Phytochemical Profiling, Anti-Inflammatory Action, and Human Gut Microbiota-Assisted Digestion of Rheum officinale Petiole and Root Extracts—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
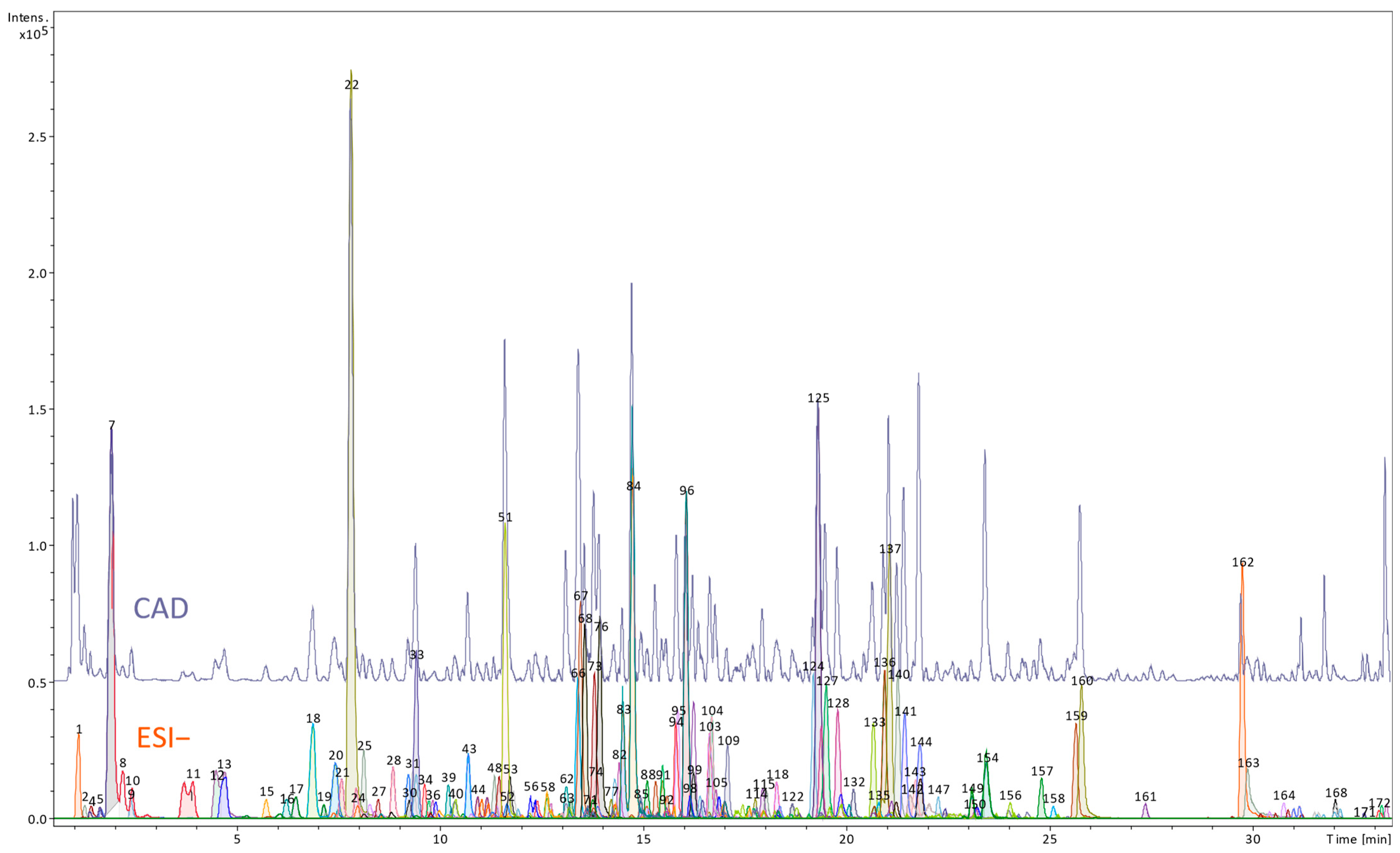
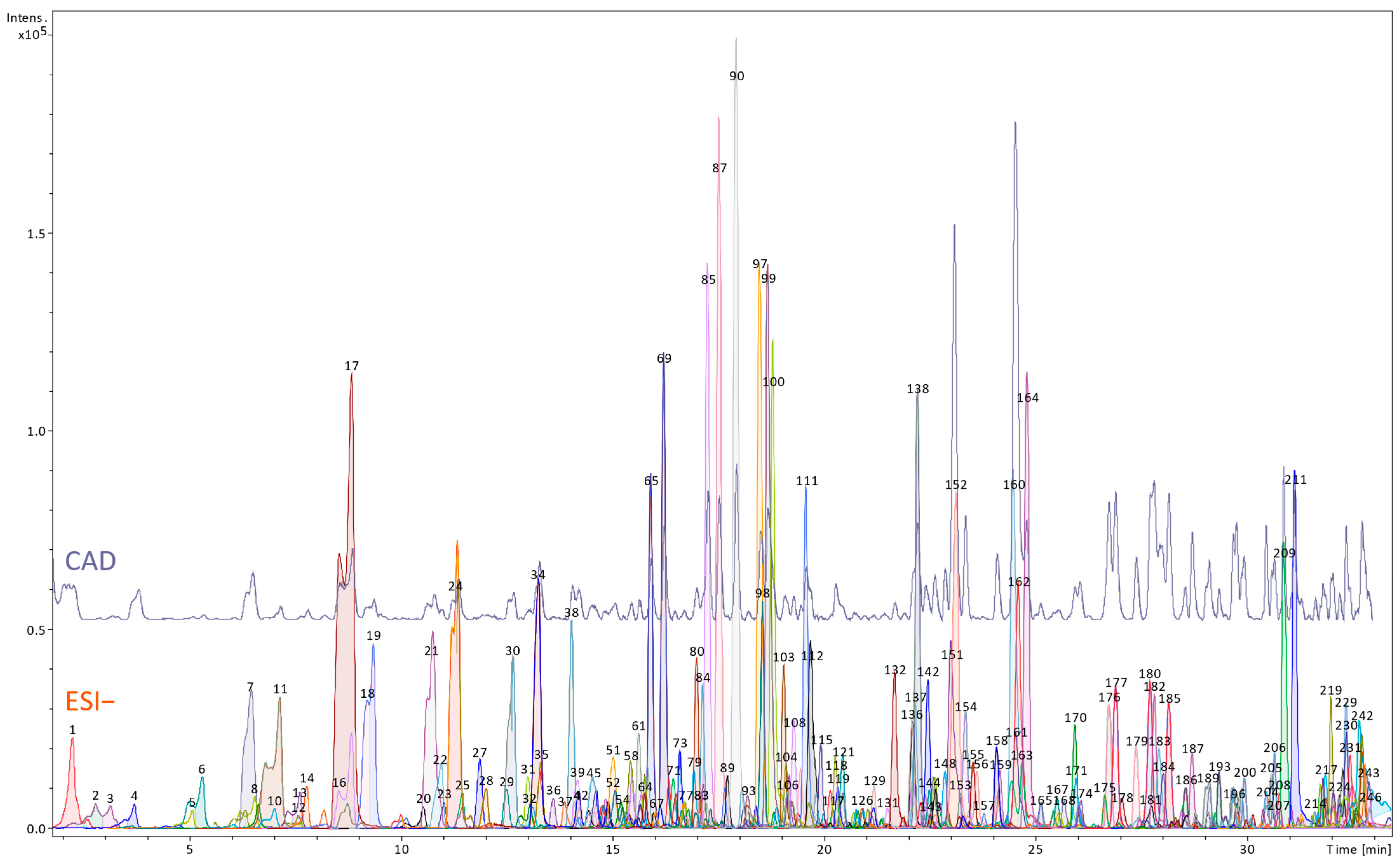
Preparation of Extracts and High-Resolution LC-MS Qualitative and Semi-Quantitative Analyses
2.3. Cell Cultures
2.4. Effects of the Examined Extracts on COX2 and ALOX5 Gene Expression in HUVECs
2.4.1. Total RNA Isolation and cDNA Synthesis
2.4.2. Real-Time—Quantitative PCR (RT-qPCR)
2.5. Evaluation of the COX-2 and 5-LOX-Inhibitory Efficiency of the Examined Extracts
2.6. Effects of the Examined Extracts on HUVEC Viability
2.7. Measurements of Cytokine Secretion from PBMCs
2.8. Cytotoxicity Assays in PBMCs Culture
2.9. Experiments on Human Gut Microbiota
2.10. Chromatographic Analysis of Extracts Metabolized by Human Gut Microbiota
2.11. Statistical Analysis
3. Results
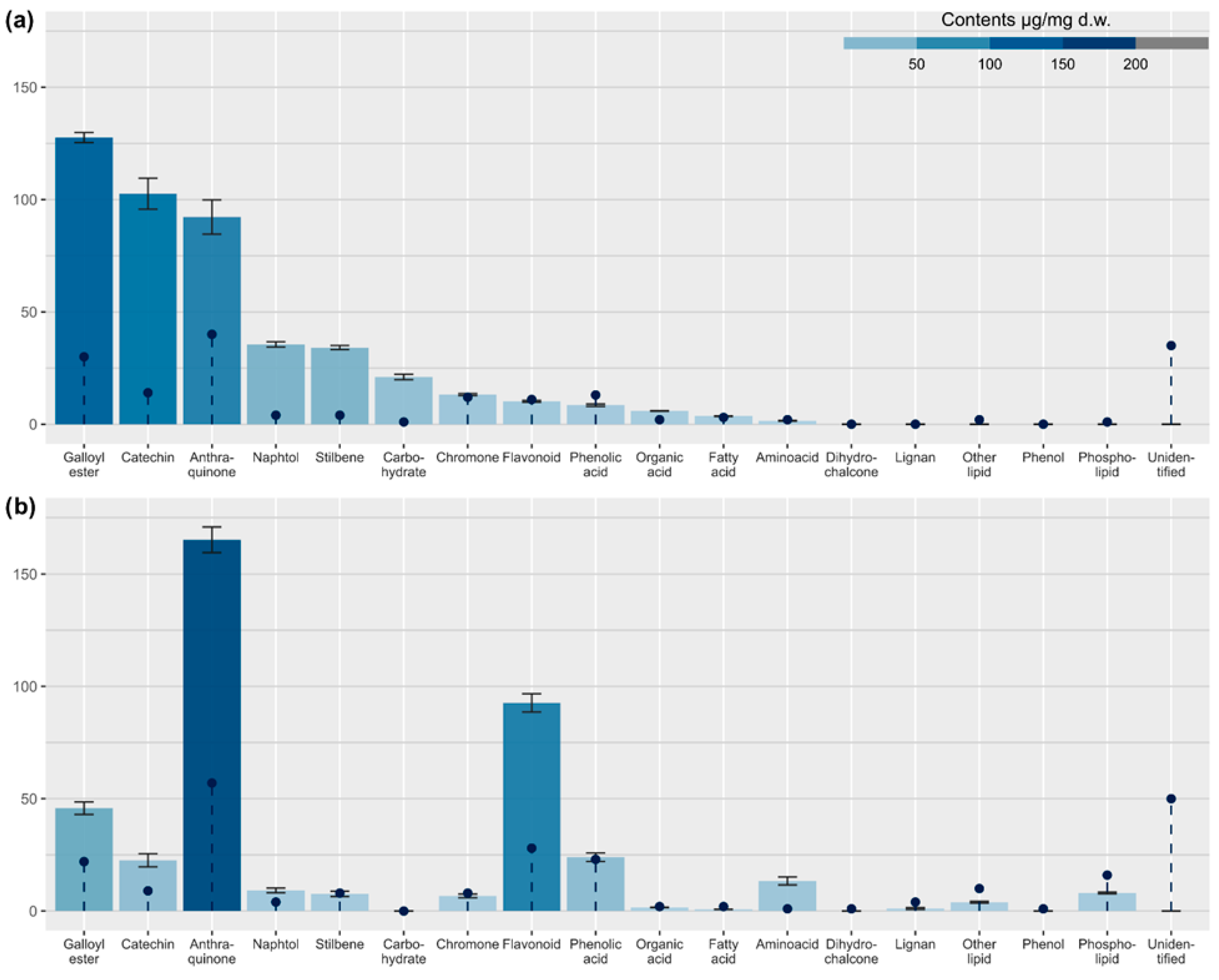
3.1. Phytochemical Profile of the Petiole and Root Extracts of R. officinale
3.2. Effects of the Examined Extracts on COX2 and ALOX5 Gene and Protein Expression in HUVECs
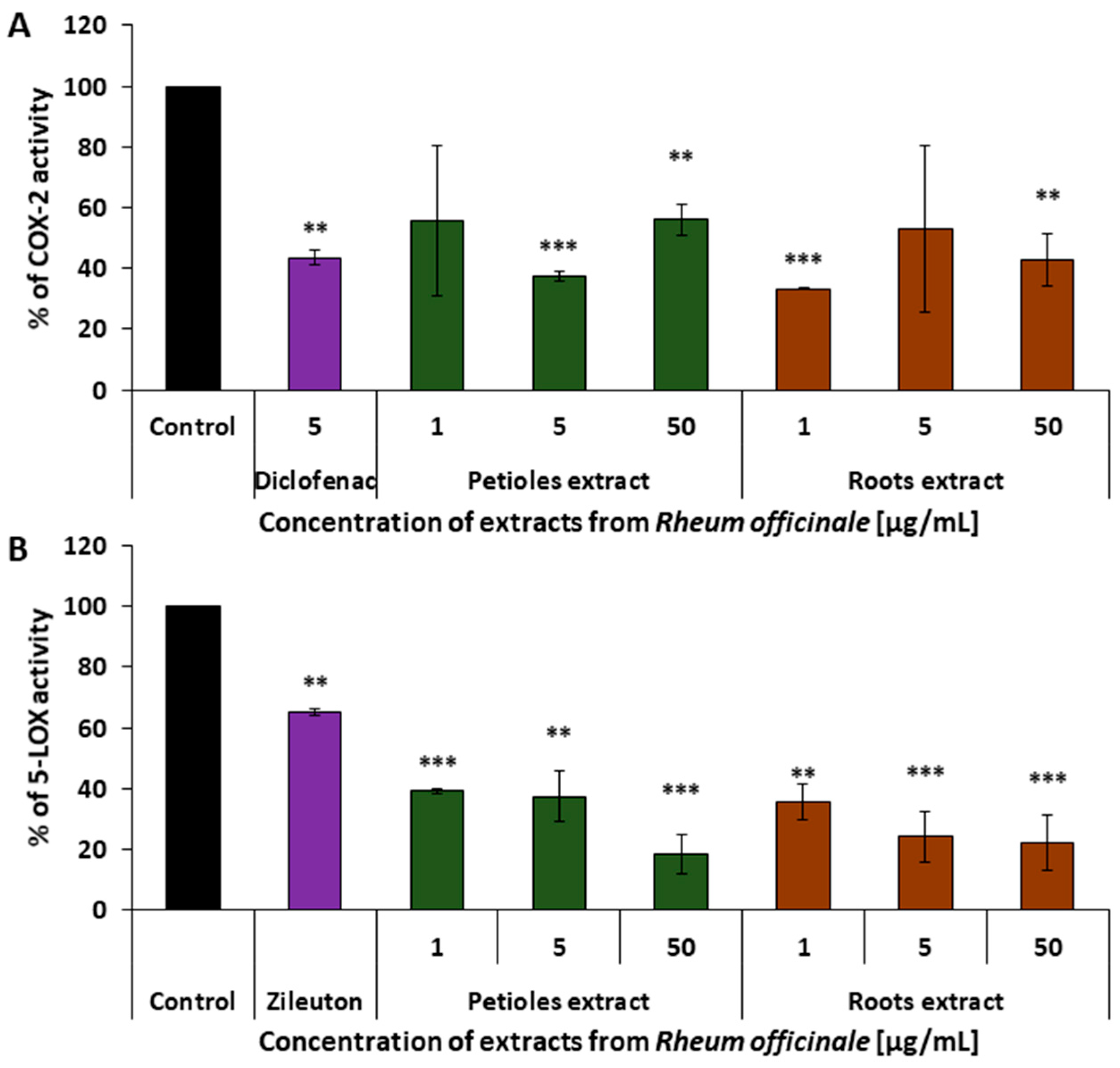
3.3. Evaluation of the COX-2 and 5-LOX-Inhibitory Ability of the Examined Extracts
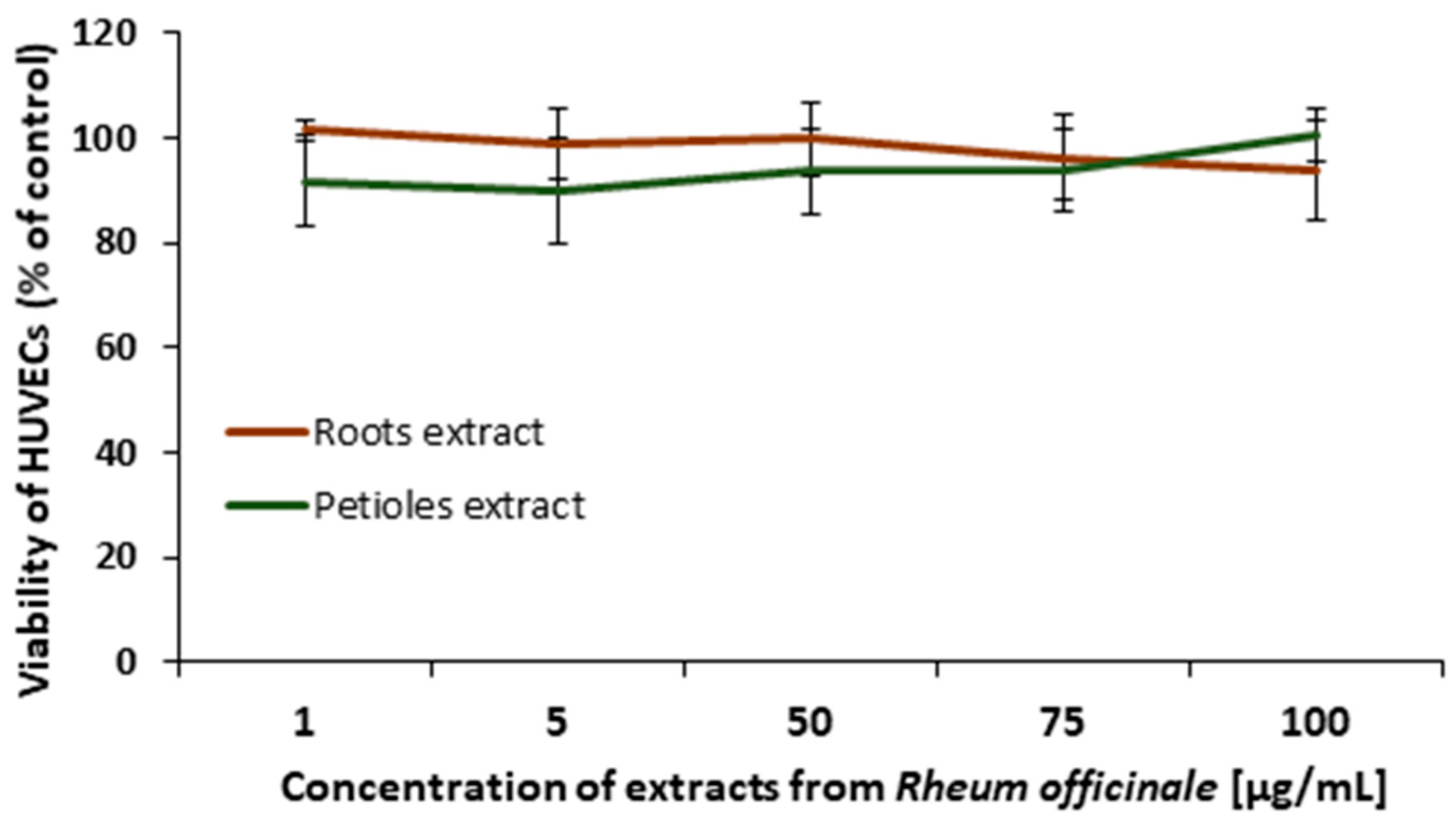
3.4. Effects of the Examined Extracts on HUVECs Viability
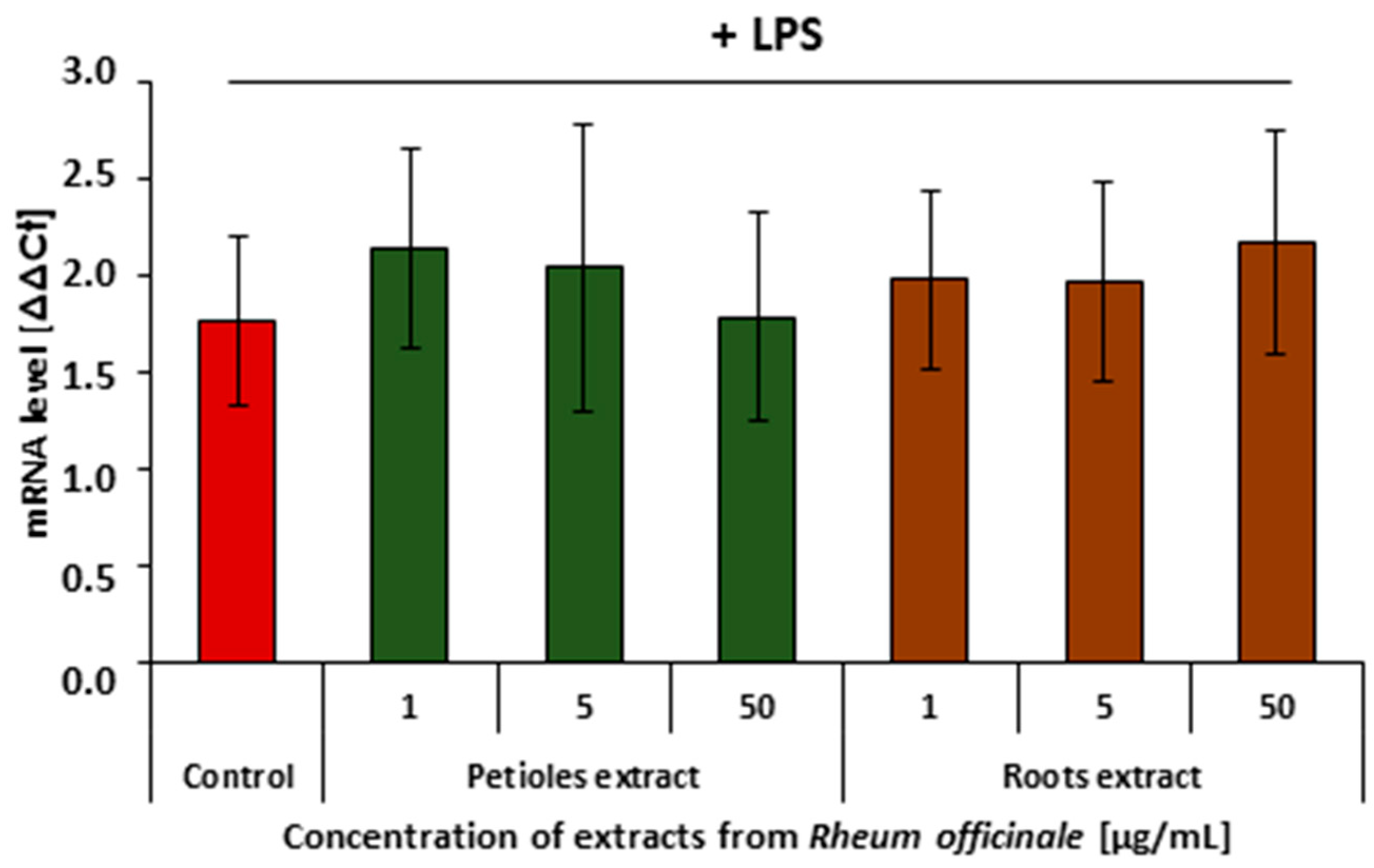
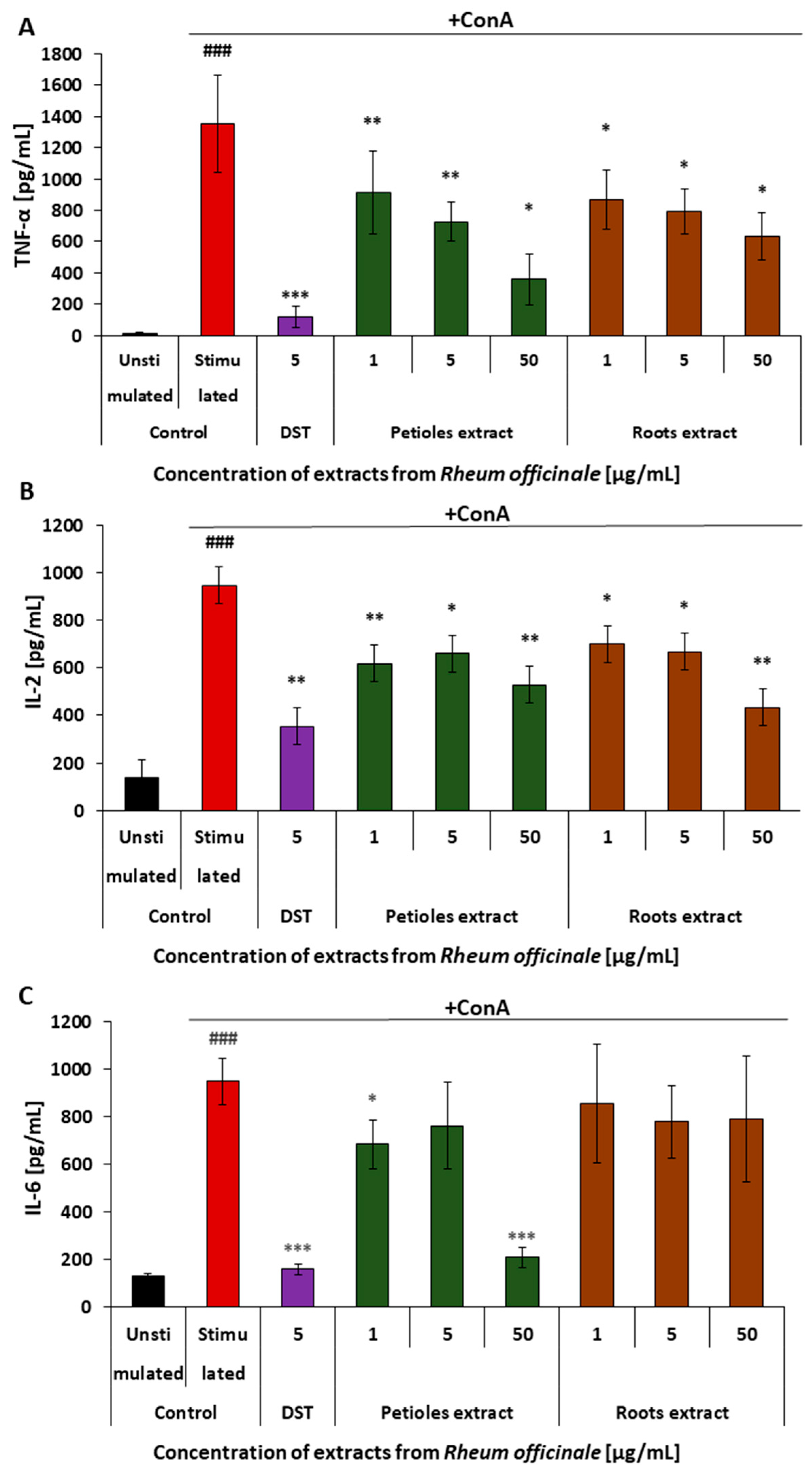
3.5. Effects of the Examined Extracts on the Inflammatory Response of the PBMCs
3.6. Cytotoxicity Evaluation in PBMCs
3.7. Metabolism of R. officinale Extracts by Human Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Xu, Z.; Zhang, G.; Lv, H.; Wang, X.; Xu, G.; Li, X.; Yang, Z.; Wang, H.; et al. Identification and action mechanism of lipid regulating components from Rhei radix et rhizoma. J. Ethnopharmacol. 2022, 292, 115179. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Fu, F.; Wu, Y.; Han, C.; Pu, W.; Wen, L.; Xia, Q.; Du, D. Rhei radix et rhizoma and its anthraquinone derivatives: Potential candidates for pancreatitis treatment. Phytomedicine 2024, 129, 155708. [Google Scholar] [CrossRef]
- Zhuang, T.; Gu, X.; Zhou, N.; Ding, L.; Yang, L.; Zhou, M. Hepatoprotection and hepatotoxicity of Chinese herb Rhubarb (Dahuang): How to properly control the “General (Jiang Jun)” in Chinese medical herb. Biomed. Pharmacother. 2020, 127, 110224. [Google Scholar] [CrossRef]
- Yang, X.; Dai, L.; Yan, F.; Ma, Y.; Guo, X.; Jenis, J.; Wang, Y.; Zhang, J.; Miao, X.; Shang, X. The phytochemistry and pharmacology of three Rheum species: A comprehensive review with future perspectives. Phytomedicine 2024, 131, 155772. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Li, W.-Y.; Chan, S.-W.; Guo, D.-J.; Yu, P.H.F. Correlation Between Antioxidative Power and Anticancer Activity in Herbs from Traditional Chinese Medicine Formulae with Anticancer Therapeutic Effect. Pharm. Biol. 2007, 45, 541–546. [Google Scholar] [CrossRef]
- Yao, M.; Li, J.; He, M.; Ouyang, H.; Ruan, L.; Huang, X.; Rao, Y.; Yang, S.; Zhou, X.; Bai, J. Investigation and identification of the multiple components of Rheum officinale Baill. using ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry and data mining strategy. J. Sep. Sci. 2021, 44, 681–690. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Ethnobotanical Uses, Phytochemistry and Pharmacology of Different Rheum Species (Polygonaceae): A Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1308. [Google Scholar] [CrossRef]
- Tang, R.C. Sources for Natural Colorants in China in: Handbook of Natural Colorants, 2nd ed.; Bechtold, T., Manian, A.P., Pham, T., Eds.; no. Renewable Resource; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 89–99. [Google Scholar] [CrossRef]
- Wang, X.M.; Hou, X.Q.; Zhang, Y.Q.; Li, Y. Distribution pattern of genuine species of rhubarb as traditional Chinese medicine. J. Med. Plants Res. 2010, 4, 1865–1876. [Google Scholar]
- Dai, L.X.; Miao, X.; Yang, X.R.; Zuo, L.P.; Lan, Z.H.; Li, B.; Shang, X.F.; Yan, F.Y.; Guo, X.; Wang, Y.; et al. High Value-Added Application of Two Renewable Sources as Healthy Food: The Nutritional Properties, Chemical Compositions, Antioxidant, and Antiinflammatory Activities of the Stalks of Rheum officinale Baill. and Rheum tanguticum Maxim. ex Regel. Front. Nutr. 2022, 8, 770264. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yang, L.; Yu, D.; Qian, Y.; Ni, H. Efficacy of Rheum officinale liquid formulation on cucumber powdery mildew. Crop Prot. 2009, 28, 1031–1035. [Google Scholar] [CrossRef]
- Xie, J.; Liu, B.; Zhou, Q.; Su, Y.; He, Y.; Pan, L.; Ge, X.; Xu, P. Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture 2008, 281, 5–11. [Google Scholar] [CrossRef]
- Liu, B.; Ge, X.; Xie, J.; Xu, P.; He, Y.; Cui, Y.; Ming, J.; Zhou, Q.; Pan, L. Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2012, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Zhao, Z.M.; Li, J.C.; Yang, G.Z.; Liu, Y.Q.; Dai, L.X.; Zhang, Z.J.; Yang, Z.G.; Miao, X.L.; Yang, C.J.; et al. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crops Prod. 2019, 137, 508–520. [Google Scholar] [CrossRef]
- Verified Market Reports. 2025. Available online: https://www.verifiedmarketreports.com/product/rheum-officinale-extract-market/ (accessed on 19 February 2025).
- Cao, Y.-J.; Pu, Z.-J.; Tang, Y.-P.; Shen, J.; Chen, Y.-Y.; Kang, A.; Zhou, G.-S.; Duan, J.-A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Jiang, J.; Chen, Y.; Peng, B.; Jin, W. Mechanism of rhubarb in the treatment of hyperlipidemia: A recent review. Open Med. 2023, 18, 20230812. [Google Scholar] [CrossRef]
- Li, W.Y.; Chan, S.W.; Guo, D.J.; Chung, M.K.; Leung, T.Y.; Yu, P.H. Water extract of Rheum officinale Baill. induces apoptosis in human lung adenocarcinoma A549 and human breast cancer MCF-7 cell lines. J. Ethnopharmacol. 2009, 124, 251–256. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Li, R.Z.; Xu, C.; Fan, X.X.; Li, J.X.; Meng, W.Y.; Wang, X.R.; Liang, T.L.; Guan, X.X.; Pan, H.D.; et al. Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine 2022, 95, 153786. [Google Scholar] [CrossRef]
- Qiao, S.; Zhang, W.; Jiang, Y.; Su, Y. Sennoside A induces autophagic death of prostate cancer via inactivation of PI3K/AKT/mTOR axis. J. Mol. Histol. 2023, 54, 645–654. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Vaziri, N.D.; Wei, F.; Cheng, X.L.; Bai, X.; Zhao, Y.Y. An integrated lipidomics and metabolomics reveal nephroprotective effect and biochemical mechanism of Rheum officinale in chronic renal failure. Sci. Rep. 2016, 6, 22151. [Google Scholar] [CrossRef]
- Bai, J.; Xie, Y.; Li, M.; Huang, X.; Guo, Y.; Sun, J.; Tang, Y.; Liu, X.; Wei, C.; Li, J.; et al. Ultrasound-assisted extraction of emodin from Rheum officinale Baill and its antibacterial mechanism against Streptococcus suis based on CcpA. Ultrason. Sonochemistry 2024, 102, 106733. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenginq activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Ke, C.Y.; Wu, W.T.; Harn, H.J.; Tseng, Y.H.; Lee, R.P. Effects of Angelica dahurica and Rheum officinale Extracts on Excisional Wound Healing in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1583031. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.P.; Lee, P.T.; Nan, F.H. Rheum officinale extract promotes the innate immunity of orange-spotted grouper (Epinephelus coioides) and exerts strong bactericidal activity against six aquatic pathogens. Fish Shellfish Immunol. 2020, 102, 117–124. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Zhao, G.; Li, B.; Qin, X.; Xu, J.; Li, X. Phytoremediation potential evaluation of three rhubarb species and comparative analysis of their rhizosphere characteristics in a Cd- and Pb-contaminated soil. Chemosphere 2022, 296, 134045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Liu, Y.R.; Tang, Z.S.; Song, Z.X.; Zhang, J.W.; Chang, B.J.; Zhao, M.L.; Xu, J. Rheum officinale Baill. Treats zebrafish embryo thrombosis by regulating NOS3 expression in the arginine biosynthesis pathway. Phytomedicine 2022, 99, 153967. [Google Scholar] [CrossRef]
- Tan, S.M.; Ho, L.N.; Wong, Y.S.; Abidin, C.Z.A.; Ong, S.A. Sustainable utilization of anthraquinone-rich Rheum officinale as electron shuttle in microbial fuel cell: Strategy for stimulating monohydric phenols degradation and bioelectricity generation. Chem. Eng. J. 2023, 475, 146423. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Ma, B.; An, L.; Zhang, L.; Li, X.; Gao, W. Active ingredients, nutrition values and health-promoting effects of above-ground parts of rhubarb: A review. Food Sci. Biotechnol. 2025, 1–16. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Gallo, G.; Savoia, C. New Insights into Endothelial Dysfunction in Cardiometabolic Diseases: Potential Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 2973. [Google Scholar] [CrossRef]
- Liudvytska, O.; Ponczek, M.B.; Ciesielski, O.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Balcerczyk, A.; Kolodziejczyk-Czepas, J. Rheum rhaponticum and Rheum rhabarbarum Extracts as Modulators of Endothelial Cell Inflammatory Response. Nutrients 2023, 15, 949. [Google Scholar] [CrossRef]
- Liudvytska, O.; Ponczek, M.B.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Balcerczyk, A.; Kolodziejczyk-Czepas, J. Effects of Rheum rhaponticum and Rheum rhabarbarum extracts on haemostatic activity of blood plasma components and endothelial cells in vitro. J. Ethnopharmacol. 2023, 315, 116562. [Google Scholar] [CrossRef]
- Liudvytska, O.; Bandyszewska, M.; Skirecki, T.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Kolodziejczyk-Czepas, J. Anti-inflammatory and antioxidant actions of extracts from Rheum rhaponticum and Rheum rhabarbarum in human blood plasma and cells in vitro. Biomed. Pharmacother. 2023, 165, 115111. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Kozachok, S.; Pecio, Ł.; Kolodziejczyk-Czepas, J.; Marchyshyn, S.; Nowak, P.; Mołdoch, J.; Oleszek, W. γ-Pyrone compounds: Flavonoids and maltol glucoside derivatives from Herniaria glabra L. collected in the Ternopil region of the Ukraine. Phytochemistry 2018, 152, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Rybaczek, D.; Wojtala, M.; Pirola, L.; Okabe, J.; El-Osta, A. Pharmacological inhibition of arginine and lysine methyltransferases induces nuclear abnormalities and suppresses angiogenesis in human endothelial cells. Biochem. Pharmacol. 2016, 121, 18–32. [Google Scholar] [CrossRef]
- Krzyzanowska-Kowalczyk, J.; Kolodziejczyk-Czepas, J.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Yunnaneic Acid B, a Component of Pulmonaria officinalis Extract, Prevents Peroxynitrite-Induced Oxidative Stress in Vitro. J. Agric. Food Chem. 2017, 65, 3827–3834. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Yin, Z.; Gao, X. On-line incubation and real-time detection by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapidly analyzing metabolites of anthraquinones in rat liver microsomes. J. Chromatogr. A 2018, 1571, 94–106. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Y.; Wang, Q.; Gao, X. Metabolism and mutual biotransformations of anthraquinones and anthrones in rhubarb by human intestinal flora using UPLC-Q-TOF/MS. J. Chromatogr. B 2019, 1104, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Peng, C.; Peng, Y.; Zhang, M.; Li, X. Analysis of metabolites of anthraquinones by human fecal bacteria using UPLC-Q-TOF-HRMS/MS. Chromatographia 2016, 79, 1593–1604. [Google Scholar] [CrossRef]
- Wang, X.M.; Ren, Y. Rheum tanguticum, an endangered medicinal plant endemic to China. J. Med. Plants Res. 2009, 3, 1195–1203. [Google Scholar]
- Wang, X.; Yang, R.; Feng, S.; Hou, X.; Zhang, Y.; Li, Y.; Ren, Y. Genetic Variation in Rheum palmatum and Rheum tanguticum (Polygonaceae), Two Medicinally and Endemic Species in China Using ISSR Markers. PLoS ONE 2012, 7, e51667. [Google Scholar] [CrossRef]
- Wang, X.M.; Hou, X.Q.; Zhang, Y.Q.; Yang, R.; Feng, S.F.; Li, Y.; Ren, Y. Genetic diversity of the endemic and medicinally important plant Rheum officinale as revealed by inter-simpe sequence repeat (ISSR) markers. Int. J. Mol. Sci. 2012, 13, 3900–3915. [Google Scholar] [CrossRef]
- Facchini, F.; Silvestri, B.; Digiesi, S.; Lucchese, A. Agri-food loss and waste management: Win-win strategies for edible discarded fruits and vegetables sustainable reuse. Innov. Food Sci. Emerg. Technol. 2023, 83, 103235. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C.; Lee, Y.Y. From waste to value: Addressing the relevance of waste recovery to agricultural sector in line with circular economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Perdana, T.; Kusnandar, K.; Perdana, H.H.; Hermiatin, F.R. Circular supply chain governance for sustainable fresh agricultural products: Minimizing food loss and utilizing agricultural waste. Sustain. Prod. Consum. 2023, 41, 391–403. [Google Scholar] [CrossRef]
- Pandey, A.K.; Thakur, S.; Mehra, R.; Kaler, R.S.S.; Paul, M.; Kumar, A. Transforming Agri-food waste: Innovative pathways toward a zero-waste circular economy. Food Chem. X 2025, 28, 102604. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Jin, C.; Qu, Y.; Xiao, X. Development and validation of a UPLC method for quality control of rhubarb-based medicine: Fast simultaneous determination of five anthraquinone derivatives. J. Pharm. Biomed. Anal. 2008, 47, 765–770. [Google Scholar] [CrossRef]
- Wei, S.Y.; Yao, W.X.; Ji, W.Y.; Wei, J.Q.; Peng, S.Q. Qualitative and quantitative analysis of anthraquinones in rhubarbs by high performance liquid chromatography with diode array detector and mass spectrometry. Food Chem. 2013, 141, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Dai, Y.; Zhou, Y.; Wang, S. Quality evaluation of rhubarb based on qualitative analysis of the HPLC fingerprint and UFLC–Q-TOF–MS/MS combined with quantitative analysis of eight anthraquinone glycosides by QAMS. Biomed. Chromatogr. 2021, 35, e5074. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Weimei, Z.; Chen, Y.; Sun, J.; Guo, F.; Hu, J.; Gao, W.; Li, X. Quality evaluation of different varieties of rhubarb based on multicomponents and bioactivity: Application to quality control in the production of rhubarb decoction pieces. Biomed. Chromatogr. 2022, 36, e5368. [Google Scholar] [CrossRef] [PubMed]
- Vanmen, C.; Jang, Y.S.; Zhu, H.M.; Lee, J.H.; Trung, T.N.; Ngoc, T.M.; Kim, Y.H.; Kang, J.S. Chemical-based species classification of Rhubarb using simultaneous determination of five bioactive substances by HPLC and LDA analysis. Phytochem. Anal. 2012, 23, 359–364. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, P.; Xu, L.; He, C.; Peng, Y.; Xiao, P. Evaluation of the content variation of anthraquinone glycosides in rhubarb by UPLC-PDA. Chem. Cent. J. 2013, 7, 170. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia 11.0; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2022. [Google Scholar]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of Phenolic Compounds in Rhubarbs Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Niu, X.; Zhu, Q.; Wang, X.; Li, S.; Sun, J.; Hua, S.; Yang, L.; Yao, W. Distinguishment of different varieties of rhubarb based on UPLC fingerprints and chemometrics. J. Pharm. Biomed. Anal. 2024, 241, 116003. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Zhang, Z.; Dai, P.; Li, P.; Li, W. Effects of adding Rheum officinale to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on renal function in patients with chronic renal failure: A meta-analysis of randomized controlled trials. Clin. Nephrol. 2018, 89, 445. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Nagayama, Y.; Tanaka, K.; Ling, Y.; Cai, S.Q.; Omote, T.; Meselhy, M.R. Comparative study of chemical constituents of rhubarb from different origins. Chem. Pharm. Bull. 2006, 54, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, L.; Hu, H.; Li, Y.; Liu, C.; Wei, S. Influence of the environmental factors on the accumulation of the bioactive ingredients in Chinese rhubarb products. PLoS ONE 2016, 11, e0154649. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Nagayama, Y.; Tanaka, K.; Ling, Y.; Basnet, P.; Meselhy, M.R. Development of a high performance liquid chromatographic method for systematic quantitative analysis of chemical constituents in rhubarb. Chem. Pharm. Bull. 2006, 54, 941–947. [Google Scholar] [CrossRef]
- Sun, M.; Li, L.; Wang, M.; van Wijk, E.; He, M.; van Wijk, R.; Koval, S.; Hankemeier, T.; van der Greef, J.; Wei, S. Effects of growth altitude on chemical constituents and delayed luminescence properties in medicinal rhubarb. J. Photochem. Photobiol. B Biol. 2016, 162, 24–33. [Google Scholar] [CrossRef]
- Au, T.T.D.; Ho, Y.L.; Chang, Y.S. Qualitative and quantitative analysis methods for quality control of rhubarb in Taiwan’s markets. Front. Pharmacol. 2024, 15, 1364460. [Google Scholar] [CrossRef]
- Gao, X.Y.; Jiang, Y.; Lu, J.Q.; Tu, P.F. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J. Chromatogr. A 2009, 1216, 2118–2123. [Google Scholar] [CrossRef]
- Tan, P.; Zhang, L.; Zhao, Y.L.; Zhang, C.E.; Niu, M.; Xiao, X.H.; Wang, J.B. A practical method for the simultaneous quantitative determination of twelve anthraquinone derivatives in rhubarb by a single-marker based on ultra-performance liquid chromatography and chemometric analysis. Anal. Methods 2016, 8, 3927–3934. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Y.; Dong, S.; Li, X.; Gao, W. Quantitative studies of rhubarb using quantitative analysis of multicomponents by single marker and response surface methodology. J. Sep. Sci. 2017, 40, 3792–3800. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Zhang, B.; Lin, Z. Quantitative analysis of multi-components by single marker—A rational method for the internal quality of Chinese herbal medicine. Integr. Med. Res. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Chen, A.; Sun, L.; Yuan, H.; Wu, A.; Lu, J.; Ma, S. Simultaneous qualitative and quantitative analysis of 11 active compounds in rhubarb using two reference substances by UHPLC. J. Sep. Sci. 2018, 41, 3686–3696. [Google Scholar] [CrossRef]
- Almeling, S.; Ilko, D.; Holzgrabe, U. Charged aerosol detection in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2012, 69, 50–63. [Google Scholar] [CrossRef]
- Liu, S.; Lu, B.; Peng, Z.; Liu, C.; Liu, Y.; Jiao, H.; Wu, D.; Li, P.; Zhao, X.; Song, S. HPLC-CAD as a supplementary method for the quantification of related structure impurities for the purity assessment of organic CRMs. Anal. Bioanal. Chem. 2023, 415, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. Studies on Rhubarb (Rhei Rhizoma). VI.1) Isolation and Characterization of Stilbenes. Chem. Pharm. Bull. 1984, 32, 3501–3517. [Google Scholar] [CrossRef]
- Smolarz, H.; Hałka, A.; Chabros, O.; Dzido, T. Rapid method for rhaponticin and deoxyrhaponticin separation and determination by TLC in Rheum rhaponticum L. and Rheum undulatum L. Acta Chromatogr. 2013, 25, 127–134. [Google Scholar] [CrossRef]
- Xu, S.; Yang, G.; Feng, F. Investigation of Distinction Chemical Markers for Rhubarb Authentication Based on High-Performance Liquid Chromatography-Time-of-Flight Mass Spectrometry and Multivariate Statistical Analysis. Food Anal. Methods 2017, 10, 3934–3946. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.J.; Han, L.; Li, Y.; Wang, X.M. Optimization of chloroplast microsatellite PCR conditions and primer screening for endangered Rheum officinale, Rheum palmatum, and Rheum tanguticum. Genet. Mol. Res. 2014, 13, 5787–5794. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar]
- Simon, L.S. Nonsteroidal anti-inflammatory drugs and their risk: A story still in development. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S1. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Chaudhry, W.R.; Akbar, A. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: A narrative review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Joshi, E.M.; Heasley, B.H.; Chordia, M.D.; Macdonald, T.L. In Vitro Metabolism of 2-Acetylbenzothiophene: Relevance to Zileuton Hepatotoxicity. Chem. Res. Toxicol. 2004, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Iaccarino, G. Inflammation and cardiovascular diseases: The most recent findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 21, 100130. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Boutari, C.; Hill, M.A.; Procaccini, C.; Matarese, G.; Mantzoros, C.S. The key role of inflammation in the pathogenesis and management of obesity and CVD. Metab. Clin. Exp. 2023, 145, 155627. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Z.; Sun, Y.; Jin, D.; Guo, L.; Li, X.; Zhao, D.; Feng, X.; Qi, W.; Zhu, H. Research Progress and Molecular Mechanisms of Endothelial Cells Inflammation in Vascular-Related Diseases. J. Inflamm. Res. 2023, 16, 3593–3617. [Google Scholar] [CrossRef]
- Nunes, C.D.R.; Arantes, M.B.; de Faria Pereira, S.M.; da Cruz, L.L.; de Souza Passos, M.; de Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Dell, M. Special issue: Anti-inflammatory activity of plant polyphenols. Biomedicines 2020, 8, 64. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, A.; Mukherjee, S.; Maji, B.K. Quercetin inhibits NF-kB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: An in vitro approach. Mol. Biol. Rep. 2024, 51, 277. [Google Scholar] [CrossRef]
- Naponelli, V.; Rocchetti, M.T.; Mangieri, D. Apigenin: Molecular Mechanisms and Therapeutic Potential against Cancer Spreading. Int. J. Mol. Sci. 2024, 25, 5569. [Google Scholar] [CrossRef]
- Lee, H.H.; Yu, J.K.; Moon, Y.S. Antioxidant and anti-inflammatory activities of different parts of rhubarb (Rheum rhabarbarum) compared with da huang root (R. officinale). Korean J. Food Preserv. 2022, 29, 186–195. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Yi, Q.; Qiao, G.; Wang, L.; Chen, L.; Fan, L.; Li, Y.; Duan, L.; Huang, L.; et al. Free total rhubarb anthraquinones protect intestinal mucosal barrier of SAP rats via inhibiting the NLRP3/caspase-1/GSDMD pyroptotic pathway. J. Ethnopharmacol. 2024, 326, 117873. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wu, J.; Lu, H.; Cheng, M.L.; Wang, B.H.; Zhu, H.L.; Liu, L.; Xie, M. Emodin suppresses oxaliplatin-induced neuropathic pain by inhibiting COX2/NF-κB mediated spinal inflammation. J. Biochem. Mol. Toxicol. 2023, 37, e23229. [Google Scholar] [CrossRef]
- Park, M.Y.; Kwon, H.J.; Sung, M.K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef]
- Sharanya, C.S.; Arun, K.G.; Sabu, A.; Haridas, M. Aloe emodin shows high affinity to active site and low affinity to two other sites to result consummately reduced inhibition of lipoxygenase. Prostaglandins Other Lipid Mediat. 2020, 150, 106453. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Vázquez, S.; Eseberri, I.; Les, F.; Pérez-Matute, P.; Herranz-López, M.; Atgié, C.; Lopez-Yus, M.; Aranaz, P.; Oteo, J.A.; Escoté, X.; et al. Polyphenols and metabolism: From present knowledge to future challenges. J. Physiol. Biochem. 2024, 80, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Espín, J.C. In Vitro Research on Dietary Polyphenols and Health: A Call of Caution and a Guide on How to Proceed. J. Agric. Food Chem. 2018, 66, 7857–7858. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Del Rio, D. Gold Standards for Realistic (Poly)phenol Research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef]
- Ansari, M.H.R.; Saher, S.; Parveen, R.; Khan, W.; Khan, I.A.; Ahmad, S. Role of gut microbiota metabolism and biotransformation on dietary natural products to human health implications with special reference to biochemoinformatics approach. J. Tradit. Complement. Med. 2022, 13, 150–160. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]


| Metabolite | Metabolite Identification | RT (min) | UV λmax (nm) | [M–H]− (m/z) | MS2 (m/z) | Source Compounds |
|---|---|---|---|---|---|---|
| Petiole Extract | ||||||
| M1 | (aloe)emodin | 73.8 | 223 | 268.87 | 240.80 | (aloe)emodin glycosides/ dianthrones |
| M2 | hydroxy-emodin | 62.2 | 221 | 285.05 | 240.78 | emodin glycosides/ dianthrones |
| M3 | acetyl-hydroxy-emodin | 71.3 | 223 | 327.39 | - | emodin glycosides/ dianthrones |
| M4 | (aloe)emodin-physcion-dianthrone hexoside | 72.0 | 222 | 685.30 | 253.79, 523.08 | (aloe)emodin-physcion-dianthrone dihexosides |
| M5 | chrysophanol isomer | 11.5 | 190 | 253.26 | 209.81 | physcion glycosides/ dianthrones |
| Root extract | ||||||
| M6 | rhein | 71.0 | 222 | 282.96 | 238.74 | rhein glycosides/ dianthrones |
| M7 | acetyl-1,3,8-trihydroxy-6-methyl-9-oxanthranol/acetyl-1,3,8-trihydroxy-6-methyl-10-oxanthranol | 66.0 | 220 | 313.15 | 268.75 | emodin/chrysophanol glycosides/dianthrones |
| M8 | sennidin A-8-O-glucoside | 65.8 | 220 | 699.22 | 223.25, 537.07 | sennoside A/ sennoside A esters |
| M9 | sennidin C/D-8-O-glucoside/ sennidin C/D-8′-O-glucoside | 64.8 | 220 | 685.21 | 223.75, 385.95, 479.05 | sennoside C/D |
| Parameters and Experimental Systems | Maximum Inhibitory Effect | ||
|---|---|---|---|
| Petiole Extract from R. officinale | Root Extract from R. officinale | ||
| Gene/protein expression and the enzyme activity tests | COX2 (gene expression)/(HUVECs) | No effect | No effect |
| COX-2 (enzyme activity) | 63% | 67% | |
| ALOX5 (gene expression)/(HUVECs) | 40% | 22% | |
| 5-LOX/(enzyme activity) | 81% | 78% | |
| Cytokine release | TNF-α/(PBMCs) | 73% | 53% |
| IL-2/(PBMCs) | 44% | 54% | |
| Il-6/(PBMCs) | 78% | No effect | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liudvytska, O.; Kowalczyk, M.; Krzyżanowska-Kowalczyk, J.; Michaś, K.; Michalak, M.; Balcerczyk, A.; Skowrońska, W.; Równicki, M.; Bazylko, A.; Olszewska, M.A.; et al. Phytochemical Profiling, Anti-Inflammatory Action, and Human Gut Microbiota-Assisted Digestion of Rheum officinale Petiole and Root Extracts—An In Vitro Study. Nutrients 2025, 17, 3455. https://doi.org/10.3390/nu17213455
Liudvytska O, Kowalczyk M, Krzyżanowska-Kowalczyk J, Michaś K, Michalak M, Balcerczyk A, Skowrońska W, Równicki M, Bazylko A, Olszewska MA, et al. Phytochemical Profiling, Anti-Inflammatory Action, and Human Gut Microbiota-Assisted Digestion of Rheum officinale Petiole and Root Extracts—An In Vitro Study. Nutrients. 2025; 17(21):3455. https://doi.org/10.3390/nu17213455
Chicago/Turabian StyleLiudvytska, Oleksandra, Mariusz Kowalczyk, Justyna Krzyżanowska-Kowalczyk, Karolina Michaś, Maria Michalak, Aneta Balcerczyk, Weronika Skowrońska, Marcin Równicki, Agnieszka Bazylko, Monika A. Olszewska, and et al. 2025. "Phytochemical Profiling, Anti-Inflammatory Action, and Human Gut Microbiota-Assisted Digestion of Rheum officinale Petiole and Root Extracts—An In Vitro Study" Nutrients 17, no. 21: 3455. https://doi.org/10.3390/nu17213455
APA StyleLiudvytska, O., Kowalczyk, M., Krzyżanowska-Kowalczyk, J., Michaś, K., Michalak, M., Balcerczyk, A., Skowrońska, W., Równicki, M., Bazylko, A., Olszewska, M. A., & Kolodziejczyk-Czepas, J. (2025). Phytochemical Profiling, Anti-Inflammatory Action, and Human Gut Microbiota-Assisted Digestion of Rheum officinale Petiole and Root Extracts—An In Vitro Study. Nutrients, 17(21), 3455. https://doi.org/10.3390/nu17213455








