Hyocholic Acid Species as the Key Modulator for Cecal Epithelial Homeostasis in Low-Birth-Weight Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Total Bile Acid Levels
2.3. Histological Analysis
2.4. Real-Time Quantitative PCR
2.5. 16S rRNA Gene Sequencing
2.6. RNA-Seq and Data Analysis
2.7. Quantitative Analysis of Cecal Metabolites
2.8. Western Blot (WB) Analysis
2.9. Immunofluorescence Staining of Cecal Tissue Sections
2.10. Statistical Analysis
3. Results
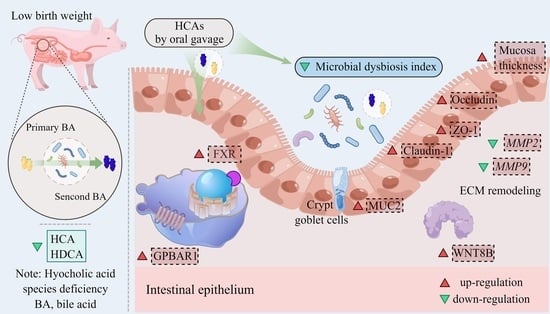
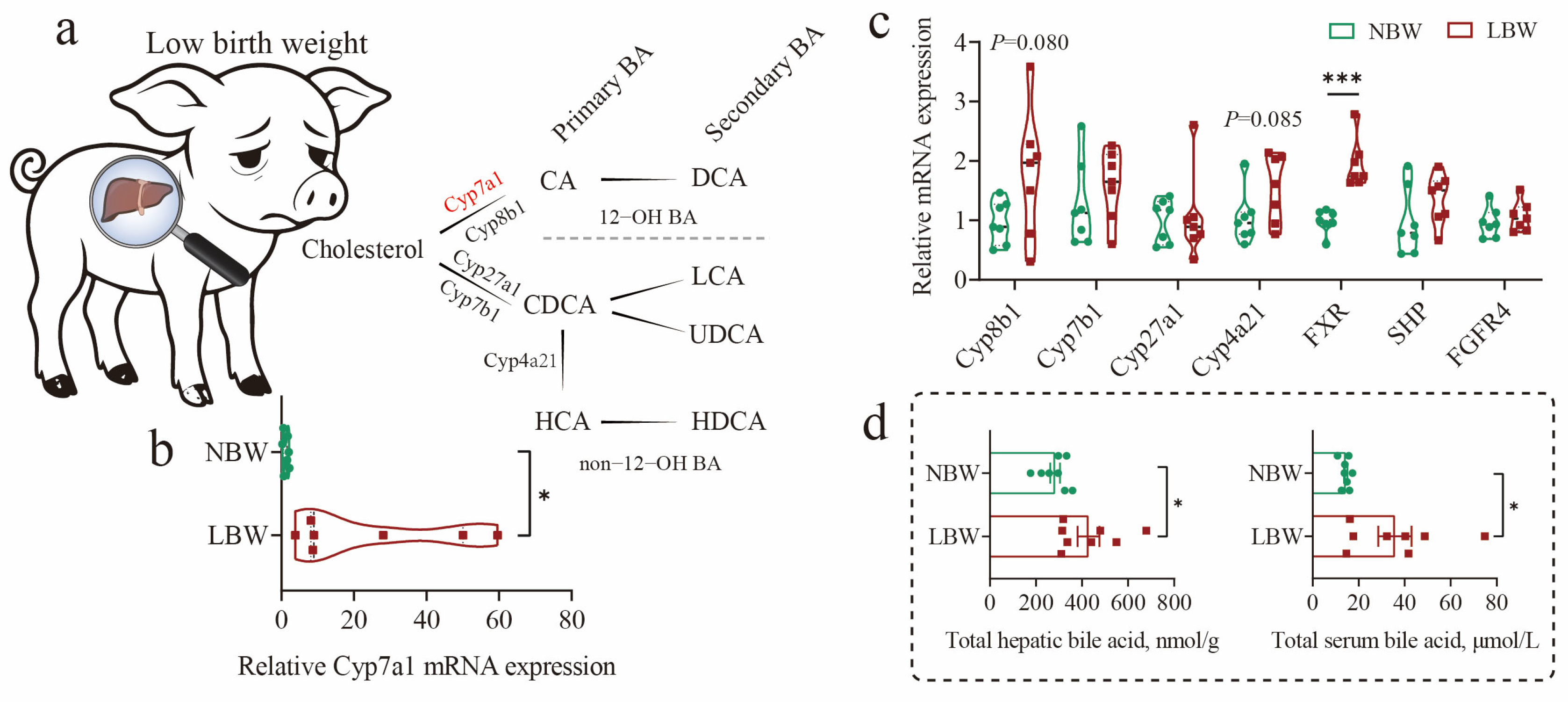
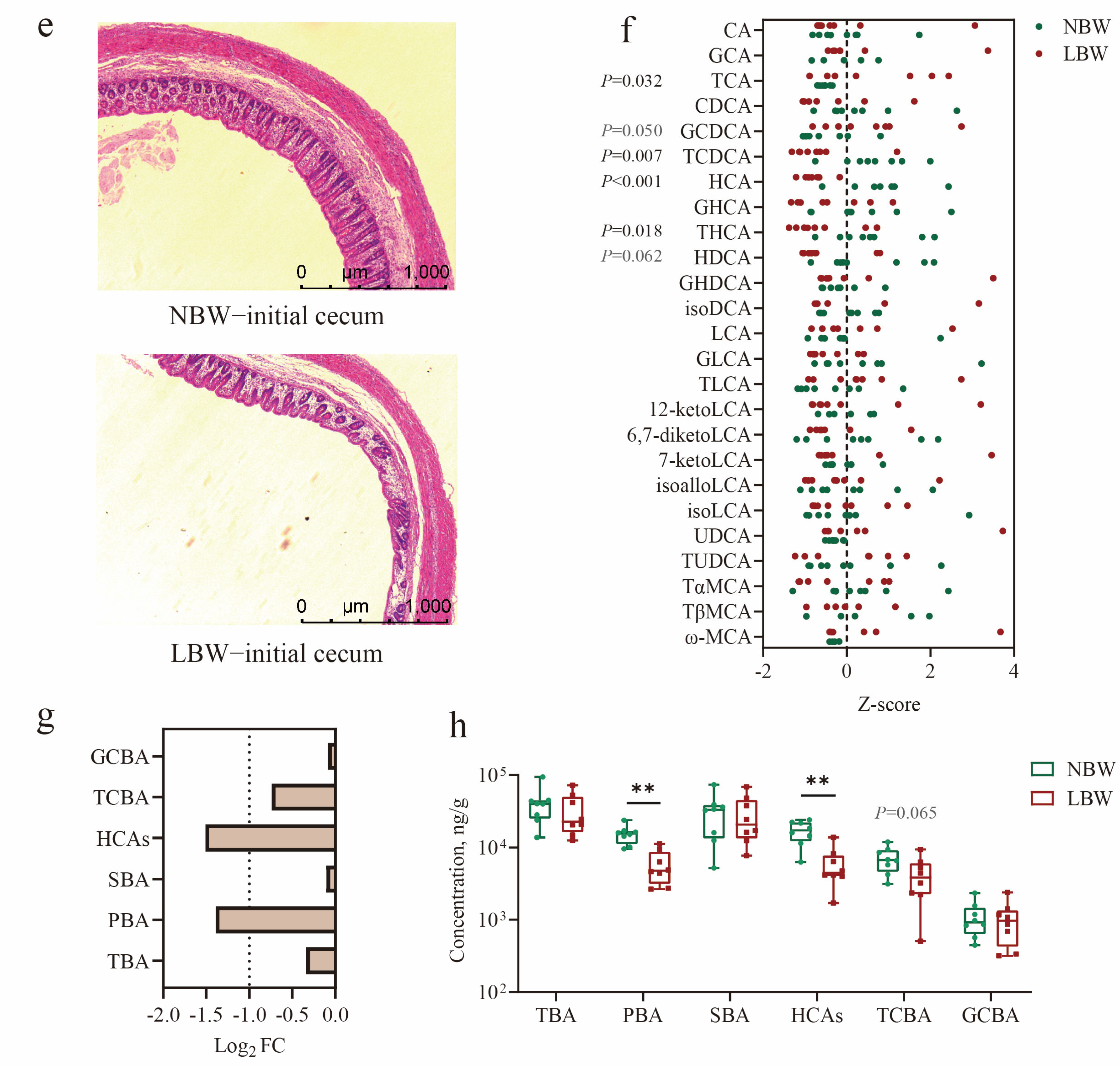
3.1. HCAs Deficiency Was Found in the Cecum of LBW Piglets
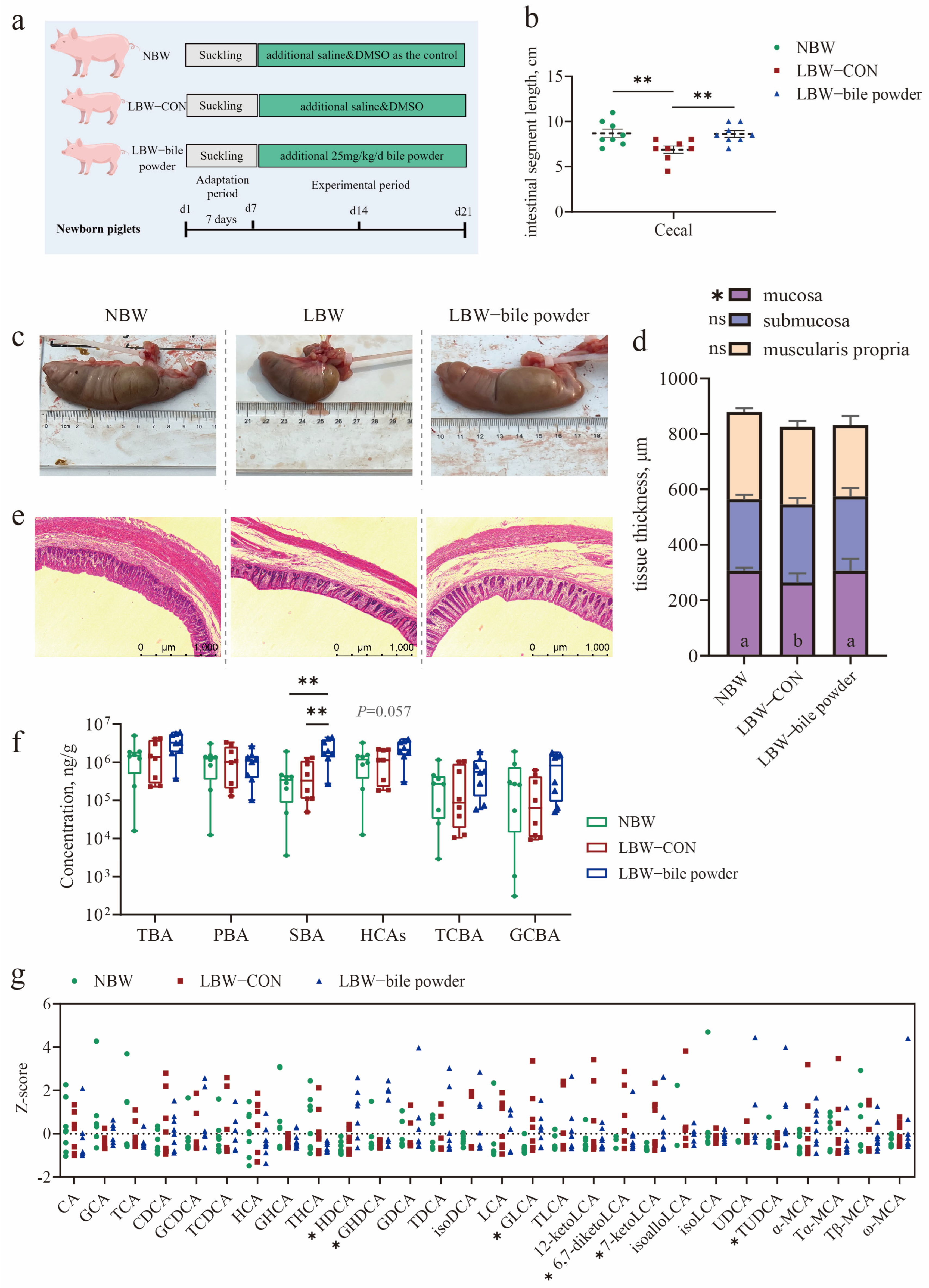
3.2. Oral Gavage of HCAs Restores the Cecal Length and Mucosal Thickness of LBW Piglets to Healthy Levels
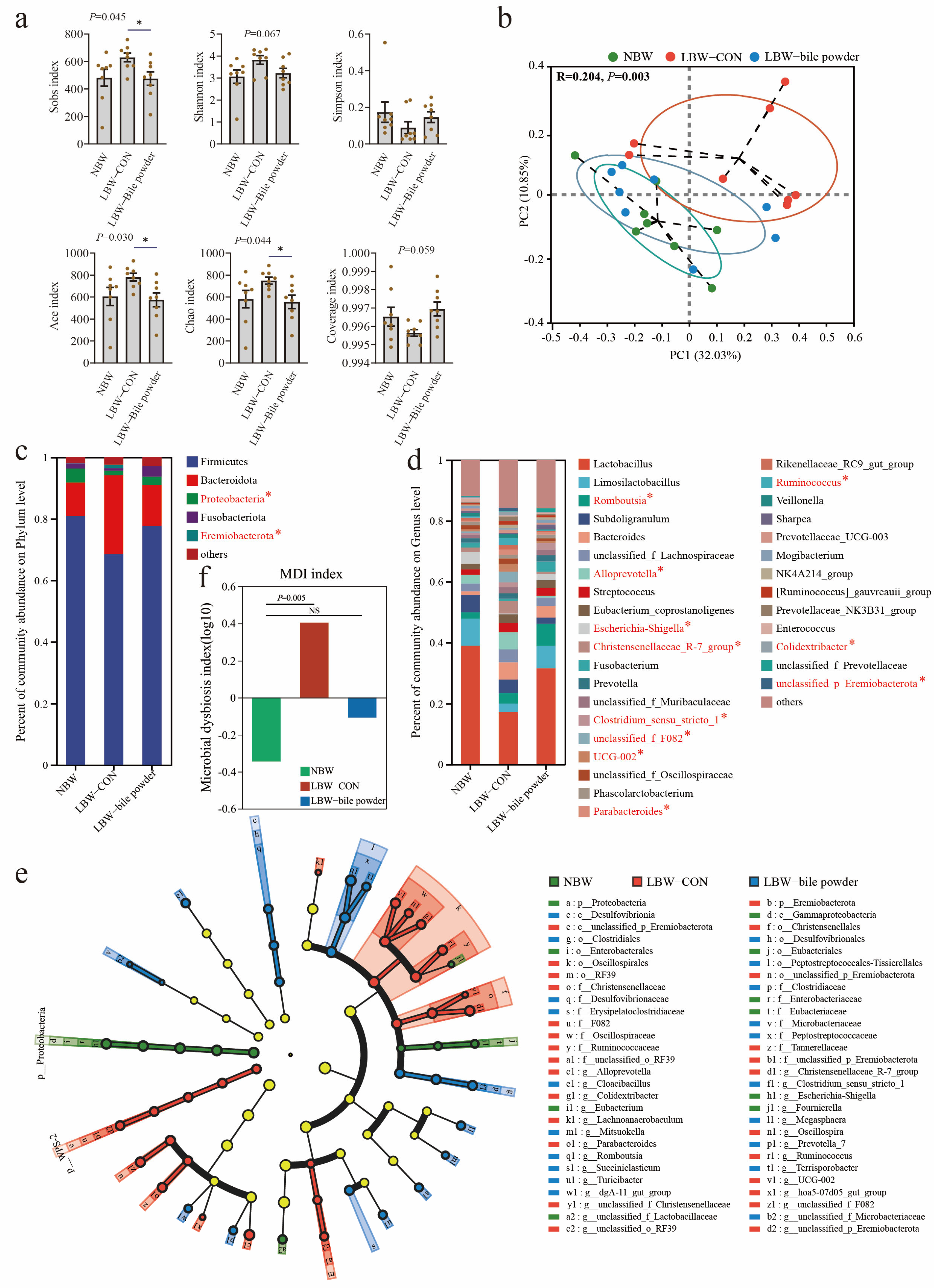
3.3. Supplementation of HCAs Induces Alterations in Gut Microbial Ecology and Composition
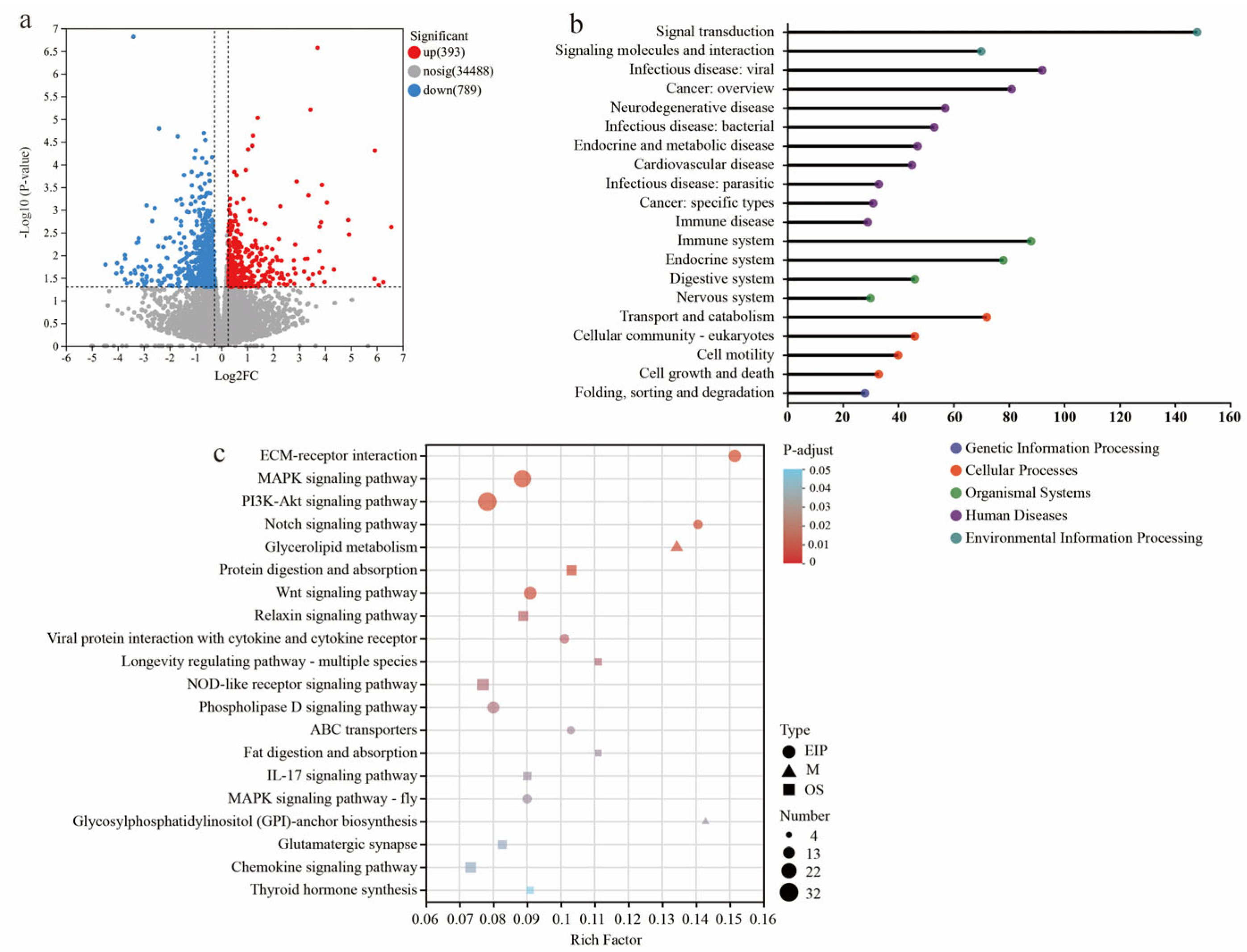
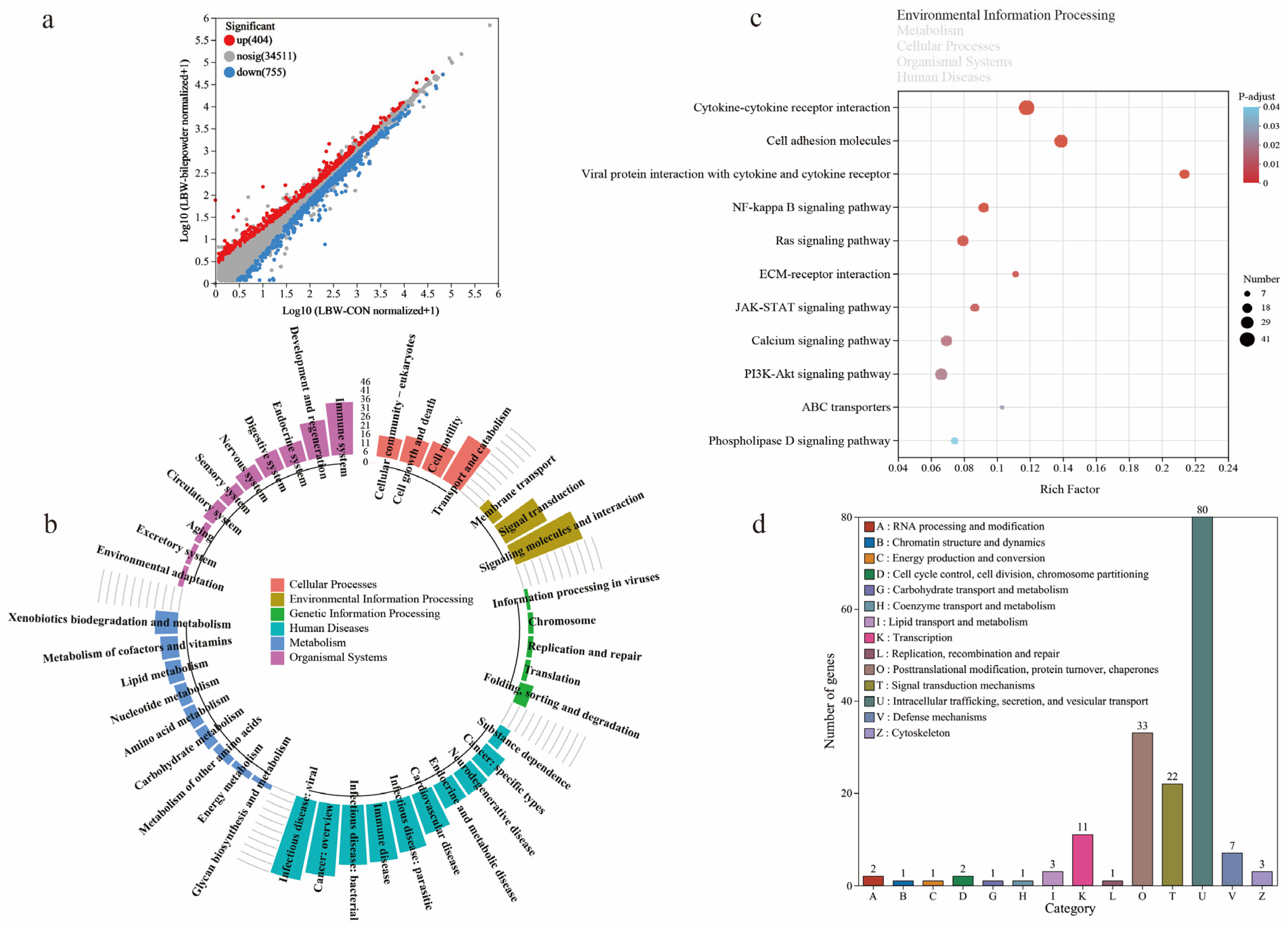
3.4. Transcriptome Profiling Reveals Changes in Cecal Barrier Function in LBW Piglets
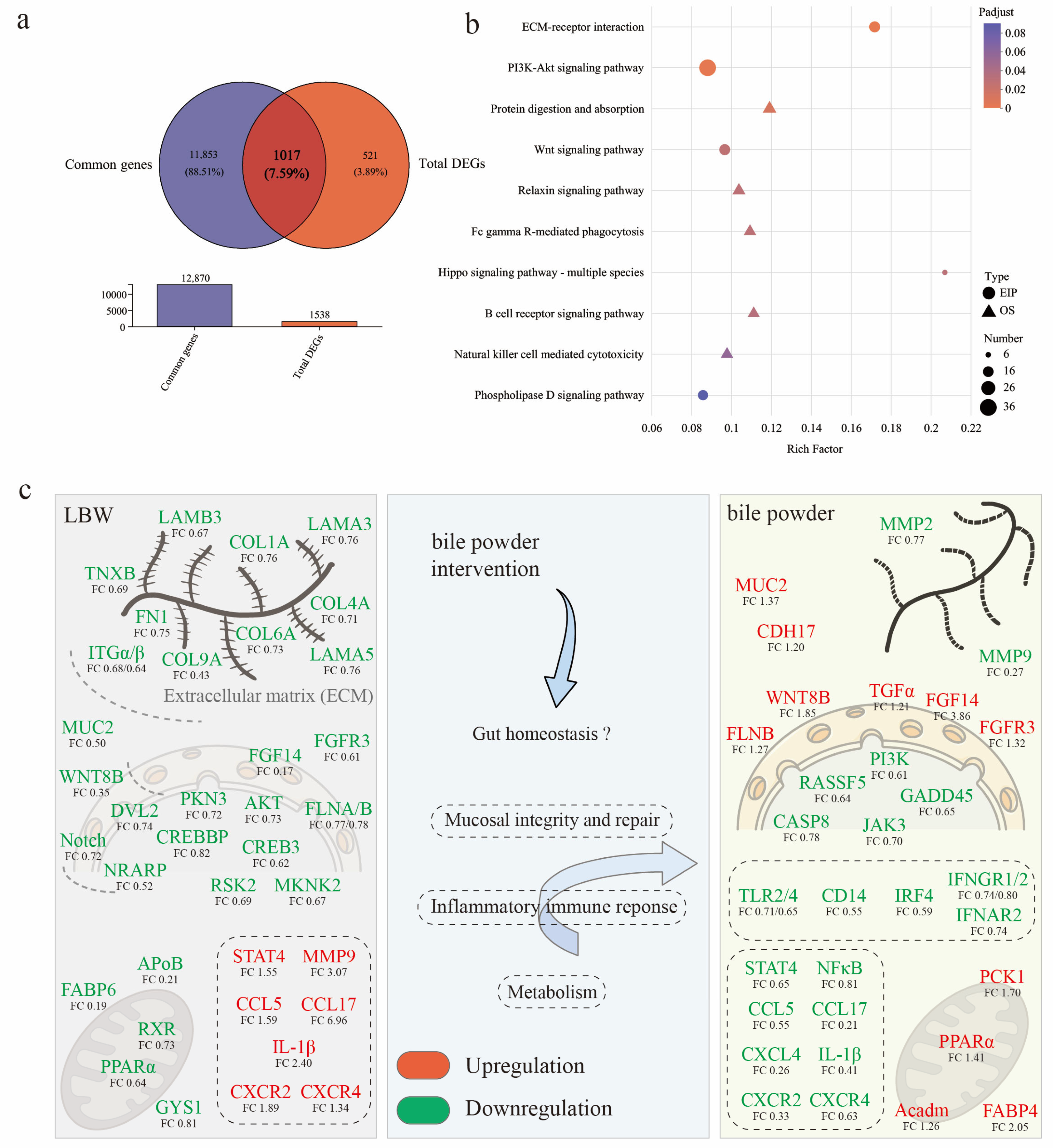
3.5. Potential Mechanisms Underlying Intestinal Barrier Function Alterations in LBW Piglets
3.6. Supplementation of HCAs Improves Epithelial Barrier Function in the Cecal Mucosa of LBW Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | bile acid |
| CYP7A1 | cholesterol 7 alpha-hydroxylase |
| CYP7B1 | oxysterol 7 alpha-hydroxylase |
| CYP8B1 | sterol 12 alpha-hydroxylase |
| CYP27A1 | sterol 27-hydroxylase |
| CYP4A21 | taurochenodeoxycholic acid 6alpha-hydroxylase |
| DEGs | differentially expressed genes |
| ECM | extracellular matrix |
| FXR | farnesoid x receptor |
| GCBA | glycine-conjugated bile acid |
| GC-MS | gas chromatography–mass spectrometry |
| GPBAR1 | G-protein-coupled bile acid receptor 1 |
| HCA | hyocholic acid |
| HDCA | hyodeoxycholic acid |
| LBW | low birth weight |
| LCA | lithocholic acid |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| MUC2 | mucin 2 |
| NBW | normal birth weight |
| OTU | operational taxonomic unit |
| PBA | primary bile acid |
| PCoA | principal coordinate analysis |
| SBA | secondary bile acid |
| SCFA | short-chain fatty acid |
| TCBA | taurine-conjugated bile acid |
| UDCA | ursodeoxycholic acid |
| WNT8B | Wnt family member 8B |
| ZO-1 | zonula occluden-1 |
References
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: A systematic review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr. Res. Rev. 2010, 23, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Borghesi, A.; Tzialla, C.; Stronati, M. IUGR and infections. Early Hum. Dev. 2014, 90, S42–S44. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Chen, Y.; Jia, P.; Ji, S.; Zhang, Y.; Wang, T. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 70. [Google Scholar] [CrossRef]
- Zhang, X.; Yun, Y.; Lai, Z.; Ji, S.; Yu, G.; Xie, Z.; Zhang, H.; Zhong, X.; Wang, T.; Zhang, L. Supplemental Clostridium butyricum modulates lipid metabolism by reshaping the gut microbiota composition and bile acid profile in IUGR suckling piglets. J. Anim. Sci. Biotechnol. 2023, 14, 36. [Google Scholar] [CrossRef]
- Monte, M.J.; Marin, J.J.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Banan, B.; Antoun, J.; Xiong, Y.; Guo, Y.; Ping, J.; Alikhan, M.; Clements, B.A.; Abumrad, N.N.; Flynn, C.R. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 2019, 156, 1041–1051.e4. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Ye, J.; Zhang, F.; Su, H.; Yang, X.; He, H.; Liu, F.; Zhu, X.; Wang, L.; Gao, P.; et al. Chenodeoxycholic Acid (CDCA) protects against the lipopolysaccharide-induced impairment of the intestinal epithelial barrier function via the FXR-MLCK pathway. J. Agric. Food Chem. 2019, 67, 8868–8874. [Google Scholar] [CrossRef]
- Xie, Q.; Khaoustov, V.I.; Chung, C.C.; Sohn, J.; Krishnan, B.; Lewis, D.E.; Yoffe, B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 2002, 36, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Li, Q.; Cai, L.; Wang, C.; Lei, X. Chemoproteomic Profiling of bile acid interacting proteins. ACS Cent. Sci. 2017, 3, 501–509. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile acids and their receptors in metabolic disorders. Prog. Lipid Res. 2021, 82, 101094. [Google Scholar] [CrossRef]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The microbiome modulating activity of bile acids. Gut Microbes 2020, 11, 979–996. [Google Scholar] [CrossRef]
- Amdi, C.; Klarlund, M.V.; Hales, J.; Thymann, T.; Hansen, C.F. Intrauterine growth-restricted piglets have similar gastric emptying rates but lower rectal temperatures and altered blood values when compared with normal-weight piglets at birth. J. Anim. Sci. 2016, 94, 4583–4590. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.; Ai, C.; Huang, S.; Zhang, H.; Guo, X.; Wang, W.; Hua, W.; Bi, H.; Wang, H. Serum metabolic profile characteristics of offspring rats before and after birth caused by prenatal caffeine exposure. Toxicology 2019, 427, 152302. [Google Scholar] [CrossRef]
- Liu, Y.; Azad, M.A.K.; Zhang, W.; Xiong, L.; Blachier, F.; Yu, Z.; Kong, X. Intrauterine growth retardation affects liver bile acid metabolism in growing pigs: Effects associated with the changes of colonic bile acid derivatives. J. Anim. Sci. Biotechnol. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhong, R.; Wu, W.; Luo, C.; Meng, Q.; Gao, Q.; Zhao, Y.; Chen, L.; Zhang, S.; Zhao, X.; et al. Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model. Microbiome 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Wu, Y.; Zhang, X.; Lu, D.; Han, D.; Zhao, J.; Zheng, X.; Zhang, S.; Ye, H.; Lian, S.; et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, Y.; He, J.; Yun, Y.; Shen, M.; Gan, Z.; Zhang, L.; Wang, T. Dietary dihydroartemisinin supplementation alleviates intestinal inflammatory injury through TLR4/NOD/NF-κB signaling pathway in weaned piglets with intrauterine growth retardation. Anim. Nutr. 2021, 7, 667–678. [Google Scholar] [CrossRef]
- Tao, S.; Bai, Y.; Li, T.; Li, N.; Wang, J. Original low birth weight deteriorates the hindgut epithelial barrier function in pigs at the growing stage. FASEB J. 2019, 33, 9897–9912. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef]
- Crosignani, A.; Setchell, K.D.; Invernizzi, P.; Larghi, A.; Rodrigues, C.M.; Podda, M. Clinical pharmacokinetics of therapeutic bile acids. Clin. Pharmacokinet. 1996, 30, 333–358. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Deng, F.; Yin, C.; Luo, C.; Xu, Y.; Chen, Y.; Zhong, R.; Tang, S.; Zhang, H.; Chen, L. Lactobacillus reuteri-mediated dietary xylooligosaccharides enhance jejunal cell survival via suppression of oxygen-dependent apoptotic processes in a pig model. iMeta 2025, 4, e70080. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wu, W.; Xie, J.; Zhang, H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016, 7, 4644–4654. [Google Scholar] [CrossRef]
- Yin, C.; Wen, X.; Dang, G.; Zhong, R.; Meng, Q.; Feng, X.; Liu, L.; Wu, S.; He, J.; Chen, L.; et al. Modulation of pectin on intestinal barrier function via changes in microbial functional potential and bile acid metabolism. J. Nutr. Biochem. 2024, 124, 109491. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, F.; Fu, Y.; Yi, X.; Feng, S.; Liu, Z.; Deng, D.; Yang, Q.; Yu, M.; Zhu, C.; et al. Tauroursodeoxycholic acid (TUDCA) improves intestinal barrier function associated with TGR5-MLCK pathway and the alteration of serum metabolites and gut bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell 2022, 29, 1366–1381. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Dai, Z.; Li, T.; Han, D.; Wang, J. Characterization of the early life microbiota development and predominant Lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front. Microbiol. 2019, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Olszewski, J.; Zabielski, R.; Skrzypek, T.; Matyba, P.; Wierzbicka, M.; Adamski, A.; Grzesiuk, E.; Sady, M.; Gajewski, Z.; Ferenc, K. Differences in intestinal barrier development between intrauterine growth restricted and normal birth weight piglets. Animals 2021, 11, 990. [Google Scholar] [CrossRef]
- Yun, Y.; Ji, S.; Yu, G.; Jia, P.; Niu, Y.; Zhang, H.; Zhang, X.; Wang, T.; Zhang, L. Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J. Anim. Sci. 2021, 99, skab255. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri CCFM8631 alleviates hypercholesterolaemia caused by the paigen atherogenic diet by regulating the gut microbiota. Nutrients 2022, 14, 1272. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Zhou, M.; Zhao, J.; Liu, H.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Zhang, Q.; Zhang, X.; Zhang, N.; Shen, Z.; Pang, Y.; Lin, X.; Liu, D.; Yang, X. Hyocholic acid: A novel therapeutic strategy for metabolic syndrome. Innov. Life 2024, 2, 100093. [Google Scholar] [CrossRef]
- Theriot, C.M.; Bowman, A.A.; Young, V.B. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 2016, 1, e00045-15. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Kinross, J.; Gibson, G.R.; Burcelin, R.; Jia, W.; Pettersson, S.; Nicholson, J.K. Therapeutic modulation of microbiota-host metabolic interactions. Sci. Transl. Med. 2012, 4, 137rv6. [Google Scholar] [CrossRef]
- Che, L.; Hu, L.; Zhou, Q.; Peng, X.; Liu, Y.; Luo, Y.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Microbial insight into dietary protein source affects intestinal function of pigs with intrauterine growth retardation. Eur. J. Nutr. 2020, 59, 327–344. [Google Scholar] [CrossRef]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum β-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef]
- Liu, Y.; Azad, M.A.K.; Ding, S.; Zhu, Q.; Blachier, F.; Yu, Z.; Gao, H.; Kong, X. Dietary bile acid supplementation in weaned piglets with intrauterine growth retardation improves colonic microbiota, metabolic activity, and epithelial function. J. Anim. Sci. Biotechnol. 2023, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhong, R.; Zhang, W.; Liu, L.; Chen, L.; Zhang, H. The potential of bile acids as biomarkers for metabolic disorders. Int. J. Mol. Sci. 2023, 24, 12123. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Jiang, R.; Zhao, A.; Wu, Q.; Kuang, J.; Sun, D.; Ren, Z.; Li, M.; Zhao, M.; et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021, 33, 791–803.e797. [Google Scholar] [CrossRef]
- Chen, X.; Lou, G.; Meng, Z.; Huang, W. TGR5: A novel target for weight maintenance and glucose metabolism. Exp. Diabetes Res. 2011, 2011, 853501. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology 2020, 159, 956–968.e9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D.; et al. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Li, Y.; Han, D.; Pi, Y.; Whitmore, M.A.; Lu, X.; Zhang, G.; Zheng, J.; et al. Strain specificity of lactobacilli with promoted colonization by galactooligosaccharides administration in protecting intestinal barriers during Salmonella infection. J. Adv. Res. 2024, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- Englund, J.I.; Ritchie, A.; Blaas, L.; Cojoc, H.; Pentinmikko, N.; Döhla, J.; Iqbal, S.; Patarroyo, M.; Katajisto, P. Laminin alpha 5 regulates mammary gland remodeling through luminal cell differentiation and Wnt4-mediated epithelial crosstalk. Development 2021, 148, dev199281. [Google Scholar] [CrossRef]
- Ugalde-Silva, P.; Gonzalez-Lugo, O.; Navarro-Garcia, F. Tight junction disruption induced by type 3 secretion system effectors injected by enteropathogenic and enterohemorrhagic Escherichia coli. Front. Cell. Infect. Microbiol. 2016, 6, 87. [Google Scholar] [CrossRef]

| Items (Unit: kg) | Groups | p-Value | ||
|---|---|---|---|---|
| NBW | LBW-CON | LBW-Bile Powder | ||
| Initial BW | 1.563 ± 0.052 a | 0.950 ± 0.120 b | 0.925 ± 0.116 b | p < 0.0001 |
| BW-7d | 2.600 ± 0.177 a | 1.550 ± 0.267 b | 1.600 ± 0.293 b | p < 0.0001 |
| BW-14d | 4.375 ± 0.410 a | 3.013 ± 0.533 b | 3.513 ± 0.673 b | p = 0.0003 |
| BW-21d | 6.300 ± 0.760 a | 4.338 ± 0.703 b | 4.538 ± 0.769 b | p < 0.0001 |
| ADG | 0.264 ± 0.052 a | 0.199 ± 0.039 b | 0.210 ± 0.039 b | p = 0.016 |
| Items (Unit: μmol/g) | Groups | p-Value | ||
|---|---|---|---|---|
| NBW | LBW-CON | LBW-Bile Powder | ||
| Acetic acid | 145.212 ± 85.099 | 108.132 ± 23.934 | 90.955 ± 36.764 | p = 0.159 |
| Propionic acid | 26.678 ± 16.485 | 24.071 ± 6.685 | 19.564 ± 8.656 | p = 0.465 |
| Butyric acid | 15.542 ± 8.733 | 12.171 ± 4.342 | 13.238 ± 6.264 | p = 0.596 |
| Isovaleric acid | 6.805 ± 4.207 | 6.109 ± 1.527 | 4.755 ± 1.840 | p = 0.347 |
| Valeric acid | 6.082 ± 4.420 | 5.127 ± 1.243 | 4.406 ± 1.562 | p = 0.498 |
| Total SCFAs | 200.319 ± 118.062 | 155.610 ± 34.882 | 132.918 ± 53.471 | p = 0.232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Liu, X.; Fang, W.; Meng, Q.; Feng, X.; Zhang, W.; Dang, G.; Zhong, R.; Chen, L.; Wang, Z.; et al. Hyocholic Acid Species as the Key Modulator for Cecal Epithelial Homeostasis in Low-Birth-Weight Piglets. Nutrients 2025, 17, 3415. https://doi.org/10.3390/nu17213415
Yin C, Liu X, Fang W, Meng Q, Feng X, Zhang W, Dang G, Zhong R, Chen L, Wang Z, et al. Hyocholic Acid Species as the Key Modulator for Cecal Epithelial Homeostasis in Low-Birth-Weight Piglets. Nutrients. 2025; 17(21):3415. https://doi.org/10.3390/nu17213415
Chicago/Turabian StyleYin, Chang, Xuan Liu, Wei Fang, Qingshi Meng, Xiaohui Feng, Weidong Zhang, Guoqi Dang, Ruqing Zhong, Liang Chen, Zirong Wang, and et al. 2025. "Hyocholic Acid Species as the Key Modulator for Cecal Epithelial Homeostasis in Low-Birth-Weight Piglets" Nutrients 17, no. 21: 3415. https://doi.org/10.3390/nu17213415
APA StyleYin, C., Liu, X., Fang, W., Meng, Q., Feng, X., Zhang, W., Dang, G., Zhong, R., Chen, L., Wang, Z., & Zhang, H. (2025). Hyocholic Acid Species as the Key Modulator for Cecal Epithelial Homeostasis in Low-Birth-Weight Piglets. Nutrients, 17(21), 3415. https://doi.org/10.3390/nu17213415