Effect of Vitamin D Supplementation on Oxidative Stress Biomarkers in Women Following Religious or Intermittent Fasting Patterns
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Anthropometric Measurements and Biochemical Analysis
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflug. Arch. 2019, 471, 83–98. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Karras, S.N.; Michalakis, K.; Tekos, F.; Skaperda, Z.; Vardakas, P.; Ziakas, P.D.; Kypraiou, M.; Anemoulis, M.; Vlastos, A.; Tzimagiorgis, G.; et al. Effects of Religious Fasting on Markers of Oxidative Status in Vitamin D-Deficient and Overweight Orthodox Nuns versus Implementation of Time-Restricted Eating in Lay Women from Central and Northern Greece. Nutrients 2024, 16, 3300. [Google Scholar] [CrossRef]
- Giaginis, C.; Mantzorou, M.; Papadopoulou, S.K.; Gialeli, M.; Troumbis, A.Y.; Vasios, G.K. Christian Orthodox Fasting as a Traditional Diet with Low Content of Refined Carbohydrates That Promotes Human Health: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1225. [Google Scholar] [CrossRef]
- Kokkinopoulou, A.; Rodopaios, N.E.; Koulouri, A.-A.; Vasara, E.; Papadopoulou, S.K.; Skepastianos, P.; Dermitzakis, E.; Hassapidou, M.; Kafatos, A.G. Impact of Christian Orthodox Church Fasting on Metabolic Syndrome Components in Adults Aged 18–49 Years. Nutrients 2023, 15, 1755. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Desmet, L.; Depoortere, I. Time-restricted eating for chronodisruption-related chronic diseases. Acta Physiol. 2023, 239, e14027. [Google Scholar] [CrossRef]
- Gabel, K.; Cienfuegos, S.; Kalam, F.; Ezpeleta, M.; Varady, K.A. Time-Restricted Eating to Improve Cardiovascular Health. Curr. Atheroscler. Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Trabelsi, K.; Ammar, A.; Boujelbane, M.A.; Puce, L.; Garbarino, S.; Scoditti, E.; Boukhris, O.; Khanfir, S.; Clark, C.C.T.; Glenn, J.M.; et al. Religious fasting and its impacts on individual, public, and planetary health: Fasting as a “religious health asset” for a healthier, more equitable, and sustainable society. Front. Nutr. 2022, 9, 1036496. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Liu, Y.; Jiang, H.; Chen, W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2021, 179, 109003. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Michalakis, K.; Kypraiou, M.; Vlastos, A.; Anemoulis, M.; Koukoulis, G.; Mouslech, Z.; Talidis, F.; Haitoglou, C.; Michos, G.; et al. Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population. Nutrients 2025, 17, 1656. [Google Scholar] [CrossRef] [PubMed]
- Ignacio-Mejía, I.; Bandala, C.; González-Zamora, J.F.; Chavez-Galan, L.; Buendia-Roldan, I.; Pérez-Torres, K.; Rodríguez-Díaz, M.Z.; Pacheco-Tobón, D.X.; Quintero-Fabián, S.; Vargas-Hernández, M.A.; et al. Association of Vitamin D Supplementation with Glutathione Peroxidase (GPx) Activity, Interleukine-6 (IL-6) Levels, and Anxiety and Depression Scores in Patients with Post-COVID-19 Condition. Int. J. Mol. Sci. 2025, 26, 4582. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Yin, Z.; Gao, J.; Wang, W.; Lu, F.; Qin, H.-M.; Mao, S. Redesigning CYP109E1 for Improving Catalytic Performance in 25-Hydroxyvitamin D3 Synthesis Through Synergistic Enhancement of Electron Transfer and NADPH Regeneration. ACS Synth. Biol. 2025, 14, 1240–1249. [Google Scholar] [CrossRef]
- McAllister, M.J.; Gonzalez, A.E.; Waldman, H.S. Impact of time restricted feeding on markers of cardiometabolic health and oxidative stress in resistance-trained firefighters. J. Strength Cond. Res. 2020, 36, 2515–2522. [Google Scholar] [CrossRef]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar] [PubMed]
- Karras, S.N.; Persynaki, A.; Petróczi, A.; Barkans, E.; Mulrooney, H.; Kypraiou, M.; Tzotzas, T.; Tziomalos, K.; Kotsa, K.; Tsioudas, A.; et al. Health benefits and consequences of the Eastern Orthodox fasting in monks of Mount Athos: A cross-sectional study. Eur. J. Clin. Nutr. 2017, 71, 743–749. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Zebekakis, P.; Makedou, K.; Kotsa, K. Different patterns of changes in free 25-hydroxyvitamin D concentrations during intermittent fasting among meat eaters and non-meat eaters and correlations with amino acid intake. Int. J. Food Sci. Nutr. 2023, 74, 257–267. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Popovic, D.S.; Adamidou, L.; Karalazou, P.; Thisiadou, K.; Zebekakis, P.; Makedou, K.; Kotsa, K. A Mediterranean Eating Pattern Combining Energy and Time-Restricted Eating Improves Vaspin and Omentin Concentrations Compared to Intermittent Fasting in Overweight Individuals. Nutrients 2023, 15, 5058. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Dimakopoulos, G.; Popovic, D.S.; Kotsa, K. Changes in dietary intake of aspartic acid during and after intermittent fasting correlate with an improvement in fasting glucose in overweight individuals. J. Diabetes 2023, 15, 181–184. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Bloomer, R.J. The impact of religious fasting on human health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef]
- Sarri, K.O.; Linardakis, M.K.; Bervanaki, F.N.; Tzanakis, N.E.; Kafatos, A.G. Greek Orthodox fasting rituals: A hidden characteristic of the Mediterranean diet of Crete. Br. J. Nutr. 2004, 92, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sarri, K.O.; Tzanakis, N.E.; Linardakis, M.K.; Mamalakis, G.D.; Kafatos, A.G. Effects of Greek Orthodox Christian Church fasting on serum lipids and obesity. BMC Public Health 2003, 3, 16. [Google Scholar] [CrossRef]
- Sarri, K.; Linardakis, M.; Codrington, C.; Kafatos, A. Does the periodic vegetarianism of Greek Orthodox Christians benefit blood pressure? Prev. Med. 2007, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Greek National Dietary Guidelines for Adults. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/greece/en/ (accessed on 25 July 2024).
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Petróczi, A.; Folkerts, D.; Kypraiou, M.; Mulrooney, H.; Naughton, D.P.; Persynaki, A.; Zebekakis, P.; Skoutas, D.; et al. Christian Orthodox fasting in practice: A comparative evaluation between Greek Orthodox general population fasters and Athonian monks. Nutrition 2019, 59, 69–76. [Google Scholar] [CrossRef]
- WHO Global Database on Body Mass Index. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 5 February 2016).
- Tanita Academy Understanding Your Measurements. Available online: http://tanita.eu/ (accessed on 25 May 2018).
- Karras, S.N.; Michalakis, K.; Katsiki, N.; Kypraiou, M.; Vlastos, A.; Anemoulis, M.; Koukoulis, G.; Mouslech, Z.; Talidis, F.; Tzimagiorgis, G.; et al. Interrelations of Leptin and Interleukin-6 in Vitamin D Deficient and Overweight Orthodox Nuns from Northern Greece: A Pilot Study. Nutrients 2025, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.; Murthy, S.; Krishna, D.; Prabhakar, M.C. Role of Free Radicals and Antioxidants in Tuberculosis Patients. Indian J. Tuberc. 2004, 51, 213–218. [Google Scholar]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers 2016, 21, 208–217. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Keles, M.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Brodsky, I.G.; Chatterjee, R.; Kim, S.H.; Pratley, R.E.; Staten, M.A.; Pittas, A.G.; D2d Research Group. Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. J. Clin. Endocrinol. Metab. 2022, 107, 230–240. [Google Scholar] [CrossRef]
- Pusceddu, I.; Farrell, C.-J.L.; Di Pierro, A.M.; Jani, E.; Herrmann, W.; Herrmann, M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin. Chem. Lab. Med. 2015, 53, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- De Leon, J.A.D.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 12, e61122. [Google Scholar] [CrossRef]
- Oyeleye, I.S.; Bolarinde, A.A.; Ademiluyi, A.O.; Oboh, G.; Ojo, O.R. Bitter Leaf (Vernonia amygdalina) and Siam Weed (Chromolaena odorata) Aqueous Extracts Alleviate Testicular Damage Induced by Plasmodium berghei in Male Mice via Modulation of Oxidative Stress Pathways. J. Ethnopharmacol. 2025, 353, 120326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, Q.; Li, D.; Chen, L.; Zhang, H.; Yi, B. Vitamin D receptor alleviates lipid peroxidation in diabetic nephropathy by regulating ACLY/Nrf2/Keap1 pathway. FASEB J. 2024, 38, e70060, Erratum in FASEB J. 2025, 39, e70343. [Google Scholar] [CrossRef]
- Zhan, D.; Zhao, J.; Shi, Q.; Lou, J.; Wang, W. 25-hydroxyvitamin D3 inhibits oxidative stress and ferroptosis in retinal microvascular endothelial cells induced by high glucose through down-regulation of miR-93. BMC Ophthalmol. 2023, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, R.; Liu, H.; Li, L.; Chen, B.; Jia, Q.; Wang, L.; Ma, R.; Tian, S.; Wang, M.; et al. Aqueous Extract of Mori Folium Exerts Bone Protective Effect Through Regulation of Calcium and Redox Homeostasis via PTH/VDR/CaBP and AGEs/RAGE/Nox4/NF-κB Signaling in Diabetic Rats. Front. Pharmacol. 2018, 9, 1239. [Google Scholar] [CrossRef]
- Cui, C.; Song, S.; Cui, J.; Feng, Y.; Gao, J.; Jiang, P. Vitamin D Receptor Activation Influences NADPH Oxidase (NOX2) Activity and Protects against Neurological Deficits and Apoptosis in a Rat Model of Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2017, 2017, 9245702. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Antanaviciute, E.M.; Drzewoski, J.; Majsterek, I.; Śliwińska, A. Vitamin D Protects Pancreatic Cancer (PC) Cells from Death and DNA Damage Induced by Oxidative Stress. Antioxidants 2025, 14, 1101. [Google Scholar] [CrossRef]
- Hoseini, R.; Rahim, H.A.; Ahmed, J.K. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: How aerobic training and vitamin D improve T2DM. BMC Complement. Med. Ther. 2022, 22, 165. [Google Scholar] [CrossRef]
- Campolo, J.; Corradi, E.; Parolini, M.; Di Guglielmo, M.L.; Rizzardi, A.; Dellanoce, C.; Tarlarini, P.; Cattaneo, M.; Scioscioli, E.; Trivella, M.G.; et al. Gender-Specific Behaviour in Obesity Stages I-II: Imbalance of Aminothiol Status and Adipomyokine Profile in Subjects with Different Insulin Resistance Severity. Oxid. Med. Cell. Longev. 2021, 2021, 9713582. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Huber, F.; Oláh, B.; Neogrády, Z.; Mátis, G. Redox metabolic disruptions in the honey bee brain following acute exposure to the pyrethroid deltamethrin. Sci. Rep. 2025, 15, 28322. [Google Scholar] [CrossRef]
- Uzun, L.; Kutlu, R.; Ataseven, A.; Aydemir, F.H.Y. Total oxidant capacity, total antioxidant capacity, ischemic modified albumin, microRNA levels, and their relationship with psoriasis area and severity index. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 501–509. [Google Scholar] [CrossRef]
- Brighenti, F.; Valtueña, S.; Pellegrini, N.; Ardigò, D.; Del Rio, D.; Salvatore, S.; Piatti, P.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef]
- Álvarez, C.M.M.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. Oxidative stress in animal models of obesity caused by hypercaloric diets: A systematic review. Life Sci. 2023, 331, 122019. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, W.; You, L.; Zhang, F.; Cao, X.; Feng, J.; Shen, D.; Li, Y.; Wang, Y.; Ji, C.; et al. Age-induced oxidative stress impairs adi-pogenesis and thermogenesis in brown fat. FEBS J. 2019, 286, 2753–2768. [Google Scholar] [CrossRef]
- Kolusari, A.; Kurdoglu, M.; Yildizhan, R.; Adali, E.; Edirne, T.; Cebi, A.; Demir, H.; Yoruk, I. Catalase activity, serum trace element and heavy metal concentrations, and Vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008, 36, 1335–1341. [Google Scholar] [CrossRef]
- Dahiri, B.; Hinojosa, M.G.; Carbonero-Aguilar, P.; Cerrillos, L.; Ostos, R.; Bautista, J.; Moreno, I. Assessment of the oxidative status in mother-child couples from Seville (Spain): A prospective cohort study. Free Radic. Biol. Med. 2023, 207, 308–319. [Google Scholar] [CrossRef]
- Frost, P. Vitamin D deficiency among northern Native Peoples: A real or apparent problem? Int. J. Circumpolar Health 2012, 71, 18001. [Google Scholar] [CrossRef] [PubMed]
- Frost, P. To supplement or not to supplement: Are Inuit getting enough vitamin D? Études Inuit Stud. 2019, 40, 271–291. [Google Scholar] [CrossRef]
- Lips, P.; Duong, T.; Oleksik, A.; Black, D.; Cummings, S.; Cox, D.; Nickelsen, T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J. Clin. Endocrinol. Metab. 2001, 86, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Snellman, G.; Melhus, H.; Gedeborg, R.; Olofsson, S.; Wolk, A.; Pedersen, N.L.; Michaëlsson, K. Seasonal genetic influence on serum 25-hydroxyvitamin D levels: A twin study. PLoS ONE 2009, 4, e7747. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, R.P.; Lowik, M.R.; van den Berg, H.; de Groot, L.; Haller, J.; van Staveren, W.; Moreiras, O. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef] [PubMed]
| Variable | Supplementation-Baseline | Supplementation-Post | p | Controls-Baseline | Controls-Post | p |
|---|---|---|---|---|---|---|
| Weight (kg) | 71.52 ± 9.85 | 70.16 ± 9.51 | 0.021 | 68.75 ± 8.62 | 69.6 ± 8.29 | 0.287 |
| BMI (kg/m2) | 27.02 ± 3.96 | 26.47 ± 3.75 | 0.194 | 26.53 ± 3.54 | 26.8 ± 3.54 | 0.476 |
| Body fat (%) | 34.71 ± 8.42 | 33.96 ± 8.23 | 0.163 | 33.12 ± 7.97 | 32.6 ± 7.93 | 0.312 |
| 25(OH)D (ng/mL) | 15.77 ± 5.21 | 31.24 ± 7.87 | 0.031 | 26.41 ± 7.56 | 28.9 ± 7.58 | 0.534 |
| TAC | 0.93 ± 0.11 | 0.97 ± 0.09 | 0.081 | 0.79 ± 0.08 | 0.79 ± 0.08 | 0.267 |
| GSH | 6.01 ± 1.55 | 5.81 ± 1.41 | 0.069 | 7.11 ± 1.74 | 6.74 ± 1.74 | 0.453 |
| TBARS | 7.32 ± 1.31 | 6.94 ± 1.21 | 0.041 | 7.4 3 ± 1.11 | 7.26 ± 1.12 | 0.634 |
| Outcome | Pearson r | 95% CI | p-Value |
|---|---|---|---|
| TAC | −0.244 | −0.58 to 0.237 | 0.3463 |
| GSH | 0.11 | −0.435 to 0.64 | 0.6748 |
| TBARS | −0.116 | −0.535 to 0.318 | 0.6572 |
| Outcome | Variable | Coef. | Std. Err. | p-Value |
|---|---|---|---|---|
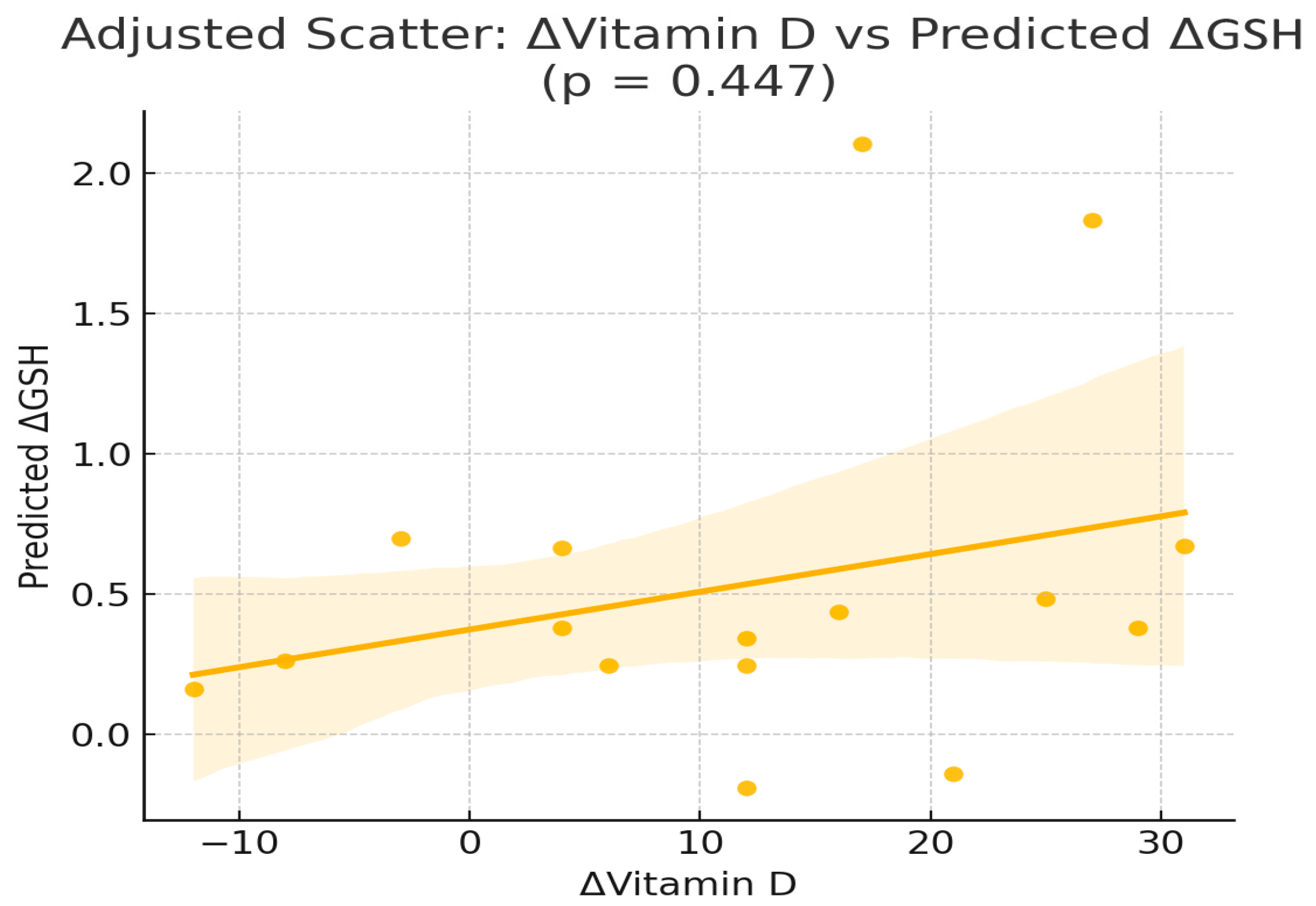
| ΔGSH | const | −1.11 | 4.58 | 0.814 |
| ΔGSH | ΔVitD | 0.05 | 0.06 | 0.447 |
| ΔGSH | AGE (y) | 0.01 | 0.05 | 0.866 |
| ΔGSH | WEIGHT (kg) | −0.0 | 0.06 | 0.948 |
| ΔGSH | BODY FAT % | −0.02 | 0.11 | 0.856 |
| ΔGSH | 25(OH)-D3 (ng/mL) | 0.06 | 0.07 | 0.375 |
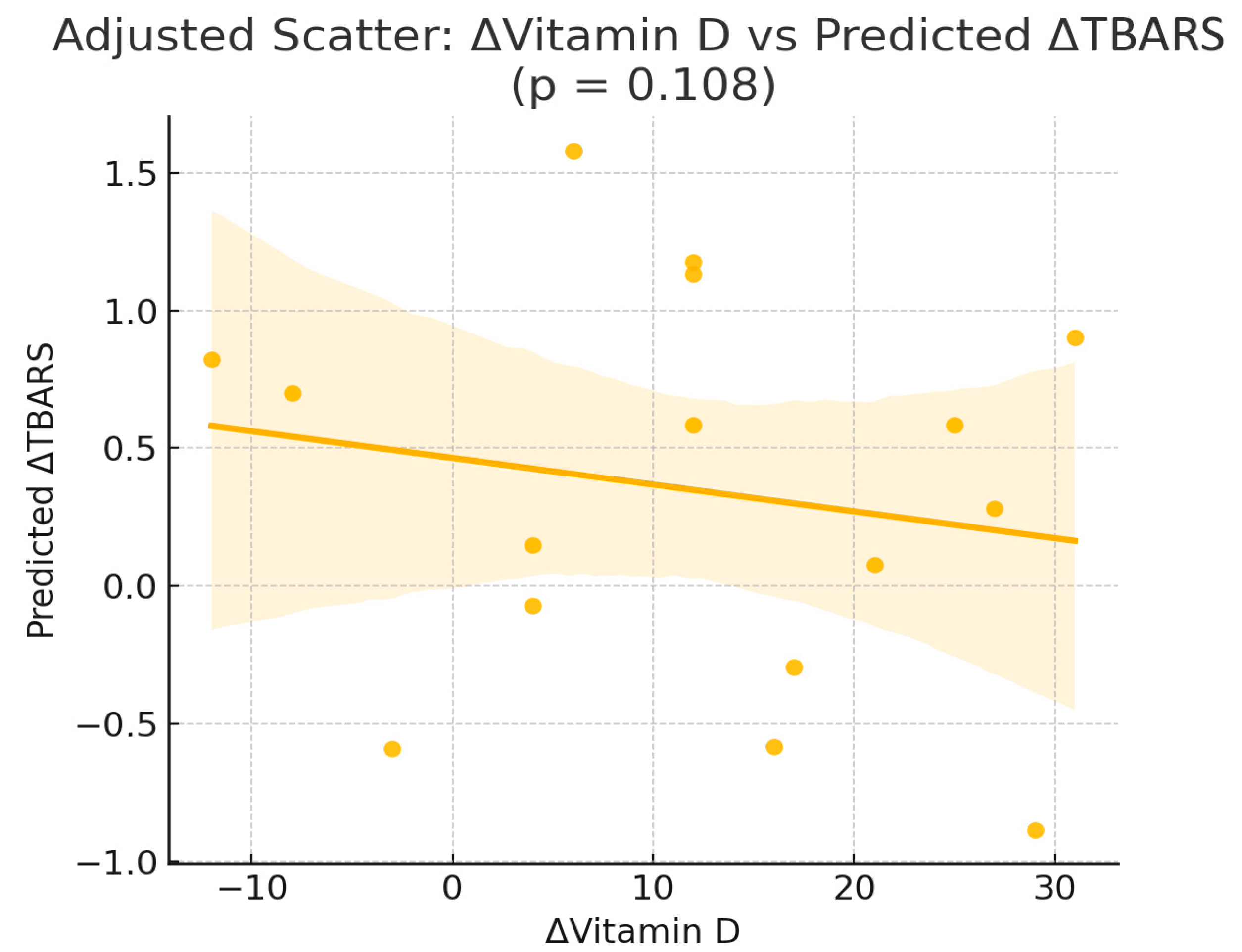
| ΔTBARS | const | −1.18 | 1.89 | 0.548 |
| ΔTBARS | ΔVitD | −0.05 | 0.03 | 0.108 |
| ΔTBARS | AGE (y) | 0.03 | 0.02 | 0.128 |
| ΔTBARS | WEIGHT (kg) | 0.08 | 0.02 | 0.011 * |
| ΔTBARS | BODY FAT % | −0.13 | 0.05 | 0.014 * |
| ΔTBARS | 25(OH)-D3 (ng/mL) | −0.03 | 0.03 | 0.246 |
| ΔTAC | const | −0.21 | 0.11 | 0.087 |
| ΔTAC | ΔVitD | 0.0 | 0.0 | 0.991 |
| ΔTAC | AGE (y) | 0.0 | 0.0 | 0.107 |
| ΔTAC | WEIGHT (kg) | 0.0 | 0.0 | 0.244 |
| ΔTAC | BODY FAT % | −0.0 | 0.0 | 0.999 |
| ΔTAC | 25(OH)-D3 (ng/mL) | 0.0 | 0.0 | 0.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karras, S.N.; Michalakis, K.; Kypraiou, M.; Anemoulis, M.; Vlastos, A.; Tzimagiorgis, G.; Haitoglou, C.; Tekos, F.; Skaperda, Z.; Vardakas, P.; et al. Effect of Vitamin D Supplementation on Oxidative Stress Biomarkers in Women Following Religious or Intermittent Fasting Patterns. Nutrients 2025, 17, 3389. https://doi.org/10.3390/nu17213389
Karras SN, Michalakis K, Kypraiou M, Anemoulis M, Vlastos A, Tzimagiorgis G, Haitoglou C, Tekos F, Skaperda Z, Vardakas P, et al. Effect of Vitamin D Supplementation on Oxidative Stress Biomarkers in Women Following Religious or Intermittent Fasting Patterns. Nutrients. 2025; 17(21):3389. https://doi.org/10.3390/nu17213389
Chicago/Turabian StyleKarras, Spyridon N., Konstantinos Michalakis, Maria Kypraiou, Marios Anemoulis, Antonios Vlastos, Georgios Tzimagiorgis, Costas Haitoglou, Fotios Tekos, Zoi Skaperda, Periklis Vardakas, and et al. 2025. "Effect of Vitamin D Supplementation on Oxidative Stress Biomarkers in Women Following Religious or Intermittent Fasting Patterns" Nutrients 17, no. 21: 3389. https://doi.org/10.3390/nu17213389
APA StyleKarras, S. N., Michalakis, K., Kypraiou, M., Anemoulis, M., Vlastos, A., Tzimagiorgis, G., Haitoglou, C., Tekos, F., Skaperda, Z., Vardakas, P., Georgopoulos, N., Papanikolaou, E. G., & Kouretas, D. (2025). Effect of Vitamin D Supplementation on Oxidative Stress Biomarkers in Women Following Religious or Intermittent Fasting Patterns. Nutrients, 17(21), 3389. https://doi.org/10.3390/nu17213389









