder (0.5–1 g/kg/day, reconstituted in water, oral/NG tube, daily × 29 days) | Isocaloric milk protein powder placebo | No difference in fever days (median 0 in both groups). Oral mucositis: Peak severity lower with colostrum (p = 0.02); fewer severe cases (3% vs. 23% in placebo). Patient-reported OMDQ: Sensitivity analysis showed lower severity in colostrum group (p = 0.009). No differences in bacteraemia, IV antibiotics, or treatment delays. | [53] |
| Widjaja (2020) | Indonesia | 48 | Oral glutamine at a dose of 400 mg/kg/day started 24 h before methotrexate for 14 days | Placebo not detailed | Oral glutamine (400 mg/kg/day) significantly reduced oral mucositis in children with ALL receiving high-dose methotrexate, with an incidence of 4.2% vs. 62.5% for placebo (p = 0.001; OR 0.026, 95% CI 0.003–0.228). No severe (grade 3–4) cases occurred in the glutamine group. Hospital stay was shorter (7.7 vs. 12 days; p = 0.005). No adverse effects were reported. | [54] |
| Badr (2023) | Lebanon | 46 | Group 1: Manuka honey (2.5 cc, swish 1 min then swallow, TID × 7 days) Group 2: Extra virgin olive oil (2.5 cc, swish 1 min then swallow, TID × 7 days) | Standard care (5 cc 3% sodium bicarbonate + 5 cc Rinsidin, swish and spit, TID × 7 days) | On day 7, both honey and olive oil groups had significantly lower OM grades vs. control (p = 0.01). Honey group had the lowest pain scores on VAS (p = 0.00), superior to both olive oil and control. Olive oil reduced pain compared to control but less than honey. Children tolerated honey better than olive oil (taste acceptance issue for olive oil) | [57] |
| Shah (2023) | India | 90 | Oral zinc gluconate syrup, 1 mg/kg/day (max 30 mg), once daily × 14 days. | Placebo syrup (matched for taste, color, and smell) | Incidence of OM: 20.5% (zinc) vs. 19.6% (placebo), p = 0.91. Severity: No significant differences between groups (p = 0.79). Onset: Slightly delayed in zinc (5.2 days) vs. placebo (3.8 days), not significant (p = 0.09). Duration: 5.6 vs. 7.1 days (p = 0.18). Hospitalization: 6.8% vs. 8.7%. Well-tolerated, no adverse effects linked to zinc. | [58] |
| Author (Year) | Country | Population (n) 1 | Intervention | Comparator | Key Findings | Reference |
|---|---|---|---|---|---|---|
| El-Housseiny (2007) | Egypt | 80 | Group A: 100 mg vitamin E (from 100 IU capsule) emptied into mouth twice daily × 5 days. Group B: 100 mg vitamin E capsule swallowed twice daily × 5 days. | Head-to-head comparison between topical and systemic vitamin E. | Topical group: 80% (24/30) healed completely; significant improvement (p < 0.001). Systemic group: No complete healing, most remained at grade ≥ 1; no significant improvement (p = 0.317). Topical vitamin E was well-tolerated; systemic administration not effective at tested dose. | [42] |
| Abdulrhman (2012) | Egypt | 90 | Group 1: Honey (0.5 g/kg, max 15 g, applied topically TID × 10 days); Group 2: HOPE mixture (honey + olive oil–propolis extract + beeswax, 0.25 g/kg, max 5 g, topically TID × 10 days) | Benzocaine 7.5% gel, TID × 10 days | Grade 2 mucositis: Recovery time significantly shorter with honey (3.6 days) vs. HOPE (4.2 days) and control (4.6 days) (p < 0.05). Grade 3 mucositis: Recovery time honey (5.4 days) vs. HOPE (5.8 days) faster than control (8.6 days, p < 0.01). Honey healed faster than both HOPE and control (p < 0.01). Adverse effects: HOPE caused transient burning in 27% due to propolis; honey was well-tolerated. | [45] |
| Khurana (2013) | India | 72 | Group II: Vitamin E (200 mg/day topical solution in glycerine, 3× daily × 7 days) Group III: Pycnogenol (pine bark extract, 1 mg/kg/day in glycerine, 3× daily × 7 days) | Group 1: Glycerine solution | Complete healing in 75% Vit E, 58.3% Pycnogenol, vs. 4.2% control (p < 0.001). OMAS: Significant reduction in scores in Vit E and Pycnogenol vs. control (p < 0.001); no difference between Vit E vs. Pycnogenol. ChIMES: Pain reduction significant in Vit E and Pycnogenol vs. control (p < 0.01 from day 4 onwards). Severe OM (grade 4): Pycnogenol less effective (no difference from control), while Vit E showed significant benefit (p = 0.006). Both agents were well-tolerated; isolated vomiting episodes likely chemo-related. | [46] |
| Tomaževič (2013) | Slovenia | 40 | 70% ethanolic extract of Chinese propolis (0.38 g per application, applied twice daily to vestibular mucosa) | Placebo solution (70% alcohol + caramel dye, matched for color, viscosity, taste) | Severe OM: 42% (propolis) vs. 48% (placebo). No significant difference in frequency, duration, or severity of severe OM (p = 0.59). | [48] |
| Al Jaouni (2017) | India | 40 | Local Saudi commercial honey, applied topically 4–6× daily to oral mucosa + saline rinse | Standard oral hygiene (lidocaine, Mycostatin, Daktarin gel, mouthwash) without honey | Grade III–IV OM: 20% honey vs. 55% control (ARR 35%, NNT = 2, p = 0.02). Candida colonization: 10% honey vs. 60% control (ARR 50%, NNT = 2, p = 0.003). Aerobic bacterial infection: 10% honey vs. 60% control (ARR 50%, NNT = 2, p = 0.003). Hospital stay (per OM episode): 7 days (honey) vs. 13 days (control), p < 0.001. Body weight: Mean gain 35% (honey) vs. 15% (control), p < 0.001. Pain: Delayed onset, reduced severity with honey. | [50] |
| Alkhouli (2019) | Syria | 22 | Topical olive oil, swabbed twice daily on oral mucosa (tongue, buccal mucosa, lips, palate) starting 2 days before chemotherapy | Sodium bicarbonate 5% solution (swab, 4× daily × 10 days) | OM developed in 3/11 olive oil vs. 11/11 sodium bicarbonate patients. Olive oil group: onset of OM significantly later (mean week 4.33 vs. 2.27; p = 0.022). Severity: OM grades significantly less severe in olive oil group from week 2 through week 8 (p < 0.01). No cases of grade 4 OM in olive oil group. | [52] |
| Alkhouli (2021) | Syria | 26 | 70% Aloe vera solution, applied topically with sponge swab 2× daily starting 3 days before chemo, continued during induction | Sodium bicarbonate 5% (swab, 4× daily × 10 days) | Aloe vera group developed OM later (mean week 4.3) vs. sodium bicarbonate (week 2.3), p = 0.001. Lower severity of OM in Aloe vera group at weeks 2, 3, 4, and 7 (p < 0.05). No differences at weeks 1, 5, 6, 8. | [55] |
| Alkhouli (2021) | Syria | 36 | Group A: Aloe vera 70% solution (swab, 4× daily × 10 days) Group B: Extra virgin olive oil (swab, 4× daily × 10 days) | Sodium bicarbonate 5% solution (swab, 4× daily × 10 days) | Aloe vera and olive oil groups both showed significant OM improvement (p = 0.007, p = 0.002). Sodium bicarbonate showed no improvement (p = 0.414). No significant difference between Aloe vera vs. olive oil, or Aloe vera vs. Sodium bicarbonate. Olive oil significantly better than sodium bicarbonate (p < 0.05). | [56] |
| Badr (2023) | Lebanon | 46 | Group 1: Manuka honey (2.5 cc, swish 1 min then swallow, TID × 7 days) Group 2: Extra virgin olive oil (2.5 cc, swish 1 min then swallow, TID × 7 days) | Standard care (5 cc 3% sodium bicarbonate + 5 cc Rinsidin, swish and spit, TID × 7 days) | On day 7, both honey and olive oil groups had significantly lower OM grades vs. control (p = 0.01). Honey group had the lowest pain scores on VAS (p = 0.00), superior to both olive oil and control. Olive oil reduced pain compared to control but less than honey. Children tolerated honey better than olive oil (taste acceptance issue for olive oil) | [57] |
| Author (Year) | Country | Population (n) 1 | Intervention | Comparator | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Oberbaum (2001) | Israel | 30 | Traumeel S oral rinse (homeopathic complex of 14 herbs/minerals), 5× daily from day +2 post-SCT for ≥14 days or until OM resolution | Placebo (saline solution, indistinguishable in appearance and taste) | Incidence: 33% Traumeel group did not develop OM vs. 7% placebo. Worsening of OM: 47% Traumeel vs. 93% placebo (p < 0.01). Mean AUC mucositis score: 10.4 (Traumeel) vs. 24.3 (Placebo), p < 0.01. Time to worsening: Significantly delayed with Traumeel (p < 0.001). Subjective symptoms (pain, dryness, dysphagia): Lower in Traumeel group. Safety: Well-tolerated; nausea led 2 patients to discontinue after one dose. | [40] |
| Sung (2007) | Canada | 16 | Topical vitamin E solution (800 mg DL-α-tocopheryl acetate in corn oil, 2 mL once daily × 14 days after each doxorubicin-containing cycle, swish and spit) | Placebo solution (corn oil carrier, identical taste/appearance) | Primary endpoint (objective mucositis score): Mean 0.2 (Vit E) vs. 0.3 (Placebo), ratio 0.90 (95% CR 0.57–1.37), probability Vit E better = 73% (not meeting pre-defined efficacy threshold >95%). Clinically significant reduction (>20%): Only 35% probability with vitamin E. Secondary outcomes: No significant differences in WHO mucositis scores, pain, swallowing difficulty, or opioid/hydration/TPN requirements. Compliance: Lower in vitamin E group (79% vs. 89%). Safety: No unexpected toxicities; oily texture led to acceptability issues. | [43] |
| Sencer (2012) | USA and Israel | 181 | Traumeel S oral rinse (complex homeopathic solution with 14 herbal/mineral components), 5× daily from day –1 to day +20 after HSCT | Placebo saline solution (identical ampoules) | Primary endpoint (AUC Walsh score): No significant difference (76.7 Traumeel vs. 67.3 Placebo, p = 0.13). WHO OM scores: No significant difference (AUC 24.4 Traumeel vs. 21.6 Placebo, p = 0.24). Narcotic use: Trend lower with Traumeel (17.7 vs. 28.5 mg/kg morphine equivalents, p = 0.2). TPN days: Similar (15.3 vs. 15.2). NG feeding: Similar (11 vs. 9 patients). Adverse events: Similar across groups (GI, cardiac, infection, bleeding, GVHD, VOD). Mortality (30 days): 17% Traumeel vs. 14% Placebo (NS). Compliance: Variable; many centers had low adherence, but subgroup analyses still showed no benefit. | [47] |
| Raphael (2014) | The Neatherlands | 29 | Caphosol (supersaturated calcium phosphate rinse), 4× daily during OM episode | Placebo (NaCl 0.9% rinse), identical in appearance and taste | Days with OM > grade 1: 9.9 (Caphosol) vs. 6.4 (Placebo), p = 0.154. Total OM duration: Trend longer in Caphosol group (15.8 vs. 10.2 days). Pain: More days with pain in Caphosol (11.3 vs. 7.3, p = 0.043). Analgesic use: Longer in Caphosol group (15.5 vs. 9.1 days, p = 0.035). Other outcomes (tube/TPN feeding, blood cultures, morphine use): No significant differences. | [49] |
| Treister (2016) | USA, Canada, Australia/NZ | 220 | Caphosol oral rinse (supersaturated calcium phosphate electrolyte solution), 4× daily from start of conditioning to day +20 post-HCT | Placebo oral rinse (0.9% saline), same schedule | Primary endpoint (duration of severe OM, WHO grade 3–4): No difference (4.5 days both arms, p = 0.99). Incidence severe OM: 63% Caphosol vs. 68% placebo (p = 0.44). Pain/OMDQ scores: No differences between groups. Opioid use: Similar incidence, dose, and duration. TPN: Required in 72% Caphosol vs. 78% placebo (p = 0.30); mean duration 11.4 vs. 13.6 days. Infections: Febrile neutropenia, invasive bacterial infection similar. Adverse events: None attributed to rinses; 3 deaths unrelated to study drug. Compliance: 72% completed ≥2 rinses/day; compliance lower in younger children. | [37] |
| Pourdeghatkar (2017) | Iran | 62 | Chamomile mouthwash (30 drops diluted in 20 mL water, swish/gargle 1 min, 3× daily for 14 days, starting 1 day before chemotherapy) | Topical mouth rinse (sucralfate, allopurinol, bicarbonate 7.5%, half-saline solution, 20 mL swish 3× daily × 14 days). | Day 7: No significant difference in OM severity between groups (p = 0.46). Day 14: Chamomile group had significantly lower OM severity vs. topical rinse group (Z = 3.23, p = 0.001). Overall, chamomile mouthwash was more effective than the comparator in reducing OM after 2 weeks. Both interventions were safe and tolerated. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horhat, R.M.; Alexandru, A.; Ivan, C.-S.; Varga, N.-I.; Suba, M.-I.; Ciurariu, E.; Susan, M.; Susan, R.; Cote, A. Nutritional and Supplemental Interventions for Prevention and Treatment of Oral Mucositis in Pediatric Oncology. Nutrients 2025, 17, 3521. https://doi.org/10.3390/nu17223521
Horhat RM, Alexandru A, Ivan C-S, Varga N-I, Suba M-I, Ciurariu E, Susan M, Susan R, Cote A. Nutritional and Supplemental Interventions for Prevention and Treatment of Oral Mucositis in Pediatric Oncology. Nutrients. 2025; 17(22):3521. https://doi.org/10.3390/nu17223521
Chicago/Turabian StyleHorhat, Razvan Mihai, Alexandru Alexandru, Cristiana-Smaranda Ivan, Norberth-Istvan Varga, Madalina-Ianca Suba, Elena Ciurariu, Monica Susan, Razvan Susan, and Adrian Cote. 2025. "Nutritional and Supplemental Interventions for Prevention and Treatment of Oral Mucositis in Pediatric Oncology" Nutrients 17, no. 22: 3521. https://doi.org/10.3390/nu17223521
APA StyleHorhat, R. M., Alexandru, A., Ivan, C.-S., Varga, N.-I., Suba, M.-I., Ciurariu, E., Susan, M., Susan, R., & Cote, A. (2025). Nutritional and Supplemental Interventions for Prevention and Treatment of Oral Mucositis in Pediatric Oncology. Nutrients, 17(22), 3521. https://doi.org/10.3390/nu17223521






