The Water Extract of Sweet Tea Alleviates LPS-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. WEL Preparation
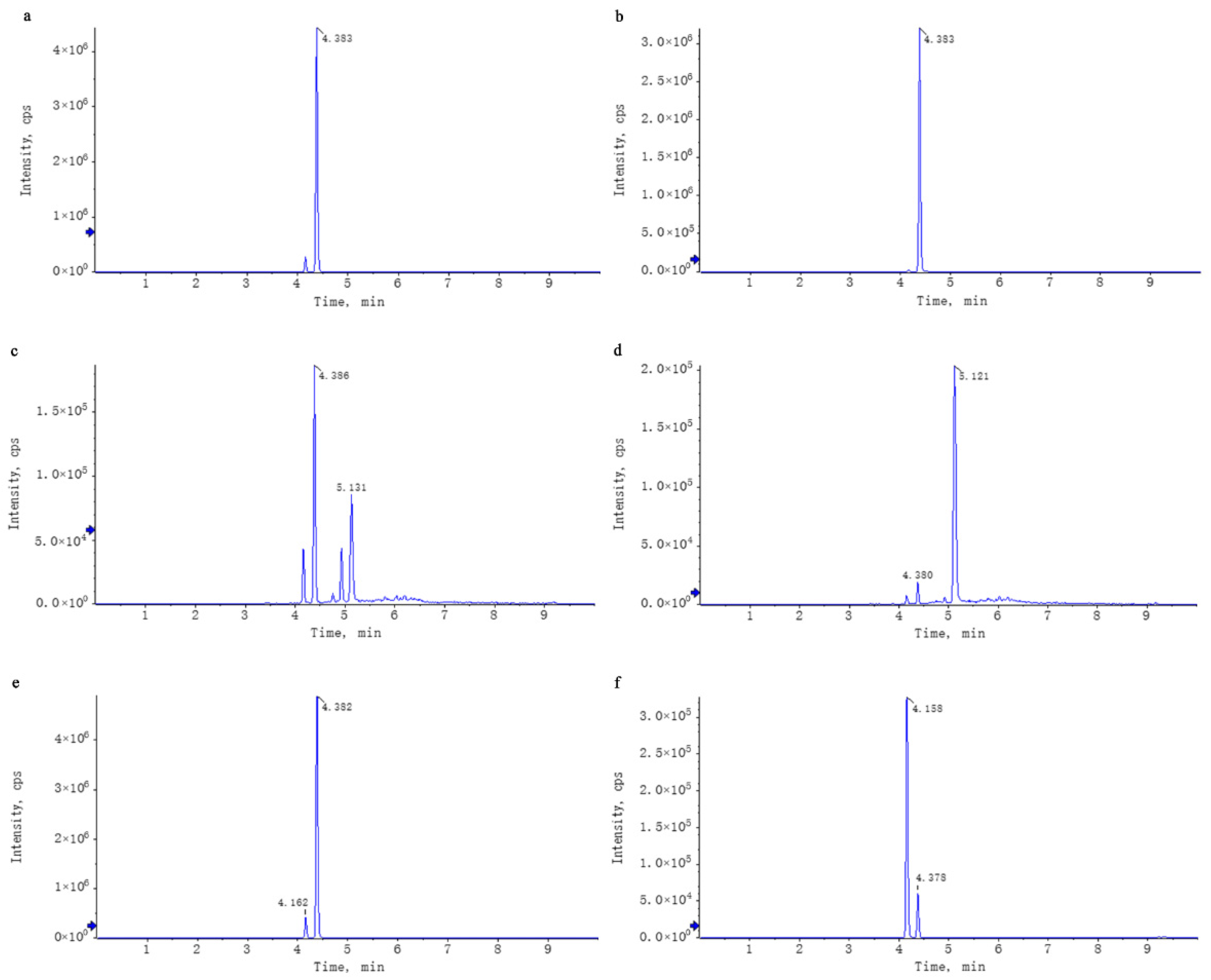
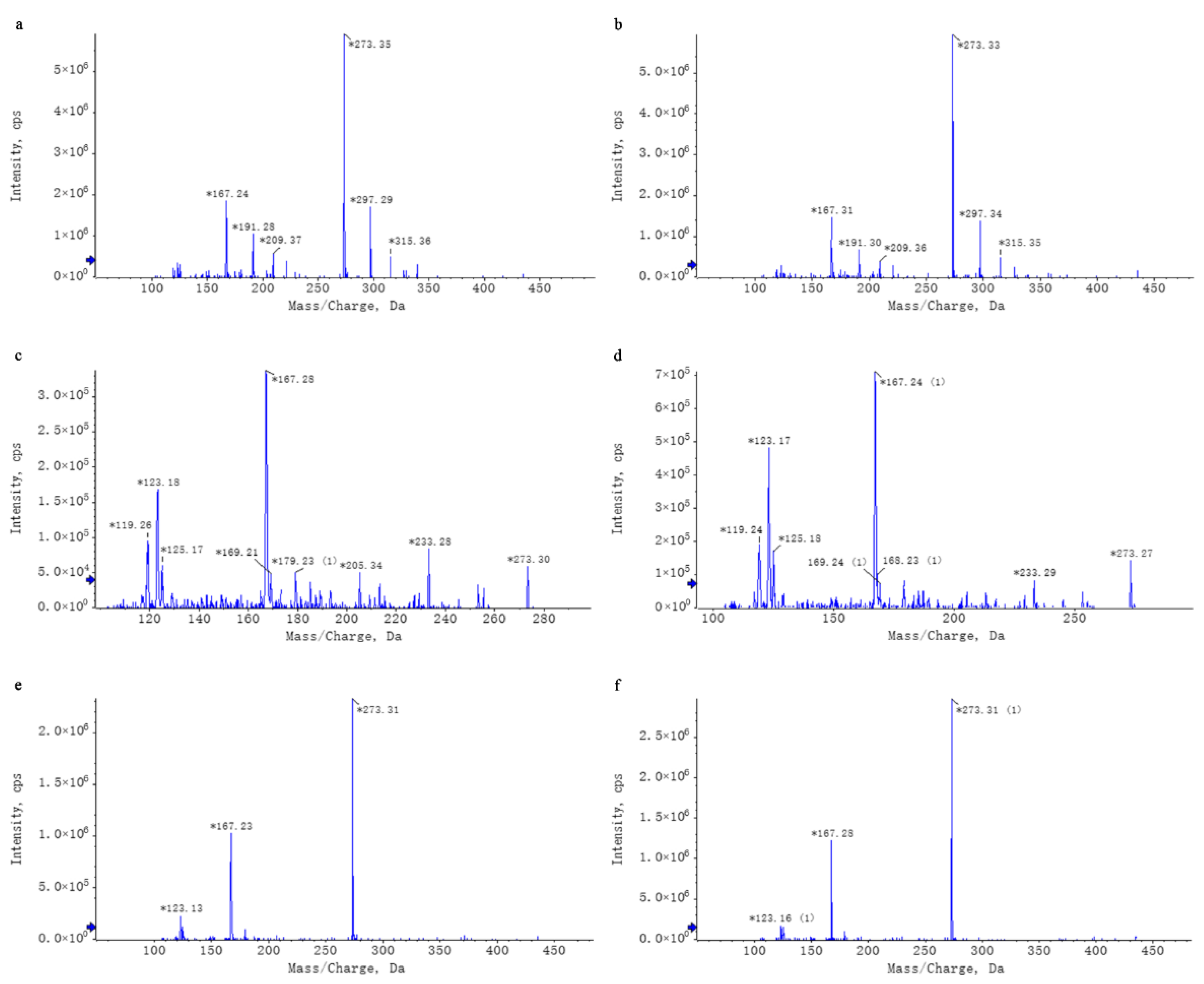
2.3. WEL Untargeted Mass Spectrometry Analysis
2.3.1. Liquid-Phase Conditions
2.3.2. Mass Spectrometry Conditions
2.3.3. Data Processing
2.4. WEL Targeted Mass Spectrometry Analysis
2.4.1. Liquid-Phase Conditions
2.4.2. Mass Spectrometry Conditions
2.4.3. Data Processing
2.5. Animal and Experimental Design
- (1)
- Control Group: The mice in the control group were administered an equal volume of normal saline by gavage once a day. On the seventh day, 2 h after the last administration, the mice were anesthetized with isoflurane. Subsequently, an equal volume of sterile water was injected into the trachea. Six hours after the injection (this injection did not cause any induced injury in this group), the mice were euthanized by cervical dislocation.
- (2)
- LPS Group: The treatment method for the mice in the LPS group was the same as that of the control group, with an equal volume of normal saline administered by gavage once a day. On the seventh day, 2 h after the last gavage of normal saline, the mice were anesthetized with isoflurane. Then, LPS (5 mg/kg) was injected into the trachea to induce injury. Six hours after the LPS injection, the mice were euthanized by cervical dislocation.
- (3)
- Dexamethasone (10 mg/kg) Group: The mice in the dexamethasone group were administered dexamethasone at a dose of 10 mg/kg by gavage once a day. On the seventh day, 2 h after the last administration of dexamethasone, the mice were anesthetized with isoflurane. Similar to the LPS group, LPS (5 mg/kg) was injected into the trachea to induce injury. Six hours after the LPS injection, the mice were euthanized by cervical dislocation. This group serves as the positive control.
- (4)
- Experimental (Low-Dose and High-Dose) Groups: The mice in the low-dose experimental group were administered WEL at a dose of 200 mg/kg by gavage once a day, while the mice in the high-dose experimental group were administered WEL at a dose of 600 mg/kg by gavage once a day. On the seventh day, 2 h after the last administration of WEL, the mice were anesthetized with isoflurane. Then, LPS (5 mg/kg) was injected into the trachea to induce injury. Six hours after the LPS injection, the mice were euthanized by cervical dislocation.
2.6. Wet/Dry Lung Weight Ratio
2.7. Detection of Inflammatory Factors in BALF
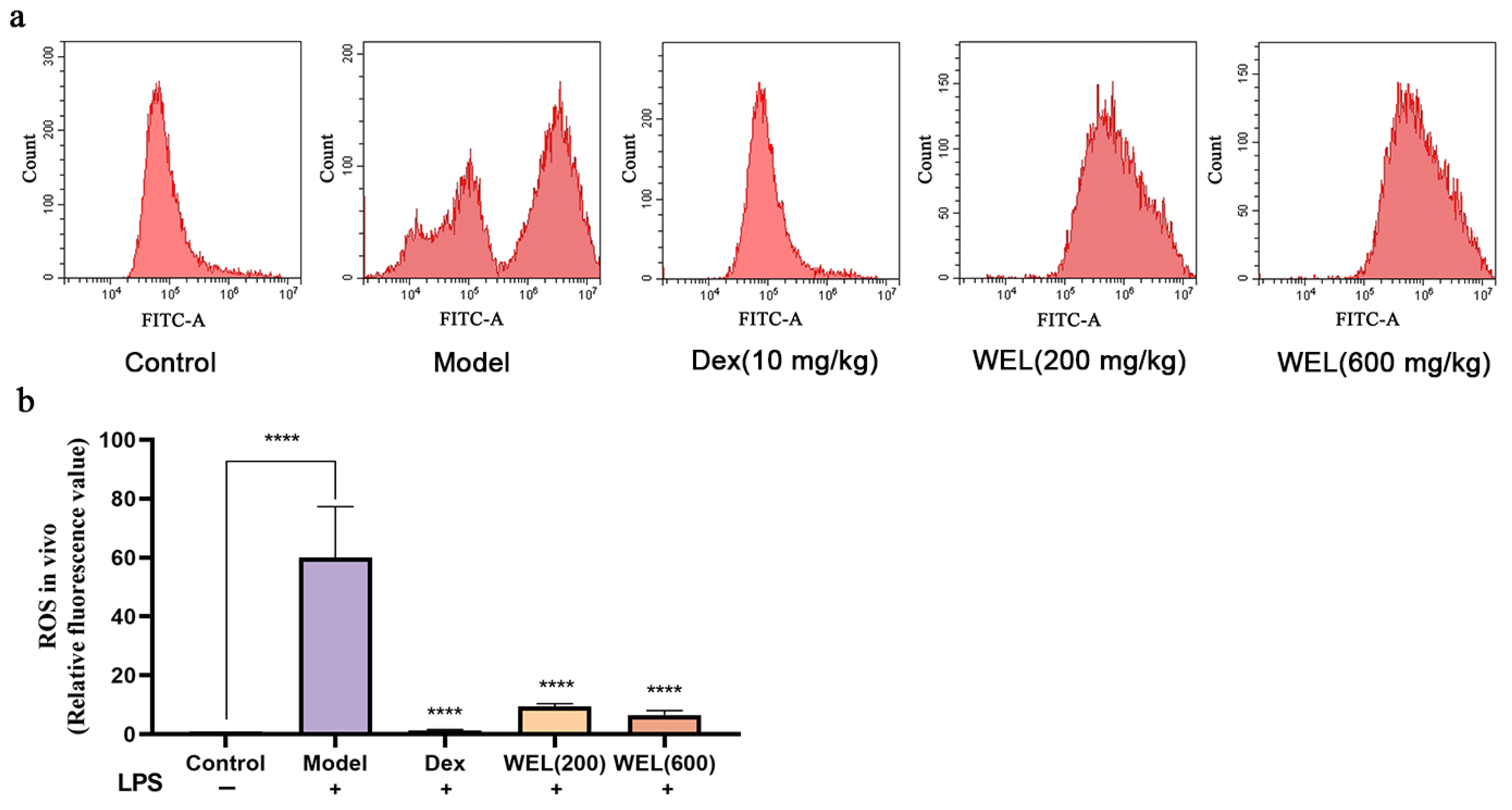
2.8. ROS and NO Content of Lung Tissue
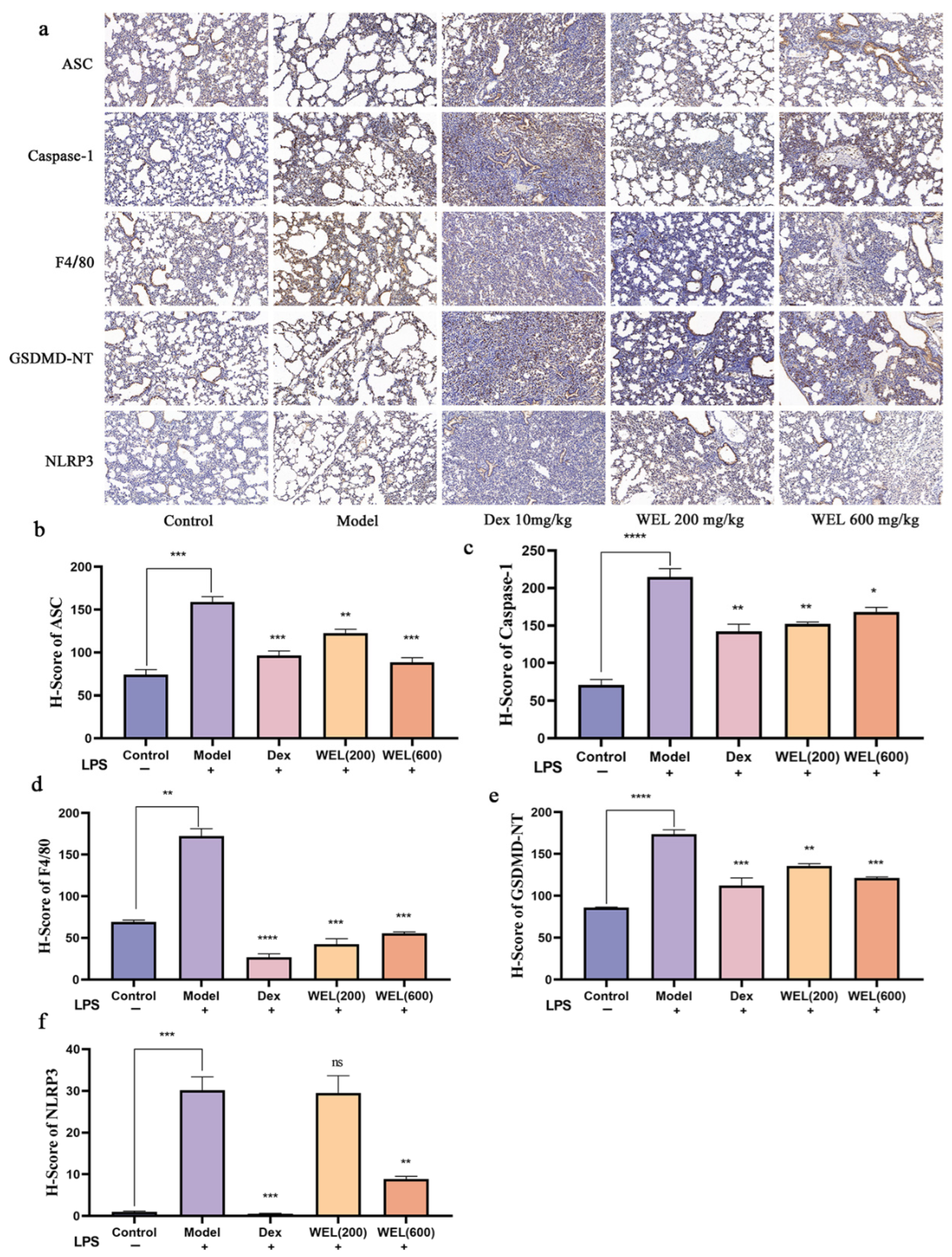
2.9. Histological Analysis and Immunohistochemistry
2.10. Cell Culture
2.11. Cell Experiments
2.11.1. Establishment of an LPS-Induced Inflammatory Model in RAW264.7 Cells
- (1)
- Control group: 2 mL of serum-free medium was added, and the cells were cultured for an additional 11 h. After that, the supernatant was discarded, the cells were washed 2–3 times with PBS, and then 2 mL of serum-free medium was added again for a further 11 h of culture.
- (2)
- Model group: 2 mL of serum-free medium was added, and the cells were cultured for 11 h. Subsequently, the supernatant was removed, the cells were washed 2–3 times with PBS, and then treated with 1 μg/mL LPS for 11 h.
- (3)
- Positive control group: 2 mL of medium containing 200 μM dexamethasone was added, and the cells were treated for 11 h. Afterward, the supernatant was discarded, the cells were washed 2–3 times with PBS, and then treated with 1 μg/mL LPS for 11 h.
- (4)
- Experimental groups: 2 mL of serum-free medium containing WEL at concentrations of 50, 100, and 150 μg/mL, respectively, was added, and the cells were cultured for 11 h. Then, the supernatant was removed, the cells were washed 2–3 times with PBS, and subsequently treated with 1 μg/mL LPS for 11 h.
2.11.2. Establishment of a Cell Pyroptosis Model Induced by the Combined Stimulation of LPS and ATP [13,14]
- (1)
- Control group: 2 mL of serum-free medium was added, and the cells were cultured for an additional 11 h. After that, the supernatant was discarded, the cells were washed 2–3 times with PBS, and then 2 mL of serum-free medium was added again for a further 11 h of culture. Subsequently, the supernatant was removed, the cells were washed 2–3 times with PBS, and 2 mL of serum-free medium was added for a 30 min treatment.
- (2)
- Model group: 2 mL of serum-free medium was added, and the cells were cultured for 11 h. Then, the supernatant was discarded, the cells were washed 2–3 times with PBS, and treated with 1 μg/mL LPS for 11 h. Afterward, the supernatant was removed, the cells were washed 2–3 times with PBS, and 2 mL of serum-free medium containing 5 mM ATP was added for a 30 min treatment.
- (3)
- Positive control group: 2 mL of medium containing 200 μM dexamethasone was added, and the cells were treated for 11 h. Then, the supernatant was discarded, the cells were washed 2–3 times with PBS, and treated with 1 μg/mL LPS for 11 h. Subsequently, the supernatant was removed, the cells were washed 2–3 times with PBS, and 2 mL of serum-free medium containing 5 mM ATP was added for a 30 min treatment.
- (4)
- Experimental groups: 2 mL of serum-free medium containing WEL at concentrations of 50, 100, and 150 μg/mL, respectively, was added, and the cells were cultured for 11 h. Then, the supernatant was discarded, the cells were washed 2–3 times with PBS, and treated with 1 μg/mL LPS for 11 h. Afterward, the supernatant was removed, the cells were washed 2–3 times with PBS, and 2 mL of serum-free medium containing 5 mM ATP was added for a 30 min treatment.
2.12. Scanning Electron Microscopy to Observe Cell Morphology
2.13. Cell NO and ROS Contents
2.14. TUNEL Staining and Immunofluorescence Staining
2.15. Statistical Analysis
3. Results
3.1. WEL Components Analysis and Principal Components Qualitative Quantification
3.2. WEL Alleviates LPS-Induced Acute Lung Injury in Mice
3.3. WEL Inhibits the Generation of Reactive Oxygen Species in the Lungs of Mice
3.4. WEL Inhibits Pyroptosis Through the NLRP3/GSDMD Pathway
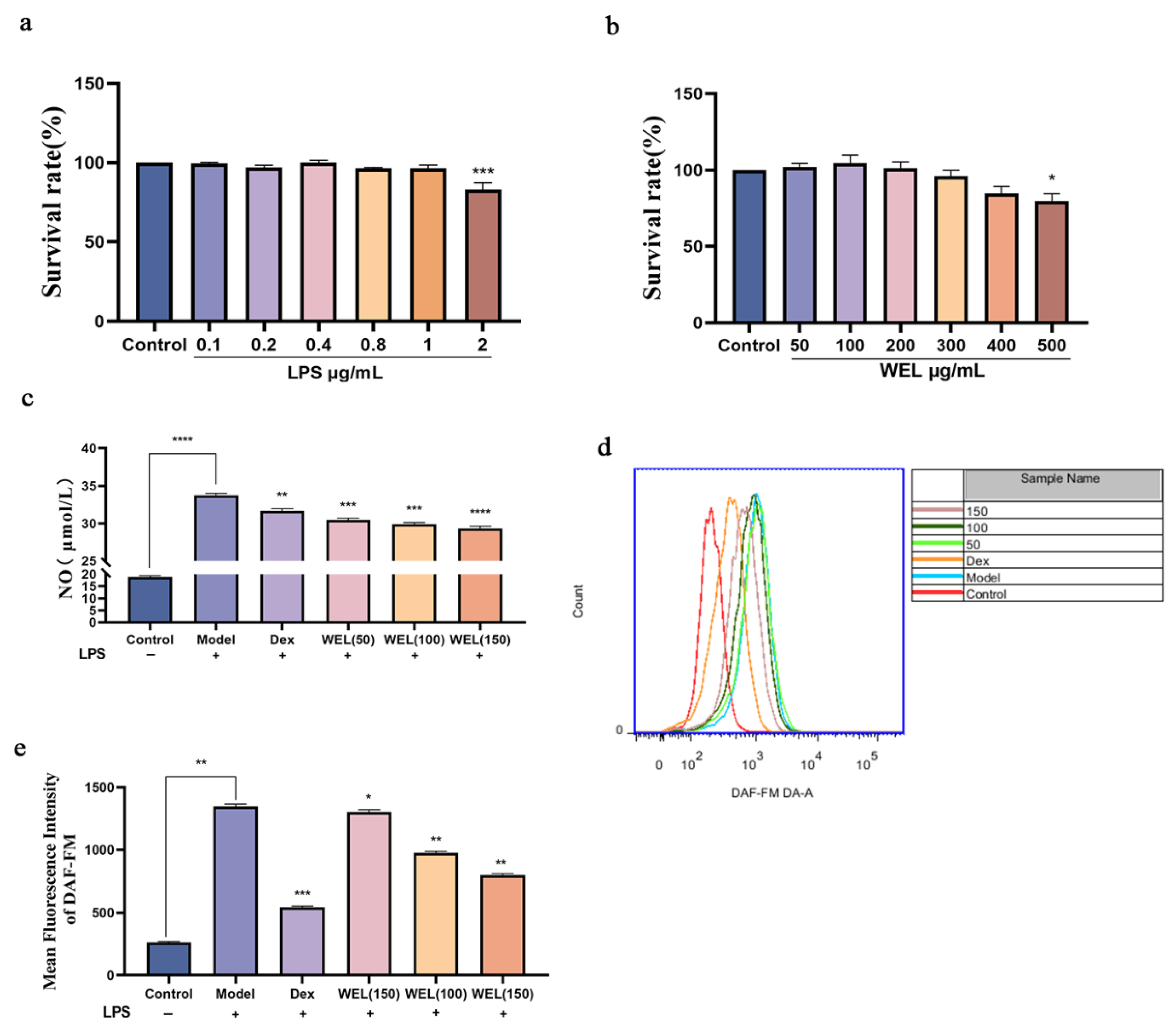
3.5. WEL Inhibits LPS-Induced RAW264.7 Inflammatory Response
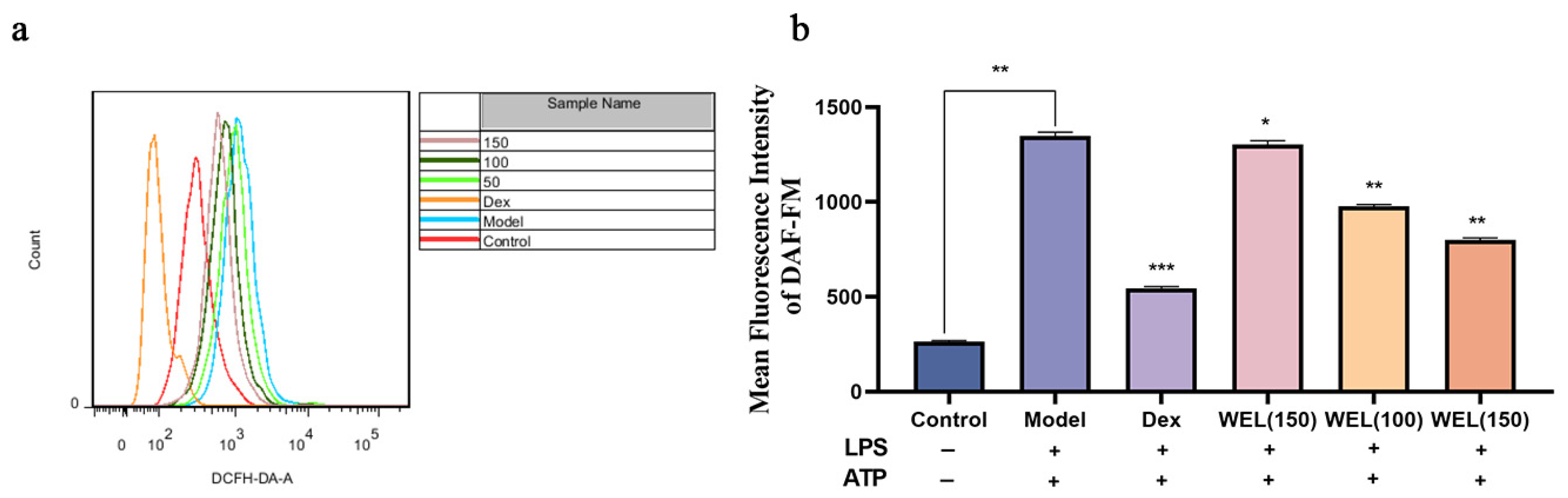
3.6. WEL Inhibits LPS-Induced Oxidative Stress in RAW264.7 Cells
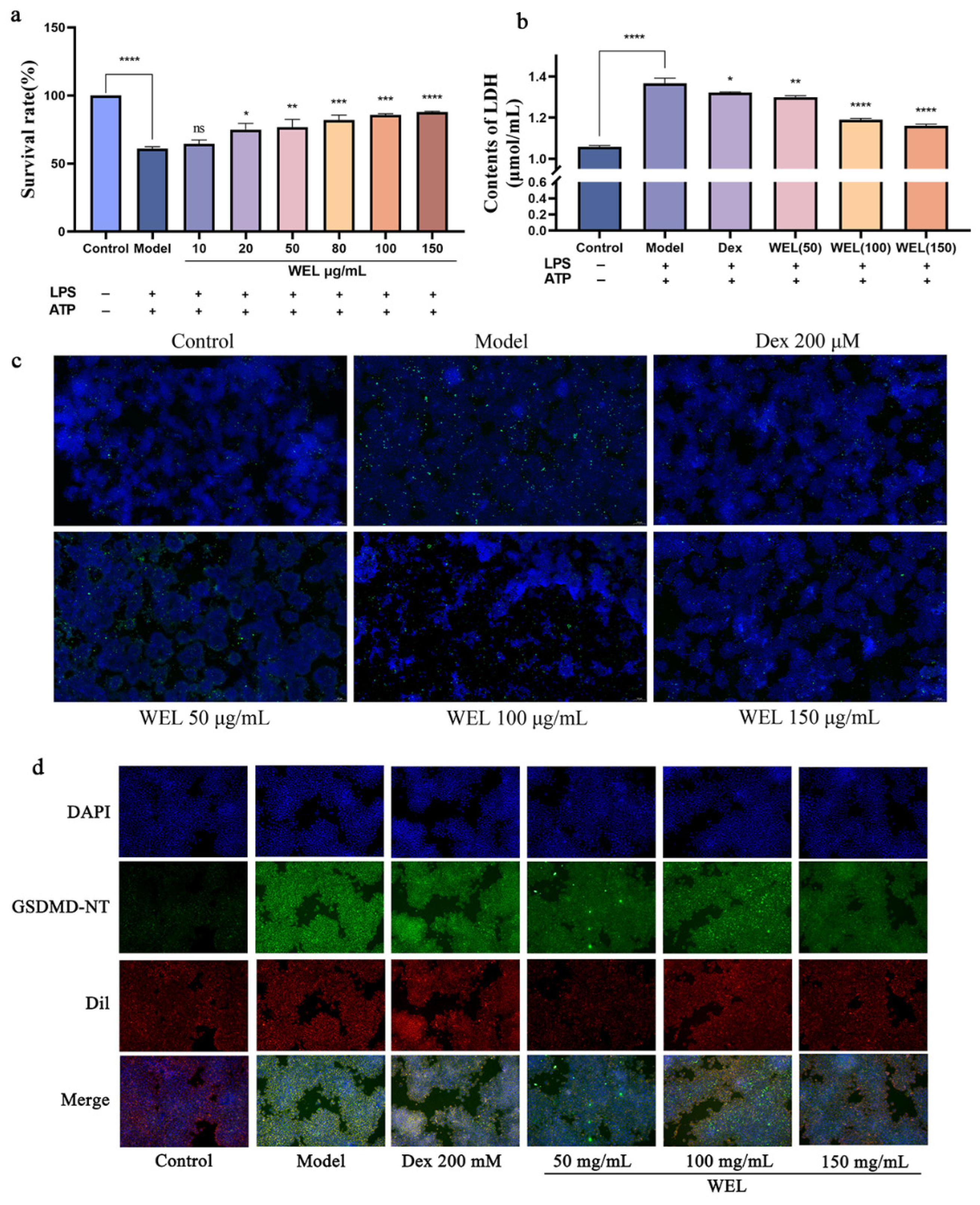
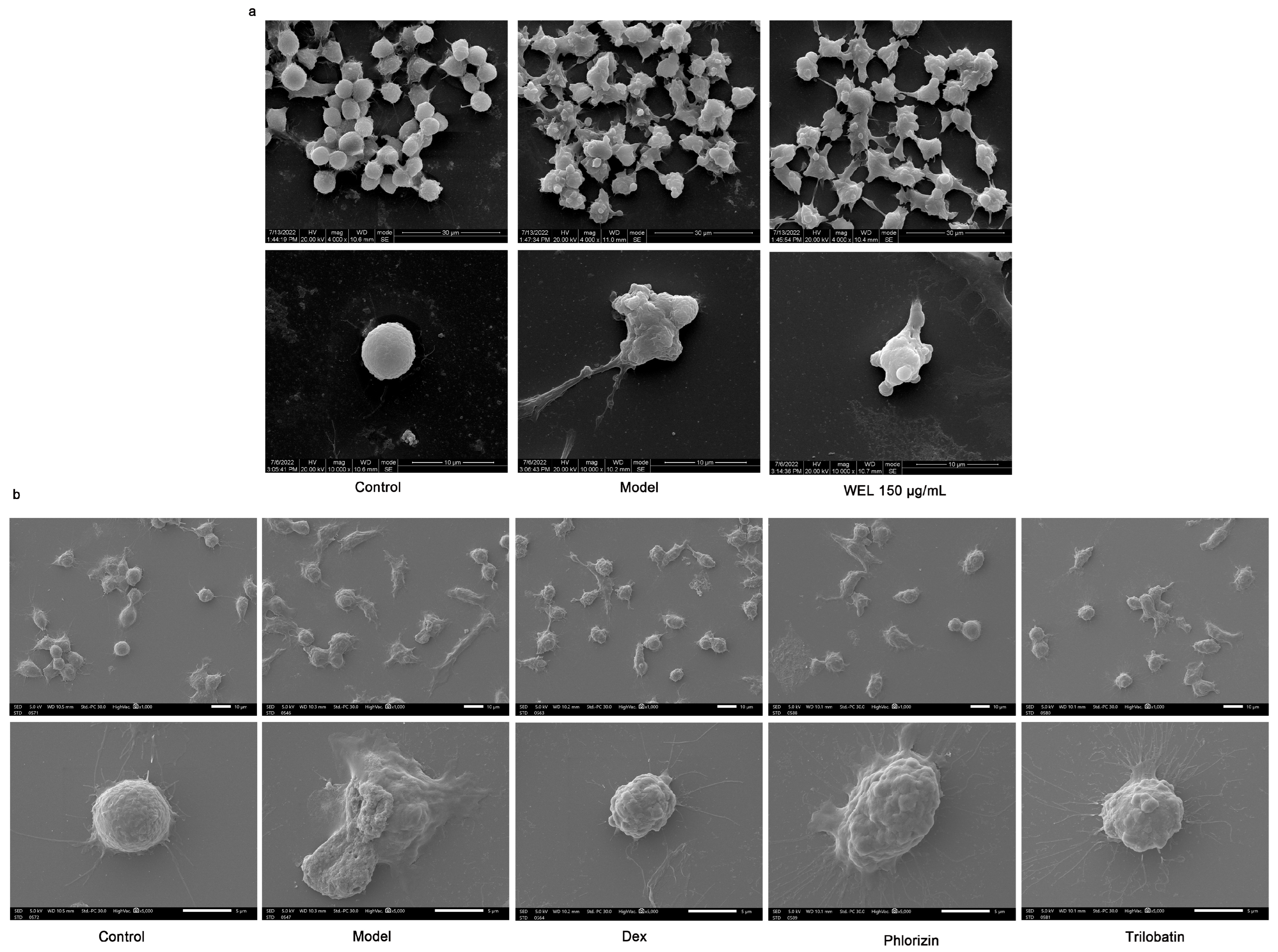
3.7. WEL Inhibits the Expression of GSDMD and Exerts Anti-Pyroptosis In Vitro
3.8. The Main Components of WEL, Trilobatin and Phlorizin Monomers, Exert Anti-Pyroptosis Effects In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Yan, J.; Wang, A.; Cao, J.; Chen, L. Apelin/APJ system: An emerging therapeutic target for respiratory diseases. Cell. Mol. Life Sci. CMLS 2020, 77, 2919–2930. [Google Scholar] [CrossRef]
- Cen, M.; Ouyang, W.; Zhang, W.; Yang, L.; Lin, X.; Dai, M.; Hu, H.; Tang, H.; Liu, H.; Xia, J.; et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021, 41, 101936. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Chen, X.M.; Yang, W.Q.; Wang, X.; Chen, C.; Qian, Z.M.; Wang, S.M.; Tang, D. Effects of natural dihydrochalcones in sweet tea (Lithocarpus polystachyus) on diabetes: A systematical review and meta-analysis of animal studies. Food Funct. 2022, 13, 5899–5913. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Liu, H.Y.; Luo, M.; Xia, Y.; Yang, X.; Li, H.A.-O.; Wu, D.T.; Sun, Q.; Geng, F.; Gan, R.A.-O. Sweet tea (Lithocarpus polystachyus rehd.) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Zhang, Z.; Yu, J.; Liu, J.A.-O.; Wu, Y. Phytochemicals and bioactive analysis of different sweet tea (Lithocarpus litseifolius [Hance] Chun) varieties. J. Food Biochem. 2021, 45, e13183. [Google Scholar] [CrossRef]
- Zhao, H.; Zhai, B.W.; Zhang, M.Y.; Huang, H.; Zhu, H.L.; Yang, H.; Ni, H.Y.; Fu, Y.J. Phlorizin from Lithocarpus litseifolius [Hance] Chun ameliorates FFA-induced insulin resistance by regulating AMPK/PI3K/AKT signaling pathway. Phytomedicine 2024, 130, 155743. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Y.; Zhang, J.; Shi, J.; Gong, Q. Lithocarpus polystachyus Rehd. leaves aqueous extract protects against hydrogen peroxide-induced SH-SY5Y cells injury through activation of Sirt3 signaling pathway. Int. J. Mol. Med. 2018, 42, 3485–3494. [Google Scholar] [CrossRef]
- He, X.A.-O.; Liu, D.; Liu, H.Y.; Wu, D.T.; Li, H.A.-O.; Zhang, X.S.; Gan, R.A.-O. Prevention of Ulcerative Colitis in Mice by Sweet Tea (Lithocarpus litseifolius) via the Regulation of Gut Microbiota and Butyric-Acid-Mediated Anti-Inflammatory Signaling. Nutrients 2022, 14, 2208. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Wu, D.D.; Pan, P.H.; Liu, B.; Su, X.L.; Zhang, L.M.; Tan, H.Y.; Cao, Z.; Zhou, Z.R.; Li, H.T.; Li, H.S.; et al. Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice. Chin. Med. J. 2015, 128, 2638–2645. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Beasley, M.B.; Franks, T.J.; Galvin, J.R.; Gochuico, B.; Travis, W.D. Acute fibrinous and organizing pneumonia: A histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch. Pathol. Lab. Med. 2002, 126, 1064–1070. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Yangzhong, X.; Zhang, X.; Zu, A.; Hou, Y.; Li, L.; Sun, S. Pyroptosis in inflammation-related respiratory disease. J. Physiol. Biochem. 2022, 78, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Zia, M.K.; Ali, S.S.; Rehman, A.A.; Ahsan, H.; Khan, F.H. Reactive oxygen species and anti-proteinases. Arch. Physiol. Biochem. 2016, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- He, X.; Qian, Y.; Li, Z.; Fan, E.K.; Li, Y.; Wu, L.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. TLR4-Upregulated IL-1β and IL-1RI Promote Alveolar Macrophage Pyroptosis and Lung Inflammation through an Autocrine Mechanism. Sci. Rep. 2016, 6, 31663. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef]
- Rocco, P.R.M.; Nieman, G.F. ARDS: What experimental models have taught us. Intensive Care Med. 2016, 42, 806–810. [Google Scholar] [CrossRef]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Mokry, J. Corticosteroids in Acute Lung Injury: The Dilemma Continues. Int. J. Mol. Sci. 2019, 20, 4765. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Zhou, C.C.; Yu, L.Y.; Wang, L.; Deng, J.L.; Tao, Y.L.; Zhang, F.; Chen, W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, H.; Xiaoyin, M.; Yang, G.; Hu, Y.; Xie, K.; Yu, Y. Autophagy Activation Improves Lung Injury and Inflammation in Sepsis. Inflammation 2019, 42, 426–439. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Liang, J.; Chen, S.X.; Huang, S.; Wu, Y.Y.; Zhou, C.J.; Jiang, D.X.; Liang, C.Y.; Yuan, H.Q.; Hou, S.Z.; Lai, X.P. Evaluation of toxicity studies of flavonoid fraction of Lithocarpus polystachyus Rehd in rodents. Regul. Toxicol. Pharmacol. 2017, 88, 283–290. [Google Scholar] [CrossRef]
- Shen, H.; Huang, L.; Dou, H.; Yang, Y.; Wu, H. Effect of Trilobatin from Lithocarpus polystachyus Rehd on Gut Microbiota of Obese Rats Induced by a High-Fat Diet. Nutrients 2021, 13, 891. [Google Scholar] [CrossRef]
- Wu, W.A.-O.; Yan, Y.; Yi, T.; Wei, Y.; Gao, J.; Gong, Q. Lithocarpus polystachyus Rehd. leaves aqueous extract inhibits learning and memory impairment in Alzheimer’s disease rats: Involvement of the SIRT6/NLRP3 signaling pathway. Ibrain 2025, 11, 228–244. [Google Scholar] [CrossRef]
- Qiu, Z.; He, Y.; Ming, H.; Lei, S.; Leng, Y.; Xia, Z.Y. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J. Diabetes Res. 2019, 2019, 8151836. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H.; Ni, H.; Dai, Q. Targeting HDAC6 attenuates nicotine-induced macrophage pyroptosis via NF-κB/NLRP3 pathway. Atherosclerosis 2021, 317, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. CMLS 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Dominic, A.; Le, N.T.; Takahashi, M. Loop Between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxid. Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]

| Time | Flow Rate (mL/min) | Mobile Phase B |
|---|---|---|
| 0 | 0.4 | 5 |
| 1 | 0.4 | 5 |
| 6 | 0.4 | 95 |
| 8 | 0.4 | 95 |
| 8.1 | 0.4 | 2 |
| 10 | 0.4 | 2 |
| Name | Condition |
|---|---|
| Acquisition Time | 10 min |
| Nebulizing Gas | 50 psi |
| Drying Gas | 50 psi |
| Curtain Gas | 35 psi |
| Ion Source Temperature | 500 °C |
| Collision Gas | 7 psi |
| Scan Mode | Negative |
| Ion Source Voltage | −4500 |
| Scan Range (MS) | 50–1000 Da |
| Time | Flow Rate (mL/min) | Mobile Phase B |
|---|---|---|
| 0 | 0.4 | 5 |
| 0.5 | 0.4 | 5 |
| 6 | 0.4 | 95 |
| 8 | 0.4 | 95 |
| 8.1 | 0.4 | 5 |
| 10 | 0.4 | 5 |
| No. | RT (min) | LC-MS [M-H]− | Molecular Formula | Tentative Identification |
|---|---|---|---|---|
| 1 | 0.73 | 192.06 | C7H12O6 | Quinic acid |
| 2 | 0.92 | 174.05 | C7H10O5 | Shikimic acid |
| 3 | 3.4 | 435.12 | C12H24O10 | Phlorizin |
| 4 | 4.24 | 154.06 | C8H10O3 | Hydroxytyrosol |
| 5 | 4.85 | 354.095 | C16H18O9 | Neochlorogenic acid |
| 6 | 5.02 | 312.04 | C13H12O9 | Caftaric acid |
| 7 | 5.22 | 449.10 | C21H21O11 | Asterin |
| 8 | 5.5 | 290.07 | C15H14O6 | (+)-Catechin |
| 9 | 5.55 | 354.09 | C16H18O9 | Chlorogenic acid |
| 10 | 5.79 | 354.09 | C16H18O9 | Cryptochlorogenic acid |
| 11 | 5.83 | 168.04 | C8H8O4 | Vanillic Acid |
| 12 | 5.84 | 180.04 | C9H8O4 | Caffeic acid |
| 13 | 6.26 | 290.07 | C15H14O6 | (-)-Epicatechin |
| 14 | 6.52 | 516.12 | C25H24O12 | 1,3-Dicaffeoylquinic acid |
| 15 | 6.74 | 786.70 | C35H46O20 | Echinacoside |
| 16 | 6.83 | 152.04 | C8H8O3 | Vanillin |
| 17 | 6.9 | 164.16 | C9H8O3 | p-Coumaric acid |
| 18 | 7.22 | 640.58 | C29H36O16 | Plantamajoside |
| 19 | 7.27 | 610.15 | C27H30O16 | Rutin |
| 20 | 7.33 | 302.00 | C14H6O8 | Ellagic acid |
| 21 | 7.38 | 194.18 | C10H10O4 | Ferulic acid |
| 22 | 7.45 | 464.09 | C21H20O12 | Isoquercitrin |
| 23 | 7.54 | 304.05 | C15H12O7 | Taxifolin |
| 24 | 7.54 | 624.20 | C29H36O15 | Verbascoside |
| 25 | 7.73 | 594.52 | C27H30O15 | Nicotiflorin |
| 26 | 7.9 | 516.12 | C25H24O12 | Isochlorogenic acid A |
| 27 | 7.9 | 516.12 | C25H24O12 | 1,5-Dicaffeoylquinic acid |
| 28 | 7.92 | 516.12 | C25H24O12 | Isochlorogenic acid B |
| 29 | 7.94 | 448.10 | C21H20O11 | Astragalin |
| 30 | 8.31 | 516.12 | C25H24O12 | Isochlorogenic acid C |
| 31 | 8.51 | 286.23 | C15H10O6 | Fisetin |
| 32 | 8.63 | 474.07 | C22H18O12 | Chicoric acid |
| 33 | 8.72 | 302.04 | C15H10O7 | Tricetin |
| 34 | 9.85 | 288.25 | C15H12O6 | Fustin |
| 35 | 9.85 | 288.06 | C15H12O6 | Eriodictyol |
| 36 | 10 | 286.04 | C15H10O6 | Luteolin |
| 37 | 10.02 | 302.04 | C15H10O7 | Quercetin |
| 38 | 10.39 | 272.06 | C15H12O5 | Naringenin |
| 39 | 10.42 | 148.05 | C15H10O5 | Apigenin |
| 40 | 10.47 | 286.04 | C15H10O6 | Kaempferol |
| 41 | 10.48 | 330.29 | C17H14O7 | Tricin |
| 42 | 10.9 | 148.05 | C9H8O2 | Cinnamic acid |
| Compounds | Linear Equation | R2 | Content in WEL (ng/μg) |
|---|---|---|---|
| trilobatin | y = 14,022.001435 x + −5658.83325 | 0.99872 | 465.5 |
| phloretin | y = 7.84933 × 104 x + 24,591.13457 | 0.99696 | 1.31 |
| phlorizin | y = 10,660.73682 x + −5404.51522 | 0.99715 | 49.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Wang, T.; Xue, H.; Zhang, Z.; Zhang, H.; Cao, Y.; Tang, L. The Water Extract of Sweet Tea Alleviates LPS-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects. Nutrients 2025, 17, 3425. https://doi.org/10.3390/nu17213425
Zheng H, Wang T, Xue H, Zhang Z, Zhang H, Cao Y, Tang L. The Water Extract of Sweet Tea Alleviates LPS-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects. Nutrients. 2025; 17(21):3425. https://doi.org/10.3390/nu17213425
Chicago/Turabian StyleZheng, Haorui, Taoyu Wang, Hairui Xue, Zihan Zhang, Hengyang Zhang, Yang Cao, and Lin Tang. 2025. "The Water Extract of Sweet Tea Alleviates LPS-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects" Nutrients 17, no. 21: 3425. https://doi.org/10.3390/nu17213425
APA StyleZheng, H., Wang, T., Xue, H., Zhang, Z., Zhang, H., Cao, Y., & Tang, L. (2025). The Water Extract of Sweet Tea Alleviates LPS-Induced Acute Lung Injury Through Anti-Inflammatory and Antioxidant Effects. Nutrients, 17(21), 3425. https://doi.org/10.3390/nu17213425







