The Effects of Semen Ziziphi Spinosae Extract on LPS-Induced Astrocyte Gene Expression and Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SZS Extract
2.2. Cell Culture and Treatments
2.3. Transcriptomics Analysis
2.4. Metabolomics Analysis
2.4.1. Sample Preparation
2.4.2. LC-MS/MS Conditions
2.4.3. Metabolomics Data Processing and Analysis
2.5. Quantitative Real-Time PCR Analysis
2.6. Detection of Cell Cycle
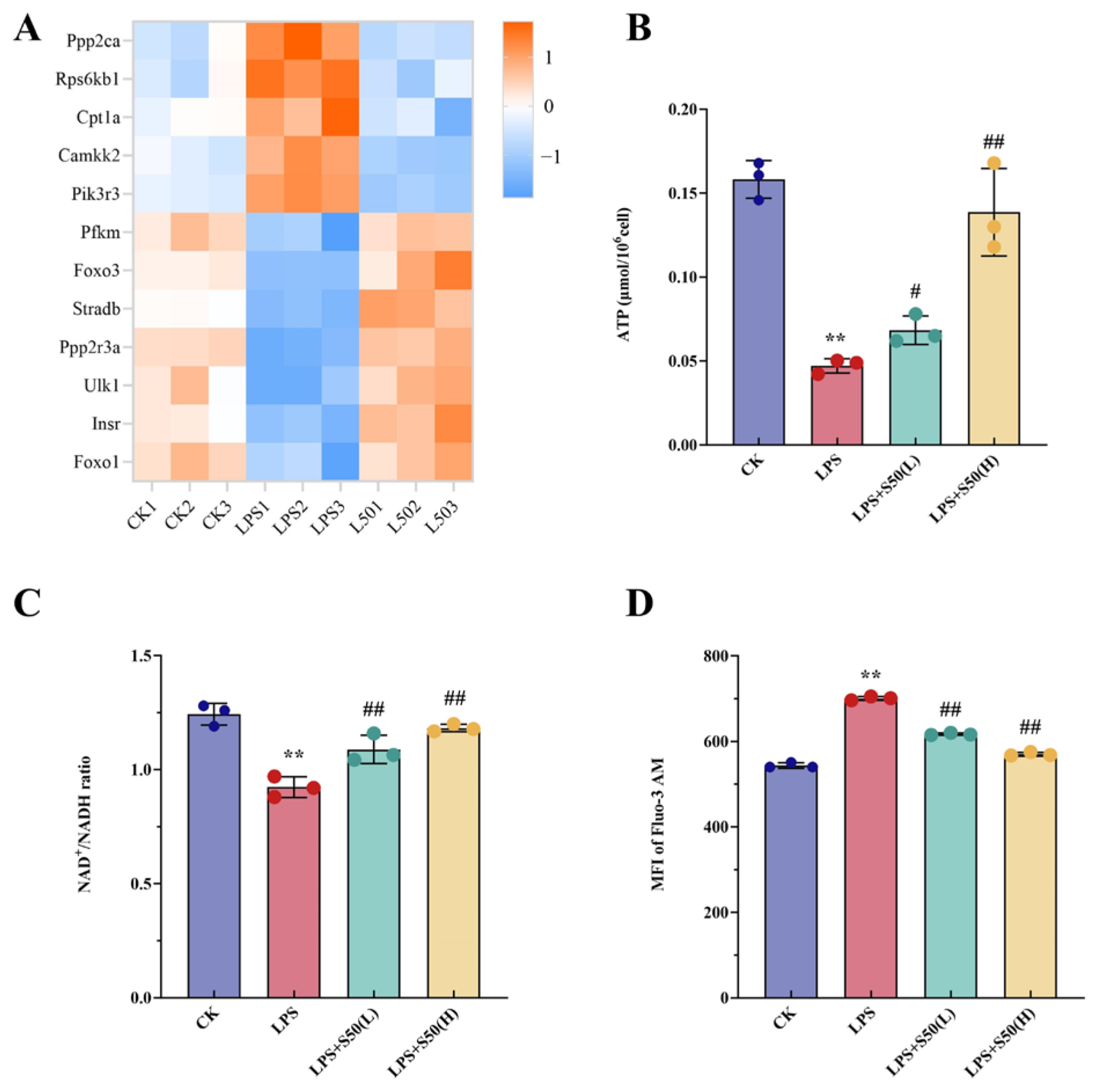
2.7. Intercellular ATP, NAD+/NADH Ratio Measurements
2.8. Measurement of Intracellular Ca2+ Level
2.9. Statistical Analysis
3. Results
3.1. Transcriptome Analysis
3.1.1. The Results of RNA Sequencing Reads
3.1.2. GO and KEGG Enrichment Analysis of DEGS
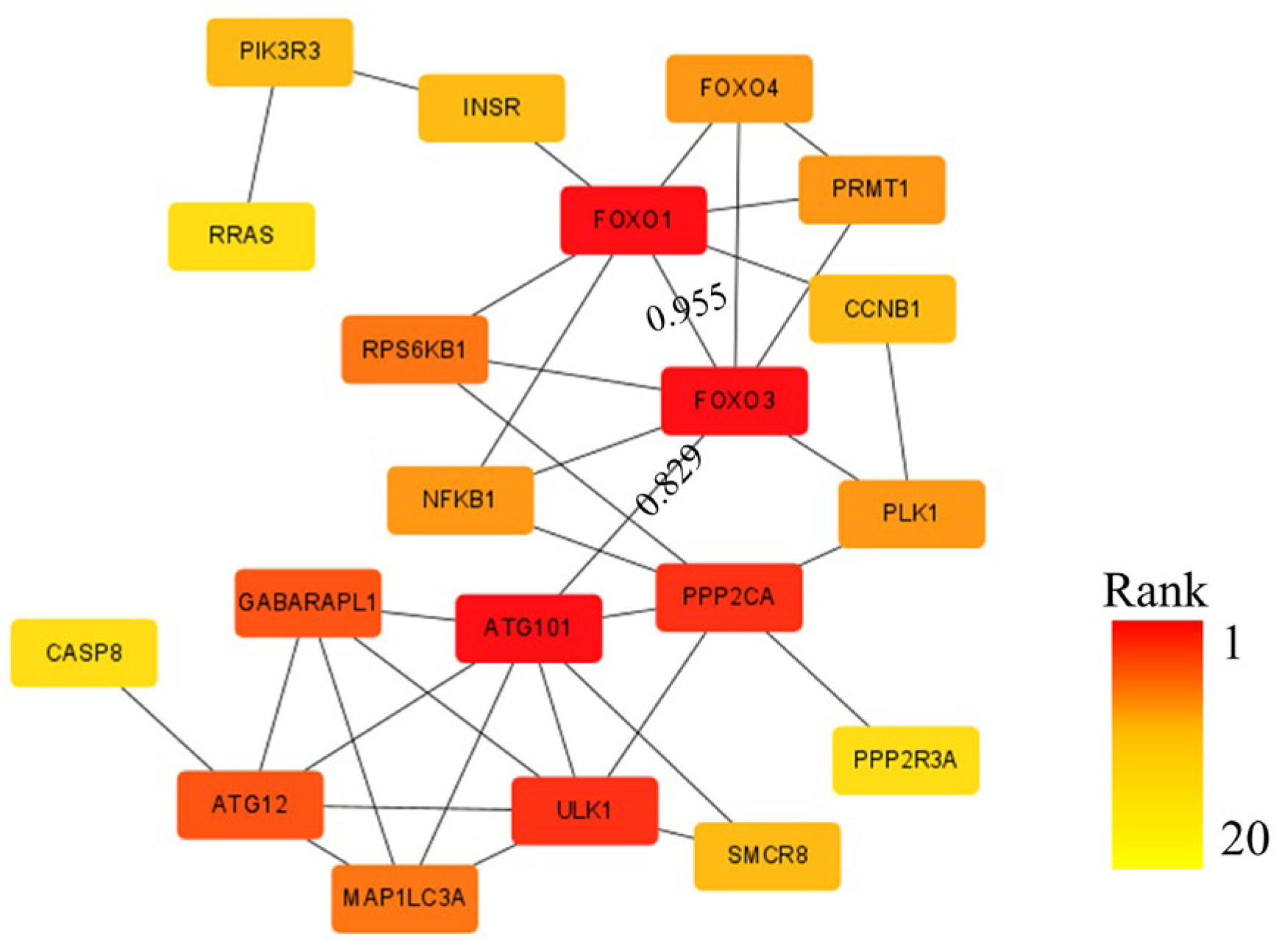
3.1.3. Identification of Potential Target Genes
3.1.4. Regulation the Pathway of AMPK Signaling Pathway
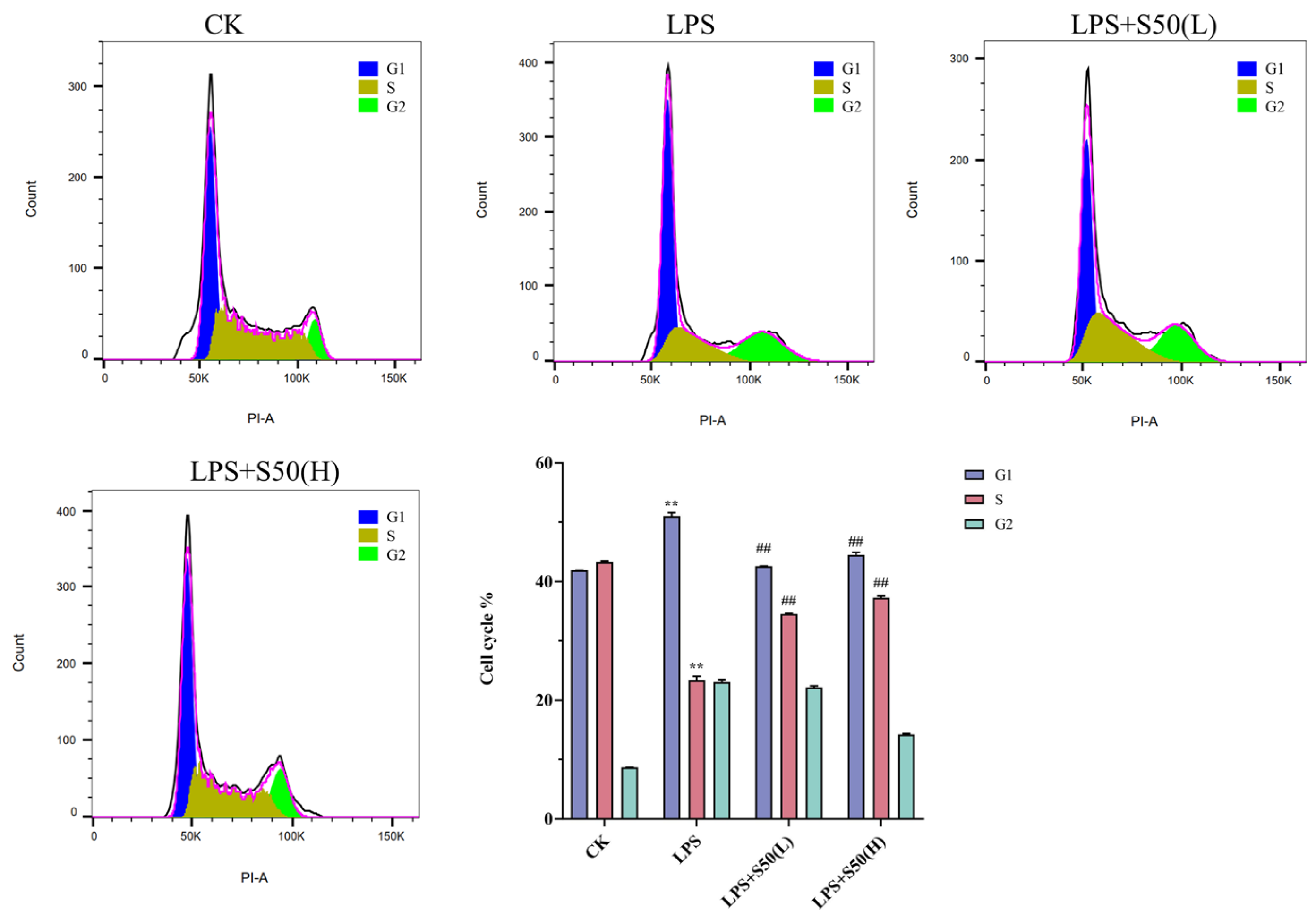
3.1.5. Effect of Different Treatment on the Cell Cycle
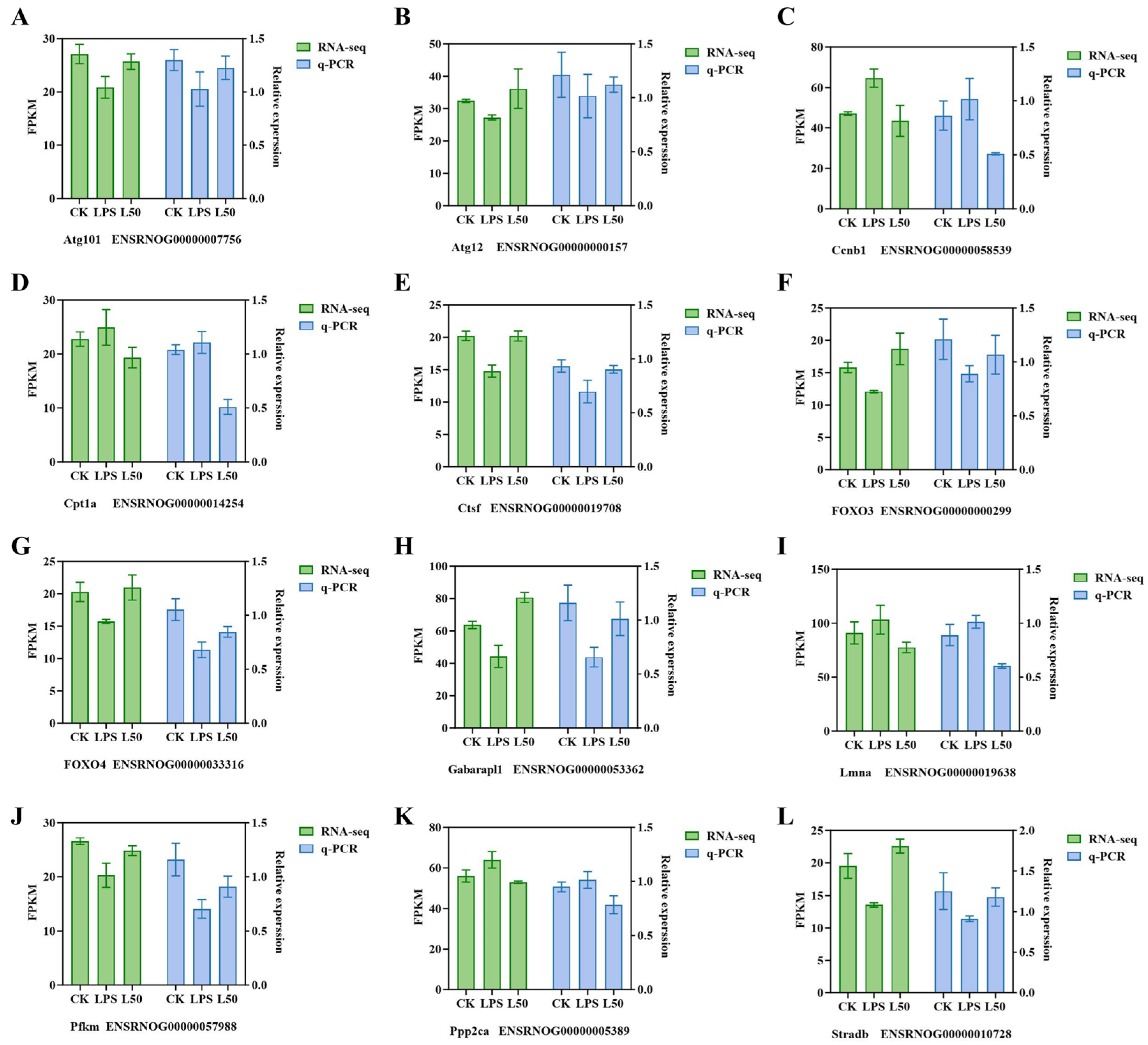
3.2. qRT-PCR Verification
3.3. Metabolomic Analysis
3.3.1. Multivariate Statistical Analysis
3.3.2. Determination of DEMs and Pathway Enrichment Analysis
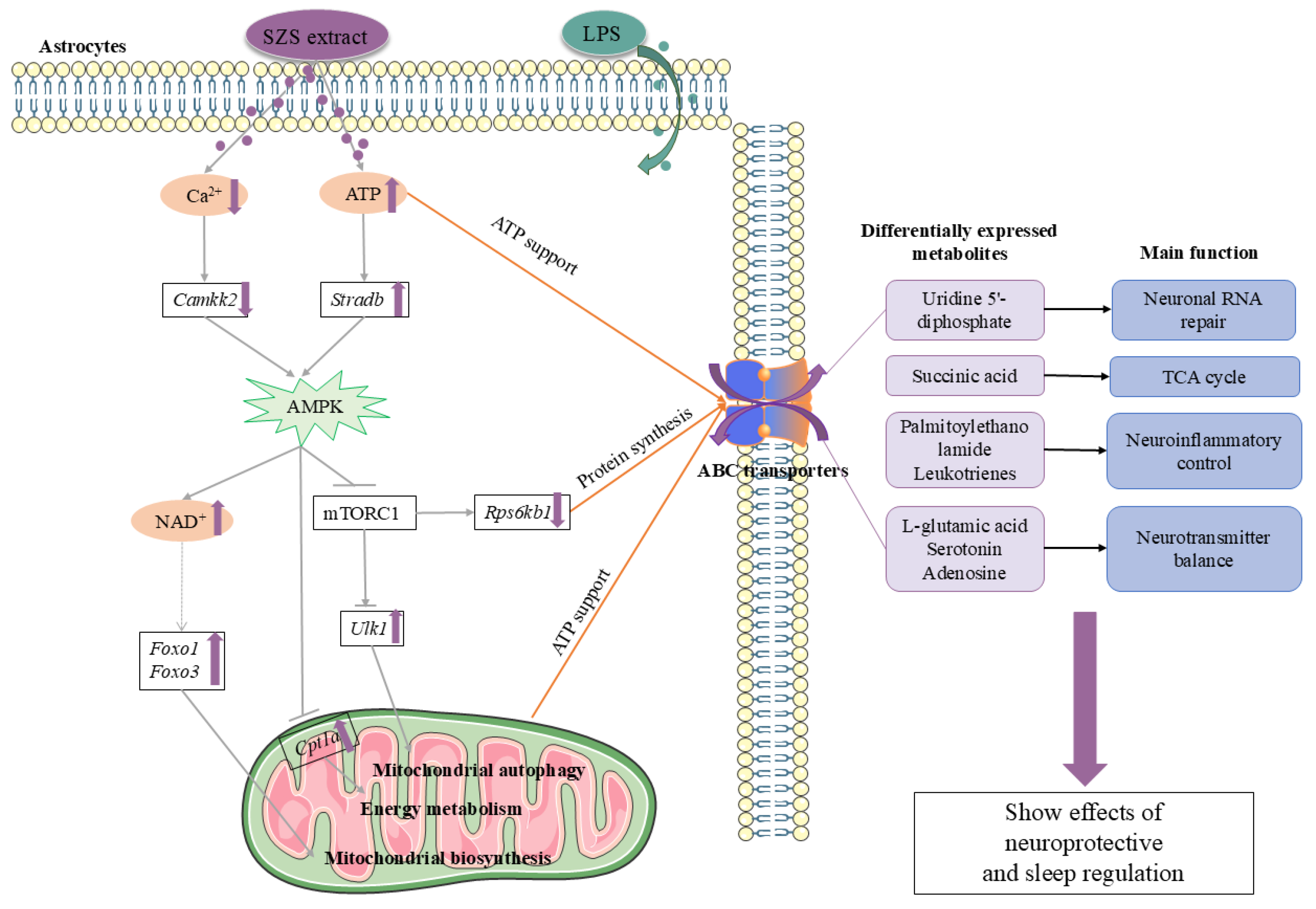
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dressle, R.J.; Spiegelhalder, K.; Schiel, J.E.; Benz, F.; Johann, A.; Feige, B.; Jernelöv, S.; Perlis, M.; Riemann, D. The Future of Insomnia Research-There’s Still Work to Be Done. J. Sleep Res. 2025, 34, e70091. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Chang, S.C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharmacal Res. 2019, 42, 416–425. [Google Scholar] [CrossRef]
- Sheng, F.Y.; Zhang, L.L.; Wang, S.S.; Yang, L.L.; Li, P. Deacetyl Ganoderic Acid F Inhibits LPS-Induced Neural Inflammation via NF-κB Pathway Both In Vitro and In Vivo. Nutrients 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Toth, L.A. Lipopolysaccharide effects on neuronal activity in rat basal forebrain and hypothalamus during sleep and waking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R620–R627. [Google Scholar] [CrossRef] [PubMed]
- Aghelan, Z.; Pashaee, S.; Abtahi, S.H.; Karima, S.; Khazaie, H.; Ezati, M.; Khodarahmi, R. Natural Immunosuppressants as a Treatment for Chronic Insomnia Targeting the Inflammatory Response Induced by NLRP3/caspase-1/IL1ß Axis Activation: A Scooping Review. J. Neuroimmune Pharmacol. 2023, 18, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Carvalhas-Almeida, C.; Serra, J.; Moita, J.; Cavadas, C.; Alvaro, A.R. Understanding neuron-glia crosstalk and biological clocks in insomnia. Neurosci. Biobehav. Rev. 2023, 147, 105100. [Google Scholar] [CrossRef]
- Palagini, L.; Geoffroy, P.A.; Miniati, M.; Perugi, G.; Biggio, G.; Marazziti, D.; Riemann, D. Insomnia, sleep loss, and circadian sleep disturbances in mood disorders: A pathway toward neurodegeneration and neuroprogression? A theoretical review. CNS Spectr. 2022, 27, 298–308. [Google Scholar] [CrossRef]
- Wu, R.; Tripathy, S.; Menon, V.; Yu, L.; Buchman, A.S.; Bennett, D.A.; De Jager, P.L.; Lim, A.S.P. Fragmentation of rest periods, astrocyte activation, and cognitive decline in older adults with and without Alzheimer’s disease. Alzheimers Dement. 2023, 19, 1888–1900. [Google Scholar] [CrossRef]
- Eldridge, S.L.S.; Teetsel, J.F.K.; Torres, R.A.; Ulrich, C.H.; Shah, V.V.; Singh, D.; Zamora, M.J.; Zamora, S.; Sater, A.K. A Focal Impact Model of Traumatic Brain Injury in Xenopus Tadpoles Reveals Behavioral Alterations, Neuroinflammation, and an Astroglial Response. Int. J. Mol. Sci. 2022, 23, 7578. [Google Scholar] [CrossRef]
- Wei, Y.H.; Zhang, Y.Q.; Sun, J.; Li, W.; Zhao, X.T.; Tian, N.; Cao, Y.X.; Xie, J.B. Modulation of the receptor for advanced glycation end products pathway by natural polyphenols: A therapeutic approach to neurodegenerative diseases. Food Biosci. 2024, 62, 105511. [Google Scholar] [CrossRef]
- Hu, N.; Zeng, C.; Cao, Y.; Li, X.H.; Bai, F.; Wang, J.H.; Yang, B.F.; Li, C.L. Therapeutic potential of Shilong Qingxue Granule and its extract against glutamate induced neural injury: Insights from in vivo and in vitro models. J. Ethnopharmacol. 2025, 342, 119396. [Google Scholar] [CrossRef]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Zydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef]
- Ma, J.; Huang, S.; Shi, L.; Shen, Y.; Gao, S.; Wu, Z. Research progress on the effect of medicine and food homology resources for sleep improvement. Heliyon 2024, 10, e40067. [Google Scholar] [CrossRef]
- Fan, W.X.; Lu, X.F.; Meng, X.L.; Lan, Y.R.; Song, B.Z.; Ma, Q.L.; Chang, L.P. Thermal Decomposition Mechanism and Kinetic Parameters of Semen Ziziphi Spinosae Based on Thermogravimetric Analysis. Asian J. Chem. 2014, 26, 4907–4911. [Google Scholar] [CrossRef]
- Wang, D.D.; Ho, C.T.; Bai, N.S. Ziziphi Spinosae Semen: An updated review on pharmacological activity, quality control, and application. J. Food Biochem. 2022, 46, e14153. [Google Scholar] [CrossRef]
- Bi, A.Q.; Liu, R.H.; Xie, M.; He, B.S.; Yan, T.X.; Du, Y.Y.; Jia, Y. Semen Ziziphi Spinosae alleviates cardiomyocyte apoptosis in rats with coronary heart disease via the AMPK/SIRT1/PGC-1α signaling pathway activation. Phytomedicine 2025, 142, 156743. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Peng, Y.T.; Shao, Y.T.; Pan, D.D.; Cheng, Q.; Jiang, Z.Z.; Qian, S.T.; Li, B.J.; Yan, M.; Zhu, X.; et al. Mitochondria-dependent apoptosis was involved in the alleviation of Jujuboside A on diabetic kidney disease-associated renal tubular injury via YY1/PGC-1α signaling. Phytomedicine 2025, 138, 156743. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, T.H.; Zhao, R.J.; Kim, Y.S.; Jung, J.Y.; Park, C.A.; Jegal, K.H.; Ku, S.K.; Kim, J.K.; Lee, C.W.; et al. Amelioration of inflammatory responses by Socheongryong-Tang, a traditional herbal medicine, in RAW 264.7 cells and rats. Int. J. Mol. Med. 2018, 41, 2771–2783. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.R.; Zhang, Y.Q.; Xie, J.B. Ziziphi Spinosae Semen: A Natural Herb Resource for Treating Neurological Disorders. Curr. Top. Med. Chem. 2022, 22, 1379–1391. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hu, J.Y.; Zhang, Y.; Peng, L.L.; Li, X.Y.; Li, C.; Wu, X.Y.; Wang, C. Epilepsy therapy beyond neurons: Unveiling astrocytes as cellular targets. Neural Regen. Res. 2026, 21, 23–38. [Google Scholar] [CrossRef]
- John, A.; Raza, H. Azadirachtin Attenuates Lipopolysaccharide-Induced ROS Production, DNA Damage, and Apoptosis by Regulating JNK/Akt and AMPK/mTOR-Dependent Pathways in Rin-5F Pancreatic Beta Cells. Biomedicines 2021, 9, 1943. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, S.; Salvesen, G.S.; Walsh, C.M. Enzymatically active single chain caspase-8 maintains T-cell survival during clonal expansion. Cell Death Differ. 2011, 18, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.L.; Dou, Q.P.; Li, D.P. Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, D.H.; Kim, D. Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun. 2023, 14, 2994. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Canbolat, E.; Cakiroglu, F.P. The importance of AMPK in obesity and chronic diseases and the relationship of AMPK with nutrition: A literature review. Crit. Rev. Food Sci. Nutr. 2023, 63, 449–456. [Google Scholar] [CrossRef]
- Shin, C.Y.; Choi, J.W.; Ryu, J.R.; Ryu, J.H.; Kim, W.; Kim, H.; Ko, K.H. Immunostimulation of rat primary astrocytes decreases intracellular ATP level. Brain Res. 2001, 902, 198–204. [Google Scholar] [CrossRef]
- Birla, H.; Xia, J.S.; Gao, X.H.; Zhao, H.; Wang, F.Y.; Patel, S.; Amponsah, A.; Bekker, A.; Tao, Y.X.; Hu, H.J. Toll-like receptor 4 activation enhances Orai1-mediated calcium signal promoting cytokine production in spinal astrocytes. Cell Calcium 2022, 105, 102619. [Google Scholar] [CrossRef]
- Gupta, S.; Afzal, M.; Agrawal, N.; Almalki, W.H.; Rana, M.; Gangola, S.; Chinni, S.V.; KumarK, B.; Ali, H.; Singh, S.K.; et al. Harnessing the FOXO-SIRT1 axis: Insights into cellular stress, metabolism, and aging. Biogerontology 2025, 26, 65. [Google Scholar] [CrossRef]
- Karagöz, M.F.; Celep, A.G.S. The effect of caloric restriction on genetical pathways. Food Sci. Hum. Wellness 2023, 12, 1450–1457. [Google Scholar] [CrossRef]
- Luengo, A.; Li, Z.Q.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell 2021, 81, 691–707.e6. [Google Scholar] [CrossRef] [PubMed]
- Schertl, P.; Braun, H.P. Respiratory electron transfer pathways in plant mitochondria. Front. Plant Sci. 2014, 5, 163. [Google Scholar] [CrossRef]
- Zhang, Z.; Che, X.; Feng, T.; Zou, J.; Chen, G.; Guo, W.; Ma, C.; Yuan, H.; Chen, J.; Xu, X. Jujuboside A improves insomnia by maintaining mitochondrial homeostasis in prefrontal neurons. Brain Res. Bull. 2025, 226, 111372. [Google Scholar] [CrossRef]
- Gibson, C.J.; Hossain, M.M.; Richardson, J.R.; Aleksunes, L.M. Inflammatory Regulation of ATP Binding Cassette Efflux Transporter Expression and Function in Microglia. J. Pharmacol. Exp. Ther. 2012, 343, 650–660. [Google Scholar] [CrossRef]
- Sáenz, J.; Alba, G.; Reyes-Quiroz, M.E.; Geniz, I.; Jiménez, J.; Sobrino, F.; Santa-María, C. Curcumin enhances LXRα in an AMP-activated protein kinase-dependent manner in human macrophages. J. Nutr. Biochem. 2018, 54, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumari, S.; Neeli, P.K.; Pulipaka, S.; Kuncha, M.; Chandra, Y.; Annamaneni, S.; Kotamraju, S. MTDHz7-mediated mTOR activation drives doxorubicin resistance in triple-negative breast cancer: Relevance of mTORC1 inhibition on chemosensitization. Cell. Signal. 2025, 132, 111864. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A.; Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog. Neurobiol. 2022, 217, 102331. [Google Scholar] [CrossRef]
- Jing, D.Q.; Hou, X.L.; Guo, X.; Zhao, X.; Zhang, K.X.; Zhang, J.W.; Kan, C.X.; Han, F.; Liu, J.L.; Sun, X.D. Astrocytes in Post-Stroke Depression: Roles in Inflammation, Neurotransmission, and Neurotrophin Signaling. Cell. Mol. Neurobiol. 2023, 43, 3301–3313. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, N.; Baysal, B.E.; Shadel, G.S. Revisiting the TCA cycle: Signaling to tumor formation. Trends Mol. Med. 2011, 17, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Chen, T.; Yao, M.; Wang, Y.; Xiao, W.; Li, B. ABC transporter and its application in synthetic biology. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2020, 36, 1754–1766. [Google Scholar] [CrossRef]
- Meng, L.S.; Xing, G.; Li, B.; Li, D.N.; Sung, X.Y.; Yan, T.C.; Li, L.; Cao, S.; Meng, X.J. Anthocyanins Extracted from Aronia melanocarpa Protect SH-SY5Y Cells against Amyloid-beta (1-42)-Induced Apoptosis by Regulating Ca2+ Homeostasis and Inhibiting Mitochondrial Dysfunction. J. Agric. Food Chem. 2018, 66, 12967–12977. [Google Scholar] [CrossRef]




| Pathway Name | CK vs. LPS | LPS vs. L50 | ||||
|---|---|---|---|---|---|---|
| p Value | Metabolites | p Value | Metabolites | |||
| Up-Regulated | Down-Regulated | Up-Regulated | Down-Regulated | |||
| ABC transporters | 0.0000308 | L-Serine; Sucrose; Biotin; Uridine; Cytidine; Iron | L-Lysine; L-Phenylalanine; L-Histidine; Spermidine; L-Isoleucine; | 0.0000905 | L-Lysine; L-Arginine; L-Phenylalanine; L-Histidine; L-Proline; L-Valine; Spermidine; L-Isoleucine; Xanthosine; Deoxyinosine; | L-Glutamic acid; Adenosine |
| Neuroactive ligand receptor interaction | - | - | 0.000552 | Uridine 5′-diphosphate; Serotonin | L-Glutamic acid; Adenosine; Leukotriene; Palmitoylethanolamide | |
| GABAergic synapse | - | - | 0.023978 | - | L-Glutamic acid; Succinic acid | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, R.; Xu, Y.; Wang, Y.; Liu, Z.; Wu, Z.; Bian, Y. The Effects of Semen Ziziphi Spinosae Extract on LPS-Induced Astrocyte Gene Expression and Metabolites. Nutrients 2025, 17, 3498. https://doi.org/10.3390/nu17223498
Ma J, Wang R, Xu Y, Wang Y, Liu Z, Wu Z, Bian Y. The Effects of Semen Ziziphi Spinosae Extract on LPS-Induced Astrocyte Gene Expression and Metabolites. Nutrients. 2025; 17(22):3498. https://doi.org/10.3390/nu17223498
Chicago/Turabian StyleMa, Jingxuan, Ru Wang, Yaping Xu, Yan Wang, Zixuan Liu, Zhaoxia Wu, and Yuanyuan Bian. 2025. "The Effects of Semen Ziziphi Spinosae Extract on LPS-Induced Astrocyte Gene Expression and Metabolites" Nutrients 17, no. 22: 3498. https://doi.org/10.3390/nu17223498
APA StyleMa, J., Wang, R., Xu, Y., Wang, Y., Liu, Z., Wu, Z., & Bian, Y. (2025). The Effects of Semen Ziziphi Spinosae Extract on LPS-Induced Astrocyte Gene Expression and Metabolites. Nutrients, 17(22), 3498. https://doi.org/10.3390/nu17223498





