Restoring Balance: The Role of Omega-3 Polyunsaturated Fatty Acids on the Gut–Brain Axis and Other Interconnected Biological Pathways to Improve Depression
Abstract
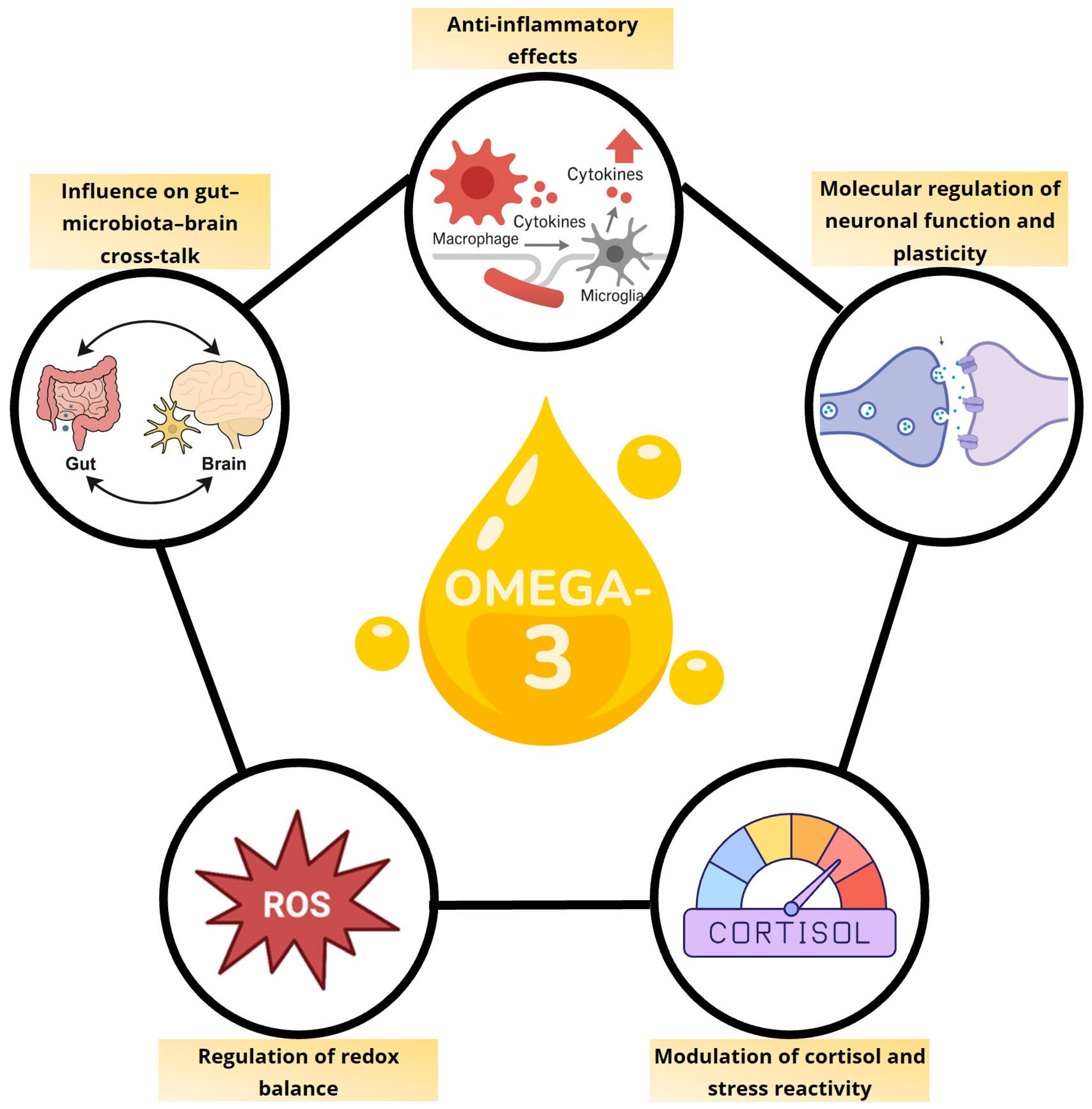
1. Introduction
2. Regulation of Microbiota–Gut–Brain Axis by Omega-3 Polyunsaturated Fatty Acids
3. Anti-Inflammatory Effects of Omega-3 Polyunsaturated Fatty Acids
4. Role of Omega-3 Polyunsaturated Fatty Acids in Brain Plasticity and Neuronal Functioning
5. Modulation of Cortisol and Stress Reactivity by Omega-3 Polyunsaturated Fatty Acids
6. Modulation of Oxidative Stress by n-3 Polyunsaturated Fatty Acids in the Pathophysiology of Depression
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| COX | Cyclooxygenase |
| CREB | cAMP Response Element-Binding Protein |
| CRP | C-Reactive Protein |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| GDNF | Glial cell line-Derived Neurotrophic Factor |
| HEPE | Hydroxy Eicosapentaenoic Acid |
| HPA | Hypothalamic–Pituitary–Adrenal |
| IL | Interleukin |
| IDO | Indoleamine 2,3-Dioxygenase |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| MAP2 | Microtubule-Associated Protein 2 |
| MDD | Major Depressive Disorder |
| n-3 PUFAs | Omega-3 Polyunsaturated Fatty Acids |
| n-6 PUFAs | Omega-6 Polyunsaturated Fatty Acids |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PFC | Prefrontal Cortex |
| PUFAs | Polyunsaturated Fatty Acids |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| RvE | Resolvin E |
| SCFA | Short Chain Fatty Acid |
| SPM | Specialized Pro-resolving Mediator |
| TLR4 | Toll-like Receptor 4 |
| TNF-α | Tumor Necrosis Factor alpha |
| TrkB | Tropomyosin receptor kinase B |
| ZO-1 | Zonula Occludens-1 |
References
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come From? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Klinedinst, N.J.; Regenold, W.T. A Mitochondrial Bioenergetic Basis of Depression. J. Bioenerg. Biomembr. 2015, 47, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- von Schacky, C. Importance of EPA and DHA Blood Levels in Brain Structure and Function. Nutrients 2021, 13, 1074. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-Analysis and Meta-Regression of Omega-3 Polyunsaturated Fatty Acid Supplementation for Major Depressive Disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Guu, T.-W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 263–273. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Chen, W.-Y.; Lin, C.; Lee, S.-H.; Chiu, C.-C.; Kuo, P.-H. The N-3 Polyunsaturated Fatty Acids Supplementation to Prevent Depression Recurrence in Patients with Late-Life Depression: A 52-Week Double-Blind, Placebo-Controlled Trial. J. Affect. Disord. 2025, 369, 8–15. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Bhatt, S.; Kanoujia, J.; Mohana Lakshmi, S.; Patil, C.R.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota−Gut−Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Bravo, M.A.; Mota-Rojas, D.; Orozco-Gregorio, H.; Pérez-Guille, B.; Soriano-Rosales, R.; Roldan-Santiago, P.; Alonso-Spilsbury, M.; Arch-Tirado, E.; Mora-Medina, P.; Martínez-Burnes, J. Evaluation of the Protective Effect of Thiamine Pyrophosphate Based on the Biochemical Analysis of Rabbit Foetuses at 30 Days of Gestation. Reprod. Toxicol. 2016, 65, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pramanik, J.; Goyal, N.; Chauhan, D.; Sivamaruthi, B.S.; Prajapati, B.G.; Chaiyasut, C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals 2023, 16, 565. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut-Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Abdel-Azim, T.; Rogers, K.; Elathamna, E.; Zandinejad, A.; Metz, M.; Morton, D. Comparison of the Marginal Fit of Lithium Disilicate Crowns Fabricated with CAD/CAM Technology by Using Conventional Impressions and Two Intraoral Digital Scanners. J. Prosthet. Dent. 2015, 114, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish Oil Enhances Intestinal Integrity and Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The Intestinal Barrier in Disorders of the Central Nervous System. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Balvers, M.; Beermann, C.; Stahl, B.; van Tol, E.A.F. Polyunsaturated Fatty Acids Support Epithelial Barrier Integrity and Reduce IL-4 Mediated Permeability in Vitro. Eur. J. Nutr. 2008, 47, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish Oil Attenuates Omega-6 Polyunsaturated Fatty Acid-Induced Dysbiosis and Infectious Colitis but Impairs LPS Dephosphorylation Activity Causing Sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppée, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-Inflammatory Effect of Lactobacillus Casei on Shigella-Infected Human Intestinal Epithelial Cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Horigome, A.; Okubo, R.; Hamazaki, K.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.Z.; Matsuoka, Y.J. Association between Blood Omega-3 Polyunsaturated Fatty Acids and the Gut Microbiota among Breast Cancer Survivors. Benef. Microbes 2019, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The Prebiotic Effects of Omega-3 Fatty Acid Supplementation: A Six-Week Randomised Intervention Trial. Gut Microbes 2021, 13, 1863133. [Google Scholar] [CrossRef]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Jones, M.K. The Multifaceted Impact of Bioactive Lipids on Gut Health and Disease. Int. J. Mol. Sci. 2024, 25, 13638. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.; Alkahtani, R.; Akbarali, H.I.; Murthy, K.S.; Grider, J.R. Stimulation of Synthesis and Release of Brain-derived Neurotropic Factor from Intestinal Smooth Muscle Cells by Substance P and Pituitary Adenylate Cyclase-activating Peptide. Neurogastroenterol. Motil. 2015, 27, 1162–1174. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The Kynurenine and Serotonin Pathway, Neopterin and Biopterin in Depressed Children and Adolescents: An Impact of Omega-3 Fatty Acids, and Association with Markers Related to Depressive Disorder. A Randomized, Blinded, Prospective Study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Toader, C.; Dobrin, N.; Costea, D.; Glavan, L.-A.; Covache-Busuioc, R.-A.; Dumitrascu, D.-I.; Bratu, B.-G.; Costin, H.-P.; Ciurea, A.V. Mind, Mood and Microbiota—Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3340. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Santos, C.F.; Bagatini, M.D.; Ignácio, Z.M. Hypothalamus-Pituitary-Adrenal and Gut-Brain Axes in Biological Interaction Pathway of the Depression. Front. Neurosci. 2025, 19, 1541075. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-Specific Effects of Docosahexaenoic Acid (DHA) on the Microbiome and Behavior of Socially-Isolated Mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jo, M.; Oh, H.; Lee, Y.; Park, Y. Synergistic Antidepressant-like Effect of n-3 Polyunsaturated Fatty Acids and Probiotics through the Brain-Gut Axis in Rats Exposed to Chronic Mild Stress. J. Nutr. Biochem. 2023, 116, 109326. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood-Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, M.G.; Benedetti, F. Inflammatory Mediators in Major Depression and Bipolar Disorder. Transl. Psychiatry 2024, 14, 247. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Crowe, T.M.; Wong, A.; et al. Omega-3 Fatty Acids for Major Depressive Disorder With High Inflammation. J. Clin. Psychiatry 2022, 83, 21m14074. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a Predictive Biomarker for Response to Omega-3 Fatty Acids in Major Depressive Disorder: A Proof-of-Concept Study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and Time-Dependent Increase in Circulating Anti-Inflammatory and pro-Resolving Lipid Mediators Following Eicosapentaenoic Acid Supplementation in Patients with Major Depressive Disorder and Chronic Inflammation. Prostaglandins Leukot. Essent. Fat Acids 2021, 164, 102219. [Google Scholar] [CrossRef]
- Qiu, T.; Li, X.; Chen, W.; He, J.; Shi, L.; Zhou, C.; Zheng, A.; Lei, Z.; Tang, C.; Yu, Q.; et al. Prospective Study on Maresin-1 and Cytokine Levels in Medication-Naïve Adolescents with First-Episode Major Depressive Disorder. Front. Psychiatry 2023, 14, 1132791. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Chen, W.; Tan, J.; Li, X.; Zheng, A.; Fu, Y.; Qiu, T. The Relationship between Serum Resolvin D1, NLRP3, Cytokine Levels, and Adolescents with First-Episode Medication-Naïve Major Depressive Disorder. BMC Psychiatry 2024, 24, 285. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhu, J.; Zou, M.; Zhang, Y.; Wu, H.; Jin, T. Specialized Pro-Resolving Lipid Mediators: A Key Player in Resolving Inflammation in Autoimmune Diseases. Sci. Bull. 2025, 70, 778–794. [Google Scholar] [CrossRef]
- Wen, J.; Satyanarayanan, S.K.; Li, A.; Yan, L.; Zhao, Z.; Yuan, Q.; Su, K.-P.; Su, H. Unraveling the Impact of Omega-3 Polyunsaturated Fatty Acids on Blood-Brain Barrier (BBB) Integrity and Glymphatic Function. Brain Behav. Immun. 2024, 115, 335–355. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A Neurotrophic and Neuroprotective Metabolite of Docosahexaenoic Acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved Spatial Learning Performance of Fat-1 Mice Is Associated with Enhanced Neurogenesis and Neuritogenesis by Docosahexaenoic Acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Laricchiuta, D.; Foti, F.; Ronci, M.; Rossi, C.; Neri, C.; Spalletta, G.; Caltagirone, C.; et al. N-3 Polyunsaturated Fatty Acids Supplementation Enhances Hippocampal Functionality in Aged Mice. Front. Aging Neurosci. 2014, 6, 220. [Google Scholar] [CrossRef]
- Martin, M.; Boulaire, M.; Lucas, C.; Peltier, A.; Pourtau, L.; Gaudout, D.; Layé, S.; Pallet, V.; Joffre, C.; Dinel, A.-L. Plant Extracts and ω-3 Improve Short-Term Memory and Modulate the Microbiota-Gut-Brain Axis in D-Galactose Model Mice. J. Nutr. 2024, 154, 3704–3717. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary Omega-3 Fatty Acids Normalize BDNF Levels, Reduce Oxidative Damage, and Counteract Learning Disability after Traumatic Brain Injury in Rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Song, C.; Shieh, C.-H.; Wu, Y.-S.; Kalueff, A.; Gaikwad, S.; Su, K.-P. The Role of Omega-3 Polyunsaturated Fatty Acids Eicosapentaenoic and Docosahexaenoic Acids in the Treatment of Major Depression and Alzheimer’s Disease: Acting Separately or Synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. Int. J. Mol. Sci. 2022, 23, 3399. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Varma, S.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Dietary Omega-3 Fatty Acid Deficiency from Pre-Pregnancy to Lactation Affects Expression of Genes Involved in Hippocampal Neurogenesis of the Offspring. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102566. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Omokawa, D.; Katakura, M.; Matsumata, M.; Aizawa, H.; Sugita, M.; Sakayori, N. Nutritional Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Increases the Number of Pyramidal Neurons in the Basolateral Amygdala and Anxiety-Related Behavior in Offspring. J. Nutr. Sci. Vitaminol. 2024, 70, 411–421. [Google Scholar] [CrossRef]
- Borsini, A.; Giacobbe, J.; Mandal, G.; Boldrini, M. Acute and Long-Term Effects of Adolescence Stress Exposure on Rodent Adult Hippocampal Neurogenesis, Cognition, and Behaviour. Mol. Psychiatry 2023, 28, 4124–4137. [Google Scholar] [CrossRef]
- McNamara, R.K.; Schurdak, J.D.; Asch, R.H.; Peters, B.D.; Lindquist, D.M. Deficits in Docosahexaenoic Acid Accrual during Adolescence Reduce Rat Forebrain White Matter Microstructural Integrity: An in Vivo Diffusion Tensor Imaging Study. Dev. Neurosci. 2018, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Katakura, M.; Hashimoto, M.; Okui, T.; Shahdat, H.M.; Matsuzaki, K.; Shido, O. Omega-3 Polyunsaturated Fatty Acids Enhance Neuronal Differentiation in Cultured Rat Neural Stem Cells. Stem Cells Int. 2013, 2013, 490476. [Google Scholar] [CrossRef]
- Borsini, A.; Stangl, D.; Jeffries, A.R.; Pariante, C.M.; Thuret, S. The Role of Omega-3 Fatty Acids in Preventing Glucocorticoid-Induced Reduction in Human Hippocampal Neurogenesis and Increase in Apoptosis. Transl. Psychiatry 2020, 10, 219. [Google Scholar] [CrossRef]
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.-P.; Pariante, C.M.; Zunszain, P.A. Rescue of IL-1β-Induced Reduction of Human Neurogenesis by Omega-3 Fatty Acids and Antidepressants. Brain Behav. Immun. 2017, 65, 230–238. [Google Scholar] [CrossRef]
- Serefko, A.; Jach, M.E.; Pietraszuk, M.; Świąder, M.; Świąder, K.; Szopa, A. Omega-3 Polyunsaturated Fatty Acids in Depression. Int. J. Mol. Sci. 2024, 25, 8675. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, T.; Hilal, L.M.; Fourrier, C.; De Smedt-Peyrusse, V.; Sans, N.; Capuron, L.; Layé, S. Nutritional Omega-3 Modulates Neuronal Morphology in the Prefrontal Cortex along with Depression-Related Behaviour through Corticosterone Secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef]
- Choi, J.-E.; Kim, E.-Y.; Park, Y. N-3 PUFA Improved Pup Separation-Induced Postpartum Depression via Serotonergic Pathway Regulated by MiRNA. J. Nutr. Biochem. 2020, 84, 108417. [Google Scholar] [CrossRef] [PubMed]
- Paduchová, Z.; Katrenčíková, B.; Vaváková, M.; Laubertová, L.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z.; Trebatická, J. The Effect of Omega-3 Fatty Acids on Thromboxane, Brain-Derived Neurotrophic Factor, Homocysteine, and Vitamin D in Depressive Children and Adolescents: Randomized Controlled Trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Radford-Smith, D.; Dunstan, I.K.; Gorlova, A.; Svirin, E.; Sheveleva, E.; Burova, A.; Morozov, S.; Lyundup, A.; Berger, G.; et al. Omega-3 Alleviates Behavioral and Molecular Changes in a Mouse Model of Stress-Induced Juvenile Depression. Neurobiol. Stress 2024, 31, 100646. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA Is More Effective than DHA to Improve Depression-Like Behavior, Glia Cell Dysfunction and Hippcampal Apoptosis Signaling in a Chronic Stress-Induced Rat Model of Depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Maffione, A.B.; Tucci, P.; Trabace, L. Depressive-like Phenotype Evoked by Lifelong Nutritional Omega-3 Deficiency in Female Rats: Crosstalk among Kynurenine, Toll-like Receptors and Amyloid Beta Oligomers. Brain Behav. Immun. 2020, 87, 444–454. [Google Scholar] [CrossRef]
- Nemeth, M.; Eisenschenk, I.; Engelmann, A.; Esser, F.M.; Kokodynska, M.; Szewczak, V.F.; Barnreiter, E.; Wallner, B.; Millesi, E. Flaxseed Oil as Omega-3 Polyunsaturated Fatty Acid Source Modulates Cortisol Concentrations and Social Dominance in Male and Female Guinea Pigs. Horm. Behav. 2021, 134, 105025. [Google Scholar] [CrossRef]
- Oravcova, H.; Katrencikova, B.; Garaiova, I.; Durackova, Z.; Trebaticka, J.; Jezova, D. Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression. Antioxidants 2022, 11, 1546. [Google Scholar] [CrossRef]
- Madison, A.A.; Belury, M.A.; Andridge, R.; Renna, M.E.; Rosie Shrout, M.; Malarkey, W.B.; Lin, J.; Epel, E.S.; Kiecolt-Glaser, J.K. Omega-3 Supplementation and Stress Reactivity of Cellular Aging Biomarkers: An Ancillary Substudy of a Randomized, Controlled Trial in Midlife Adults. Mol. Psychiatry 2021, 26, 3034–3042. [Google Scholar] [CrossRef]
- Jahangard, L.; Hedayati, M.; Abbasalipourkabir, R.; Haghighi, M.; Ahmadpanah, M.; Faryadras, M.; Mikoteit, T.; Sadeghi Bahmani, D.; Brand, S. Omega-3-Polyunsatured Fatty Acids (O3PUFAs), Compared to Placebo, Reduced Symptoms of Occupational Burnout and Lowered Morning Cortisol Secretion. Psychoneuroendocrinology 2019, 109, 104384. [Google Scholar] [CrossRef]
- Bajpai, A. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Pandya, C.D.; Howell, K.R.; Pillai, A. Antioxidants as Potential Therapeutics for Neuropsychiatric Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and Oxidative Stress: Results from a Meta-Analysis of Observational Studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ferriani, L.O.; Silva, D.A.; Molina, M.D.C.B.; Mill, J.G.; Brunoni, A.R.; da Fonseca, M.d.J.M.; Moreno, A.B.; Benseñor, I.M.; de Aguiar, O.B.; Barreto, S.M.; et al. Associations of Depression and Intake of Antioxidants and Vitamin B Complex: Results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J. Affect. Disord. 2022, 297, 259–268. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of Oxidative Stress in Depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Yager, S.; Forlenza, M.J.; Miller, G.E. Depression and Oxidative Damage to Lipids. Psychoneuroendocrinology 2010, 35, 1356–1362. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Piperi, C.; Psarra, V.; Lea, R.W.; Kalofoutis, A. Increased Plasma Levels of 8-Iso-PGF2alpha and IL-6 in an Elderly Population with Depression. Psychiatry Res. 2008, 161, 59–66. [Google Scholar] [CrossRef]
- Forlenza, M.J.; Miller, G.E. Increased Serum Levels of 8-Hydroxy-2’-Deoxyguanosine in Clinical Depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Wang, D.; Gao, Y.; Wang, Q.; Wang, T.; Wang, G.; Peng, D.; Qiao, Y.; Zhou, J.; et al. Effects of Omega-3 PUFAs on Lipid Profiles and Antioxidant Response in Depressed Adolescents: A Metabolomic and Lipidomic Study. Redox Biol. 2025, 82, 103617. [Google Scholar] [CrossRef]
- Mazereeuw, G.; Lanctôt, K.L.; Chau, S.A.; Swardfager, W.; Herrmann, N. Effects of ω-3 Fatty Acids on Cognitive Performance: A Meta-Analysis. Neurobiol. Aging 2012, 33, e17–e29. [Google Scholar] [CrossRef]
- Baek, D.; Park, Y. Association between Erythrocyte N-3 Polyunsaturated Fatty Acids and Biomarkers of Inflammation and Oxidative Stress in Patients with and without Depression. Prostaglandins Leukot. Essent. Fat Acids 2013, 89, 291–296. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Harris, W.S.; Falcón, L.M.; Ordovás, J.M.; Lai, C.-Q.; Tucker, K.L. The Omega-3 Index Is Inversely Associated with Depressive Symptoms among Individuals with Elevated Oxidative Stress Biomarkers. J. Nutr. 2016, 146, 758–766. [Google Scholar] [CrossRef]
- Réus, G.Z.; Maciel, A.L.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; dos Santos, T.R.; Darabas, A.C.; Parzianello, M.; Matos, D.; Abatti, M.; et al. ω-3 and Folic Acid Act against Depressive-like Behavior and Oxidative Damage in the Brain of Rats Subjected to Early- or Late-Life Stress. Nutrition 2018, 53, 120–133. [Google Scholar] [CrossRef]
- Naveen, S.; Siddalingaswamy, M.; Singsit, D.; Khanum, F. Anti-depressive Effect of Polyphenols and Omega-3 Fatty Acid from Pomegranate Peel and Flax Seed in Mice Exposed to Chronic Mild Stress. Psychiatry Clin. Neurosci. 2013, 67, 501–508. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Tsao, Y.-Y.; Leung, Y.-M.; Su, K.-P. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for ω-3 Fatty Acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef]
- Golpour, P.; Nourbakhsh, M.; Mazaherioun, M.; Janani, L.; Nourbakhsh, M.; Yaghmaei, P. Improvement of NRF2 Gene Expression and Antioxidant Status in Patients with Type 2 Diabetes Mellitus after Supplementation with Omega-3 Polyunsaturated Fatty Acids: A Double-Blind Randomised Placebo-Controlled Clinical Trial. Diabetes Res. Clin. Pract. 2020, 162, 108120. [Google Scholar] [CrossRef]
- Trifunovic, S.; Stevanovic, I.; Milosevic, A.; Ristic, N.; Janjic, M.; Bjelobaba, I.; Savic, D.; Bozic, I.; Jakovljevic, M.; Tesovic, K.; et al. The Function of the Hypothalamic–Pituitary–Adrenal Axis During Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Front. Neurosci. 2021, 15, 649485. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Vieira, R.; et al. Dynamic Regulation of Mitochondrial Function by Glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the Hypothalamic-Pituitary-Adrenal Stress Axis Induces Cellular Oxidative Stress. Front. Neurosci. 2014, 8, 456. [Google Scholar] [CrossRef]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef]
- Guillot, N.; Caillet, E.; Laville, M.; Calzada, C.; Lagarde, M.; Véricel, E. Increasing Intakes of the Long-chain Ω-3 Docosahexaenoic Acid: Effects on Platelet Functions and Redox Status in Healthy Men. FASEB J. 2009, 23, 2909–2916. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Lallès, J.-P. Intestinal Alkaline Phosphatase: Novel Functions and Protective Effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A Host-Microbiome Interaction Mediates the Opposing Effects of Omega-6 and Omega-3 Fatty Acids on Metabolic Endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Alijani, S.; Hahn, A.; Harris, W.S.; Schuchardt, J.P. Bioavailability of EPA and DHA in Humans—A Comprehensive Review. Prog. Lipid Res. 2025, 97, 101318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cillis, F.; Begni, V.; Genini, P.; Leo, D.; Riva, M.A.; Cattaneo, A. Restoring Balance: The Role of Omega-3 Polyunsaturated Fatty Acids on the Gut–Brain Axis and Other Interconnected Biological Pathways to Improve Depression. Nutrients 2025, 17, 3426. https://doi.org/10.3390/nu17213426
De Cillis F, Begni V, Genini P, Leo D, Riva MA, Cattaneo A. Restoring Balance: The Role of Omega-3 Polyunsaturated Fatty Acids on the Gut–Brain Axis and Other Interconnected Biological Pathways to Improve Depression. Nutrients. 2025; 17(21):3426. https://doi.org/10.3390/nu17213426
Chicago/Turabian StyleDe Cillis, Floriana, Veronica Begni, Patrizia Genini, Daniele Leo, Marco Andrea Riva, and Annamaria Cattaneo. 2025. "Restoring Balance: The Role of Omega-3 Polyunsaturated Fatty Acids on the Gut–Brain Axis and Other Interconnected Biological Pathways to Improve Depression" Nutrients 17, no. 21: 3426. https://doi.org/10.3390/nu17213426
APA StyleDe Cillis, F., Begni, V., Genini, P., Leo, D., Riva, M. A., & Cattaneo, A. (2025). Restoring Balance: The Role of Omega-3 Polyunsaturated Fatty Acids on the Gut–Brain Axis and Other Interconnected Biological Pathways to Improve Depression. Nutrients, 17(21), 3426. https://doi.org/10.3390/nu17213426







