High Salt Intake and Atherosclerosis Progression—Not Only via Blood Pressure: A Narrative Review
Abstract
1. Atherosclerotic Cardiovascular Disease
2. Salt Intake and ASCVD: Global Perspective
3. Level of Salt Intake and ASCVD Risk
4. Pathophysiological Mechanisms
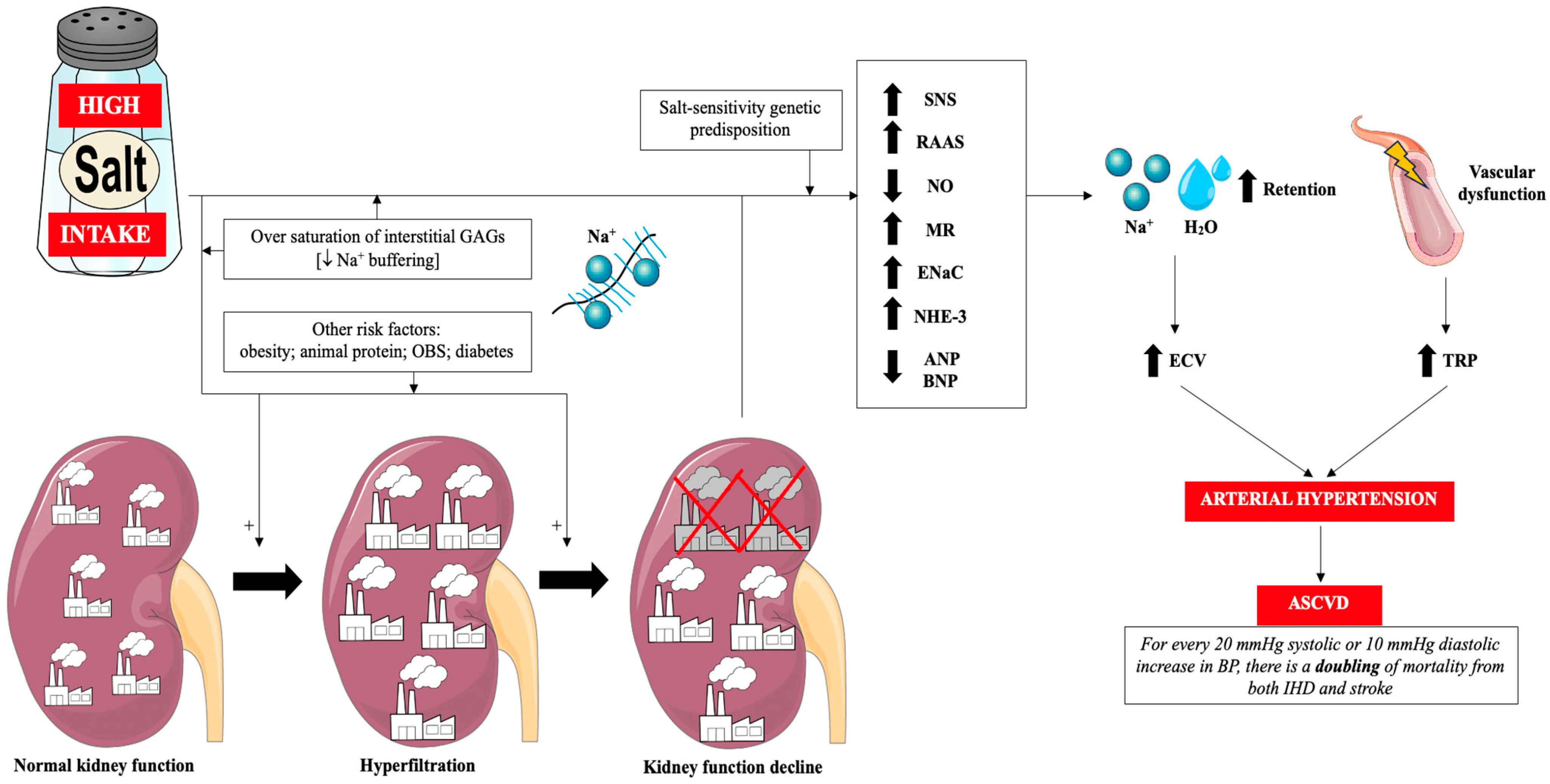
4.1. Classic Mechanism: Salt → Arterial Hypertension → ASCVD
4.2. Impact on Metabolic Disorders
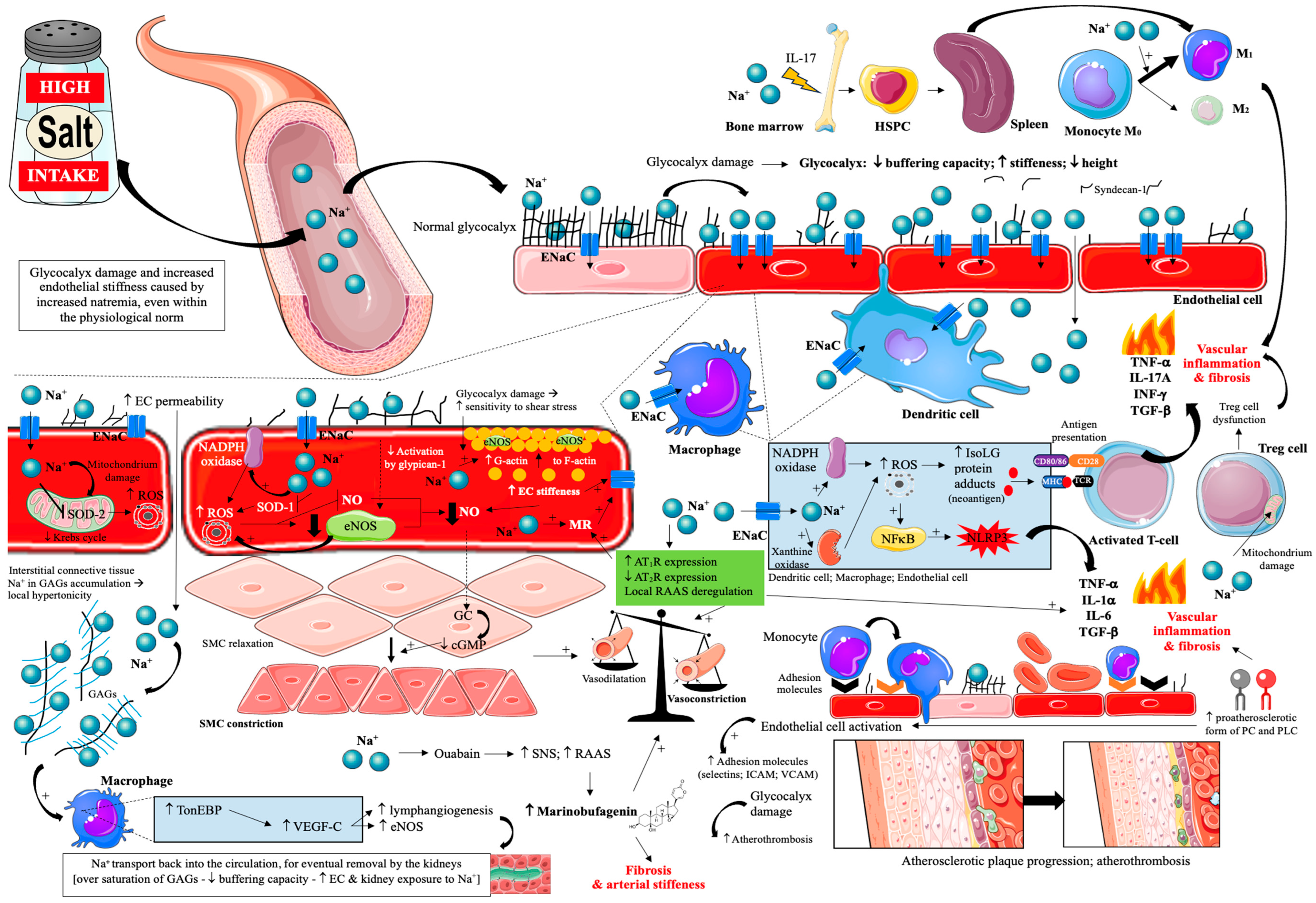
4.3. Impact on Endothelial Cells
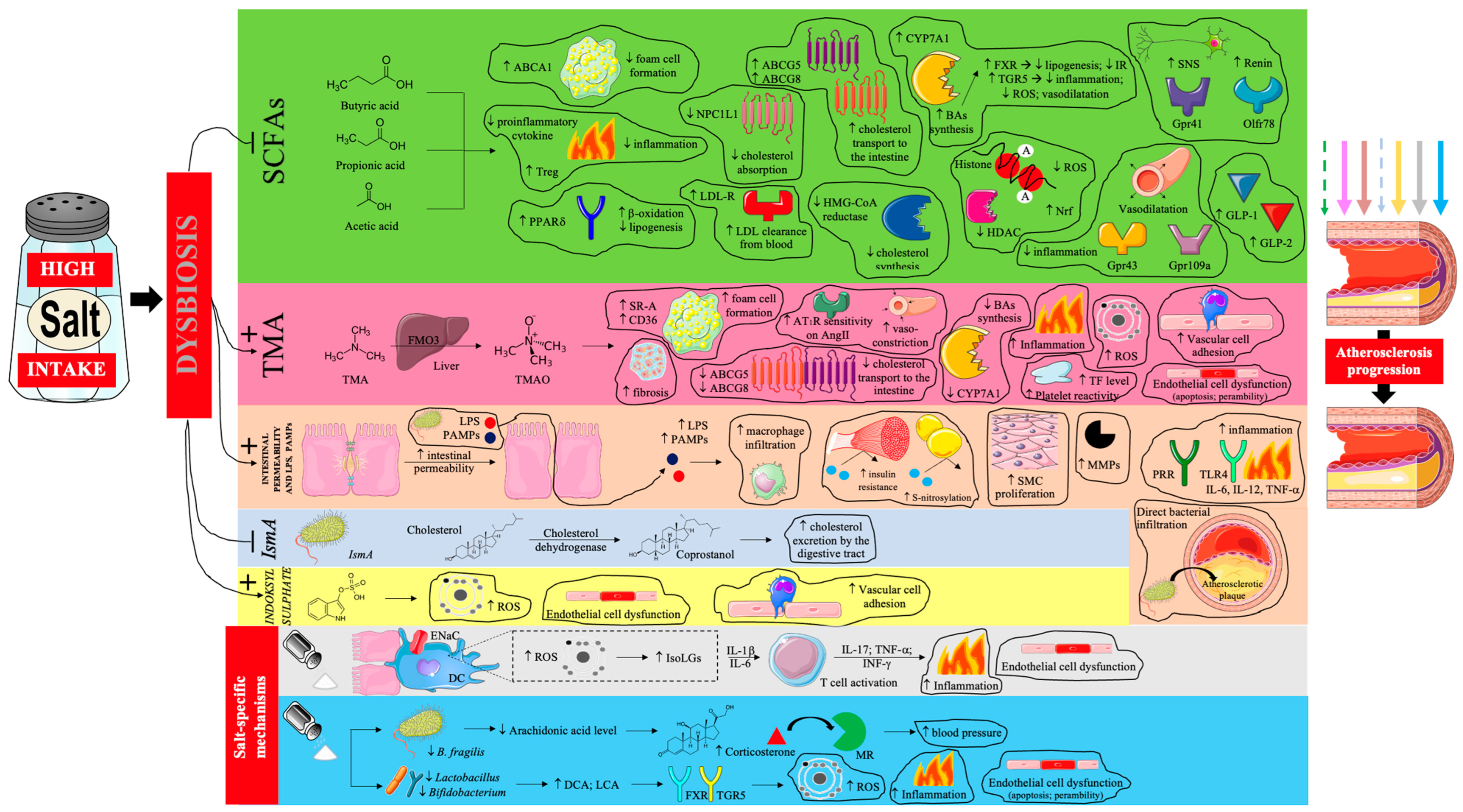
4.4. Impact on Gut Microbiota
4.5. Impact on Immune System
5. Conclusions and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| IHD | Ischemic heart disease |
| PAD | Peripheral artery disease |
| CVD | Cardiovascular disease |
| COVID-19 | Coronavirus disease 2019 |
| RR | Relative risk |
| 95% CI | 95% confidence interval |
| WHO | World Health Organization |
| ESC | European Society of Cardiology |
| HR | Hazard ratio |
| RAAS | Renin-angiotensin-aldosterone system |
| DASH | Dietary approach to stop hypertension |
| cIMT | Carotid intima-media thickness |
| cf-PWV | Pulse wave velocity |
| AIx | Augmentation index |
| SNS | Sympathetic nervous system |
| MR | Mineralocorticoid receptor |
| CKD | Chronic kidney disease |
| GAGs | Glycosaminoglycans |
| ECs | Endothelial cells |
| OR | Odds ratio |
| eNOS | Endothelial nitric oxide synthase |
| NO | Nitric oxide |
| TRP | Transient receptor potential |
| ENaC | Epithelial sodium channel |
| SCFA | Short-chain fatty acid |
| TMA | Trimethylamine |
| PAMPs | Pathogen-associated molecular patterns |
| BMI | Body mass index |
| NHE-3 | Sodium-hydrogen antiporter 3 |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| IRS-1 | Insulin receptor substrate 1 |
| GLUT-4 | Glucotransporter type 4 |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| GLP-1/2 | Glucagon-like peptide ½ |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| FXR | Farnesoid X receptor |
| PPARα | Peroxisome proliferator-activated receptor α |
| SDI | Socioeconomic development index |
| TRP | Transient receptor potential channel |
| TMAO | Trimethylamine N-oxide |
| FMD | Flow-mediated dilation |
| VSMC | Vascular smooth muscle cell |
| NCX | Na+-Ca2+ exchanger |
| TGF-β1 | Transforming growth factor β1 |
| Src | Tyrosine kinase |
| PC | Phosphatidylcholine |
| LPC | Lysophosphatidylcholine |
| DCA | Deoxycholic acid |
| LCA | Lithocholic acid |
| TGR5 | Takeda G protein-coupled receptor 5 |
| LPS | Lipopolysaccharide |
| IL-17 | Interleukin 17 |
| IL-23 | Interleukin 23 |
| HSPC | Hematopoietic stem and progenitor cells |
| NFκB | Nuclear factor κ B |
| IL-1β | Interleukin 1β |
| CHIP | Clonal hematopoiesis of indeterminate potential |
References
- Banach, M.; Surma, S.; Toth, P.P.; endorsed by the International Lipid Expert Panel (ILEP). 2023: The year in cardiovascular disease—The year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch. Med. Sci. 2023, 19, 1602–1615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. https://doi.org/10.1016/j.jacc.2021.02.039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Hu, M.; Liu, H.; Zhang, X.; Li, H.; Zhou, F.; Liu, Y.M.; Lei, F.; Qin, J.J.; Zhao, Y.C.; et al. Global Burden of Disease Study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab. 2021, 33, 1943–1956.e2. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, 32, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Makover, M.E.; Surma, S.; Banach, M.; Toth, P.P. Eliminating atherosclerotic cardiovascular disease residual risk. Eur. Heart J. 2023, 44, 4731–4733. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.H.; Yaroch, A.L.; Blanck, H.M. Reported Changes in Eating Habits Related to Less Healthy Foods and Beverages during the COVID-19 Pandemic among US Adults. Nutrients 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burnatowska, E.; Surma, S.; Olszanecka-Glinianowicz, M. Relationship between Mental Health and Emotional Eating during the COVID-19 Pandemic: A Systematic Review. Nutrients 2022, 14, 3989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surma, S.; Gajos, G. Alcohol, health loss, and mortality: Can wine really save the good name of moderate alcohol consumption? Pol. Arch. Intern. Med. 2024, 134, 16708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pu, L.; Zhao, T.; Wang, L.; Shu, C.; Xu, S.; Sun, J.; Zhang, R.; Han, L. Global Burden of Cardiovascular Disease from 1990 to 2019 Attributable to Dietary Factors. J. Nutr. 2023, 153, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, N.; Huang, M.; Li, Y.; Lu, Y.; Li, H.; Wu, L. Global burden of disease from high-sodium diets, 1990–2021: Analysis of GBD 2021 data. Front. Nutr. 2025, 12, 1617644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, W.; Chen, L.; Hu, T.; Sun, N.; Wei, X.; Zhang, Z. Global, regional and national ischemic heart disease burden attributable to a high-sodium diet in 204 countries, 1990–2019: A systematic analysis for the global burden of disease study. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 104016. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Xuan, S.; Wang, M.; Yang, S.; Wu, Q.; Tang, Z. Global, regional, and national burden of ischemic heart disease associated with diet high in sodium from 1990 to 2021, and its projections to 2035: A systematic analysis of the global burden of disease study 2021. Front. Nutr. 2025, 12, 1630331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egan, B.M.; Lackland, D.T.; Sutherland, S.E.; Rakotz, M.K.; Williams, J.; Commodore-Mensah, Y.; Jones, D.W.; Kjeldsen, S.E.; Campbell, N.R.C.; Parati, G.; et al. PERSPECTIVE—The Growing Global Benefits of Limiting Salt Intake: An urgent call from the World Hypertension League for more effective policy and public health initiatives. J. Hum. Hypertens. 2025, 39, 241–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contreras Navarro, A.; Gallagher, K.; Griffin, S.; Leydon, C.L.; Perry, I.J.; Harrington, J.M. Systematic Review on the Impact of Salt-Reduction Initiatives by Socioeconomic Position to Address Health Inequalities in Adult Populations. Nutr. Rev. 2025, 83, e1090–e1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karlsson, K.; Rådholm, K.; Dunford, E.; Wu, J.H.Y.; Neal, B.; Sundström, J. Sodium content in processed food items in Sweden compared to other countries: A cross-sectional multinational study. Front. Public Health 2023, 11, 1182132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, H.S.; Cai, Q.; Yang, J.J.; Lipworth, L.; Cai, H.; Yu, D.; Steinwandel, M.D.; Gupta, D.K.; Blot, W.J.; Zheng, W.; et al. Sodium Intake and Cause-Specific Mortality Among Predominantly Low-Income Black and White US Residents. JAMA Netw. Open. 2024, 7, e243802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surma, S.; Szyndler, A.; Narkiewicz, K. Salt and arterial hypertension—Epidemiological, pathophysiological and preventive aspects. Arter. Hypertens. 2020, 24, 148–158. [Google Scholar] [CrossRef]
- Surma, S.; Romańczyk, M.; Bańkowski, E. The role of limiting sodium intake in the diet—From theory to practice. Folia Cardiol. 2020, 15, 227–235. [Google Scholar] [CrossRef]
- Surma, S.; Francuz, T.; Ciechanowski, K. High Salt Intake and Cardiovascular Disease: An Overview. In Handbook of Public Health Nutrition; Preedy, V.R., Patel, V.B., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Y.; Wang, M.; Liu, J.; Bu, X.; Mu, J.; Lu, J. Global cardiovascular diseases burden attributable to high sodium intake from 1990 to 2019. J. Clin. Hypertens. 2023, 25, 868–879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Yeh, T.L.; Shih, M.C.; Tu, Y.K.; Chien, K.L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Yang, X.; Zhang, X.; Li, Y.; Zhao, X.; Ren, L.; Wang, L.; Gu, C.; Zhu, Z.; Han, Y. Dietary salt intake and coronary atherosclerosis in patients with prehypertension. J. Clin. Hypertens. 2014, 16, 575–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, D.; Li, H.M.; Li, C.X.; Zhou, B. 24-Hour Urinary Sodium Excretion Association with Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Biomed. Environ. Sci. 2022, 35, 921–930. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; O’Leary, N.; Yin, L.; Liu, X.; Swaminathan, S.; Khatib, R.; Rosengren, A.; et al. Joint association of urinary sodium potassium excretion with cardiovascular events mortality: Prospective cohort study. BMJ 2019, 364, l772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337, Erratum in Eur. Heart J. 2022, 43, 4468. https://doi.org/10.1093/eurheartj/ehac458. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Data Portal; World Health Organization: Geneva, Switzerland, 2022; Available online: https://ncdportal.org/ (accessed on 8 September 2025).
- Rybicka, I.; Nunes, M.L. Benefit and risk assessment of replacing of sodium chloride by other salt/substances in industrial seafood products. EFSA J. 2022, 20 (Suppl. 1), e200420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972, Erratum in Lancet 2021, 397, 2466. https://doi.org/10.1016/S0140-6736(21)01342-8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mente, A.; O’Donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, H.; Wang, X.; Li, X.; Heianza, Y.; Qi, L. Adding Salt to Foods and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, M.A.; van Raaij, J.M.; Geleijnse, J.M.; Breda, J.; Boshuizen, H.C. Health gain by salt reduction in europe: A modelling study. PLoS ONE 2015, 10, e0118873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graudal, N.; Jürgens, G.; Baslund, B.; Alderman, M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am. J. Hypertens. 2014, 27, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; Dagenais, G.; Lear, S.; McQueen, M.; Diaz, R.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet 2016, 388, 465–475, Erratum in Lancet 2021, 397, 1350. https://doi.org/10.1016/S0140-6736(21)00727-3. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhao, B.; Inoue-Choi, M.; Liao, L.M.; Graubard, B.I.; Weinstein, S.J.; Albanes, D.; Huang, J. Sex-specific associations between sodium and potassium intake and overall and cause-specific mortality: A large prospective U.S. cohort study, systematic review, and updated meta-analysis of cohort studies. BMC Med. 2024, 22, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messerli, F.H.; Hofstetter, L.; Bangalore, S. Salt and heart disease: A second round of “bad science”? Lancet 2018, 392, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Naser, A.M.; He, F.J.; Rahman, M.; Campbell, N.R.C. Spot Urine Formulas to Estimate 24-Hour Urinary Sodium Excretion Alter the Dietary Sodium and Blood Pressure Relationship. Hypertension 2021, 77, 2127–2137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Li, J.; Rao, J.; Lai, W.; Wei, Q.; Li, H.; Li, Y.; Peng, H.; Zhang, J. Joint effects of sodium intake and circadian rhythm of urinary sodium excretion on prognosis of chronic kidney disease: A prospective study. Ther. Adv. Chronic Dis. 2025, 16, 20406223251344474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huynh, K. Sodium and potassium intake for CV health. Nat. Rev. Cardiol. 2022, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, X.; Zhang, X.; Huang, L.; Xiong, S.; Li, Z.; Zhao, Y.; Zhou, B.; Yin, X.; Xu, B.; Wu, Y.; et al. Salt Substitution and Recurrent Stroke and Death: A Randomized Clinical Trial. JAMA Cardiol. 2025, 10, 343–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knauss, H.M.; Kovell, L.C.; Miller, E.R., 3rd; Appel, L.J.; Mukamal, K.J.; Plante, T.B.; Juraschek, S.P. Dietary sodium reduction lowers 10-year atherosclerotic cardiovascular disease risk score: Results from the DASH-sodium trial. Am. J. Prev. Cardiol. 2025, 22, 100980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surma, S.; Pruc, M.; Szarpak, Ł.; Banach, M. Dietary salt on vascular function—A meta-analysis. Arch. Med. Sci. 2025, 21, 1631–1635. [Google Scholar] [CrossRef]
- Basdeki, E.D.; Karatzi, K.; Manios, Y.; Sfikakis, P.P.; Argyris, A.A.; Protogerou, A.D. Dietary Sodium Consumption and 3-Year Progression of Subclinical Arterial Damage in Adults with Cardiovascular Risk Factors. Nutrients 2025, 17, 808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juraschek, S.P.; Miller, E.R., 3rd; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banach, M.; Surma, S. Dietary salt intake and atherosclerosis: An area not fully explored. Eur. Heart J. Open 2023, 3, oead025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaques, D.A.; Wuerzner, G.; Ponte, B. Sodium Intake as a Cardiovascular Risk Factor: A Narrative Review. Nutrients 2021, 13, 3177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edwards, D.G.; Farquhar, W.B. Chapter 53—Dietary salt and arterial stiffness. In Textbook of Arterial Stiffness and Pulsatile Hemodynamics in Health and Disease; Academic Press: Cambridge, MA, USA, 2022; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323913911000534 (accessed on 8 September 2025).
- Surma, S.; Lewek, J.; Sobierajski, T.; Banach, M. The importance of dietary salt in the process of atherosclerosis. The results of the international survey. Circulation 2024, 150 (Suppl. 1), A4145926. [Google Scholar] [CrossRef]
- Wuopio, J.; Ling, Y.T.; Orho-Melander, M.; Engström, G.; Ärnlöv, J. The association between sodium intake and coronary and carotid atherosclerosis in the general Swedish population. Eur. Heart J. Open. 2023, 3, oead024, Erratum in Eur. Heart J. Open. 2023, 3, oead062. https://doi.org/10.1093/ehjopen/oead062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, H.; Xue, Q.; Wang, X.; Li, X.; Franco, O.H.; Li, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Adding salt to foods and hazard of premature mortality. Eur. Heart J. 2022, 43, 2878–2888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Wang, Y.; Lu, X.; Wang, H.; Sun, J.; Wang, X. Association of salt added to food with risk of cardiovascular diseases: A 2-sample Mendelian randomization study. Medicine 2025, 104, e41543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Laurenzi, M. Sodium intake and kidney function in the general population: An observational, population-based study. Clin. Kidney J. 2020, 14, 647–655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kataoka, H.; Nitta, K.; Hoshino, J. Glomerular hyperfiltration and hypertrophy: An evaluation of maximum values in pathological indicators to discriminate “diseased” from “normal”. Front. Med. 2023, 10, 1179834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maaliki, D.; Itani, M.M.; Itani, H.A. Pathophysiology and genetics of salt-sensitive hypertension. Front. Physiol. 2022, 13, 1001434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mills, K.T.; Chen, J.; Yang, W.; Appel, L.J.; Kusek, J.W.; Alper, A.; Delafontaine, P.; Keane, M.G.; Mohler, E.; Ojo, A.; et al. Sodium Excretion and the Risk of Cardiovascular Disease in Patients with Chronic Kidney Disease. JAMA 2016, 315, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jhee, J.H.; Park, H.C.; Choi, H.Y. Skin Sodium and Blood Pressure Regulation. Electrolyte Blood Press. 2022, 20, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chachaj, A.; Stanimirova, I.; Chabowski, M.; Gomułkiewicz, A.; Hodurek, P.; Glatzel-Plucińska, N.; Olbromski, M.; Piotrowska, A.; Kuzan, A.; Grzegrzółka, J.; et al. Sodium accumulation in the skin is associated with higher density of skin lymphatic vessels in patients with arterial hypertension. Adv. Med. Sci. 2023, 68, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Burley, G.; Li, L.C.; Lin, S.; Shi, Y.C. The role of dietary salt in metabolism and energy balance: Insights beyond cardiovascular disease. Diabetes Obes. Metab. 2023, 25, 1147–1161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Ma, H.; Kou, M.; Tang, R.; Xue, Q.; Li, X.; Harlan, T.S.; Heianza, Y.; Qi, L. Dietary Sodium Intake and Risk of Incident Type 2 Diabetes. Mayo. Clin. Proc. 2023, 98, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, C.; Kim, O.Y.; Lee, Y.M.; Yoon, M.O.; Lee, M. The association between dietary sodium intake and obesity in adults by sodium intake assessment methods: A review of systematic reviews and re-meta-analysis. Nutr. Res. Pract. 2023, 17, 175–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.J.; Wang, H.W.; Cheon, S.Y.; Lee, H.J.; Hwang, K.M.; Yoon, H.S. Associations of Obesity and Dyslipidemia with Intake of Sodium, Fat, and Sugar among Koreans: A Qualitative Systematic Review. Clin. Nutr. Res. 2016, 5, 290–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soltani, S.; Kolahdouz Mohammadi, R.; Shab-Bidar, S.; Vafa, M.; Salehi-Abargouei, A. Sodium status and the metabolic syndrome: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Feng, H.; Zhang, N.; Yang, Z. High salt diet causally increases metabolic dysfunction-associated steatotic liver disease risk: A bidirectional mendelian randomization study. Nutr. Res. 2025, 136, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Leng, X.Y.; Dong, Y.; Ma, Y.H.; Xu, W.; Cao, X.P.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Modifiable risk factors for carotid atherosclerosis: A meta-analysis and systematic review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wuopio, J.; Yi-Ting, L.; Dekkers, K.F.; Fall, T.; Smith, J.G.; Larsson, A.; Engström, G.; Orho-Melander, M.; Johnson, L.S.; Ärnlöv, J. The metabolic signature of salt intake: A cross-sectional analysis from the SCAPIS-study. Nutr. Metab. 2025, 22, 104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shehata, M.F. Role of the IRS-1 and/or -2 in the pathogenesis of insulin resistance in Dahl salt-sensitive (S) rats. Heart Int. 2009, 4, e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; He, F.J.; MacGregor, G.A. High salt intake: Independent risk factor for obesity? Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Li, J.; Yu, Y.; Song, Y. A positive association between dietary sodium intake and obesity and central obesity: Results from the National Health and Nutrition Examination Survey 1999–2006. Nutr. Res. 2018, 55, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kibayashi, E.; Nakade, M. Dietary Salt Restriction Practices Contribute to Obesity Prevention in Middle-Aged and Older Japanese Adults. Nutrients 2025, 17, 536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Z.; Luo, J.; Lv, M.; Li, Q.; Ke, W.; Niu, X.; Zhang, Z. Characteristics and evaluation of atherosclerotic plaques: An overview of state-of-the-art techniques. Front. Neurol. 2023, 14, 1159288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suckling, R.J.; He, F.J.; Markandu, N.D.; MacGregor, G.A. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int. 2012, 81, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Suckling, R.J.; Swift, P.A.; He, F.J.; Markandu, N.D.; MacGregor, G.A. Altering plasma sodium concentration rapidly changes blood pressure during haemodialysis. Nephrol. Dial. Transpl. 2013, 28, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Surma, S.; Więcek, A. Hyponatremia in patients with arterial hypertension: Pathophysiology and management. Arch. Med. Sci. 2023, 19, 1630–1645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; White, J.; Guo, L.; Zhao, X.; Wang, J.; Smart, E.J.; Li, X.A. Salt inactivates endothelial nitric oxide synthase in endothelial cells. J. Nutr. 2009, 139, 447–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, C.; Browne, L.D.; Gilligan, R.; Galvin, R.; Glynn, L.; Walsh, C.; Stack, A.G. Impact of serum sodium concentrations, and effect modifiers on mortality in the Irish Health System. BMC Nephrol. 2023, 24, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fels, J.; Jeggle, P.; Liashkovich, I.; Peters, W.; Oberleithner, H. Nanomechanics of vascular endothelium. Cell Tissue Res. 2014, 355, 727–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, A.; Tomita, H.; Okada, H. Form follows function: The endothelial glycocalyx. Transl. Res. 2022, 247, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sembajwe, L.F.; Ssekandi, A.M.; Namaganda, A.; Muwonge, H.; Kasolo, J.N.; Kalyesubula, R.; Nakimuli, A.; Naome, M.; Patel, K.P.; Masenga, S.K.; et al. Glycocalyx-Sodium Interaction in Vascular Endothelium. Nutrients 2023, 15, 2873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patik, J.C.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Mechanisms of Dietary Sodium-Induced Impairments in Endothelial Function and Potential Countermeasures. Nutrients 2021, 13, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oberleithner, H. Vascular endothelium: A vulnerable transit zone for merciless sodium. Nephrol. Dial. Transpl. 2014, 29, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Idzerda, N.M.A.; Ettema, E.M.; Kuipers, J.; Dam, W.; van den Born, J.; Franssen, C.F.M. An acute rise of plasma Na+ concentration associates with syndecan-1 shedding during hemodialysis. Am. J. Physiol. Ren. Physiol. 2020, 319, F171–F177. [Google Scholar] [CrossRef] [PubMed]
- Vinaiphat, A.; Pazhanchamy, K.; JebaMercy, G.; Ngan, S.C.; Leow, M.K.; Ho, H.H.; Gao, Y.G.; Lim, K.L.; Richards, A.M.; de Kleijn, D.P.V.; et al. Endothelial Damage Arising from High Salt Hypertension Is Elucidated by Vascular Bed Systematic Profiling. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflug. Arch. 2011, 462, 519–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dewan, S.M.R.; Meem, S.S.; Proma, A.Y.; Shahriar, M. Dietary Salt Can Be Crucial for Food-Induced Vascular Inflammation. Clin. Pathol. 2024, 17, 2632010X241228039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. Endothelial function is impaired after a high-salt meal in healthy subjects. Am. J. Clin. Nutr. 2011, 93, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.L.; Brian, M.S.; Ramick, M.G.; Lennon-Edwards, S.; Edwards, D.G.; Farquhar, W.B. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J. Appl. Physiol. 2015, 118, 1510–1515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shenouda, N.; Ramick, M.G.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. High dietary sodium augments vascular tone and attenuates low-flow mediated constriction in salt-resistant adults. Eur. J. Appl. Physiol. 2020, 120, 1383–1389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oberleithner, H.; Kusche-Vihrog, K.; Schillers, H. Endothelial cells as vascular salt sensors. Kidney Int. 2010, 77, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Wiecek, A. Food Products That May Cause an Increase in Blood Pressure. Curr. Hypertens. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J. Cell Mol. Med. 2017, 21, 1457–1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fels, J.; Jeggle, P.; Kusche-Vihrog, K.; Oberleithner, H. Cortical actin nanodynamics determines nitric oxide release in vascular endothelium. PLoS ONE 2012, 7, e41520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.H.; Nijst, P.; Kiefer, K.; Tang, W.H. Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications. Curr. Heart Fail. Rep. 2017, 14, 117–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linz, P.; Santoro, D.; Renz, W.; Rieger, J.; Ruehle, A.; Ruff, J.; Deimling, M.; Rakova, N.; Muller, D.N.; Luft, F.C.; et al. Skin sodium measured with 23Na MRI at 7.0 T. NMR Biomed. 2015, 28, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Deacon, C.; King, A.J.; Machin, D.R. Microcirculatory and glycocalyx properties are lowered by high-salt diet but augmented by Western diet in genetically heterogeneous mice. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H328–H335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strauss, M.; Smith, W.; Fedorova, O.V.; Schutte, A.E. The Na+K+-ATPase Inhibitor Marinobufagenin and Early Cardiovascular Risk in Humans: A Review of Recent Evidence. Curr. Hypertens. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paczula, A.; Wiecek, A.; Piecha, G. Cardiotonic Steroids-A Possible Link Between High-Salt Diet and Organ Damage. Int. J. Mol. Sci. 2019, 20, 590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, M.K.S.; Murphy, A.J. A high-salt diet promotes atherosclerosis by altering haematopoiesis. Nat. Rev. Cardiol. 2023, 20, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Côrte-Real, B.F.; Hamad, I.; Arroyo Hornero, R.; Geisberger, S.; Roels, J.; Van Zeebroeck, L.; Dyczko, A.; van Gisbergen, M.W.; Kurniawan, H.; Wagner, A.; et al. Sodium perturbs mitochondrial respiration and induces dysfunctional Tregs. Cell Metab. 2023, 35, 299–315.e8. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Afsar, R.E. Mitochondrial Damage and Hypertension: Another Dark Side of Sodium Excess. Curr. Nutr. Rep. 2023, 12, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Crestani, S.; Gasparotto Júnior, A.; Marques, M.C.; Sullivan, J.C.; Webb, R.C.; da Silva-Santos, J.E. Enhanced angiotensin-converting enzyme activity and systemic reactivity to angiotensin II in normotensive rats exposed to a high-sodium diet. Vascul. Pharmacol. 2014, 60, 67–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wenstedt, E.F.; Verberk, S.G.; Kroon, J.; Neele, A.E.; Baardman, J.; Claessen, N.; Pasaoglu, Ö.T.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight. 2019, 4, e130508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawicka-Smiarowska, E.; Bondarczuk, K.; Bauer, W.; Niemira, M.; Szalkowska, A.; Raczkowska, J.; Kwasniewski, M.; Tarasiuk, E.; Dubatowka, M.; Lapinska, M.; et al. Gut Microbiome in Chronic Coronary Syndrome Patients. J. Clin. Med. 2021, 10, 5074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Mou, X.; Li, J.; Li, M.; Ye, C.; Gao, X.; Liu, X.; Ma, Y.; Xu, Y.; Zhong, Y. Alterations in gut microbiota and host transcriptome of patients with coronary artery disease. BMC Microbiol. 2023, 23, 320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, D.; Jia, W.; Wang, W.; Liao, L.; Xu, B.; Gong, L.; Dong, H.; Zhong, L.; Yang, J. Causality of the gut microbiome and atherosclerosis-related lipids: A bidirectional Mendelian Randomization study. BMC Cardiovasc. Disord. 2024, 24, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, Y.; Xu, M.; Huang, N.; Yuan, Z. Meta-analysis of the effect of probiotics or synbiotics on the risk factors in patients with coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1154888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choroszy, M.; Sobieszczańska, B.; Litwinowicz, K.; Łaczmański, Ł.; Chmielarz, M.; Walczuk, U.; Roleder, T.; Radziejewska, J.; Wawrzyńska, M. Co-toxicity of Endotoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis. Nutrients 2022, 14, 424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Lang, F.; Liu, D. High-Salt Diet and Intestinal Microbiota: Influence on Cardiovascular Disease and Inflammatory Bowel Disease. Biology 2024, 13, 674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-Salt Diet Has a Certain Impact on Protein Digestion and Gut Microbiota: A Sequencing and Proteome Combined Study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fagunwa, O.; Davies, K.; Bradbury, J. The Human Gut and Dietary Salt: The Bacteroides/Prevotella Ratio as a Potential Marker of Sodium Intake and Beyond. Nutrients 2024, 16, 942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jama, H.A.; Marques, F.Z. Don’t Take It with a Pinch of Salt: How Sodium Increases Blood Pressure via the Gut Microbiota. Circ. Res. 2020, 126, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shui, X.; Liang, Z.; Huang, Z.; Qi, Y.; He, Y.; Chen, C.; Luo, H.; Lei, W. Gut microbiota metabolites as integral mediators in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 46, 936–948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trehan, S.; Singh, G.; Bector, G.; Jain, P.; Mehta, T.; Goswami, K.; Chawla, A.; Jain, A.; Puri, P.; Garg, N. Gut Dysbiosis and Cardiovascular Health: A Comprehensive Review of Mechanisms and Therapeutic Potential. Cureus 2024, 16, e67010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; He, F.J.; Dong, Y.; Huang, Y.; Wang, C.; Harshfield, G.A.; Zhu, H. Modest Sodium Reduction Increases Circulating Short-Chain Fatty Acids in Untreated Hypertensives: A Randomized, Double-Blind, Placebo-Controlled Trial. Hypertension 2020, 76, 73–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Hu, J. Unraveling the gut microbiota’s role in salt-sensitive hypertension: Current evidences and future directions. Front. Cardiovasc. Med. 2024, 11, 1410623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Samarraie, A.; Pichette, M.; Rousseau, G. Role of the Gut Microbiome in the Development of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 5420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elijovich, F.; Laffer, C.L.; Sahinoz, M.; Pitzer, A.; Ferguson, J.F.; Kirabo, A. The Gut Microbiome, Inflammation, and Salt-Sensitive Hypertension. Curr. Hypertens. Rep. 2020, 22, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, S.; Jiang, H.; Zhuo, C.; Jiang, W. Trimethylamine/Trimethylamine-N-Oxide as a Key Between Diet and Cardiovascular Diseases. Cardiovasc. Toxicol. 2021, 21, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Flaig, B.; Garza, R.; Singh, B.; Hamamah, S.; Covasa, M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients 2023, 15, 228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, X.; Jin, J.; Su, X.; Yin, X.; Gao, J.; Wang, X.; Zhang, S.; Bu, P.; Wang, M.; Zhang, Y.; et al. Intestinal Flora Modulates Blood Pressure by Regulating the Synthesis of Intestinal-Derived Corticosterone in High Salt-Induced Hypertension. Circ. Res. 2020, 126, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, A.I.; Govindaraju, K.; Lokanathan, Y.; Said, N.A.B.M.; Ibrahim, B. Exploring the Potential of Stem Cells in Modulating Gut Microbiota and Managing Hypertension. Stem Cells Dev. 2025, 34, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Surma, S. Gut microbiota and arterial hypertension—A narrative review. Arch. Med. Sci. 2025; in press. [Google Scholar]
- Libby, P.; Oren, O.; Small, A.M. Clonal Hematopoiesis of Indeterminate Potential. JAMA Cardiol. 2025, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Zekavat, S.M.; Uddin, M.M.; Pirruccello, J.; Niroula, A.; Gibson, C.; Griffin, G.K.; Libby, P.; Ebert, B.L.; Bick, A.; et al. Association of Diet Quality with Prevalence of Clonal Hematopoiesis and Adverse Cardiovascular Events. JAMA Cardiol. 2021, 6, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Peng, X.L.; Chen, Z.X.; Qi, L.M.; Deng, T.T.; Xia, L.N. Immune Dysregulation Orchestrated by High-Salt Diet: Mechanistic Insights into Disease Pathogenesis. Nutr. Diet. Suppl. 2024, 16, 147–164. [Google Scholar] [CrossRef]
- Surma, S.; Lewandowski, Ł.; Momot, K.; Sobierajski, T.; Lewek, J.; Okopień, B.; Banach, M. Mapping the Cognitive Architecture of Health Beliefs: A Multivariate Conditional Network of Perceived Salt-Related Disease Risks. Nutrients 2025, 17, 2728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czerniuk, M.R.; Surma, S.; Romańczyk, M.; Nowak, J.M.; Wojtowicz, A.; Filipiak, K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis-Plaque Destabilization? From the Teeth to a Coronary Event. Biology 2022, 11, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surma, S.; Romańczyk, M.; Witalińska-Łabuzek, J.; Czerniuk, M.R.; Łabuzek, K.; Filipiak, K.J. Periodontitis, Blood Pressure, and the Risk and Control of Arterial Hypertension: Epidemiological, Clinical, and Pathophysiological Aspects-Review of the Literature and Clinical Trials. Curr. Hypertens. Rep. 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surma, S.; Lip, G.Y.H. Periodontitis and Atrial Fibrillation. Eur. J. Prev. Cardiol. 2025; zwaf626. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Colin-Ramirez, E.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Alemayehu, W.; McAlister, F.A.; Arcand, J.; et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): An international, open-label, randomised, controlled trial. Lancet 2022, 399, 1391–1400, Erratum in Lancet 2022, 400, 1194. https://doi.org/10.1016/S0140-6736(22)01892-X. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| System/Compartment | Predominant Phenotype Under High Sodium | Dominant Biological Drivers | Salient Features/Readouts | Net Effect on Atherogenesis |
|---|---|---|---|---|
| Hemodynamic regulation | Sustained pressor load (arterial hypertension) | Renal sodium and water retention; SNS/RAAS activation; MR signalling; ↓ NO bioavailability; ENaC and NHE-3 overactivation; ↓ ANP and BNP levels | ↑ TPR and vascular tone; endothelial vasoconstrictor bias | ↑ Atherogenesis via pressure-mediated vascular injury |
| Kidney | Glomerular stress and injury | Chronic hyperfiltration; reduced GAG buffering; renal sodium overload | Glomerulosclerosis; declining filtration; CKD progression | Renal–vascular amplification of atherogenic risk |
| Vascular endothelium—glycocalyx | Glycocalyx thinning/fragmentation | Oversaturation of interstitial proteoglycans; sodium accumulation at the surface | ↓ Buffering; ↑ exposure of EC to Na+; increased cell–blood element interactions | Loss of endothelial protection; thrombosis-prone interface |
| Vascular endothelium—mechanics/ion channels | Endothelial stiffening with ↑ ENaC expression | Mechanical shear sensitivity; altered sodium handling | Reduced NO generation; VSMC contraction bias; vasoconstriction | Impaired vasodilation; plaque-promoting hemodynamics |
| Vascular endothelium—oxidative and immune signalling | Pro-oxidative, pro-inflammatory signalling | ROS; IsoLG neoantigen formation; NFκB and NLRP3 activation | Leukocyte activation; cytokine release; fibrotic remodelling | Acceleration of vascular inflammation and lesion growth |
| Vascular endothelium—adhesion and permeability | Upregulated adhesion and barrier leak | ↑ ICAM/VCAM/selectins; junctional disruption; ↑ proatherosclerotic PC and LPC form | Enhanced monocyte recruitment and transmigration | Foam cell formation and plaque expansion |
| Vascular endothelium—mitochondria and survival | Mitochondrial injury and apoptosis | Oxidative damage; energy stress | ↑ Endothelial permeability; cell loss | Unstable endothelium; pro-atherogenic milieu |
| Vascular endothelium—vasoactive systems | Local neurohormonal dysregulation | RAAS imbalance; cardiotonic steroids (e.g., marinobufagenin) | Vasoconstriction, fibrosis, arterial stiffening | Adverse remodelling that favours atherosclerosis |
| Interstitial matrix/proteoglycans | Buffering capacity exhaustion | GAG oversaturation with sodium | Greater sodium delivery to EC, kidney, and other organs | ↑ Sodium toxicity; amplification of vascular injury |
| Gut microbiota—SCFA axis | SCFA depletion and signalling loss | Diet–microbiome disruption under high salt | Reduced anti-inflammatory/antioxidant tone; BP dysregulation; altered lipid handling | Withdrawal of protective effects against atherogenesis |
| Gut microbiota—TMAO pathway | ↑ TMAO generation | Microbial conversion of dietary precursors | Foam cell promotion; dyslipoproteinaemia; endothelial stress; prothrombotic tendency | ↑ Plaque formation and event risk |
| Gut barrier and translocation | Increased intestinal permeability | LPS/PAMPs translocation; bacterial products | Systemic inflammation; insulin resistance; macrophage vascular infiltration | Inflammation-driven atherosclerosis |
| Gut-derived/host metabolites | Adverse metabolite signalling | IsmA (↓ coprostanol → ↓ cholesterol excretion); indoxyl sulfate; arachidonic acid/corticosterone → MR overactivation; ↑ DCA and LCA levels | Oxidative stress; endothelial activation/adhesion | Pro-atherogenic metabolic programming |
| Innate/adaptive immunity—T cell axis | Th17 skewing and Treg impairment | ↑ IL-17/IL-23 signalling; mitochondrial stress in immune cells | Pro-inflammatory vascular milieu | Heightened vascular inflammation |
| Hematopoiesis and clonal dynamics | Myeloid-biased inflammation | CHIP-related mutations foster proinflammatory clones | Exaggerated monocyte output and activation | Chronic inflammatory drive to plaque growth |
| Monocyte/macrophage polarization | Shift toward M1-like phenotype | Sodium-driven innate immune reprogramming | Amplified vascular inflammation and foam cell formation | Lesion progression and destabilisation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surma, S.; Okopień, B.; Murphy, A.J.; Banach, M. High Salt Intake and Atherosclerosis Progression—Not Only via Blood Pressure: A Narrative Review. Nutrients 2025, 17, 3464. https://doi.org/10.3390/nu17213464
Surma S, Okopień B, Murphy AJ, Banach M. High Salt Intake and Atherosclerosis Progression—Not Only via Blood Pressure: A Narrative Review. Nutrients. 2025; 17(21):3464. https://doi.org/10.3390/nu17213464
Chicago/Turabian StyleSurma, Stanisław, Bogusław Okopień, Andrew J. Murphy, and Maciej Banach. 2025. "High Salt Intake and Atherosclerosis Progression—Not Only via Blood Pressure: A Narrative Review" Nutrients 17, no. 21: 3464. https://doi.org/10.3390/nu17213464
APA StyleSurma, S., Okopień, B., Murphy, A. J., & Banach, M. (2025). High Salt Intake and Atherosclerosis Progression—Not Only via Blood Pressure: A Narrative Review. Nutrients, 17(21), 3464. https://doi.org/10.3390/nu17213464








