The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization
Abstract
1. Introduction
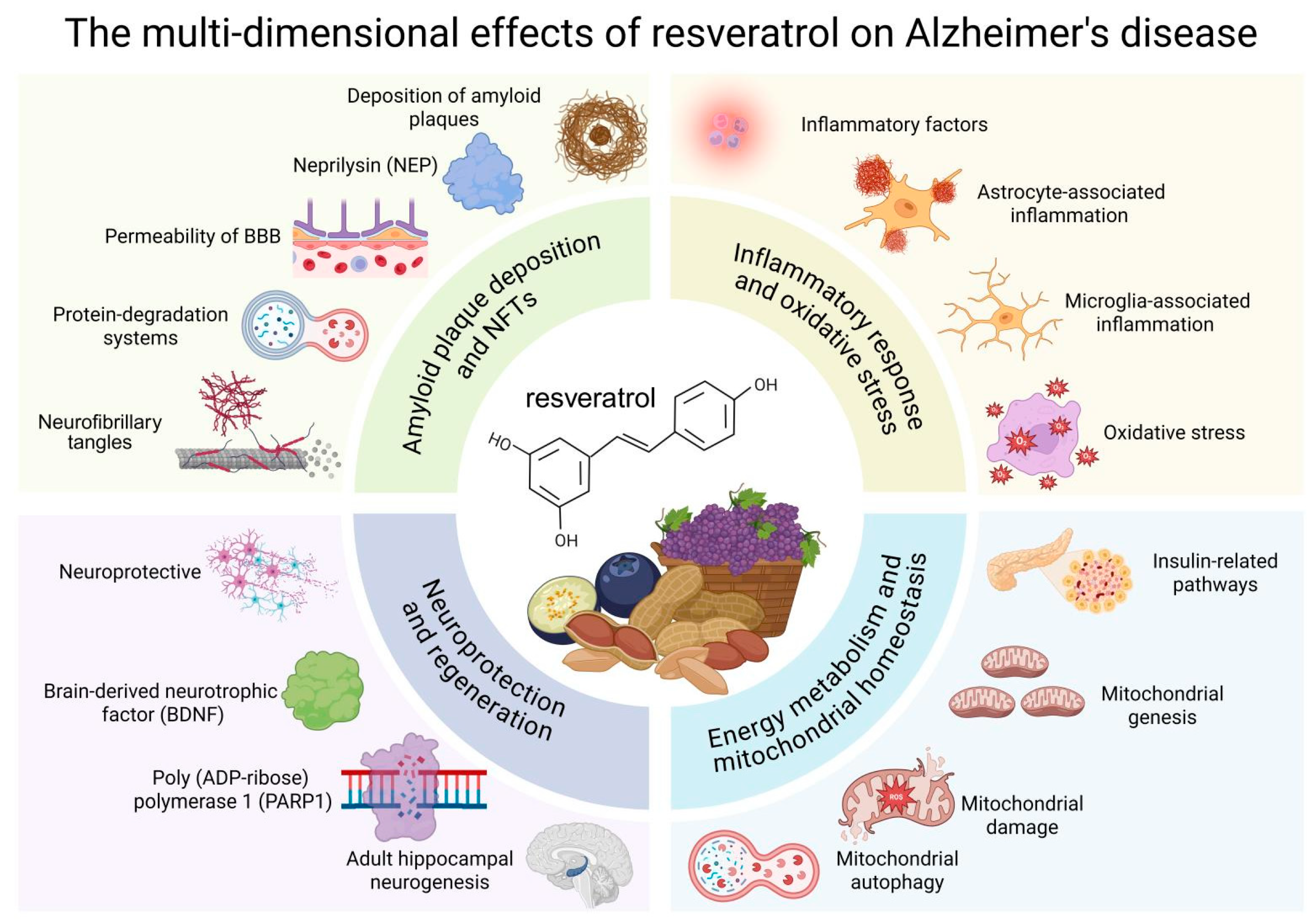
2. Mechanisms of Resveratrol in AD
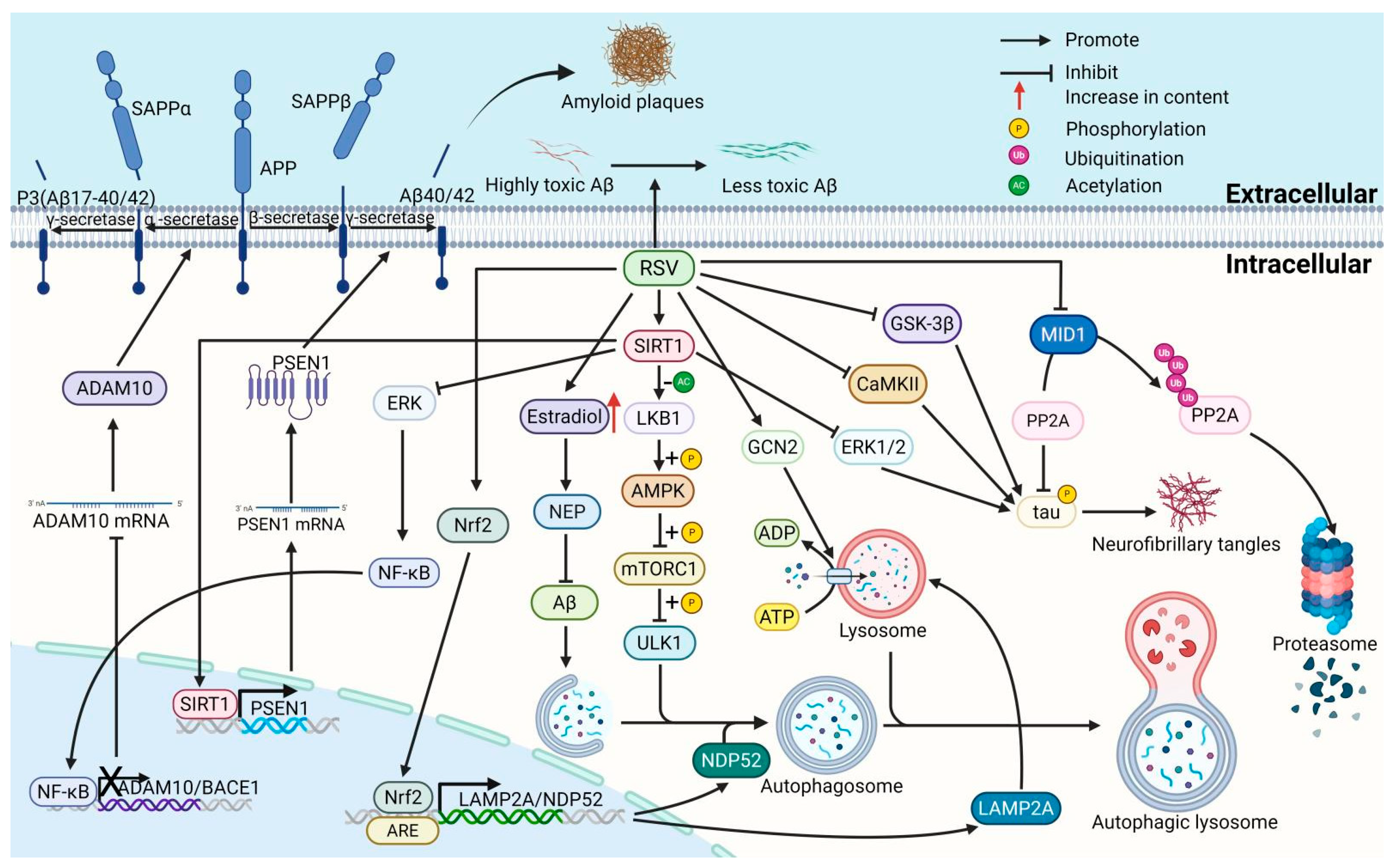
2.1. Effect of Resveratrol on Amyloid Plaque Deposition and Neurofibrillary Tangles in AD
2.1.1. Resveratrol Reduces Amyloid Plaque Deposition by Modulating Enzymes Involved in Aβ Production
2.1.2. Resveratrol Reduces Amyloid Plaque Deposition by Neprilysin (NEP) Involved in Aβ Clearance
2.1.3. Resveratrol Reduces Amyloid Plaque Deposition by Modulating BBB Permeability
2.1.4. Resveratrol Reduces Amyloid Plaque Deposition by Modulating Protein-Degradation Systems
2.1.5. Resveratrol Reduces Amyloid Plaque Deposition by Diminishing Aβ Protein Aggregation
2.1.6. Resveratrol Suppresses Neurofibrillary Tangles (NFTs) by Inhibiting the Hyperphosphorylation of Tau Protein
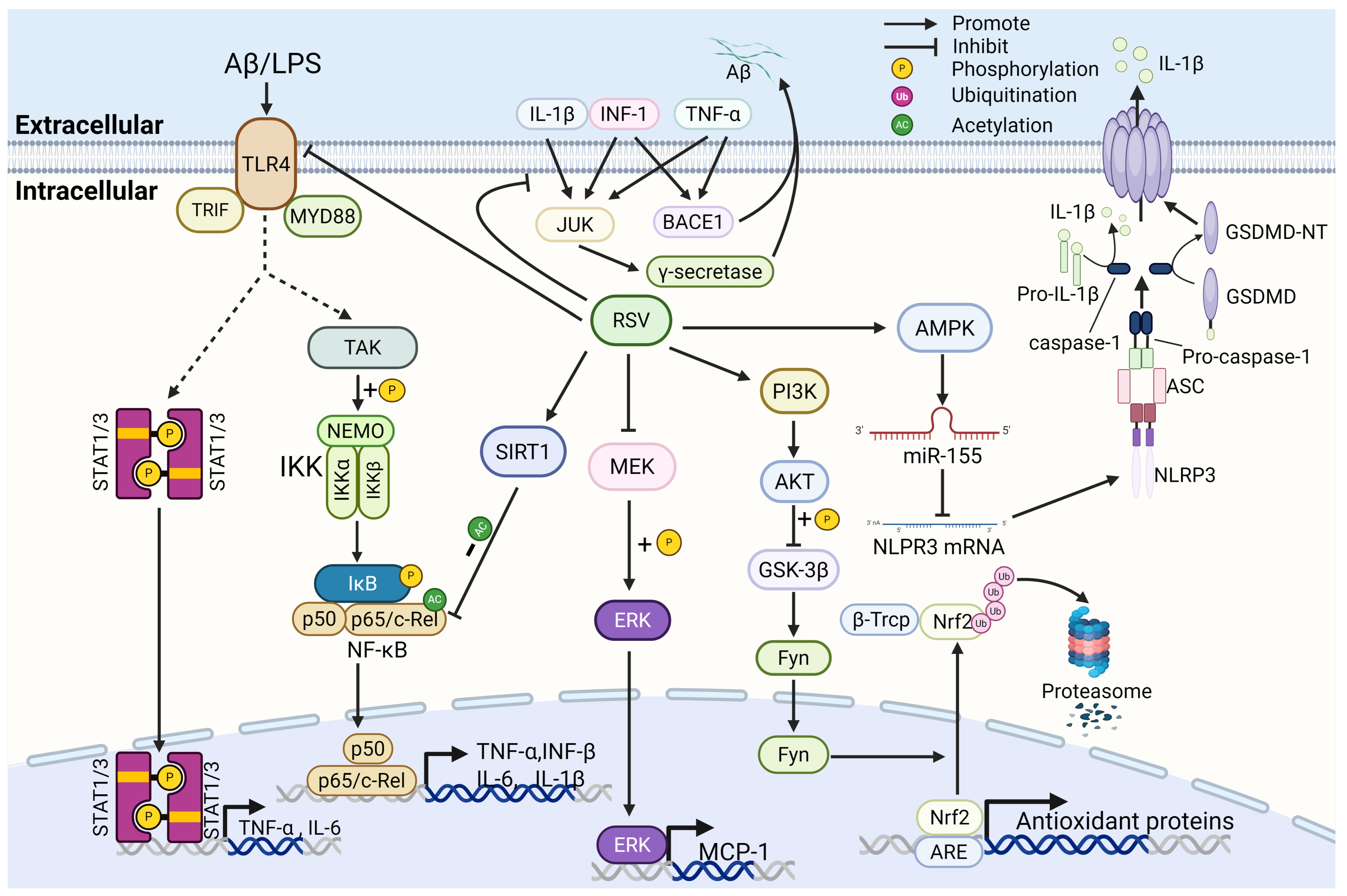
2.2. Effects of Resveratrol on Neuroinflammation and Oxidative Stress in AD
2.2.1. Resveratrol Reduces Amyloid Plaque Deposition by Down-Regulating Inflammatory Cytokines
2.2.2. Resveratrol Suppresses Inflammation-Related Signaling Pathways and the Production of Inflammatory Mediators
2.2.3. Resveratrol Inhibits Astrocyte-Associated Inflammation
2.2.4. Resveratrol Inhibits Microglia-Associated Inflammation
2.2.5. Resveratrol Inhibits Oxidative Stress-Related Pathways
2.3. Effect of Resveratrol on Energy Metabolism and Mitochondrial Homeostasis in AD
2.3.1. Resveratrol Improves Energy Metabolism by Modulating Insulin-Related Pathways
2.3.2. Resveratrol Promotes Mitochondrial Homeostasis by Modulating Mitochondrial Biogenesis and Mitophagy
Mitochondrial Genesis
Mitochondrial Autophagy
Lysosomal Acidification and the Formation of Autophagy Lysosomes
2.4. Effect of Resveratrol on Neuroprotective and Regenerative Effects in AD
2.4.1. Resveratrol Exerts Neuroprotective Effects by Promoting the Production of BDNF
2.4.2. Resveratrol Exerts Neuroprotective Effects by Modulating PARP1 Activity
2.4.3. Resveratrol Promotes Adult Hippocampal Neurogenesis
3. Strategy Optimization of Resveratrol in the Treatment of AD
3.1. Combination Therapies
3.2. Resveratrol Derivatives
3.3. Advanced Drug Delivery Systems
3.4. Intranasal Delivery
3.5. Lipid-Based Nanoparticles
3.6. Targeted Crosslinking
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ACC | Acetyl-CoA carboxylase |
| AHN | Adult hippocampal neurogenesis |
| ALP | Autophagy-lysosomal pathway |
| AMPK | AMP-activated protein kinase |
| Aph-1 | Anterior pharynx defective-1 |
| APP | Amyloid precursor protein |
| ARE | Antioxidant response elements |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATG5 | Autophagy-related protein 5 |
| AUC | Area under the curve |
| Aβ | Amyloid β-protein |
| BACE1 | β-site APP cleavage enzyme |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| Ca2+/CaMKKβ | Calcium/calmodulin-dependent protein kinase kinase-β |
| CALCOCO2/NDP52 | Nuclear dot protein 52 |
| CAT | Catalase |
| CCL2 | Chemokine ligand 2 |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| Cul3 | Cullin 3 |
| DHS | 4,4′-dihydroxyastragalus |
| DPN | Diabetic peripheral neuropathy |
| ERRα | Estrogen-related receptor α |
| fAβ42 | fibril Aβ42 |
| FGF-2 | Fibroblast growth factor-2 |
| GABARAPL1 | Gamma-aminobutyric acid receptor-associated protein-like 1 |
| GCN2 | General control nonderepressible 2 |
| GCS | γ-glutamylcysteine synthetase |
| GFAP | Glial fibrillary acidic protein |
| GLUT4 | Glucose transporter 4 |
| GSDMD | Gasdermin D |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase |
| GST | Glutathione S-transferase |
| hNSC | Human neural stem cells |
| HO-1 | Heme oxygenase-1 |
| IFN-γ | Interferon-γ |
| IGF-I | Insulin-like growth factor-1 |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| iNOS | Inducible NO synthase |
| IR | Insulin receptor |
| JNK | Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LIR | LC3-interacting region |
| LKB1 | Liver kinase B1 |
| LRP1 | Lipoprotein receptor-associated protein 1 |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDC | Macrophage-derived chemokine |
| MEL | Melatonin |
| MMP-9 | Matrix metalloproteinase-9 |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | mTOR complex 1 |
| MyD88 | Primary response gene 88 |
| NFTs | Neurofibrillary tangles |
| NF-κB | Nuclear Factor kappa-B |
| NMDA | N-Methyl-D-Aspartate |
| NO | Nitric oxide |
| NPC | Neural progenitor cell |
| NRF1/2 | Nuclear respiratory factor 1/2 |
| Nrf2/NFE2L2 | Nuclear factor erythroid 2-related factor 2 |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PDE | Phosphodiesterase |
| PDE4 | Phosphodiesterase 4 |
| Pen-2 | Presenilin enhancer-2 |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator1-α |
| PGE2 | Prostaglandin E2 |
| PHFs | Paired helical filaments |
| PI3K | Phosphatidylinositol 3-kinase |
| PINK1 | PTEN-induced putative kinase 1 |
| PP2A | Protein phosphatase 2A |
| PSEN1 | Presenilin1 |
| RAGE | Receptor for advanced glycation end |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| sAPPβ | Soluble APP beta protein |
| SIRT1 | Sirtuin 1 |
| sLRP1 | Soluble form of LRP1 |
| sMafs | small Maf proteins |
| SOCS1 | Suppressor of cytokine signaling 1 |
| SP | Senile plaques |
| SPION | Superparamagnetic iron oxide nanoparticles |
| SQSTM1/p62 | Sequestosome 1 |
| STAT1 | Activator of transcription 1 |
| TBK1 | TANK-binding kinase 1 |
| TCA | Tricarboxylic acid |
| TFAM | Promoters of mitochondrial transcription factor A |
| TFB1M | Mitochondrial transcription factor B1 |
| TFB2M | Mitochondrial transcription factor B2 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| TRX | Thioredoxin |
| TTR | Transthyretin |
| TXNIP | Thioredoxin-interacting protein |
| ULK1 | Serine/threonine-protein kinase 1 |
| UPS | Ubiquitin-proteasome system |
| xCT | Xc−cystine/glutamate transporter |
| YY1 | Yin Yang 1 |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Lefranc-Jullien, S.; Sunyach, C.; Checler, F. APPepsilon, the epsilon-secretase-derived N-terminal product of the beta-amyloid precursor protein, behaves as a type I protein and undergoes alpha-, beta-, and gamma-secretase cleavages. J. Neurochem. 2006, 97, 807–817. [Google Scholar] [CrossRef]
- Monteiro, K.L.C.; Dos Santos Alcântara, M.G.; Freire, N.M.L.; Brandão, E.M.; do Nascimento, V.L.; Dos Santos Viana, L.M.; de Aquino, T.M.; da Silva-Júnior, E.F. BACE-1 Inhibitors Targeting Alzheimer’s Disease. Curr. Alzheimer. Res. 2023, 20, 131–148. [Google Scholar] [CrossRef]
- De Strooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 2003, 38, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.; Dileo, J.; Hallas, B.; Horowitz, J.; Leheste, J. Silent information regulator 1 mediates hippocampal plasticity through presenilin1. Neuroscience 2011, 179, 32–40. [Google Scholar] [CrossRef]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Solberg, N.O.; Chamberlin, R.; Vigil, J.R.; Deck, L.M.; Heidrich, J.E.; Brown, D.C.; Brady, C.I.; Jagt, T.A.V.; Garwoo, M.; Bisoffi, M.; et al. Optical and SPION-enhanced MR imaging shows that trans-stilbene inhibitors of NF-κB concomitantly lower Alzheimer’s disease plaque formation and microglial activation in AβPP/PS-1 transgenic mouse brain. J. Alzheimers Dis. 2014, 40, 191–212. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Thonda, S.; Puttapaka, S.N.; Kona, S.V.; Kalivendi, S.V. Extracellular-Signal-Regulated Kinase Inhibition Switches APP Processing from β- to α-Secretase under Oxidative Stress: Modulation of ADAM10 by SIRT1/NF-κB Signaling. ACS Chem. Neurosci. 2021, 12, 4175–4186. [Google Scholar] [CrossRef]
- Apelt, J.; Ach, K.; Schliebs, R. Aging-related down-regulation of neprilysin, a putative beta-amyloid-degrading enzyme, in trans-genic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of beta-amyloid plaques. Neurosci. Lett. 2003, 339, 183–186. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Bayan, Y. Possible role of resveratrol targeting estradiol and neprilysin pathways in lipopolysaccharide model of Alzheimer disease. Adv. Exp. Med. Biol. 2015, 822, 107–118. [Google Scholar] [PubMed]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Walker, D.; Lue, L.F.; Paul, G.; Patel, A.; Sabbagh, M.N. Receptor for advanced glycation endproduct modulators: A new therapeutic target in Alzheimer’s disease. Expert. Opin. Investig. Drugs 2015, 24, 393–399. [Google Scholar] [CrossRef]
- Zhao, H.F.; Li, N.; Wang, Q.; Cheng, X.J.; Li, X.M.; Liu, T.T. Resveratrol decreases the insoluble Aβ1–42 level in hippocampus and protects the integrity of the blood–brain barrier in AD rats. Neuroscience 2015, 310, 641–649. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Wasser, C.R.; Herz, J. Functional Roles of the Interaction of APP and Lipoprotein Receptors. Front. Mol. Neurosci. 2017, 10, 54. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Kamiie, J.; Nezu, Y.; Terasaki, T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 2007, 103, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Alemi, M.; Silva, S.C.; Santana, I.; Cardoso, I. Transthyretin stability is critical in assisting beta amyloid clearance– Relevance of transthyretin stabilization in Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Rodrigues, D.; Alemi, M.; Silva, S.C.; Ribeiro, C.A.; Cardoso, I. Resveratrol administration increases transthyretin protein levels, ameliorating AD features: The importance of transthyretin tetrameric stability. Mol. Med. 2016, 22, 597–607. [Google Scholar] [CrossRef]
- Wolfe, D.M.; Lee, J.H.; Kumar, A.; Lee, S.; Orenstein, S.J.; Nixon, R.A. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur. J. Neurosci. 2013, 37, 1949–1961. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef]
- Ohta, K.; Mizuno, A.; Ueda, M.; Li, S.; Suzuki, Y.; Hida, Y.; Hayakawa-Yano, Y.; Itoh, M.; Ohta, E.; Kobori, M.; et al. Autophagy impairment stimulates PS1 expression and gam-ma-secretase activity. Autophagy 2010, 6, 345–352. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Arias, E.; Díaz-Carretero, A.; Cuervo, A.M.; Cuadrado, A. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy 2018, 14, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V.W. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014, 5, 3496. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Benito-Cuesta, I.; Ordóñez-Gutiérrez, L.; Wandosell, F. AMPK activation does not enhance autophagy in neurons in contrast to MTORC1 inhibition: Different impact on β-amyloid clearance. Autophagy 2021, 17, 656–671. [Google Scholar] [CrossRef]
- García-Juan, M.; Ordóñez-Gutiérrez, L.; Wandosell, F. Clearance of β-amyloid mediated by autophagy is enhanced by MTORC1 inhibition but not AMPK activation in APP/PSEN1 astrocytes. Glia 2024, 72, 588–606. [Google Scholar] [CrossRef]
- Jang, B.G.; Lee, J.; Choi, B.; Koh, Y.H.; Kim, M.J. Unexpected beta-amyloid production by middle doses of resveratrol through stabilization of APP protein and AMPK-mediated inhibition of trypsin-like proteasome activity in a cell model of Alzheimer’s disease. Food. Chem. Toxicol. 2021, 152, 112185. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.-P.; Yang, S.-G.; Wang, Y.-J.; Zhang, X.; Du, X.-T.; Sun, X.-X.; Zhao, M.; Huang, L.; Liu, R.-T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. NeuroToxicology 2009, 30, 986–995. [Google Scholar] [CrossRef]
- Ge, J.-F.; Qiao, J.-P.; Qi, C.-C.; Wang, C.-W.; Zhou, J.-N. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Int. 2012, 61, 1192–1201. [Google Scholar] [CrossRef]
- Ladiwala, A.R.A.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Aβ into off-pathway conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef]
- Albani, D.; Polito, L.; Batelli, S.; De Mauro, S.; Fracasso, C.; Martelli, G.; Colombo, L.; Manzoni, C.; Salmona, M.; Caccia, S.; et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009, 110, 1445–1456. [Google Scholar] [CrossRef]
- Sinsky, J.; Pichlerova, K.; Hanes, J. Tau Protein Interaction Partners and Their Roles in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2021, 22, 9207. [Google Scholar] [CrossRef]
- Du, L.-L.; Xie, J.-Z.; Cheng, X.-S.; Li, X.-H.; Kong, F.-L.; Jiang, X.; Ma, Z.-W.; Wang, J.-Z.; Chen, C.; Zhou, X.-W. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age 2014, 36, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Gu, J.; Dai, W.; Jin, N.; Chu, D.; Huang, Q.; Liu, F.; Qian, W. Sirt1 enhances tau exon 10 inclusion and improves spatial memory of Htau mice. Aging 2018, 10, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front. Neurosci. 2016, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Dias-Carvalho, A.; Sá, S.I.; Carvalho, F.; Fernandes, E.; Costa, V.M. Inflammation as common link to progressive neurological diseases. Arch. Toxicol. 2024, 98, 95–119. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wang, B.J.; Cheng, H.T.; Kuo, L.H.; Wolfe, M.S. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 2004, 279, 49523–49532. [Google Scholar] [CrossRef]
- Zhao, J.; O’Connor, T.; Vassar, R. The contribution of activated astrocytes to Aβ production: Implications for Alzheimer’s disease pathogenesis. J. Neuroinflammation 2011, 8, 150. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflam. 2017, 14, 1. [Google Scholar] [CrossRef]
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Bellaver, B.; Souza, D.G.; Bobermin, L.D.; Souza, D.O.; Gonçalves, C.-A.; Quincozes-Santos, A. Resveratrol Protects Hippocampal Astrocytes Against LPS-Induced Neurotoxicity Through HO-1, p38 and ERK Pathways. Neurochem. Res. 2015, 40, 1600–1608. [Google Scholar] [CrossRef]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.-S.; Zhan, D.; Chen, Y.-L.; He, Y.; Liu, J.; Zhang, Z.-J.; Sun, J.; et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS ONE 2012, 7, e44107. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.O.; Park, H.J.; Kang, J.L.; Kim, H.; Chong, Y.H. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J. Neurochem. 2010, 112, 1477–1487. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Ruan, L.; Kong, Y.; Mou, H.; Zhang, Z.; Wang, Z.; Wang, J.M.; Le, Y. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J. Neuroinflamm. 2010, 7, 46. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, L.; Shi, D.L.; Song, X.H.; Shen, Y.L.; Zheng, M.Z.; Wang, L.-L. Resveratrol Attenuates Subacute Systemic Inflammation-Induced Spatial Memory Impairment via Inhibition of Astrocyte Activation and Enhancement of Synaptophysin Expression in the Hippocampus. Ann. Clin. Lab. Sci. 2017, 47, 17–24. [Google Scholar]
- Scuderi, C.; Stecca, C.; Bronzuoli, M.R.; Rotili, D.; Valente, S.; Mai, A.; Steardo, L. Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer’s disease. Front. Pharmacol. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Souza, D.G.; Souza, D.O.; Quincozes-Santos, A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol. Vitro 2014, 28, 479–484. [Google Scholar] [CrossRef]
- Uddin, M.S.; Lim, L.W. Glial cells in Alzheimer’s disease: From neuropathological changes to therapeutic implications. Ageing Res. Rev. 2022, 78, 101622. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends. Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Frappier, J.; Dea, D.; Théroux, L.; Poirier, J. TLR4 Gene Expression and Pro-Inflammatory Cytokines in Alzheimer’s Disease and in Response to Hippocampal Deafferentation in Rodents. J. Alzheimer’s Dis. 2018, 63, 1547–1556. [Google Scholar] [CrossRef]
- Balducci, C.; Frasca, A.; Zotti, M.; La Vitola, P.; Mhillaj, E.; Grigoli, E.; Iacobellis, M.; Grandi, F.; Messa, M.; Colombo, L.; et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav. Immun. 2017, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodríguez, M.; de la Fuente, C.; García-Durillo, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflammation 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific inhibition of MyD88-Independent Signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Eltutan, B.I.; Isci, K.B.; Genc, S. Resveratrol Inhibits NLRP3 Inflammasome-Induced Pyroptosis and miR-155 Expression in Microglia Through Sirt1/AMPK Pathway. Neurotox. Res. 2021, 39, 1812–1829. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L. Resveratrol Suppresses Aβ-Induced Microglial Activation Through the TXNIP/TRX/NLRP3 Signaling Pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free. Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef]
- Kanninen, K.; Heikkinen, R.; Malm, T.; Rolova, T.; Kuhmonen, S.; Leinonen, H.; Ylä-Herttuala, S.; Tanila, H.; Levonen, A.-L.; Koistinaho, M.; et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16505–16510. [Google Scholar] [CrossRef]
- Huang, T.-C.; Lu, K.-T.; Wo, Y.-Y.P.; Wu, Y.-J.; Yang, Y.-L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef]
- Kong, D.; Yan, Y.; He, X.-Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef]
- Arús, B.A.; Souza, D.G.; Bellaver, B.; Souza, D.O.; Gonçalves, C.-A.; Quincozes-Santos, A.; Bobermin, L.D. Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol. Cell. Biochem. 2017, 428, 67–77. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Jaiswal, A.K. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. Faseb J. 2011, 25, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β1–42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Nicol, C.J.; Cheng, Y.-C. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem. Int. 2018, 115, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- Akhtar, A.; Sah, S.P. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem. Int. 2020, 135, 104707. [Google Scholar] [CrossRef]
- Dahiya, M.; Yadav, M.; Goyal, C.; Kumar, A. Insulin resistance in Alzheimer’s disease: Signalling mechanisms and therapeutics strategies. Inflammopharmacology 2025, 33, 1817–1831. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose Transporters in Brain: In Health and in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J. Pathol. 2011, 225, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Pektaş, M.B.; Koca, H.B.; Tosun, M.; Koca, T. Resveratrol improves hepatic insulin signaling and reduces the inflammatory response in streptozotocin-induced diabetes. Gene 2015, 570, 213–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Saucedo, L.J.; Ru, B.; Edgar, B.A.; Pan, D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003, 5, 578–581. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Carnevalli, L.S.; Masuda, K.; Frigerio, F.; Le Bacquer, O.; Um, S.H.; Gandin, V.; Topisirovic, I.; Sonenberg, N.; Thomas, G.; Kozma, S.C. S6K1 plays a critical role in early adipocyte differentiation. Dev Cell. 2010, 18, 763–774. [Google Scholar] [CrossRef]
- Pan, X.; Liu, C.; Wang, X.; Zhao, M.; Zhang, Z.; Zhang, X.; Wang, C.; Song, G. Resveratrol improves palmitic acid-induced insulin resistance via the DDIT4/mTOR pathway in C2C12 cells. Mol. Med. Rep. 2023, 28, 181. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.Q.; Ooi, L.; Piller, S.C.; Münch, G. Proenergetic effects of resveratrol in the murine neuronal cell line Neuro2a. Mol. Nutr. Food Res. 2013, 57, 1901–1907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lam, Y.Y.; Peterson, C.M.; Ravussin, E. Resveratrol vs. calorie restriction: Data from rodents to humans. Exp. Gerontol. 2013, 48, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Z.; Han, X.; Li, S.; Jiang, X.; Chen, S.; Zhang, J.; Lu, H. Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1400. [Google Scholar] [CrossRef]
- Moawad, M.H.E.D.; Serag, I.; Alkhawaldeh, I.M.; Abbas, A.; Sharaf, A.; Alsalah, S.; Sadeq, M.A.; Shalaby, M.M.M.; Hefnawy, M.T.; Abouzid, M.; et al. Exploring the Mechanisms and Therapeutic Approaches of Mitochondrial Dysfunction in Alzheimer’s Disease: An Educational Literature Review. Mol. Neurobiol. 2024, 62, 6785–6810. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.S.; Zhang, H.; D’Amico, D.; Moullan, N.; Potenza, F.; Schmid, A.W.; Rietsch, S.; et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef]
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Edward, O.J.; Anastasia, K. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Koyano, F.; Okatsu, K.; Kosako, H.; Tamura, Y.; Go, E.; Kimura, M.; Kimura, Y.; Tsuchiya, H.; Yoshihara, H.; Hirokawa, T.; et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 2014, 510, 162–166. [Google Scholar] [CrossRef]
- Moore, A.S.; Holzbaur, E.L.F. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proc. Natl. Acad. Sci. USA 2016, 113, E3349–E3358. [Google Scholar] [CrossRef] [PubMed]
- Yamano, K.; Youle, R.J. Two different axes CALCOCO2-RB1CC1 and OPTN-ATG9A initiate PRKN-mediated mitophagy. Autophagy 2020, 16, 2105–2107. [Google Scholar] [CrossRef]
- Richter, B.; Sliter, D.A.; Herhaus, L.; Stolz, A.; Wang, C.; Beli, P.; Zaffagnini, G.; Wild, P.; Martens, S.; Wagner, S.A.; et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc. Natl. Acad. Sci. USA 2016, 113, 4039–4044. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Wang, C.; Bunker, E.; Hao, L.; Maric, D.; Schiavo, G.; Randow, F.; Youle, R.J. Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy. Mol. Cell 2019, 74, 347–362.e6. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.-P.K.; de Cabo, R.; Mattison, J.A. Resveratrol Blunts Mitochondrial Loss in Slow and Mixed Skeletal Muscle Phenotypes of Non-Human Primates following a Long-Term High Fat/Sugar Diet. J. Diet. Suppl. 2023, 20, 563–581. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Petukhov, A.V.; Aksenov, N.D.; Pospelov, V.A. Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discov. 2019, 5, 61. [Google Scholar] [CrossRef]
- Hung, C.-M.; Lombardo, P.S.; Malik, N.; Brun, S.N.; Hellberg, K.; Van Nostrand, J.L.; Garcia, D.; Baumgart, J.; Diffenderfer, K.; Asara, J.M.; et al. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci. Adv. 2021, 7, eabg4544. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Mitophagy: Molecular Mechanisms, New Concepts on Parkin Activation and the Emerging Role of AMPK/ULK1 Axis. Cells 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, M.; Lu, X.; Lei, Y.; Sun, J.; Ren, M.; Xu, T.; Lin, H. Resveratrol alleviates Mono-2-ethylhexyl phthalate-induced mitophagy, ferroptosis, and immunological dysfunction in grass carp hepatocytes by regulating the Nrf2 pathway. J. Environ. Manag. 2024, 371, 123235. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Q.; Dong, B.; Geng, H.; Wang, Y.; Han, D.; Zhu, X.; Liu, H.; Zhang, Z.; Yang, Y.; et al. Resveratrol alleviates lipopolysaccharide-induced liver injury by inducing SIRT1/P62-mediated mitophagy in gibel carp (Carassius gibelio). Front. Immunol. 2023, 14, 1177140. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.-T.; Wu, J.-C.; Qin, Z.-H.; Xiang, M.; Lin, F. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 2019, 25, 796–807. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Zirden, L.C.; Puustinen, P.; Blanchette, P.; Jeong, H.; Dejgaard, K.; Siegel, P.M.; Pause, A. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 2021, 17, 3957–3975. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; DePinho, R.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, W.-Y.; Mao, Y.W.; Gräff, J.; Guan, J.-S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.-H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Li, W.-F.; Li, F.; Zhang, Z.; Dai, Y.-D.; Xu, A.-L.; Qi, C.; Gao, J.-M.; Gao, J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Pan, X.; Chen, J.; Wang, L.; Wang, W.; Cheng, R.; Wu, F.; Feng, X.; Yu, Y.; et al. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 2016, 7, 17380–17392. [Google Scholar] [CrossRef]
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules 2018, 23, 2328. [Google Scholar] [CrossRef]
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Kolthur-Seetharam, U.; Dantzer, F.; McBurney, M.W.; de Murcia1, G.; Sassone-Corsi, P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 2006, 5, 873–877. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Massey, J.C.; Hope, M.C., 3rd; Zhou, X.; Keene, C.D.; Ma, T.; Wyatt, M.D.; Stewart, J.A.; Sajish, S.M. Cis- and trans-resveratrol have opposite effects on histone serine-ADP-ribosylation and tyrosine induced neurodegeneration. Nat. Commun. 2022, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Strosznajder, J.B.; Czapski, G.A.; Adamczyk, A.; Strosznajder, R.P. Poly(ADP-ribose) Polymerase-1 in Amyloid Beta Toxicity and Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 78–84. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; Macià, A.; Motilva, M.; Catalán, Ú.; Solà, R. Endothelial Cells Deconjugate Resveratrol Metabolites to Free Resveratrol: A Possible Role in Tissue Factor Modulation. Mol. Nutr. Food Res. 2019, 63, e1800715. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci. Rep. 2015, 5, srep08075. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Gao, L.; Zhao, J.; Cai, Y.; Huang, J.; Jing, S.; Bao, X.; Wang, Y.; Gao, J.; et al. Protective effects of resveratrol on the inhibition of hippocampal neurogenesis induced by ethanol during early postnatal life. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1298–1310. [Google Scholar] [CrossRef]
- Moriya, J.; Chen, R.; Yamakawa, J.-I.; Sasaki, K.; Ishigaki, Y.; Takahashi, T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol. Pharm. Bull. 2011, 34, 354–359. [Google Scholar] [CrossRef]
- Park, H.R.; Kong, K.H.; Yu, B.P.; Mattson, M.P.; Lee, J. Resveratrol inhibits the proliferation of neural progenitor cells and hippo-campal neurogenesis. J. Biol. Chem. 2012, 287, 42588–42600. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Toledo, E.M. The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Mol. Neurodegener. 2008, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Magdesian, M.H.; Carvalho, M.M.V.F.; Mendes, F.A.; Saraiva, L.M.; Juliano, M.A.; Juliano, L.; Garcia-Abreu, J.; Ferreira, S.T. Amyloid-β binds to the extracellular cysteine-rich domain of frizzled and inhibits Wnt/β-catenin signaling. J. Biol. Chem. 2008, 283, 9359–9368. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Tapia-Rojas, C. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1705–1710. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Bai, L.; Liu, R.; Ba, Y.; Zhang, H.; Cheng, X.; Zhou, G.; Huang, H. Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ. Toxicol. 2021, 36, 1664–1673. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, S.; Yao, X.; Xu, L.; Hu, J.; Yin, C.; Chen, J.; Xu, H. Resveratrol promotes axonal regeneration after spinal cord injury through activating Wnt/β-catenin signaling pathway. Aging 2021, 13, 23603–23619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, Z.-H.; Chen, C.; Chen, Y.; Jia, S.-Z.; Liu, Q.; Ni, J.-Z.; Song, G.-L. Selenomethionine promoted hippocampal neurogenesis via the PI3K-Akt-GSK3β-Wnt pathway in a mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2017, 485, 6–15. [Google Scholar] [CrossRef]
- Xu, S.; Sun, F.; Ren, L.; Yang, H.; Tian, N.; Peng, S. Resveratrol controlled the fate of porcine pancreatic stem cells through the Wnt/β-catenin signaling pathway mediated by Sirt1. PLoS ONE 2017, 12, e0187159. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Suresh, P.K.; Joy, T.; Ullal, S.D.; Manjrekar, P.A.; Murlimanju, B.V.; Sharma, B.G.; Massand, A.; Agrawal, A. Outcome of resveratrol and resveratrol with donepezil combination on the β-amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. 3 Biotech 2024, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khodaie, N.; Tajuddin, N.; Mitchell, R.M.; Neafsey, E.J.; Collins, M.A. Combinatorial Preconditioning of Rat Brain Cultures with Subprotective Ethanol and Resveratrol Concentrations Promotes Synergistic Neuroprotection. Neurotox. Res. 2018, 34, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xia, X.; Rui, Y.; Zhang, Z.; Qin, L.; Han, S.; Wan, Z. The combination of 1α,25dihydroxyvitaminD3 with resveratrol improves neuronal degeneration by regulating endoplasmic reticulum stress, insulin signaling and inhibiting tau hyperphosphorylation in SH-SY5Y cells. Food Chem. Toxicol. 2016, 93, 32–40. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, J.N.; Kim, M.K.; Lee, J.; Ignarro, L.J.; Kim, H.; Shin, C.Y.; Han, S. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: A possible role in neuroprotection. J. Pineal Res. 2010, 50, 110–123. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, H.-J.; Shin, C.Y.; Han, S.-H. Melatonin Potentiates the Neuroprotective Properties of Resveratrol Against Beta-Amyloid-Induced Neurodegeneration by Modulating AMP-Activated Protein Kinase Pathways. J. Clin. Neurol. 2010, 6, 127–137. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Chahla, M.N.A.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Xiang, X.; Lin, H.-S. Determination of naturally occurring resveratrol analog trans-4,4′-dihydroxystilbene in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study. Anal. Bioanal. Chem. 2015, 407, 5793–5801. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, K.W.; Lee, H.J. Protective Effects of piceatannol against Beta-Amyloid–Induced neuronal cell death. Ann. N. Y. Acad. Sci. 2007, 1095, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, J.E.; Kang, N.J.; Lee, K.W.; Lee, H.J. Piceatannol attenuates 4-hydroxynonenal-induced apoptosis of PC12 cells by blocking activation of c-Jun N-terminal kinase. Ann. N. Y. Acad. Sci. 2009, 1171, 176–182. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, F.; Zhang, X.; Liu, S.; Mu, P. SIRT1 Is Involved in the Neuroprotection of Pterostilbene Against Amyloid β 25–35-Induced Cognitive Deficits in Mice. Front. Pharmacol. 2022, 13, 877098. [Google Scholar] [CrossRef]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. BioFactors 2018, 44, 83–90. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.-H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. A 2018, 106, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer’s Disease by Regulating Sirt1/miRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, M.; Sheng, R.; Ma, Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ 1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 174–186. [Google Scholar] [CrossRef]
- Abbas, H.; Gad, H.A.; Khattab, M.A.; Mansour, M. The Tragedy of Alzheimer’s Disease: Towards Better Management via Resveratrol-Loaded Oral Bilosomes. Pharmaceutics 2021, 13, 1635. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, Z.; Wu, X.; Mei, J.; Zheng, G. Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol de-livery: Impact on insulin resistance and gut microbiota in obesity-related Alzheimer’s disease. Carbohydr. Polym. 2023, 310, 120714. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Cazarin, C.A.; da Silva, P.B.; Dos Santos, K.P.; da Rocha, M.C.O.; Báo, S.N.; De-Souza, M.M.; Chorilli, M. Intranasal in situ gelling liquid crystal for delivery of resveratrol ameliorates memory and neuroinflammation in Alzheimer’s disease. Nanomedicine 2023, 51, 102689. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic iron oxide loaded chitosan coated bilosomes for magnetic nose to brain targeting of resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; Silva, T.d.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef]
- Yadav, A.; Sunkaria, A.; Singhal, N.; Sandhir, R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 239–254. [Google Scholar] [CrossRef]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-loaded polymeric micelles protect cells from Aβ-induced oxidative stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Chen, M.-H.; Liu, X.-Z.; Qu, X.-W.; Guo, R.-B.; Zhang, L.; Kong, L.; Yu, Y.; Liu, Y.; Zang, J.; Li, X.-Y.; et al. ApoE-modified liposomes encapsulating resveratrol and salidroside alleviate manifestations of Alzheimer’s disease in APP/PS-1 mice. Drug Dev. Ind. Pharm. 2023, 49, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chu, X.; Cui, L.; Fu, S.; Gao, C.; Li, Y.; Sun, B. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020, 27, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Sheng, D.; Guo, Q.; Wang, P.; Xu, S.; Qian, K.; Li, Y.; Cheng, Y.; Wang, L.; Lu, W.; et al. Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer’s disease. Biomaterials 2020, 238, 119844. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dong, Y.; Cao, Z.; Ji, Y.; Cheng, X.; Zheng, X. The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization. Nutrients 2025, 17, 3451. https://doi.org/10.3390/nu17213451
Liu Y, Dong Y, Cao Z, Ji Y, Cheng X, Zheng X. The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization. Nutrients. 2025; 17(21):3451. https://doi.org/10.3390/nu17213451
Chicago/Turabian StyleLiu, Yichen, Yadan Dong, Zhen Cao, Yixuan Ji, Xiaoxin Cheng, and Xu Zheng. 2025. "The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization" Nutrients 17, no. 21: 3451. https://doi.org/10.3390/nu17213451
APA StyleLiu, Y., Dong, Y., Cao, Z., Ji, Y., Cheng, X., & Zheng, X. (2025). The Multi-Dimensional Action Map of Resveratrol Against Alzheimer’s Disease: Mechanism Integration and Treatment Strategy Optimization. Nutrients, 17(21), 3451. https://doi.org/10.3390/nu17213451



