The Changes in Plasmalogens: Chemical Diversity and Nutritional Implications—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
3. Structural Changes in Plasmalogens
3.1. Oxidation and Oxidative Degradation
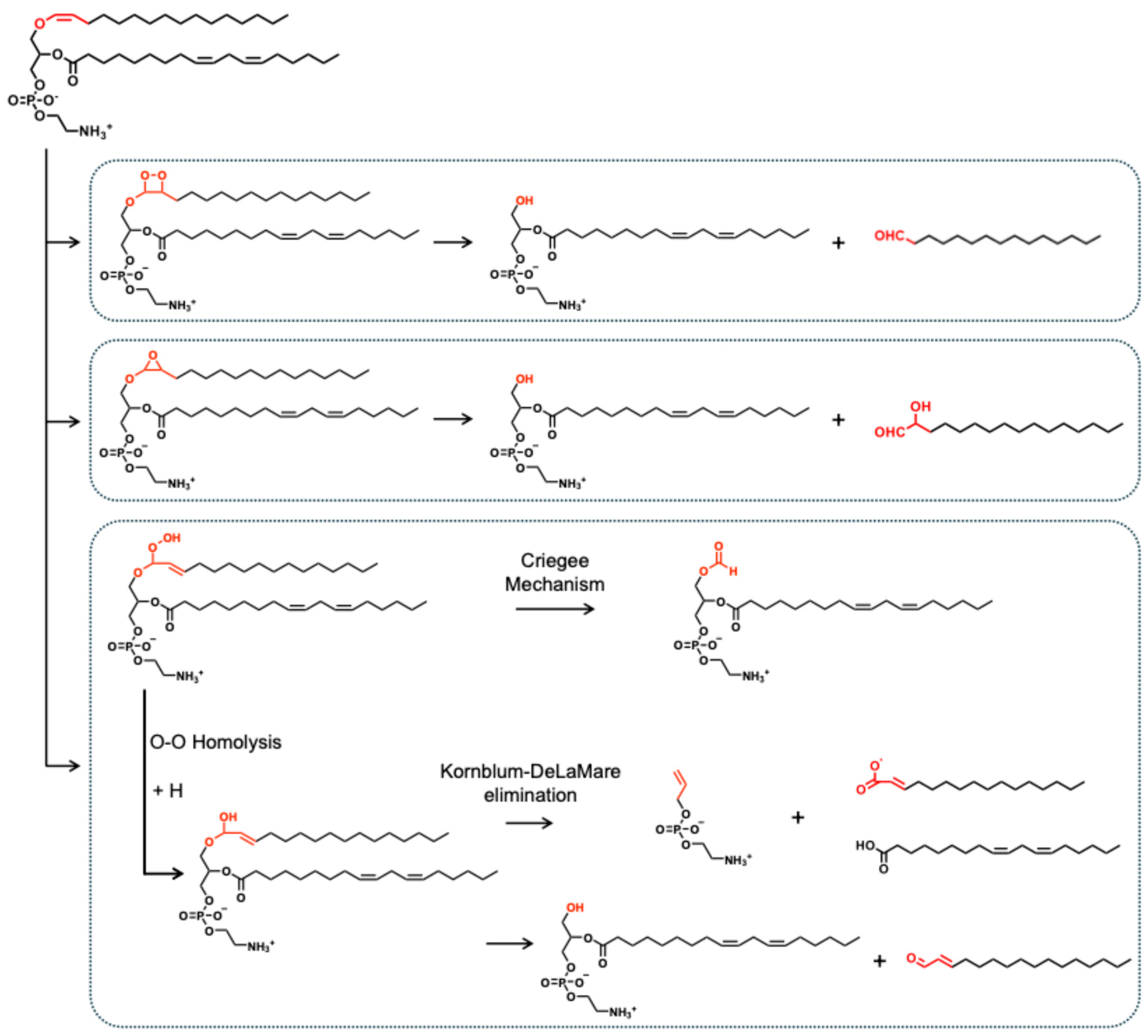
3.1.1. Oxidative Cleavage at the Vinyl Ether Bond of the sn-1 Position
3.1.2. Oxidation of Unsaturated Fatty Acyls in the sn-2 Position of Plasmalogens
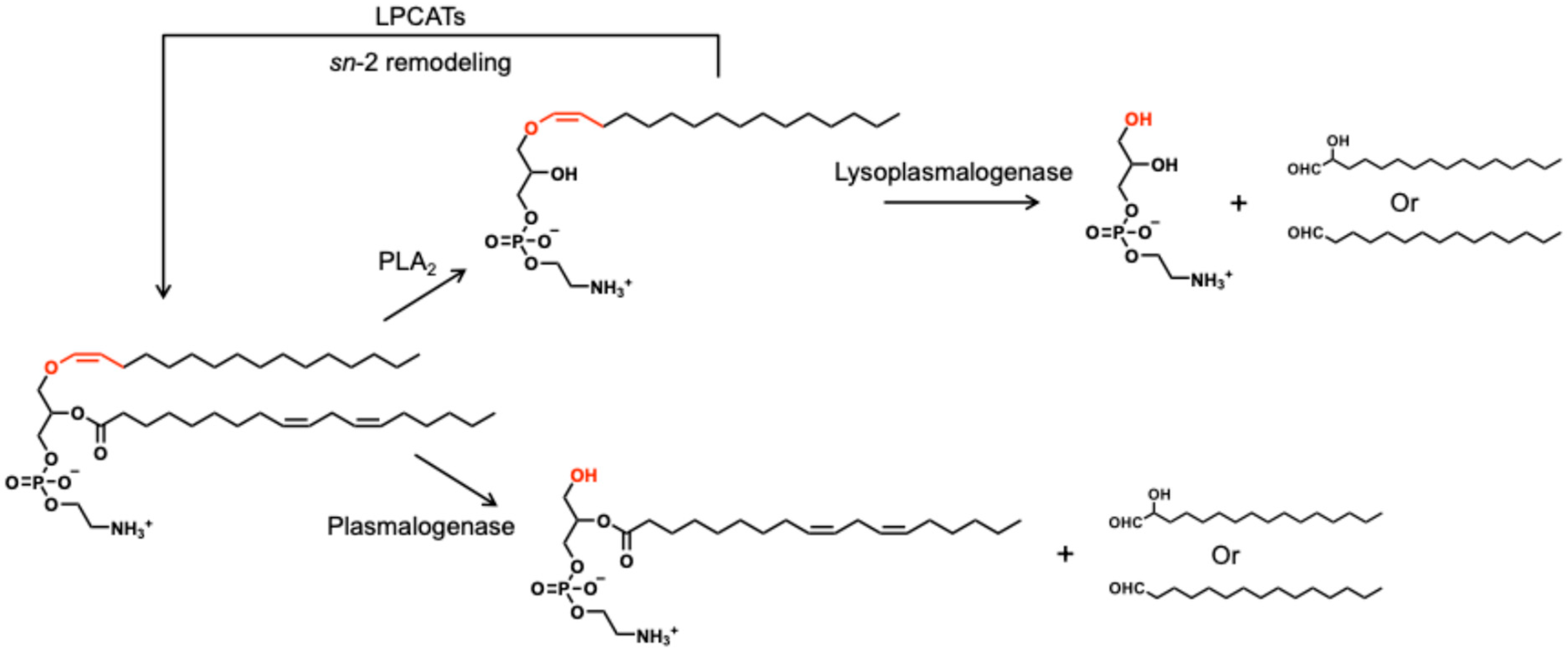
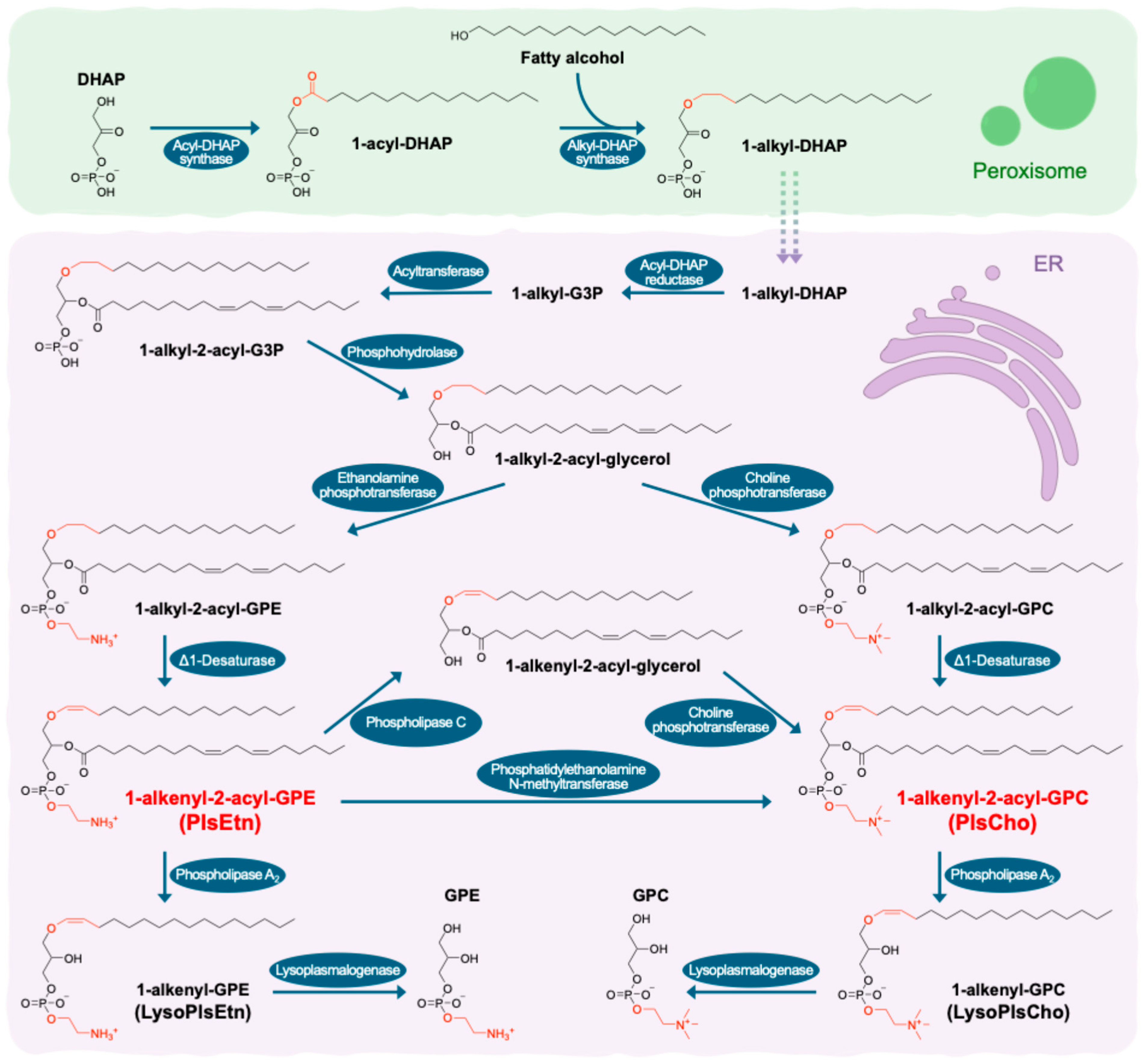
3.2. Enzymatic Degradation and Remodeling
4. Variations in Plasmalogens
4.1. Physiological and Clinical Parameters
4.2. Genetic Diseases with Inherited Plasmalogen Deficiency
4.3. Hepatic Metabolic Dysfunction
4.4. Neurodegenerative Diseases
4.5. Neurodevelopmental Disorders
4.6. Cancers
4.7. Infectious Diseases
4.8. Lifestyles
5. Exogenous Sources of Plasmalogens for Nutritional Supplements
6. Limitations, Challenges, and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ARA | Arachidonic acid |
| CPT | Choline phosphotransferase |
| DHA | Docosahexaenoic acid |
| DHAP | Dihydroxyacetone phosphate |
| EPA | Eicosapentaenoic acid |
| EPT | Ethanolamine phosphotransferase |
| ER | Endoplasmic reticulum |
| G3P | Glycerol-3-phosphate |
| GPC | Glycerophosphocholine |
| GPE | Glycerophosphoethanolamine |
| HDL | High-density lipoprotein |
| HPLC | High-performance liquid chromatography |
| HR-MS | High-resolution mass spectrometry |
| LPC | Lysophosphatidyl choline |
| LPE | Lysophosphatidyl ethanolamine |
| LPCAT | Lysophosphatidylcholine acyltransferase |
| LPLAT | Lysophospholipid acyltransferases |
| MS | Mass spectrometry |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| PC | Phosphatidylcholine |
| PD | Parkinson’s disease |
| PE | Phosphatidylethanolamine |
| PEMT | Phosphatidylethanolamine N-methyltransferase |
| PLA | Phospholipase A |
| PLC | Phospholipase C |
| PlsCho | Plasmalogen choline |
| PlsEtn | Plasmalogen ethanolamine |
| PRT | Plasmalogen replacement therapy |
| PUFA | Polyunsaturated fatty acyl or polyunsaturated fatty acid |
| RCDP | Rhizomelic Chondrodysplasia Punctata |
| ROS | Reactive oxygen species |
| SCZ | Schizophrenia |
| UV | Ultraviolet |
References
- Wu, Y.; Chen, Z.; Jia, J.; Chiba, H.; Hui, S.-P. Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs. Foods 2021, 10, 124. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and Functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; De Oliveira Carvalho, P. Plasmalogen Lipids: Functional Mechanism and Their Involvement in Gastrointestinal Cancer. Lipids Health Dis. 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Pradas, I.; Jové, M.; Huynh, K.; Puig, J.; Ingles, M.; Borras, C.; Viña, J.; Meikle, P.J.; Pamplona, R. Exceptional Human Longevity Is Associated with a Specific Plasma Phenotype of Ether Lipids. Redox Biol. 2019, 21, 101127. [Google Scholar] [CrossRef]
- Honsho, M.; Fujiki, Y. Regulation of Plasmalogen Biosynthesis in Mammalian Cells and Tissues. Brain Res. Bull. 2023, 194, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Brites, P.; Waterham, H.R.; Wanders, R.J.A. Functions and Biosynthesis of Plasmalogens in Health and Disease. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2004, 1636, 219–231. [Google Scholar] [CrossRef]
- Latorre, E.; Collado, M.P.; Fernández, I.; Aragonés, M.D.; Catalán, R.E. Signaling Events Mediating Activation of Brain Ethanolamine Plasmalogen Hydrolysis by Ceramide. Eur. J. Biochem. 2003, 270, 36–46. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of Plasmalogen Lipids in Health and Disease. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Brosche, T.; Brueckmann, M.; Haase, K.K.; Sieber, C.; Bertsch, T. Decreased Plasmalogen Concentration as a Surrogate Marker of Oxidative Stress in Patients Presenting with Acute Coronary Syndromes or Supraventricular Tachycardias. Clin. Chem. Lab. Med. 2007, 45, 689. [Google Scholar] [CrossRef]
- Dorninger, F.; Berger, J.; Honsho, M. Editorial: Solving the Plasmalogen Puzzle—From Basic Science to Clinical Application. Front. Cell Dev. Biol. 2023, 11, 1137868. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Boateng, N.A.S.; Ma, H. Ultrasound-Induced Lipid Peroxidation: Effects on Phenol Content and Extraction Kinetics and Antioxidant Activity of Tartary Buckwheat (Fagopyrum Tataricum) Water Extract. Food Biosci. 2020, 37, 100719. [Google Scholar] [CrossRef]
- Johnson, D.R.; Decker, E.A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid Peroxidation: Mechanisms, Inhibition, and Biological Effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Liu, L.; Zhao, Y.; Bai, F.; Wang, J.; Gao, R.; Xu, X. The Influence Mechanism of Phospholipids Structure and Composition Changes Caused by Oxidation on the Formation of Flavor Substances in Sturgeon Caviar. Food Chem. 2024, 460, 140585. [Google Scholar] [CrossRef] [PubMed]
- Leßig, J.; Fuchs, B. Plasmalogens in Biological Systems: Their Role in Oxidative Processes in Biological Membranes, Their Contribution to Pathological Processes and Aging and Plasmalogen Analysis. Curr. Med. Chem. 2009, 16, 2021–2041. [Google Scholar] [CrossRef] [PubMed]
- Broniec, A.; Klosinski, R.; Pawlak, A.; Wrona-Krol, M.; Thompson, D.; Sarna, T. Interactions of Plasmalogens and Their Diacyl Analogs with Singlet Oxygen in Selected Model Systems. Free Radic. Biol. Med. 2011, 50, 892–898. [Google Scholar] [CrossRef]
- Faria, R.L.; Prado, F.M.; Junqueira, H.C.; Fabiano, K.C.; Diniz, L.R.; Baptista, M.S.; Di Mascio, P.; Miyamoto, S. Plasmalogen Oxidation Induces the Generation of Excited Molecules and Electrophilic Lipid Species. PNAS Nexus 2024, 3, 216. [Google Scholar] [CrossRef]
- Engelmann, B. Plasmalogens: Targets for Oxidants and Major Lipophilic Antioxidants. Biochem. Soc. Trans. 2004, 32, 147–150. [Google Scholar] [CrossRef]
- Stadelmann-Ingrand, S.; Favreliere, S.; Fauconneau, B.; Mauco, G.; Tallineau, C. Plasmalogen Degradation by Oxidative Stress: Production and Disappearance of Specific Fatty Aldehydes and Fatty α-Hydroxyaldehydes. Free Radic. Biol. Med. 2001, 31, 1263–1271. [Google Scholar] [CrossRef]
- Khaselev, N.; Murphy, R.C. Structural Characterization of Oxidized Phospholipid Products Derived from Arachidonate-Containing Plasmenyl Glycerophosphocholine. J. Lipid Res. 2000, 41, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zemski Berry, K.A.; Murphy, R.C. Free Radical Oxidation of Plasmalogen Glycerophosphocholine Containing Esterified Docosahexaenoic Acid: Structure Determination by Mass Spectrometry. Antioxid. Redox Signal. 2005, 7, 157–169. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, J.; Wu, Y.; Chiba, H.; Hui, S.-P. LC/MS Analysis of Storage-Induced Plasmalogen Loss in Ready-to-Eat Fish. Food Chem. 2022, 383, 132320. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Farooqui, T.; Farooqui, A.A. Involvement of Cytosolic Phospholipase A2, Calcium Independent Phospholipase A2 and Plasmalogen Selective Phospholipase A2 in Neurodegenerative and Neuropsychiatric Conditions. Curr. Med. Chem. 2010, 17, 2746–2763. [Google Scholar] [CrossRef]
- Lv, J.; Ma, J.; Liu, Y.; Li, P.; Wang, D.; Geng, Z.; Xu, W. Lipidomics Analysis of Sanhuang Chicken during Cold Storage Reveals Possible Molecular Mechanism of Lipid Changes. Food Chem. 2023, 417, 135914. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoon, Y.C.; Im, H.; Son, Y.; Kim, M.; Saha, A.; Choi, C.; Lee, J.; Lee, S.; Kim, J.H.; et al. Adipocyte Lysoplasmalogenase TMEM86A Regulates Plasmalogen Homeostasis and Protein Kinase A-Dependent Energy Metabolism. Nat. Commun. 2022, 13, 4084. [Google Scholar] [CrossRef]
- Wu, L.-C.; Pfeiffer, D.R.; Calhoon, E.A.; Madiai, F.; Marcucci, G.; Liu, S.; Jurkowitz, M.S. Purification, Identification, and Cloning of Lysoplasmalogenase, the Enzyme That Catalyzes Hydrolysis of the Vinyl Ether Bond of Lysoplasmalogen. J. Biol. Chem. 2011, 286, 24916–24930. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Fu, P.; Ramchandran, R.; Ha, A.W.; Putherickal, V.; Sudhadevi, T.; Harijith, A.; Schumacher, F.; Kleuser, B.; Natarajan, V. S1P and Plasmalogen Derived Fatty Aldehydes in Cellular Signaling and Functions. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2020, 1865, 158681. [Google Scholar] [CrossRef]
- Watschinger, K. Plasmalogen. Quo Vadis? J. Lipid Res. 2025, 66, 100814. [Google Scholar] [CrossRef]
- Thomas, C.; Jalil, A.; Magnani, C.; Ishibashi, M.; Queré, R.; Bourgeois, T.; Bergas, V.; Ménégaut, L.; Patoli, D.; Le Guern, N.; et al. LPCAT3 Deficiency in Hematopoietic Cells Alters Cholesterol and Phospholipid Homeostasis and Promotes Atherosclerosis. Atherosclerosis 2018, 275, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cong, P.-X.; Cui, J.; Jiang, S.; Xu, J.; Xue, C.-H.; Huang, Q.-R.; Zhang, T.-T.; Wang, Y.-M. Digestion, Absorption, and Metabolism Characteristics of EPA-Enriched Phosphoethanolamine Plasmalogens Based on Gastrointestinal Functions in Healthy Mice. J. Agric. Food Chem. 2019, 67, 12786–12795. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Epand, R.M. Plasmalogen Replacement Therapy. Membranes 2021, 11, 838. [Google Scholar] [CrossRef]
- Labadaridis, I.; Moraitou, M.; Theodoraki, M.; Triantafyllidis, G.; Sarafidou, J.; Michelakakis, H. Plasmalogen Levels in Full-term Neonates. Acta Paediatr. 2009, 98, 640–642. [Google Scholar] [CrossRef]
- Beyene, H.B.; Olshansky, G.; T. Smith, A.A.; Giles, C.; Huynh, K.; Cinel, M.; Mellett, N.A.; Cadby, G.; Hung, J.; Hui, J.; et al. High-Coverage Plasma Lipidomics Reveals Novel Sex-Specific Lipidomic Fingerprints of Age and BMI: Evidence from Two Large Population Cohort Studies. PLoS Biol. 2020, 18, e3000870. [Google Scholar] [CrossRef]
- Donovan, E.L.; Pettine, S.M.; Hickey, M.S.; Hamilton, K.L.; Miller, B.F. Lipidomic Analysis of Human Plasma Reveals Ether-Linked Lipids That Are Elevated in Morbidly Obese Humans Compared to Lean. Diabetol. Metab. Syndr. 2013, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Maeba, R.; Maeda, T.; Kinoshita, M.; Takao, K.; Takenaka, H.; Kusano, J.; Yoshimura, N.; Takeoka, Y.; Yasuda, D.; Okazaki, T.; et al. Plasmalogens in Human Serum Positively Correlate with High- Density Lipoprotein and Decrease with Aging. J. Atheroscler. Thromb. 2007, 14, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mayneris--Perxachs, J.; Meikle, P.; Mousa, A.; Naderpoor, N.; Fernández--Real, J.M.; De Courten, B. Novel Relationship Between Plasmalogen Lipid Signatures and Carnosine in Humans. Mol. Nutr. Food Res. 2021, 65, 2100164. [Google Scholar] [CrossRef]
- Jefferies, J.L. Barth Syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 198–205. [Google Scholar] [CrossRef]
- Kimura, T.; Kimura, A.; Berno, B.; Ren, M.; Schlame, M.; Epand, R.M. Content of Plasmalogen Lipids Markedly Decreases in Barth Syndrome. Biophys. J. 2016, 110, 84a. [Google Scholar] [CrossRef][Green Version]
- Kimura, T.; Kimura, A.K.; Ren, M.; Monteiro, V.; Xu, Y.; Berno, B.; Schlame, M.; Epand, R.M. Plasmalogen Loss Caused by Remodeling Deficiency in Mitochondria. Life Sci. Alliance 2019, 2, e201900348. [Google Scholar] [CrossRef]
- Bams-Mengerink, A.M.; Koelman, J.H.; Waterham, H.; Barth, P.G.; Poll-The, B.T. The Neurology of Rhizomelic Chondrodysplasia Punctata. Orphanet J. Rare Dis. 2013, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Brodde, A.; Braverman, N.E.; Moser, A.B.; Just, W.W.; Forss-Petter, S.; Brügger, B.; Berger, J. Homeostasis of Phospholipids—The Level of Phosphatidylethanolamine Tightly Adapts to Changes in Ethanolamine Plasmalogens. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2015, 1851, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Duker, A.L.; Niiler, T.; Eldridge, G.; Brereton, N.H.; Braverman, N.E.; Bober, M.B. Growth Charts for Individuals with Rhizomelic Chondrodysplasia Punctata. Am. J. Med. Genet. A 2017, 173, 108–113. [Google Scholar] [CrossRef]
- Itzkovitz, B.; Jiralerspong, S.; Nimmo, G.; Loscalzo, M.; Horovitz, D.D.G.; Snowden, A.; Moser, A.; Steinberg, S.; Braverman, N. Functional Characterization of Novel Mutations in GNPAT and AGPS, Causing Rhizomelic Chondrodysplasia Punctata (RCDP) Types 2 and 3. Hum. Mutat. 2012, 33, 189–197. [Google Scholar] [CrossRef]
- Jang, J.E.; Park, H.; Yoo, H.J.; Baek, I.; Yoon, J.E.; Ko, M.S.; Kim, A.; Kim, H.S.; Park, H.; Lee, S.E.; et al. Protective Role of Endogenous Plasmalogens against Hepatic Steatosis and Steatohepatitis in Mice. Hepatology 2017, 66, 416–431. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Fuda, H.; Tsukui, T.; Wu, X.; Shen, N.; Saito, N.; Chiba, H.; Hui, S.-P. Oxidative Stress Linked Organ Lipid Hydroperoxidation and Dysregulation in Mouse Model of Nonalcoholic Steatohepatitis: Revealed by Lipidomic Profiling of Liver and Kidney. Antioxidants 2021, 10, 1602. [Google Scholar] [CrossRef]
- Ma, D.W.L.; Arendt, B.M.; Hillyer, L.M.; Fung, S.K.; McGilvray, I.; Guindi, M.; Allard, J.P. Plasma Phospholipids and Fatty Acid Composition Differ between Liver Biopsy-Proven Nonalcoholic Fatty Liver Disease and Healthy Subjects. Nutr. Diabetes 2016, 6, e220. [Google Scholar] [CrossRef]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The Plasma Lipidomic Signature of Nonalcoholic Steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef]
- Tiwari-Heckler, S.; Gan-Schreier, H.; Stremmel, W.; Chamulitrat, W.; Pathil, A. Circulating Phospholipid Patterns in NAFLD Patients Associated with a Combination of Metabolic Risk Factors. Nutrients 2018, 10, 649. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the Neglected Regulatory and Scavenging Lipid Species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Darwish, W.S.; Terada, K.; Chiba, H.; Hui, S.-P. Choline and Ethanolamine Plasmalogens Prevent Lead-Induced Cytotoxicity and Lipid Oxidation in HepG2 Cells. J. Agric. Food Chem. 2019, 67, 7716–7725. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From Peroxisomal Disorders to Common Neurodegenerative Diseases—The Role of Ether Phospholipids in the Nervous System. FEBS Lett. 2017, 591, 2761–2788. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A. Book Review: Plasmalogens: Workhorse Lipids of Membranes in Normal and Injured Neurons and Glia. Neuroscientist 2001, 7, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Holtzman, D.M.; McKeel, D.W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-Targeted Lipidomics of CSF and Frontal Cortex Grey and White Matter in Control, Mild Cognitive Impairment, and Alzheimer’s Disease Subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, X.; Chiba, H.; Hui, S.-P. Investigating Oxidized Lipids in an Omics Way: Oxidative Lipidomics in Biological Applications Using Mass Spectrometry. Med. Mass Spectrom. 2022, 6, 72–84. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Grösgen, S.; Riemenschneider, M.; Tanila, H.; Grimm, H.S.; Hartmann, T. From Brain to Food: Analysis of Phosphatidylcholins, Lyso-Phosphatidylcholins and Phosphatidylcholin–Plasmalogens Derivates in Alzheimer’s Disease Human Post Mortem Brains and Mice Model via Mass Spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. [Google Scholar] [CrossRef]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimers Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Kou, J.; Kovacs, G.G.; Höftberger, R.; Kulik, W.; Brodde, A.; Forss-Petter, S.; Hönigschnabl, S.; Gleiss, A.; Brügger, B.; Wanders, R.; et al. Peroxisomal Alterations in Alzheimer’s Disease. Acta Neuropathol. 2011, 122, 271–283. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral Ethanolamine Plasmalogen Deficiency: A Logical Causative Factor in Alzheimer’s Disease and Dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef]
- Huynh, K.; Lim, W.L.F.; Giles, C.; Jayawardana, K.S.; Salim, A.; Mellett, N.A.; Smith, A.A.T.; Olshansky, G.; Drew, B.G.; Chatterjee, P.; et al. Concordant Peripheral Lipidome Signatures in Two Large Clinical Studies of Alzheimer’s Disease. Nat. Commun. 2020, 11, 5698. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma Phospholipids Identify Antecedent Memory Impairment in Older Adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the Levels of Amyloid-β, Phospholipid Hydroperoxide, and Plasmalogen in the Blood of Patients with Alzheimer’s Disease: Possible Interactions between Amyloid-β and These Lipids. J. Alzheimers Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipid Alterations in the Earliest Clinically Recognizable Stage of Alzheimers Disease: Implication of the Role of Lipids in the Pathogenesis of Alzheimers Disease. Curr. Alzheimer Res. 2005, 2, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sheikh, A.M.; Haque, M.A.; Osago, H.; Sakai, H.; Shibly, A.Z.; Yano, S.; Michikawa, M.; Hossain, S.; Tabassum, S.; et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021, 11, 1603. [Google Scholar] [CrossRef]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of Blood Plasmalogens and Clinical Symptoms in Parkinson’s Disease by Oral Administration of Ether Phospholipids: A Preliminary Report. Park. Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe Alterations in Lipid Composition of Frontal Cortex Lipid Rafts from Parkinson’s Disease and Incidental Parkinson’s Disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Bargiela, D.; Chinnery, P.F. Mitochondria in Neuroinflammation—Multiple Sclerosis (MS), Leber Hereditary Optic Neuropathy (LHON) and LHON-MS. Neurosci. Lett. 2019, 710, 132932. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Melo, T.; Monteiro, A.; Paiva, A.; Domingues, P.; Domingues, M.R. Serum Phospholipidomics Reveals Altered Lipid Profile and Promising Biomarkers in Multiple Sclerosis. Arch. Biochem. Biophys. 2021, 697, 108672. [Google Scholar] [CrossRef] [PubMed]
- Geroldinger-Simić, M.; Bögl, T.; Himmelsbach, M.; Sepp, N.; Buchberger, W. Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis. Diagnostics 2021, 11, 2116. [Google Scholar] [CrossRef]
- Cai, H.Q.; Catts, V.S.; Webster, M.J.; Galletly, C.; Liu, D.; O’Donnell, M.; Weickert, T.W.; Weickert, C.S. Increased Macrophages and Changed Brain Endothelial Cell Gene Expression in the Frontal Cortex of People with Schizophrenia Displaying Inflammation. Mol. Psychiatry 2020, 25, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, T.; Sun, L.; Zhao, Z.; Qi, X.; Zhou, K.; Cao, Y.; Wang, X.; Qiu, Y.; Su, M.; et al. Potential Metabolite Markers of Schizophrenia. Mol. Psychiatry 2013, 18, 67–78. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, D.; Hu, X.; Jiang, J.; Qing, Y.; Yang, X.; Cui, G.; Wang, P.; Zhang, J.; et al. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr. Bull. 2022, 48, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.H.; Foley, K.A.; Mepham, J.R.; Tichenoff, L.J.; Possmayer, F.; MacFabe, D.F. Altered Brain Phospholipid and Acylcarnitine Profiles in Propionic Acid Infused Rodents: Further Development of a Potential Model of Autism Spectrum Disorders. J. Neurochem. 2010, 113, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Wiest, M.M.; German, J.B.; Harvey, D.J.; Watkins, S.M.; Hertz-Picciotto, I. Plasma Fatty Acid Profiles in Autism: A Case-Control Study. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 221–227. [Google Scholar] [CrossRef]
- Bell, J.G.; MacKinlay, E.E.; Dick, J.R.; MacDonald, D.J.; Boyle, R.M.; Glen, A.C.A. Essential Fatty Acids and Phospholipase A2 in Autistic Spectrum Disorders. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 201–204. [Google Scholar] [CrossRef]
- Lee, G.B.; Lee, J.C.; Moon, M.H. Plasma Lipid Profile Comparison of Five Different Cancers by Nanoflow Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2019, 1063, 117–126. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Huang, C.; Li, N.; Zou, L.; Chia, S.E.; Chen, S.; Yu, K.; Ling, Q.; Cheng, Q.; et al. Comparison of Hepatic and Serum Lipid Signatures in Hepatocellular Carcinoma Patients Leads to the Discovery of Diagnostic and Prognostic Biomarkers. Oncotarget 2018, 9, 5032–5043. [Google Scholar] [CrossRef]
- Qiu, B.; Zandkarimi, F.; Saqi, A.; Castagna, C.; Tan, H.; Sekulic, M.; Miorin, L.; Hibshoosh, H.; Toyokuni, S.; Uchida, K.; et al. Fatal COVID-19 Pulmonary Disease Involves Ferroptosis. Nat. Commun. 2024, 15, 3816. [Google Scholar] [CrossRef]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Snider, J.M.; You, J.K.; Wang, X.; Snider, A.J.; Hallmark, B.; Zec, M.M.; Seeds, M.C.; Sergeant, S.; Johnstone, L.; Wang, Q.; et al. Group IIA Secreted Phospholipase A2 Is Associated with the Pathobiology Leading to COVID-19 Mortality. J. Clin. Investig. 2021, 131, e149236. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.B.; Huynh, K.; Wang, T.; Paul, S.; Cinel, M.; Mellett, N.A.; Olshansky, G.; Meikle, T.G.; Watts, G.F.; Hung, J.; et al. Development and Validation of a Plasmalogen Score as an Independent Modifiable Marker of Metabolic Health: Population Based Observational Studies and a Placebo-Controlled Cross-over Study. Ebiomedicine 2024, 105, 105187. [Google Scholar] [CrossRef]
- Middlekauff, H.R.; William, K.J.; Su, B.; Haptonstall, K.; Araujo, J.A.; Wu, X.; Kim, J.; Sallam, T. Changes in Lipid Composition Associated with Electronic Cigarette Use. J. Transl. Med. 2020, 18, 379. [Google Scholar] [CrossRef]
- Paul, S.; Smith, A.A.T.; Culham, K.; Gunawan, K.A.; Weir, J.M.; Cinel, M.A.; Jayawardana, K.S.; Mellett, N.A.; Lee, M.K.S.; Murphy, A.J.; et al. Shark Liver Oil Supplementation Enriches Endogenous Plasmalogens and Reduces Markers of Dyslipidemia and Inflammation. J. Lipid Res. 2021, 62, 100092. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Zhang, M.; Chiba, H.; Hui, S.-P. Plasmalogen Profiling in Porcine Brain Tissues by LC-MS/MS. Foods 2023, 12, 2990. [Google Scholar] [CrossRef]
- Yunoki, K.; Kukino, O.; Nadachi, Y.; Fujino, T.; Ohnishi, M. Separation and Determination of Functional Complex Lipids from Chicken Skin. J. Am. Oil Chem. Soc. 2008, 85, 427–433. [Google Scholar] [CrossRef]
- Mawatari, S.; Katafuchi, T.; Miake, K.; Fujino, T. Dietary Plasmalogen Increases Erythrocyte Membrane Plasmalogen in Rats. Lipids Health Dis. 2012, 11, 161. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Honjo, A.; Otoki, Y.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Analysis of Plasmalogen Species in Foodstuffs. Lipids 2016, 51, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kanehama, A.; Kashiwagi, A.; Yamada, M.; Nishimukai, M. Lymphatic Absorption of Microbial Plasmalogens in Rats. Front. Cell Dev. Biol. 2022, 10, 836186. [Google Scholar] [CrossRef]
- Kolek, J.; Patáková, P.; Melzoch, K.; Sigler, K.; Řezanka, T. Changes in Membrane Plasmalogens of Clostridium Pasteurianum during Butanol Fermentation as Determined by Lipidomic Analysis. PLoS ONE 2015, 10, e0122058. [Google Scholar] [CrossRef][Green Version]
- Jackson, D.R.; Cassilly, C.D.; Plichta, D.R.; Vlamakis, H.; Liu, H.; Melville, S.B.; Xavier, R.J.; Clardy, J. Plasmalogen Biosynthesis by Anaerobic Bacteria: Identification of a Two-Gene Operon Responsible for Plasmalogen Production in Clostridium perfringens. ACS Chem. Biol. 2021, 16, 6–13. [Google Scholar] [CrossRef]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and Detection of Plasmalogen in Marine Invertebrates by High-Performance Liquid Chromatography with Evaporative Light-Scattering Detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Haroon, J.; Kling, M.A.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Front. Cell Dev. Biol. 2022, 10, 864842. [Google Scholar] [CrossRef]
- Sun, J.-T.; Wang, Z.-M.; Zhou, L.-H.; Yang, T.-T.; Zhao, D.; Bao, Y.-L.; Wang, S.-B.; Gu, L.-F.; Chen, J.-W.; Shan, T.-K.; et al. PEX3 Promotes Regenerative Repair after Myocardial Injury in Mice through Facilitating Plasma Membrane Localization of ITGB3. Commun. Biol. 2024, 7, 795. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. EBioMedicine 2017, 17, 199–205. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; McWalter, K.; Te Brinke, H.; IJlst, L.; Mooijer, P.M.; Ruiter, J.P.N.; Van Lint, A.E.M.; Pras-Raves, M.; Wever, E.; Millan, F.; et al. An Autosomal Dominant Neurological Disorder Caused by de Novo Variants in FAR1 Resulting in Uncontrolled Synthesis of Ether Lipids. Genet. Med. 2021, 23, 740–750. [Google Scholar] [CrossRef]
- Liakh, I.; Sledzinski, T.; Kaska, L.; Mozolewska, P.; Mika, A. Sample Preparation Methods for Lipidomics Approaches Used in Studies of Obesity. Molecules 2020, 25, 5307. [Google Scholar] [CrossRef] [PubMed]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holčapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for Good Practice in MS-Based Lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Lackner, K.; Wohlfarter, Y.; Sailer, S.; Zschocke, J.; Werner, E.R.; Watschinger, K.; Keller, M.A. Unequivocal Mapping of Molecular Ether Lipid Species by LC–MS/MS in Plasmalogen-Deficient Mice. Anal. Chem. 2020, 92, 11268–11276. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Watschinger, K.; Werner, E.R.; Keller, M.A. Tricky Isomers—The Evolution of Analytical Strategies to Characterize Plasmalogens and Plasmanyl Ether Lipids. Front. Cell Dev. Biol. 2022, 10, 864716. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Dong, C.; Chen, L.; Song, M.; Zhou, X.; Lv, D.; Li, Q. The Changes in Plasmalogens: Chemical Diversity and Nutritional Implications—A Narrative Review. Nutrients 2025, 17, 3497. https://doi.org/10.3390/nu17223497
Chen Z, Dong C, Chen L, Song M, Zhou X, Lv D, Li Q. The Changes in Plasmalogens: Chemical Diversity and Nutritional Implications—A Narrative Review. Nutrients. 2025; 17(22):3497. https://doi.org/10.3390/nu17223497
Chicago/Turabian StyleChen, Zhen, Chen Dong, Lin Chen, Meiling Song, Xinxin Zhou, Depeng Lv, and Quancai Li. 2025. "The Changes in Plasmalogens: Chemical Diversity and Nutritional Implications—A Narrative Review" Nutrients 17, no. 22: 3497. https://doi.org/10.3390/nu17223497
APA StyleChen, Z., Dong, C., Chen, L., Song, M., Zhou, X., Lv, D., & Li, Q. (2025). The Changes in Plasmalogens: Chemical Diversity and Nutritional Implications—A Narrative Review. Nutrients, 17(22), 3497. https://doi.org/10.3390/nu17223497