Fisetin Inhibits Periodontal Pathogen-Induced EMT in Oral Squamous Cell Carcinoma via the Wnt/β-Catenin Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial and Cell Culture
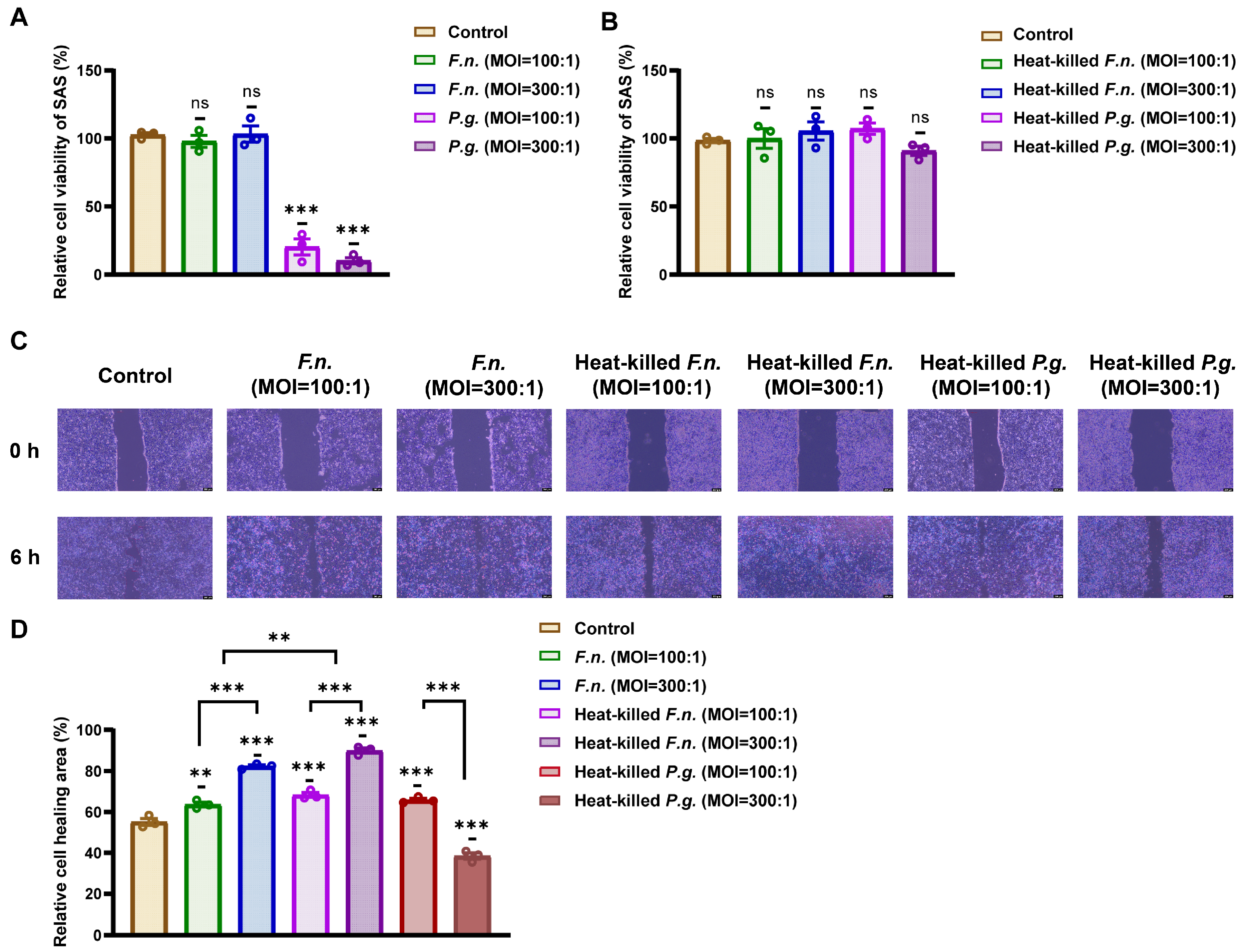
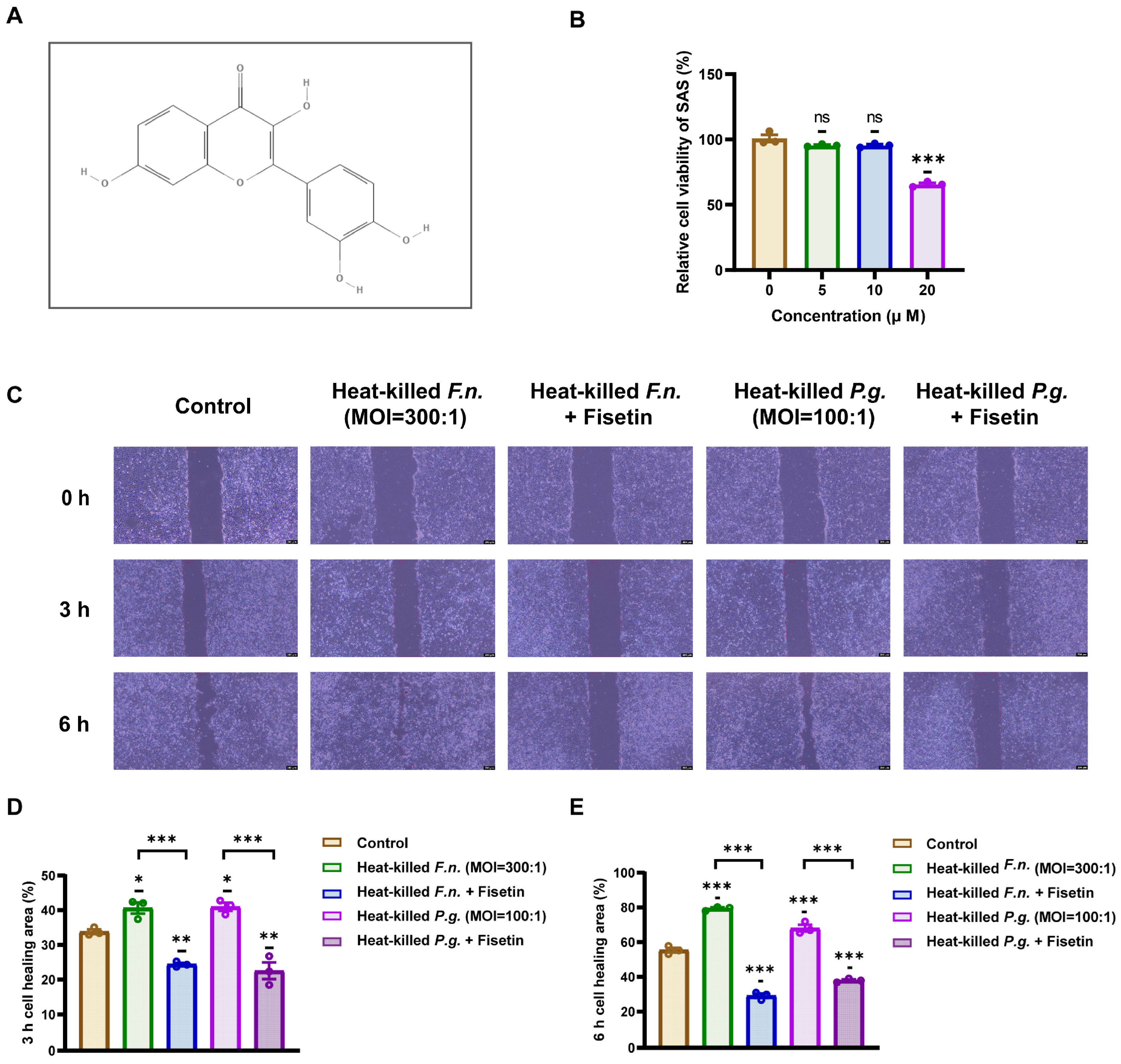
2.3. Cell Viability Assay
2.4. Wound-Healing Assay
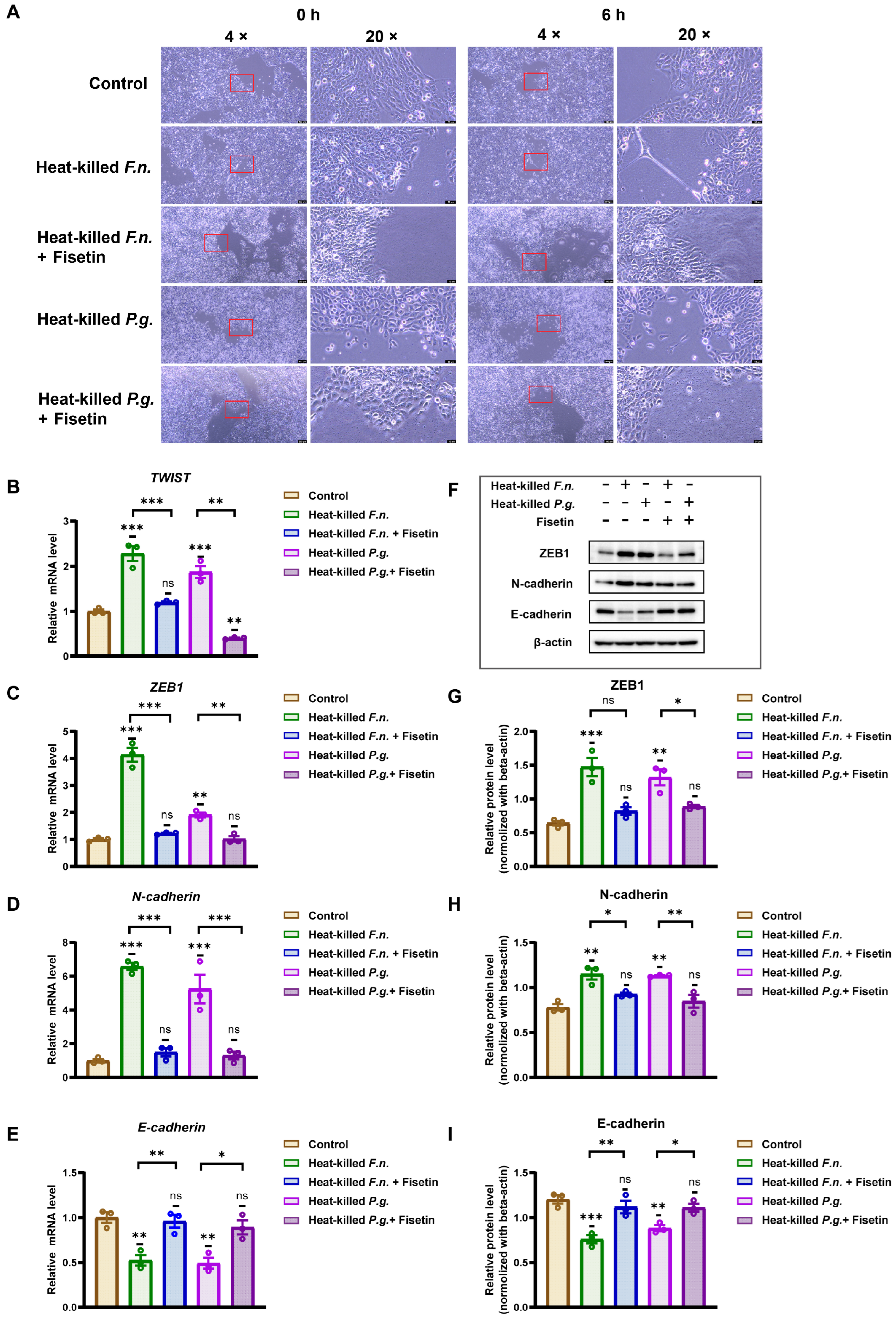
2.5. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction
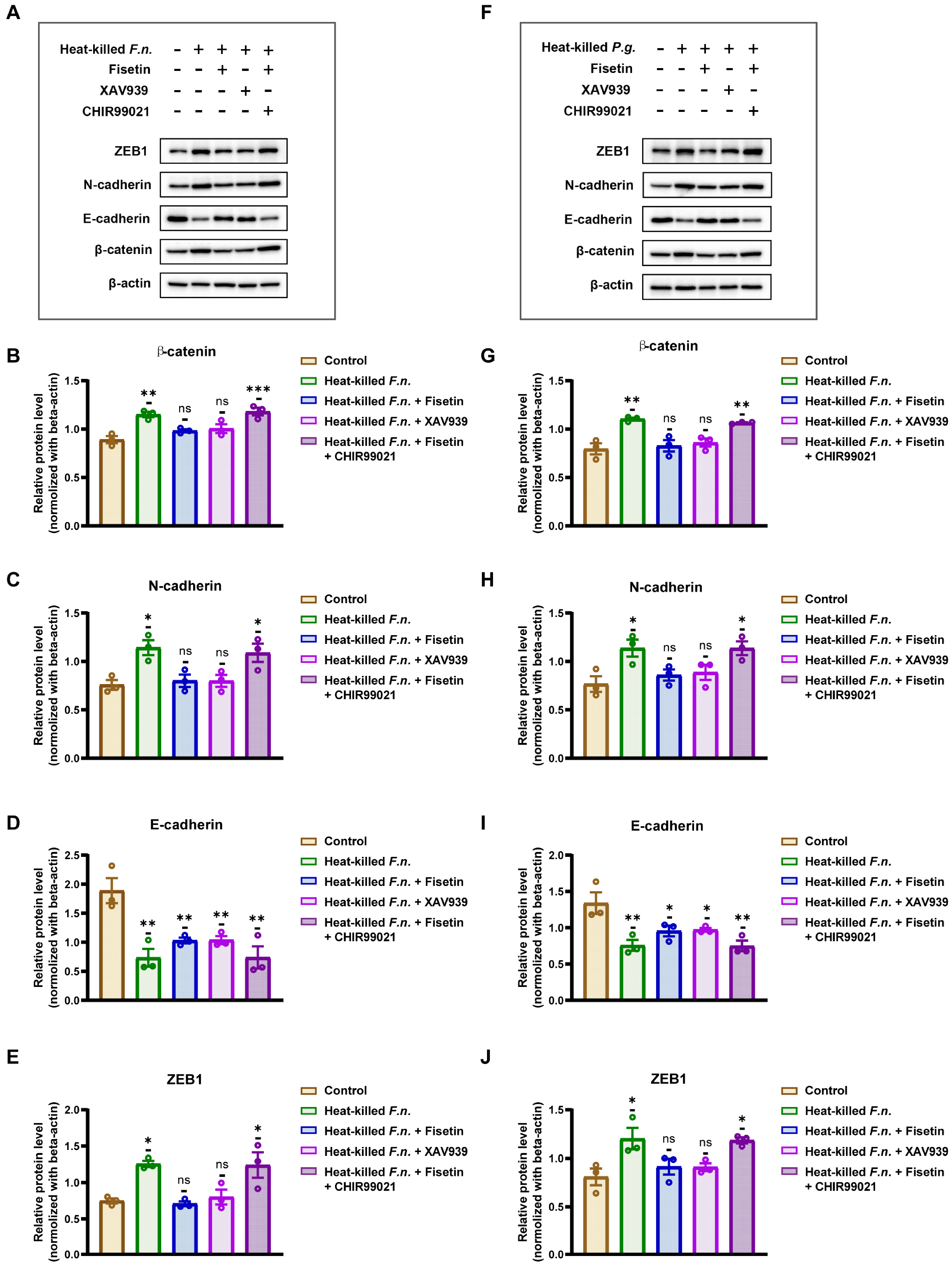
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Heat-Killed F. nucleatum and P. gingivalis Promote OSCC Cell Migration
3.2. Fisetin Inhibits OSCC Cell Migration Induced by Heat-Killed Periodontal Pathogens
3.3. Heat-Killed Forms of F. nucleatum and P. gingivalis Can Induce the EMT Process in OSCC Cells, but Fisetin Inhibits This Effect
3.4. Fisetin Inhibits Periodontal Pathogen-Induced EMT Processes in OSCC via the Wnt/β-Catenin Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rotta, R.F.; Rosa, E.A.; Milani, V.; Dias, N.R.; Masterson, D.; da Silva, E.N.; Zara, A. The cost of oral cancer: A systematic review. PLoS ONE 2022, 17, e0266346. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Zhang, Q.; Guo, Z.M.; Chen, W.K.; Liu, W.W.; Chen, Y.F.; Li, Q.L.; Liu, X.K.; Li, H.; Ou-Yang, D.; et al. Trends in clinical features and survival of oral cavity cancer: Fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag. Res. 2018, 10, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Wei, X.; Liu, R.; Li, W.; Yu, Q.; Yang, Q.T.; Li, T. Advances in research regarding epithelial-mesenchymal transition and prostate cancer. Front. Cell Dev. Biol. 2025, 13, 1583255. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Ciani, L.; Libonati, A.; Dri, M.; Pomella, S.; Campanella, V.; Barillari, G. About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview. Int. J. Mol. Sci. 2024, 25, 5083. [Google Scholar] [CrossRef]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int. J. Cancer 2019, 145, 775–784. [Google Scholar] [CrossRef]
- Zang, W.; Geng, F.; Liu, J.; Wang, Z.; Zhang, S.; Li, Y.; Lu, Z.; Pan, Y. Porphyromonas gingivalis potentiates stem-like properties of oral squamous cell carcinoma by modulating SCD1-dependent lipid synthesis via NOD1/KLF5 axis. Int. J. Oral Sci. 2025, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K.L. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J. Oral Microbiol. 2020, 13, 1849493. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Fujiwara, N.; Mouri, Y.; Kisoda, S.; Yoshida, K.; Yoshida, K.; Yumoto, H.; Ozaki, K.; Ishimaru, N.; Kudo, Y. Conversion from epithelial to partial-EMT phenotype by Fusobacterium nucleatum infection promotes invasion of oral cancer cells. Sci. Rep. 2021, 11, 14943. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Flores, T.; Betancur, D.; Peña-Oyarzún, D.; Torres, V.A. Wnt/β-Catenin Signaling in Oral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 4682. [Google Scholar] [CrossRef]
- Da, J.; Wang, X.; Li, L.; Xu, Y. Fusobacterium nucleatum Promotes Cisplatin-Resistance and Migration of Oral Squamous Carcinoma Cells by Up-Regulating Wnt5a-Mediated NFATc3 Expression. Tohoku J. Exp. Med. 2021, 253, 249–259. [Google Scholar] [CrossRef]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin-In Search of Better Bioavailability-From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological effects and mechanisms of fisetin in cancer: A promising anti-cancer agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Shelton, R.M.; Landini, G.; Cooper, P.R.; Milward, M.R. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adhes. Migr. 2018, 12, 127–137. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Mashimo, C.; Nambu, T.; Maruyama, H.; Takigawa, H.; Okinaga, T. Resveratrol is an inhibitory polyphenol of epithelial-mesenchymal transition induced by Fusobacterium nucleatum. Arch. Oral Biol. 2024, 160, 105897. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, Y.; Shi, X.; Zhao, Y.; Tang, Y.; Liu, S.; Zhu, X. Porphyromonas gingivalis outer membrane vesicles augments proliferation and metastasis of oral squamous cell carcinoma cells. BMC Oral Health 2025, 25, 701. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, Y.; Yin, Y.; Wang, L.; Yang, H.; Luo, S.; Zheng, Q.; Pan, Y.; Zhang, D. Potential role of epithelial-mesenchymal transition induced by periodontal pathogens in oral cancer. J. Cell. Mol. Med. 2024, 28, e18064. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.P. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer 2011, 11, 49. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Katz, J.; Yang, Q.B.; Zhang, P.; Potempa, J.; Travis, J.; Michalek, S.M.; Balkovetz, D.F. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 2002, 70, 2512–2518. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Yu, J.; Chen, T.; Wu, Y.; Shi, L.; Li, Q.; Wu, J.; Fu, X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 2017, 8, 31802–31814. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Yao, Q.; Liu, Y.; Du, S.; Liu, A.; Guo, Z.; Sun, A.; Ruan, J.; Chen, L.; Ye, C.; et al. IL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int. J. Oncol. 2012, 40, 1171–1179. [Google Scholar] [PubMed]
- Zhao, Z.; Wang, S.; Lin, Y.; Miao, Y.; Zeng, Y.; Nie, Y.; Guo, P.; Jiang, G.; Wu, J. Epithelial-mesenchymal transition in cancer: Role of the IL-8/IL-8R axis. Oncol. Lett. 2017, 13, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eichhorn, P.J.A.; Thiery, J.P. TGF-β, EMT, and resistance to anti-cancer treatment. Semin. Cancer Biol. 2023, 97, 1–11. [Google Scholar] [CrossRef]
- Herath, T.D.; Darveau, R.P.; Seneviratne, C.J.; Wang, C.Y.; Wang, Y.; Jin, L. Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated Nf-κB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PLoS ONE 2013, 8, e58496. [Google Scholar] [CrossRef]
- Laheij, A.M.; van Loveren, C.; Deng, D.; de Soet, J.J. The impact of virulence factors of Porphyromonas gingivalis on wound healing in vitro. J. Oral Microbiol. 2015, 7, 27543. [Google Scholar] [CrossRef]
- Yee, M.; Kim, A.; Alpagot, T.; Düzgüneş, N.; Konopka, K. Porphyromonas gingivalis stimulates IL-18 secretion in human monocytic THP-1 cells. Microbes Infect. 2012, 14, 684–689. [Google Scholar] [CrossRef]
- Berker, E.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Blocking proinflammatory cytokine release modulates peripheral blood mononuclear cell response to Porphyromonas gingivalis. J. Periodontol. 2013, 84, 1337–1345. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Tabasum, S.; Singh, R.P. Fisetin suppresses migration, invasion and stem-cell-like phenotype of human non-small cell lung carcinoma cells via attenuation of epithelial to mesenchymal transition. Chem. Biol. Interact. 2019, 303, 14–21. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, W.; Dang, M.; Yan, M.; Chen, Z. Fisetin induces apoptosis in breast cancer MDA-MB-453 cells through degradation of HER2/neu and via the PI3K/Akt pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22268. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Trigun, S.K. Fisetin induces apoptosis in colorectal cancer cells by suppressing autophagy and down-regulating nuclear factor erythroid 2-related factor 2 (Nrf2). J. Cell. Biochem. 2023, 124, 1289–1308. [Google Scholar] [CrossRef] [PubMed]
- Agraval, H.; Sharma, J.R.; Prakash, N.; Yadav, U.C.S. Fisetin suppresses cigarette smoke extract-induced epithelial to mesenchymal transition of airway epithelial cells through regulating COX-2/MMPs/β-catenin pathway. Chem. Biol. Interact. 2022, 351, 109771. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Long, H.J.; Miao, X.Y.; Liu, G.L.; Yao, H.L. Fisetin inhibits liver cancer growth in a mouse model: Relation to dopamine receptor. Oncol. Rep. 2017, 38, 53–62. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Karunarathne, W.; Park, S.R.; Choi, Y.H.; Park, E.K.; Jin, C.Y.; Yu, H.; Jo, W.S.; Lee, K.T.; Kim, G.Y. GSK-3β-Targeting Fisetin Promotes Melanogenesis in B16F10 Melanoma Cells and Zebrafish Larvae Through β-Catenin Activation. Int. J. Mol. Sci. 2020, 21, 312. [Google Scholar] [CrossRef]
- Fu, X.; Ma, J.; Ma, F.; Guo, S.; Wang, X.; Li, Y.; Tang, Y.; Qi, J.; Zhang, W.; Ye, L. Misp-mediated enhancement of pancreatic cancer growth through the Wnt/β-catenin signaling pathway is suppressed by Fisetin. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167515. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Taheri, N.; Ramadan, M.F.; Mustafa, Y.F.; Alkhayyat, S.; Sergeevna, K.N.; Alsaab, H.O.; Hjazi, A.; Molavi Vasei, F.; Daneshvar, S. A comprehensive view on the fisetin impact on colorectal cancer in animal models: Focusing on cellular and molecular mechanisms. Anim. Models Exp. Med. 2024, 7, 591–605. [Google Scholar] [CrossRef]
- Henschke, A.; Grześkowiak, B.; Ivashchenko, O.; Sánchez-Cerviño, M.C.; Coy, E.; Moya, S. Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect. Int. J. Mol. Sci. 2025, 26, 7489. [Google Scholar] [CrossRef]
- Youssef, J.R.; Boraie, N.A.; Ismail, F.A.; Bakr, B.A.; Allam, E.A.; Agami, M.A.; El-Moslemany, R.M. Mannosylated fisetin/carveol lipid nanocapsules: Brain-targeted dual therapy for modulation of epileptogenesis and cognitive deficits. Drug Deliv. Transl. Res. 2025. [Google Scholar] [CrossRef]
| Gene Symbol | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| E-cadherin | CGAGAGCTACACGTTCACGG | GGGTGTCGAGGGAAAAATAGG |
| N-cadherin | TCAGGCGTCTGTAGAGGCTT | ATGCACATCCTTCGATAAGACTG |
| ZEB1 | CACATGCGATTACATTCTGGAG | CGTGCTCATTCGAGAGGATT |
| TWIST | TGCGGAAGATCATCCCCACG | GCTGCAGCTTGCCATCTTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Takigawa, H.; Maruyama, H.; Nambu, T.; Mashimo, C.; Okinaga, T. Fisetin Inhibits Periodontal Pathogen-Induced EMT in Oral Squamous Cell Carcinoma via the Wnt/β-Catenin Pathway. Nutrients 2025, 17, 3522. https://doi.org/10.3390/nu17223522
Zhang R, Takigawa H, Maruyama H, Nambu T, Mashimo C, Okinaga T. Fisetin Inhibits Periodontal Pathogen-Induced EMT in Oral Squamous Cell Carcinoma via the Wnt/β-Catenin Pathway. Nutrients. 2025; 17(22):3522. https://doi.org/10.3390/nu17223522
Chicago/Turabian StyleZhang, Ruoyao, Hiroki Takigawa, Hugo Maruyama, Takayuki Nambu, Chiho Mashimo, and Toshinori Okinaga. 2025. "Fisetin Inhibits Periodontal Pathogen-Induced EMT in Oral Squamous Cell Carcinoma via the Wnt/β-Catenin Pathway" Nutrients 17, no. 22: 3522. https://doi.org/10.3390/nu17223522
APA StyleZhang, R., Takigawa, H., Maruyama, H., Nambu, T., Mashimo, C., & Okinaga, T. (2025). Fisetin Inhibits Periodontal Pathogen-Induced EMT in Oral Squamous Cell Carcinoma via the Wnt/β-Catenin Pathway. Nutrients, 17(22), 3522. https://doi.org/10.3390/nu17223522







