Beneficial Effects of Long-Lasting Bicarbonate–Sulfate–Calcium–Magnesium Water Intake on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Outcomes via Impacting Intestinal Permeability (IP), IP-Related Systemic Inflammation, and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.2.1. Randomization and Intervention
2.2.2. Specialist-Prescribed Controlled Nutritional Regimen
2.2.3. General Compliance Assessment
2.3. Assessment of Anthropometrical, Clinical, and Biochemical Variables
2.4. Abdominal Ultrasound and Transient Elastography
2.5. Assessment of Intestinal Permeability Markers
2.6. Assessment of Systemic Inflammation Markers
2.7. Assessment of Systemic Oxidative Stress and Antioxidant Capacity
2.8. Statistical Analysis
3. Results
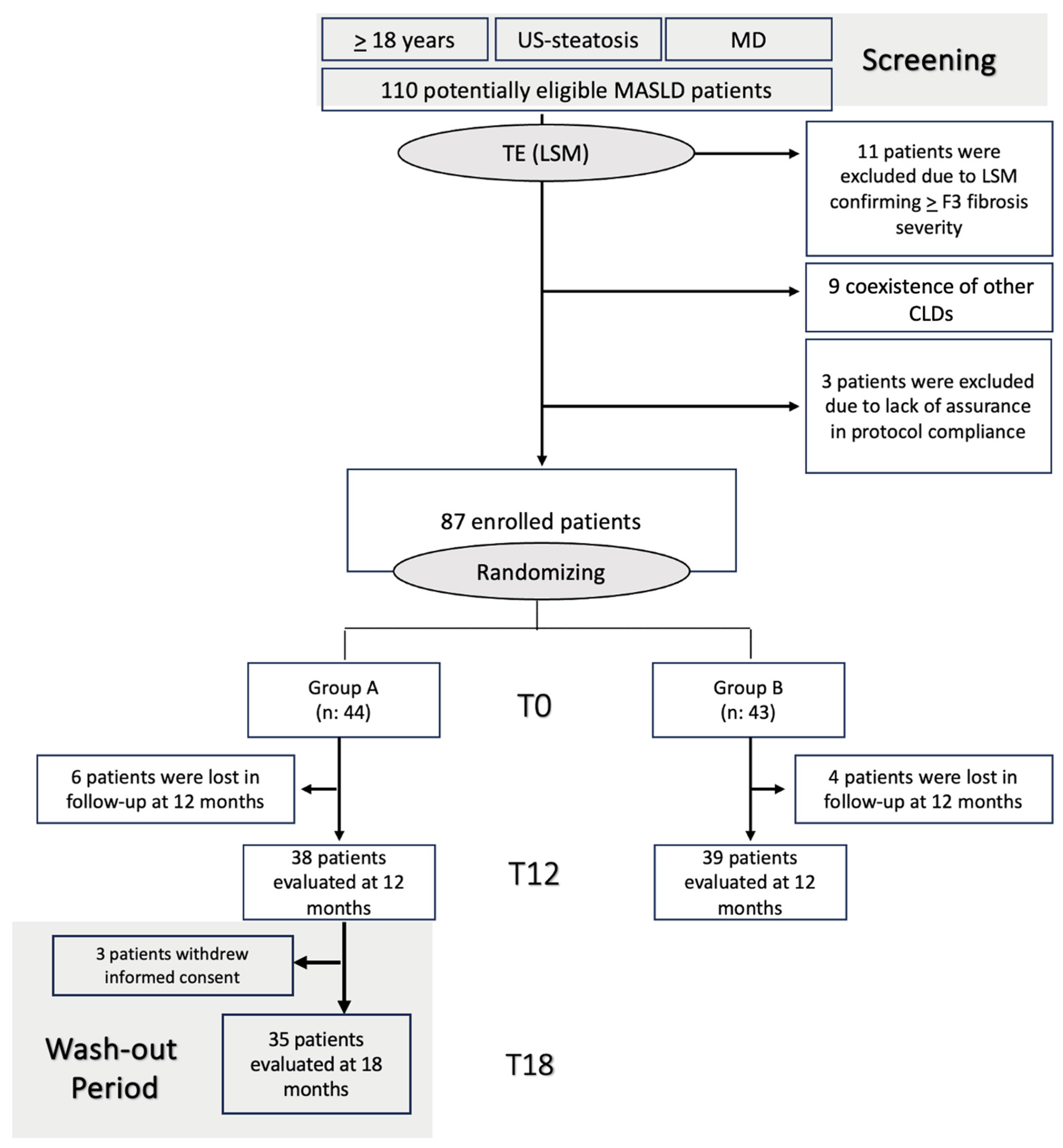
3.1. Enrollment of MASLD Patients, Follow-Up, and Compliance Evaluation
3.2. Baseline Evaluations: Characteristics of the Study Groups
3.3. 12-Month Follow-Up Evaluations
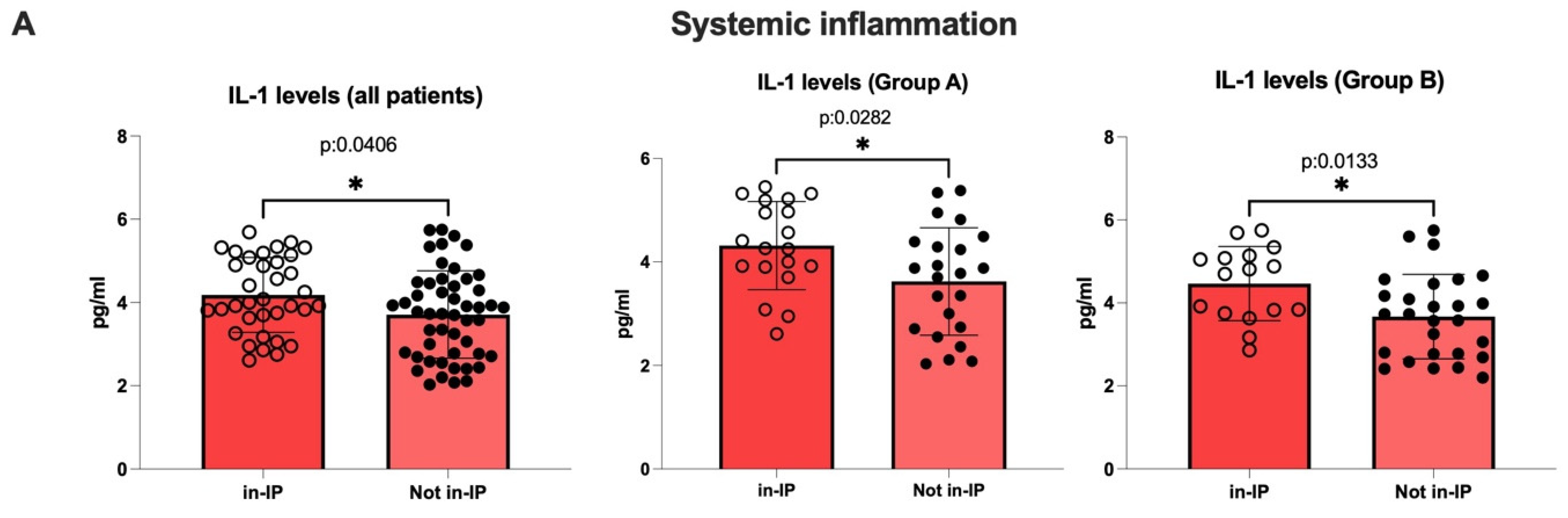
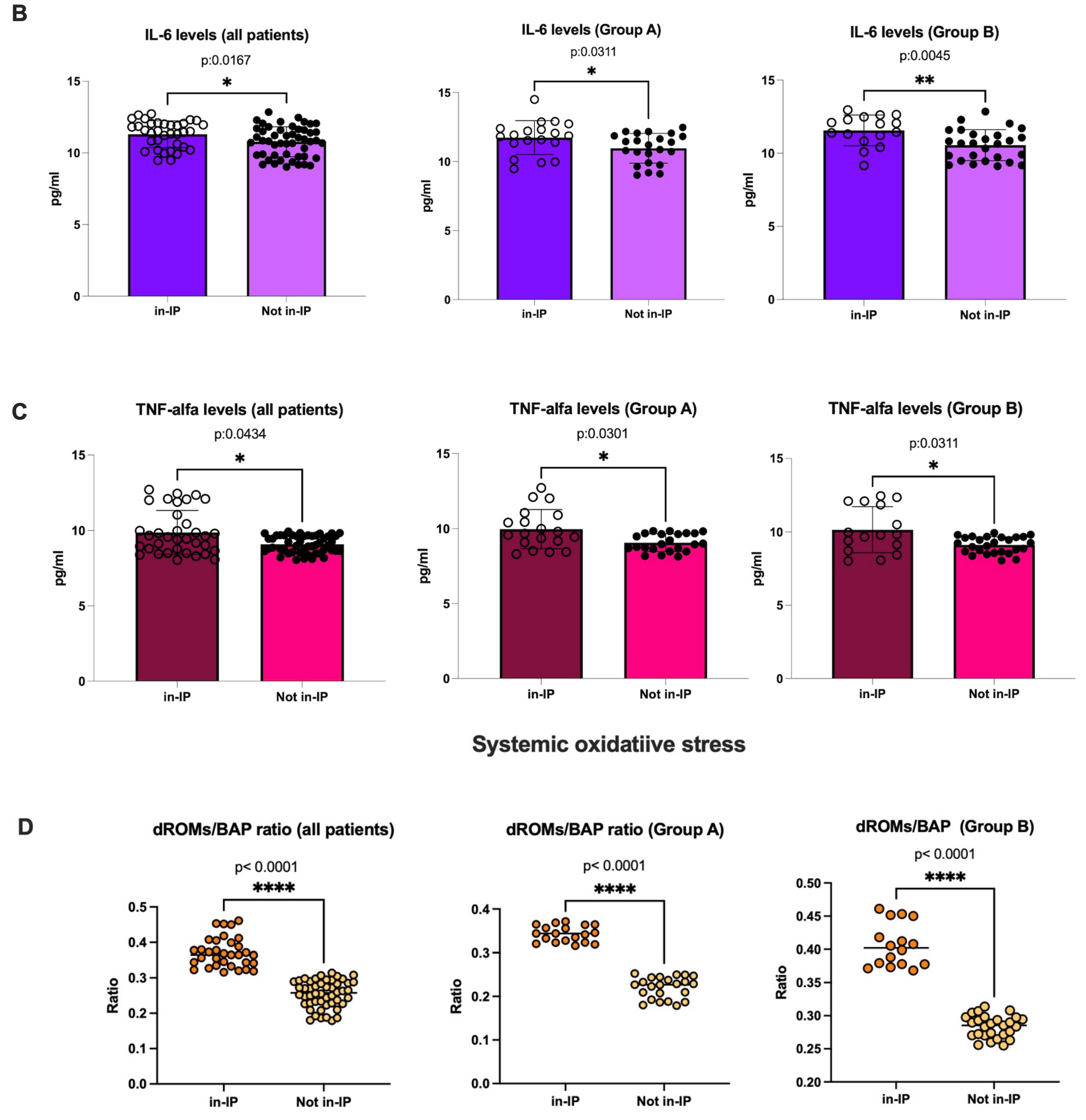
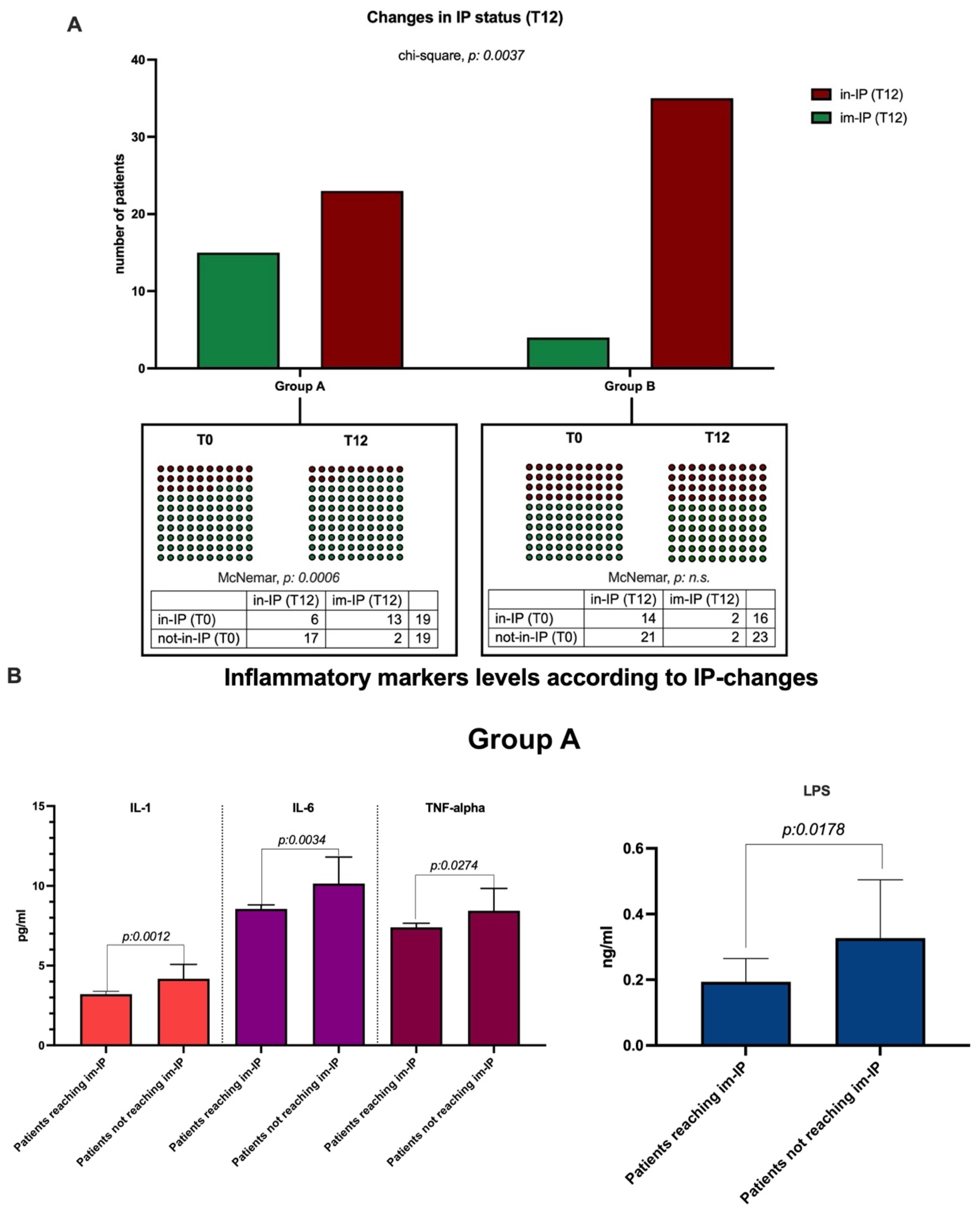
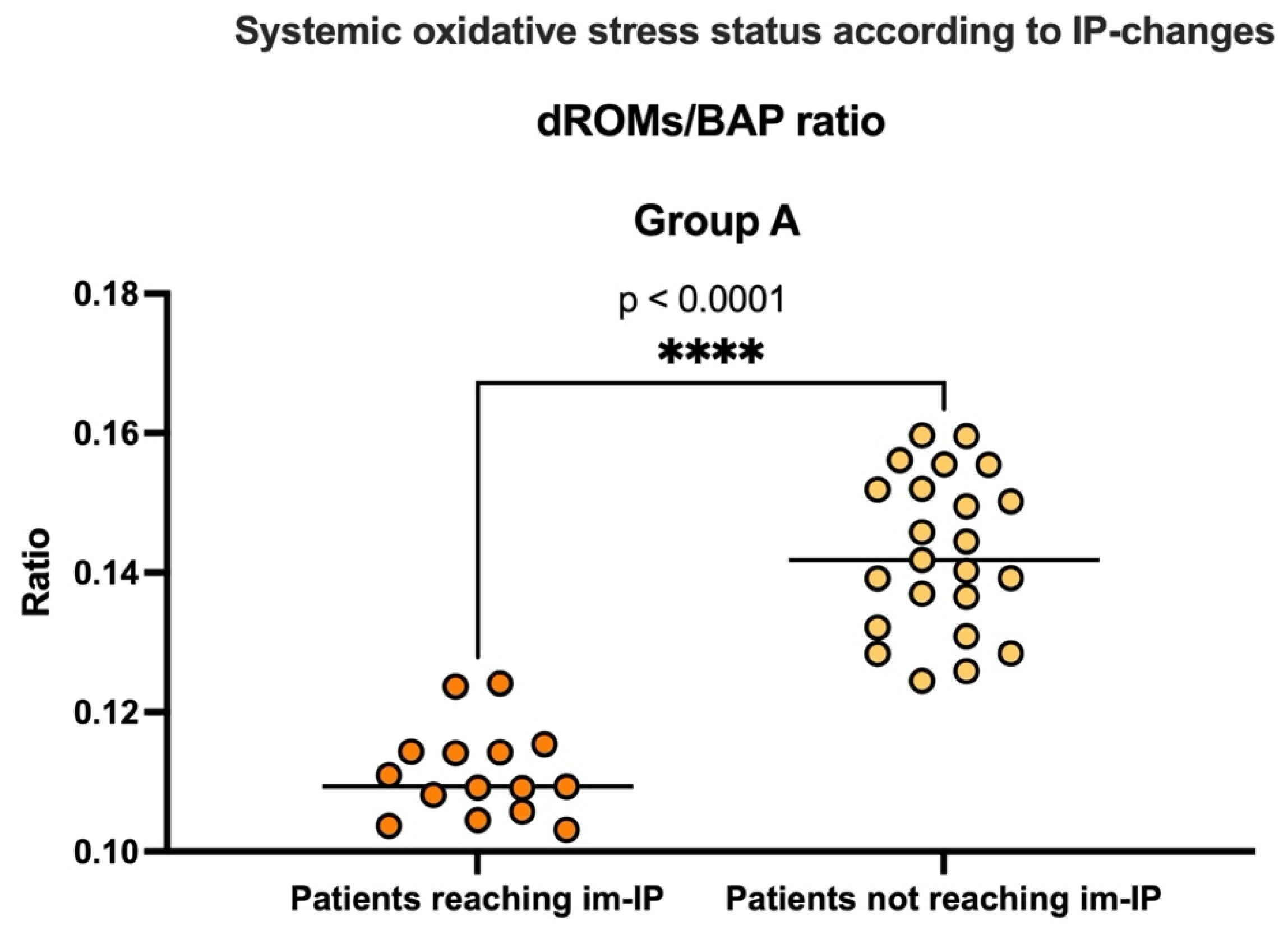
3.3.1. Evaluation of Intestinal Permeability, Systemic Inflammation, and Oxidative Stress
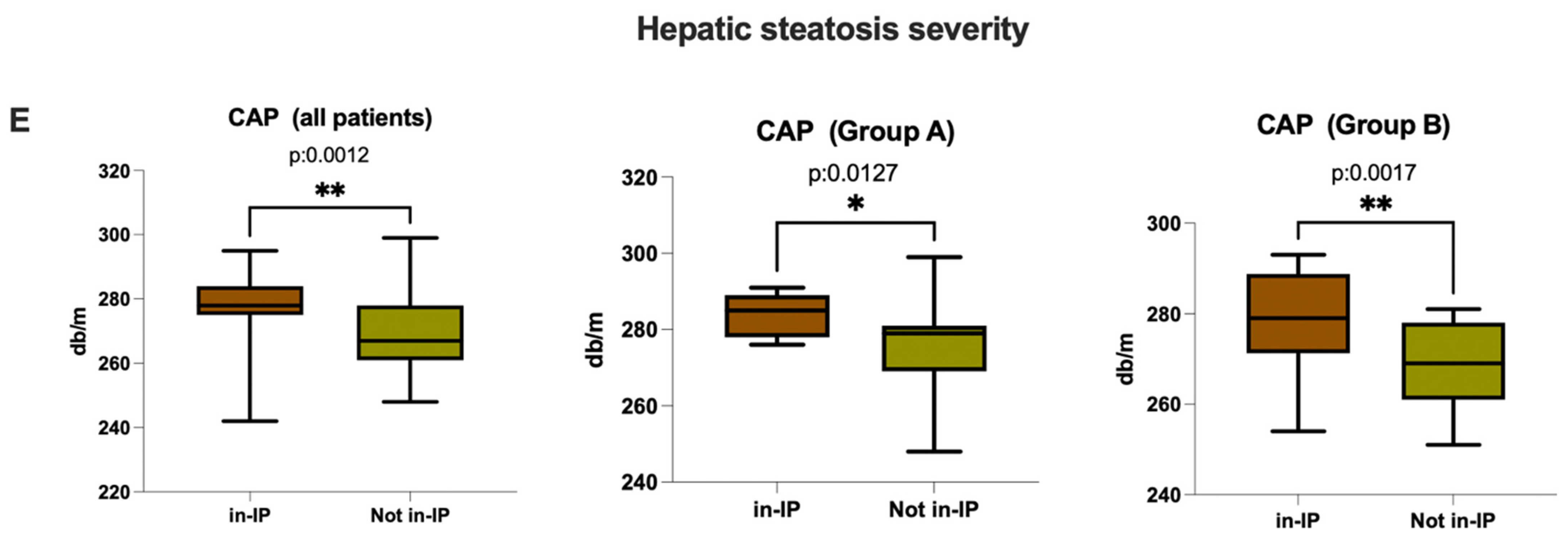
3.3.2. Evaluation of Clinical Outcomes: Biochemical and Clinical Variable Modifications
3.4. End of Water Wash-Out Period Evaluations
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BAP | Biological Antioxidant Potential |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CRP | C-Reactive Protein |
| d-ROMs | Reactive Oxygen Metabolites Test |
| γGT | Gamma-Glutamyl Transferase |
| GLP-1 | Glucagon-Like Peptide-1 |
| HDL | High-Density Lipoprotein Cholesterol |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| im-IP | Impaired Intestinal Permeability |
| in-IP | Intact Intestinal Permeability |
| IP | Intestinal Permeability |
| LDL | Low-Density Lipoprotein Cholesterol |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MD | Metabolic Dysfunction |
| PLT | platelet |
| PYY | Peptide Tyrosine–Tyrosine |
| RCF | Relative Centrifugal Force |
| SI | Systemic Inflammation |
| SOS | Systemic Oxidative Stress |
| TG | Triglycerides |
| U-CARR | Carratelli Units (unit of d-ROMs test) |
References
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Romeo, M.; Di Nardo, F.; Vaia, P.; Napolitano, C.; Ventriglia, L.; Coppola, A.; Silvestrin, A.; Olivieri, S.; Federico, A. FLAME: Training and Validating a Newly Conceived Model Incorporating Alpha-Glutathione-S-Transferase Serum Levels for Predicting Advanced Hepatic Fibrosis and Acute Cardiovascular Events in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2025, 26, 761. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Damman, C.J.; Carr, R.M. Metabolic Dysfunction-Associated Steatotic Liver Disease and the Gut Microbiome: Pathogenic Insights and Therapeutic Innovations. J. Clin. Investig. 2025, 135, e186423. [Google Scholar] [CrossRef]
- Fasano, A. All Disease Begins in the (Leaky) Gut: Role of Zonulin-Mediated Gut Permeability in the Pathogenesis of Some Chronic Inflammatory Diseases. F1000Research 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Rosso, C.; Ribaldone, D.G.; Dughera, F.; Fagoonee, S.; Astegiano, M.; Pellicano, R. Physiopathology of intestinal barrier and the role of zonulin. Minerva Biotechnol. Biomol. Res. 2019, 31, 83–92. [Google Scholar] [CrossRef]
- Bahitham, W.; Banoun, Y.; Aljahdali, M.; Almuaiqly, G.; Bahshwan, S.M.; Aljahdali, L.; Sanai, F.M.; Rosado, A.S.; Sergi, C.M. “Trust Your Gut”: Exploring the Connection between Gut Microbiome Dysbiosis and the Advancement of Metabolic Associated Steatosis Liver Disease (MASLD)/Metabolic Associated Steatohepatitis (MASH): A Systematic Review of Animal and Human Studies. Front. Nutr. 2025, 12, 1637071. [Google Scholar] [CrossRef]
- Dissayabutra, T.; Chuaypen, N.; Somnark, P.; Boonkaew, B.; Udomkarnjananun, S.; Kittiskulnam, P.; Charoenchittang, P.; Prombutara, P.; Tangkijvanich, P. Characterization of Gut Dysbiosis and Intestinal Barrier Dysfunction in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease and Chronic Kidney Disease: A Comparative Study. Sci. Rep. 2025, 15, 15481. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, Z.; Chu, M. Insights Into Intestinal Flora in Metabolic Dysfunction-Associated Steatotic Liver Disease. FASEB J. 2025, 39, e70932. [Google Scholar] [CrossRef]
- Gravina, A.G.; Romeo, M.; Pellegrino, R.; Tuccillo, C.; Federico, A.; Loguercio, C. Just Drink a Glass of Water? Effects of Bicarbonate–Sulfate–Calcium–Magnesium Water on the Gut–Liver Axis. Front. Pharmacol. 2022, 13, 869446. [Google Scholar] [CrossRef]
- Romeo, M.; Dallio, M.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Martinelli, G.; Federico, P.; Olivieri, S.; Iodice, P.; Federico, A. The Role of the Gut-Biliary-Liver Axis in Primary Hepatobiliary Liver Cancers: From Molecular Insights to Clinical Applications. J. Pers. Med. 2025, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-del-Campo, N.; Castelnuovo, G.; Ribaldone, D.G.; Caviglia, G.P. Fecal and Circulating Biomarkers for the Non-Invasive Assessment of Intestinal Permeability. Diagnostics 2023, 13, 1976. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Romeo, M.; Silvestrin, A.; Senese, G.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Coppola, A.; Federico, P.; Dallio, M.; Federico, A. From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines 2025, 13, 2004. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Gravina, A.G.; Masarone, M.; Larussa, T.; Abenavoli, L.; Persico, M.; Loguercio, C.; Federico, A. Nutrigenomics and Nutrigenetics in Metabolic- (Dysfunction) Associated Fatty Liver Disease: Novel Insights and Future Perspectives. Nutrients 2021, 13, 1679. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Kupczyk, D.; Bilski, R.; Szeleszczuk, Ł.; Mądra-Gackowska, K.; Studzińska, R. The Role of Diet in Modulating Inflammation and Oxidative Stress in Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis. Nutrients 2025, 17, 1603. [Google Scholar] [CrossRef]
- Barnich, N.; Rodrigues, M.; Sauvanet, P.; Chevarin, C.; Denis, S.; Le Goff, O.; Faure-Imbert, D.; Hanh, T.; Roques, C.F.; Chassaing, B.; et al. Beneficial Effects of Natural Mineral Waters on Intestinal Inflammation and the Mucosa-Associated Microbiota. Int. J. Mol. Sci. 2021, 22, 4336. [Google Scholar] [CrossRef]
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and Safety of a Natural Mineral Water Rich in Magnesium and Sulphate for Bowel Function: A Double-Blind, Randomized, Placebo-Controlled Study. Eur. J. Nutr. 2017, 56, 491–499. [Google Scholar] [CrossRef]
- Carpino, G.; Overi, D.; Onori, P.; Franchitto, A.; Cardinale, V.; Alvaro, D.; Gaudio, E. Effect of Calcium-Sulphate-Bicarbonate Water in a Murine Model of Non-Alcoholic Fatty Liver Disease: A Histopathology Study. Int. J. Mol. Sci. 2022, 23, 10065. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Romeo, M.; Dallio, M.; Nardo, F.D.; Napolitano, C.; Vaia, P.; Coppola, A.; Ventriglia, L.; Silvestrin, A.; Basile, C.; Federico, A. Systemic Oxidative Stress Correlates with Sarcopenia and Pruritus Severity: Two Independent Relationships Simultaneously Burdening the Quality of Life in Patients with Primary Biliary Cholangitis. Dig. Liver Dis. 2025, 57, S16. [Google Scholar] [CrossRef]
- Imatoh, T.; Kamimura, S.; Tanihara, S. Moderate Oxidative Stress and High Antioxidative Activity Are Associated with Steatosis in Japanese Males. Clin. Transl. Sci. 2013, 6, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Masuda, T.; Tasaki, M.; Ando, Y.; Ueda, M. Serum Diacron-Reactive Oxygen Metabolites (d-ROMs) and Biological Antioxidant Potential (BAP) in Patients with ATTR-PN. Amyloid 2019, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Mądra-Gackowska, K.; Szewczyk-Golec, K.; Gackowski, M.; Woźniak, A.; Kędziora-Kornatowska, K. Evaluation of Selected Parameters of Oxidative Stress and Adipokine Levels in Hospitalized Older Patients with Diverse Nutritional Status. Antioxidants 2023, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Yi, D.; Qiu, F.; Wu, L.; Tang, Y.; Wang, N. Mediterranean Diet Affects the Metabolic Outcome of Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Nutr. 2023, 10, 1225946. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary Carbohydrates and Fats in Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Cipullo, M.; Coppola, A.; Mammone, S.; Di Gioia, G.; Masarone, M.; Persico, M.; Serviddio, G.; et al. The Influence of Acute Lifestyle Changes on NAFLD Evolution in a Multicentre Cohort: A Matter of Body Composition. Nutr. Diabetes 2024, 14, 33. [Google Scholar] [CrossRef]
- Salgado, A.L.F.d.A.; Carvalho, L.d.; Oliveira, A.C.; Santos, V.N.d.; Vieira, J.G.; Parise, E.R. Insulin Resistance Index (HOMA-IR) in the Differentiation of Patients with Non-Alcoholic Fatty Liver Disease and Healthy Individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef]
- Boursier, J.; Zarski, J.-P.; de Ledinghen, V.; Rousselet, M.-C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of Reliability Criteria for Liver Stiffness Evaluation by Transient Elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A Novel VCTETM Guided Ultrasonic Attenuation Measurement for the Evaluation of Hepatic Steatosis: Preliminary Study and Validation in a Cohort of Patients with Chronic Liver Disease from Various Causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The Controlled Attenuation Parameter (CAP): A Novel Tool for the Non-Invasive Evaluation of Steatosis Using Fibroscan. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 13–20. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sanyal, A.J.; Engebretsen, K.A.; Kliers, I.; Østergaard, L.; Vanni, D.; Bugianesi, E.; Rinella, M.E.; Roden, M.; Ratziu, V. Semaglutide 2.4 Mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment. Pharmacol. Ther. 2024, 60, 1525–1533. [Google Scholar] [CrossRef]
- Knezović, E.; Hefer, M.; Blažanović, S.; Petrović, A.; Tomičić, V.; Srb, N.; Kirner, D.; Smolić, R.; Smolić, M. Drug Pipeline for MASLD: What Can Be Learned from the Successful Story of Resmetirom. Curr. Issues Mol. Biol. 2025, 47, 154. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, Immunity, and the Microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the Barriers: The Role of Gut Homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef]
- Asse Intestino-Fegato: Il Ruolo Del Microbiota Intestinale e Dei Suoi Metaboliti Nella Progressione Della Malattia Epatica Steatosica Associata a Disfunzione Metabolica—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/40336226/ (accessed on 19 September 2025).
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.-D.; Delzenne, N.M.; Muccioli, G.G.; Clément, K.; Cani, P.D. From Correlation to Causality: The Case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct Signatures of Gut Microbiome and Metabolites Associated with Significant Fibrosis in Non-Obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two Routes of Metabolic Cross-Feeding between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Perazza, F.; Leoni, L.; Selvatici, B.; Girolami, F.; Bonalumi, A.; Beretta, A.; Ferri, S.; Petroni, M.L.; Piscaglia, F.; Ravaioli, F.; et al. Dietary Strategies to Modulate Gut Microbiota in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2025, 17, 1906. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Yen, S.; Gomez Castro, P.; Misheva, M.; Jebb, S.A.; Aveyard, P.; Tomlinson, J.W.; Mozes, F.E.; Cobbold, J.F.; Johnson, J.S.; et al. Changes in Intestinal Permeability and Gut Microbiota Following Diet-Induced Weight Loss in Patients with Metabolic Dysfunction-Associated Steatohepatitis and Liver Fibrosis. Gut Microbes 2024, 16, 2392864. [Google Scholar] [CrossRef]

| Demographic, Anthropometric, and Clinical Data | |||
|---|---|---|---|
| Variables | Group A (n. 44) | Group B (n. 43) | p-Value * |
| Sex (%, male) | 23 (52.27%) | 27 (62.79%) | n.s. ** |
| Age (years) (mean ± SD) | 56.05 ± 15.69 | 50.88 ± 18.82 | n.s. |
| Smoke (%, yes) | 19 (43.18%) | 20 (46.51%) | n.s. ** |
| Type 2 diabetes mellitus (%, yes) | 21 (47.72%) | 18 (41.86%) | n.s. ** |
| Obesity (%, yes) | 20 (45.45%) | 18 (41.86%) | n.s.** |
| Body Mass Index (BMI) (mean ± SD) | 29.82 ± 3.02 | 29.49 ± 2.77 | n.s. |
| Essential arterial hypertension (%, yes) | 29 (65.91%) | 27 (62.79%) | n.s. ** |
| Dyslipidemia (%, yes) | 26 (59.09%) | 24 (55.81%) | n.s. ** |
| Biochemical Data | |||
| Variables (Mean ± SD) | Group A (n. 44) | Group B (n. 43) | p-Value * |
| Aspartate aminotransferase (AST) (U/L) (n.v. 10–40) | 53.20 ± 15.18 | 49.33 ± 9.21 | n.s. |
| Alanine aminotransferase (ALT) (U/L) (n.v. 7–45) | 59.81 ± 13.89 | 51.53 ± 17.05 | n.s. |
| Gammaglutamil-transferase (GGT) (U/L) (n.v. 18–60) | 77.64 ± 42.27 | 81.26 ± 64.69 | n.s. |
| Alkaline Phosphatase (ALP) (U/L) (n.v. 44–145) | 96.43 ± 15.52 | 89.19 ± 19.59 | n.s. |
| Platelet (PLT) count (mm3) (n.v. 150–400) | 221.1 ± 79.03 | 254.9 ± 38.38 | n.s. |
| Bilirubin (mg/dL) (n.v. 0.3–1.2) | 1.03 ± 0.07 | 0.89 ± 0.21 | n.s. |
| Albumin (g/dL) (n.v. 3.5–5.0) | 4.08 ± 0.38 | 4.21 ± 0.29 | n.s. |
| High-sensitivity CRP (mg/L) (n.v. < 2.0) | 2.11 ± 0.46 | 1.85 ± 0.65 | n.s. |
| High-density lipoprotein (HDL) (mg/dL) (n.v. > 45) | 40.48 ± 7.59 | 39.84 ± 10.43 | n.s. |
| Low-density lipoprotein (LDL) (mg/dL) (n.v. < 120) | 135.8 ± 33.31 | 144.2 ± 35.97 | n.s. |
| Triglycerides (mg/dL) (n.v. < 150) | 166.8 ± 37.48 | 179.5 ± 35.97 | n.s. |
| Fasting Plasma Glucose (FPG) (mg/dL) (n.v. 70–99) | 127.9 ± 14.86 | 129.4 ± 18.31 | n.s. |
| Insulin (microu/L) (n.v. 2–11) | 13.89 ± 3.41 | 11.74 ± 2.52 | n.s. |
| HOMA-IR (n.v. < 2.5) | 3.66 ± 1.83 | 3.79 ± 1.13 | n.s. |
| Non-Invasive Tools Assessing Liver Disease Progression Status | |||
| Variables (Mean ± SD) | Group A (n. 44) | Group B (n. 43) | p-Value * |
| Liver Stiffness Measurement (LSM) (kPa) | 7.77 ± 1.39 | 7.16 ± 1.83 | n.s. |
| Controlled Attenuation Parameter (CAP) (db/m) | 278.1 ± 10.44 | 277.1 ± 10.31 | n.s. |
| Intestinal Permeability Markers | |||
| Variables (Mean ± SD) | Group A (n. 44) | Group B (n. 43) | p-Value * |
| Fecal zonulin (ng/mL) (n.v. < 110) | 136.4 ± 45.71 | 126.4 ± 42.78 | 0.04 |
| Serum occludin (ng/mL) (n.v. > 100) | 248.5 ± 28.23 | 246.4 ± 32.12 | n.s. |
| Serum claudin-1 (ng/mL) (n.v. > 1) | 1.01 ± 0.27 | 1.09 ± 0.29 | n.s. |
| Serum (LPBp) (microg/mL) (n.v. < 10) | 12.11 ± 4.57 | 11.12 ± 3.47 | n.s. |
| Systemic Inflammation Assessment | |||
| Variables | Group A (n. 44) | Group B (n. 43) | p-Value * |
| Serum LPS (ng/mL) (n.v. < 0.1) | 0.55 ± 0.27 | 0.52 ± 0.26 | n.s. |
| Interleukin (IL)-1β (pg/mL) (n.v. < 3) | 3.88 ± 1.12 | 3.82 ± 0.99 | n.s. |
| Interleukin (IL)-6 (pg/mL) (n.v. < 10) | 11.20 ± 0.97 | 10.58 ± 1.06 | n.s. |
| Tumor Necrosis Factor-alpha (pg/mL) (n.v. < 8.1) | 9.04 ± 0.60 | 9.03 ± 0.61 | n.s. |
| Systemic Oxidative Stress Assessment | |||
| Variables | Group A (n. 44) | Group B (n. 43) | p-Value * |
| dROMs (CARR-U) (n.v. < 300) | 501.3 ± 109.4 | 513.2 ± 99.26 | n.s. |
| BAP (mmol/L) (n.v. > 2200) | 1714.1 ± 168.2 | 1684.2 ± 184.5 | n.s. |
| dROMs/BAP ratio (n.v. < 0.1) | 0.29 ± 0.08 | 0.30 ± 0.06 | n.s. |
| Severe systemic oxidative stress imbalance (%) | 39/44 (88.63%) | 39/43 (90.69%) | n.s. ** |
| Biochemical Data | ||||||
|---|---|---|---|---|---|---|
| Variables (Mean ± SD) | Group A (n. 44) T0 | Group A (n. 38) T12 | p-Value * | Group B (n. 43) T0 | Group B (n. 39) T12 | p-Value * |
| AST (U/L) (n.v. 10–40) | 53.20 ± 15.18 | 47.00 ± 4.49 | 0.023 | 49.33 ± 9.21 | 47.23 ± 3.36 | n.s. |
| ALT (U/L) (n.v. 7–45) | 59.81 ± 13.89 | 41.08 ± 4.41 | <0.0001 | 51.53 ± 17.05 | 51.92 ± 3.93 | n.s. |
| GGT (U/L) (n.v. 18–60) | 77.64 ± 42.27 | 51.79 ± 4.45 | <0.0001 | 81.26 ± 64.69 | 109.1 ± 16.77 | n.s. |
| PLT count (mm3) (n.v. 150–400) | 221.1 ± 79.03 | 244.2 ± 25.78 | n.s. | 254.9 ± 38.38 | 244.8 ± 2.95 | n.s. |
| Bilirubin (mg/dL) (n.v. 0.3-1.2) | 1.03 ± 0.07 | 1.18 ± 0.08 | n.s. | 0.89 ± 0.21 | 1.21 ± 0.07 | n.s. |
| Albumin (g/dL) (n.v. 3.5–5.0) | 4.08 ± 0.38 | 4.01 ± 0.06 | n.s. | 4.21 ± 0.29 | 3.99 ± 0.05 | n.s. |
| Hs-CRP (mg/L) (n.v. < 2.0) | 2.11 ± 0.46 | 1.51 ± 0.30 | <0.0001 | 1.85 ± 0.65 | 1.81 ± 0.17 | n.s. |
| HDL (mg/dL) (n.v. > 45) | 40.48 ± 7.59 | 51.29 ± 2.25 | <0.0001 | 39.84 ± 10.43 | 40.41 ± 2.99 | n.s. |
| LDL (mg/dL) (n.v. < 120) | 135.8 ± 33.31 | 99.68 ± 11.58 | <0.0001 | 144.2 ± 35.97 | 125.7 ± 13.54 | n.s. |
| Triglycerides (mg/dL) (n.v. < 150) | 166.8 ± 37.48 | 161.6 ± 17.24 | n.s. | 179.5 ± 35.97 | 161.7 ± 14.41 | n.s. |
| FPG (mg/dL) (n.v. 70–99) | 127.9 ± 14.86 | 123.7 ± 7.59 | n.s. | 129.4 ± 18.31 | 122.9 ± 12.21 | n.s. |
| Insulin (microu/L) (n.v. 2–11) | 13.89 ± 3.41 | 9.21 ± 0.73 | <0.0001 | 11.74 ± 2.52 | 12.38 ± 1.07 | n.s. |
| HOMA-IR (n.v.< 2.5) | 3.66 ± 1.83 | 2.81 ± 0.29 | n.s. | 3.79 ± 1.13 | 3.16 ± 0.28 | n.s. |
| Non-Invasive Tools Assessing Liver Disease Progression Status | ||||||
| Variables (Mean ± SD) | Group A (n. 44) T0 | Group A (n. 38) T12 | p-Value * | Group B (n. 43) T0 | Group B (n. 39) T12 | p-Value * |
| LSM (kPa) | 7.77 ± 1.39 | 7.47 ± 0.60 | n.s. | 7.16 ± 1.83 | 7.45 ± 0.56 | n.s. |
| CAP (db/m) | 278.1 ± 10.44 | 264.8 ± 2.67 | <0.0001 | 277.1 ± 10.31 | 279.4 ± 2.63 | n.s. |
| Intestinal Permeability Markers | ||||||
| Variables (Mean ± SD) | Group A (n. 44) T0 | Group A (n. 38) T12 | p-Value * | Group B (n. 43) T0 | Group B (n. 39) T12 | p-Value * |
| Fecal zonulin (ng/mL) (n.v. < 110) | 136.4 ± 45.71 | 112.3 ± 12.01 | 0.0163 | 126.4 ± 42.78 | 135.2 ± 12.18 | n.s. |
| Serum occluding (ng/mL) (n.v. > 100) | 248.5 ± 28.23 | 290.1 ± 5.47 | <0.0001 | 246.4 ± 32.12 | 249.3 ± 11.23 | n.s. |
| Serum claudin-1 (ng/mL) (n.v. > 1) | 1.01 ± 0.27 | 1.41 ± 0.05 | <0.0001 | 1.09 ± 0.29 | 0.96 ± 0.17 | n.s. |
| Serum (LPBp) (µg/mL) (n.v. < 10) | 8.74 ± 1.81 | 7.42 ± 2.21 | <0.0001 | 9.02 ± 1.75 | 12.08 ± 1.51 | n.s. |
| Systemic Inflammation Assessment | ||||||
| Variables | Group A (n. 44) T0 | Group A (n. 38) T12 | p-Value * | Group B (n. 43) T0 | Group B (n. 39) T12 | p-Value * |
| Serum LPS (ng/mL) (n.v < 0.1) | 0.55 ± 0.27 | 0.19 ± 0.06 | <0.0001 | 0.52 ± 0.26 | 0.59 ± 0.05 | n.s. |
| IL-1β (pg/mL) (n.v. < 3) | 3.88 ± 1.12 | 3.21 ± 0.18 | 0.0012 | 3.82 ± 0.99 | 3.80 ± 0.06 | n.s. |
| IL-6 (pg/mL) (n.v. < 10) | 11.20 ± 0.97 | 8.53 ± 0.28 | <0.0001 | 10.58 ± 1.06 | 10.51 ± 0.27 | n.s. |
| TNF-alpha (pg/mL) (n.v. < 8.1) | 9.04 ± 0.60 | 7.44 ± 0.28 | <0.0001 | 9.03 ± 0.61 | 9.92 ± 0.31 | n.s. |
| Systemic Oxidative Stress Assessment | ||||||
| Variables | Group A (n. 44) T0 | Group A (n. 38) T12 | p-Value * | Group B (n. 43) T0 | Group B (n. 39) T12 | p-Value * |
| dROMs (CARR-U) (n.v. < 300) | 501.3 ± 109.4 | 264.9 ± 31.58 | <0.0001 | 513.2 ± 99.26 | 553.1 ± 29.14 | n.s. |
| BAP (mmol/L) (n.v. > 2200) | 1714.1 ± 168.2 | 1898 ± 55.84 | <0.0001 | 1684.2 ± 184.5 | 1604 ± 64.40 | n.s. |
| dROMs/BAP ratio (n.v. < 0.1) | 0.29 ± 0.08 | 0.13 ± 0.01 | <0.0001 | 0.30 ± 0.06 | 0.34 ± 0.24 | n.s. |
| Variable | Unadjusted OR [95% CI] | p-Value | Adjusted OR [95% CI] | p-Value |
|---|---|---|---|---|
| Age (years) | 0.681 [0.48–0.96] | 0.312 | – | – |
| BMI (Kg/m2) | 0.193 [0.11–0.22] | 0.298 | – | – |
| Baseline CAP (dB/m) | 1.094 [0.95–1.31] | 0.087 | – | – |
| Type 2 Diabetes Mellitus | 2.382 [2.21–2.79] | 0.0015 | n.s. | n.s. |
| Dyslipidemia | 1.613 [1.45–1.79] | 0.006 | n.s. | n.s. |
| Physical exercise (h/day) | 0.351 [0.26–0.68] | 0.028 | n.s. | n.s. |
| Water intake (compliance) | 2.529 [2.35–2.91] | <0.0001 | 2.185 [2.01–2.34] | 0.001 |
| Improved IP | 1.790 [1.31–2.04] | <0.0001 | 1.267 [1.14–1.89] | 0.021 |
| IL-1β ΔT0–T12 | 1.491 [1.23–1.66] | <0.0001 | 1.153 [1.09–1.27] | 0.030 |
| IL-6 ΔT0–T12 | 1.172 [1.10–1.42] | <0.0001 | 1.124 [1.07–1.23] | 0.039 |
| TNF-α ΔT0–T12 | 1.195 [1.11–1.54] | <0.0001 | 1.173 [1.09–1.33] | 0.004 |
| LPS ΔT0–T12 | 1.082 [1.02–1.39] | <0.0001 | 1.279 [1.20–1.41] | 0.002 |
| dROMs/BAP ratio ΔT0–T12 | 1.189 [1.13–1.61] | <0.0001 | 1.162 [1.11–1.32] | 0.005 |
| Biochemical Data | |||
|---|---|---|---|
| Variables (Mean ± SD) | Group A (n. 38) T12 | Group A (n. 35) T18 | p-Value * |
| Aspartate aminotransferase (AST) (U/L) (n.v. 10–40) | 47.00 ± 4.49 | 48.83 ± 1.21 | n.s. |
| Alanine aminotransferase (ALT) (U/L) (n.v. 7–45) | 41.08 ± 4.41 | 42.80 ± 1.28 | n.s. |
| Gammaglutamil-transferase (GGT) (U/L) (n.v. 18–60) | 51.79 ± 4.45 | 52.71 ± 0.95 | n.s. |
| Alkaline Phosphatase (ALP) (U/L) (n.v. 44–145) | 91.39 ± 4.78 | 92.71 ± 1.29 | n.s. |
| Platelet (PLT) count (mm3) (n.v. 150–400) | 244.2 ± 25.78 | 248.9 ± 6.23 | n.s. |
| Bilirubin (mg/dL) (n.v. 0.3–1.2) | 1.18 ± 0.08 | 1.13 ± 0.04 | n.s. |
| Albumin (g/dL) (n.v. 3.5–5.0) | 4.01 ± 0.06 | 4.15 ± 0.02 | n.s. |
| High-sensitivity CRP (mg/L) (n.v. < 2.0) | 1.51 ± 0.30 | 1.67 ± 0.08 | n.s. |
| High-density lipoprotein (HDL) (mg/dL) (n.v. > 45) | 51.29 ± 2.25 | 50.77 ± 1.62 | n.s. |
| Low-density lipoprotein (LDL) (mg/dL) (n.v. < 120) | 99.68 ± 11.58 | 93.66 ± 3.15 | n.s. |
| Triglycerides (mg/dL) (n.v. < 150) | 161.6 ± 17.24 | 167.5 ± 1.42 | n.s. |
| Fasting Plasma Glucose (FPG) (mg/dL) (n.v. 70–99) | 123.7 ± 7.59 | 126.5 ± 2.05 | n.s. |
| Insulin (microu/l) (n.v. 2–11) | 9.21 ± 0.73 | 10.24 ± 0.47 | n.s. |
| HOMA-IR (n.v. < 2.5) | 2.81 ± 0.29 | 3.01 ± 0.17 | n.s. |
| Non-Invasive Tools Assessing Liver Disease Progression Status | |||
| Variables (Mean ± SD) | Group A (n. 38) T12 | Group A (n. 35) T18 | p-Value * |
| Liver Stiffness Measurement (LSM) (kPa) | 7.47 ± 0.60 | 7.41 ± 0.80 | n.s. |
| Controlled Attenuation Parameter (CAP) (db/m) | 264.8 ± 2.67 | 263.7 ± 1.66 | n.s. |
| Intestinal permeability markers | |||
| Variables (Mean ± SD) | Group A (n. 38) T12 | Group A (n. 35) T18 | p-Value * |
| Fecal zonulin (ng/mL) (n.v. 15–110) | 112.3 ± 12.01 | 109.7 ± 5.72 | n.s. |
| Serum occludin (ng/mL) (n.v. > 100) | 290.1 ± 5.47 | 290.4 ± 3.03 | n.s. |
| Serum claudin-1 (ng/mL) (n.v. > 1) | 1.41 ± 0.05 | 1.472 ± 0.01 | n.s. |
| Serum (LPBp) (microg/mL) (n.v. 0.5–10) | 7.42 ± 2.21 | 8.42 ± 0.39 | n.s. |
| Systemic Inflammation Assessment | |||
| Variables | Group A (n. 38) T12 | Group A (n. 35) T18 | p-Value * |
| Serum LPS (ng/mL) (n.v. < 0.1) | 0.19 ± 0.06 | 0.18 ± 0.02 | n.s. |
| Interleukin (IL)-1β (pg/mL) (n.v. < 3) | 3.21 ± 0.18 | 3.22 ± 0.07 | n.s. |
| Interleukin (IL)-6 (pg/mL) (n.v. < 10) | 8.53 ± 0.28 | 8.42 ± 0.16 | n.s. |
| Tumor Necrosis Factor-alpha (pg/mL) (n.v. < 8.1) | 7.44 ± 0.28 | 7.39 ± 0.12 | n.s. |
| Systemic Oxidative Stress Assessment | |||
| Variables | Group A (n. 38) T12 | Group A (n. 35) T18 | p-Value * |
| dROMs(CARR-U) (n.v. < 300) | 264.9 ± 31.58 | 255.3 ± 17.41 | n.s. |
| BAP (mmol/L) (n.v. > 2200) | 1898 ± 55.84 | 1877 ± 27.32 | n.s. |
| dROMs/BAP ratio (n.v. < 0.1) | 0.13 ± 0.01 | 0.14 ± 0.02 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallio, M.; Romeo, M.; Di Nardo, F.; Senese, G.; Silvestrin, A.; Coppola, A.; Napolitano, C.; Vaia, P.; Basile, C.; Martinelli, G.; et al. Beneficial Effects of Long-Lasting Bicarbonate–Sulfate–Calcium–Magnesium Water Intake on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Outcomes via Impacting Intestinal Permeability (IP), IP-Related Systemic Inflammation, and Oxidative Stress. Nutrients 2025, 17, 3452. https://doi.org/10.3390/nu17213452
Dallio M, Romeo M, Di Nardo F, Senese G, Silvestrin A, Coppola A, Napolitano C, Vaia P, Basile C, Martinelli G, et al. Beneficial Effects of Long-Lasting Bicarbonate–Sulfate–Calcium–Magnesium Water Intake on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Outcomes via Impacting Intestinal Permeability (IP), IP-Related Systemic Inflammation, and Oxidative Stress. Nutrients. 2025; 17(21):3452. https://doi.org/10.3390/nu17213452
Chicago/Turabian StyleDallio, Marcello, Mario Romeo, Fiammetta Di Nardo, Giusy Senese, Alessia Silvestrin, Annachiara Coppola, Carmine Napolitano, Paolo Vaia, Claudio Basile, Giuseppina Martinelli, and et al. 2025. "Beneficial Effects of Long-Lasting Bicarbonate–Sulfate–Calcium–Magnesium Water Intake on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Outcomes via Impacting Intestinal Permeability (IP), IP-Related Systemic Inflammation, and Oxidative Stress" Nutrients 17, no. 21: 3452. https://doi.org/10.3390/nu17213452
APA StyleDallio, M., Romeo, M., Di Nardo, F., Senese, G., Silvestrin, A., Coppola, A., Napolitano, C., Vaia, P., Basile, C., Martinelli, G., Gregorio, A. D., & Federico, A. (2025). Beneficial Effects of Long-Lasting Bicarbonate–Sulfate–Calcium–Magnesium Water Intake on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Related Outcomes via Impacting Intestinal Permeability (IP), IP-Related Systemic Inflammation, and Oxidative Stress. Nutrients, 17(21), 3452. https://doi.org/10.3390/nu17213452









