α-Ketoglutarate Ameliorates Sarcopenia in D-Galactose-Induced Aging Mice by Modulating Protein Homeostasis and Optimizing Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents
2.2. Mice
2.3. Animal Experiment Design
2.4. Body Composition
2.5. Muscle Endurance Test
2.6. Maximal Running Speed Test (Muscle Explosiveness)
2.7. Grip Strength Test
2.8. Body Temperature
2.9. H&E Staining
2.10. Transmission Electron Micrographs (TEMs)
2.11. Gene Expression Detection
2.12. Western Blot Analysis
2.13. ROS Measurements
2.14. MDA Measurements
2.15. Inflammatory Factors Measurements
2.16. Statistical Analysis
3. Results
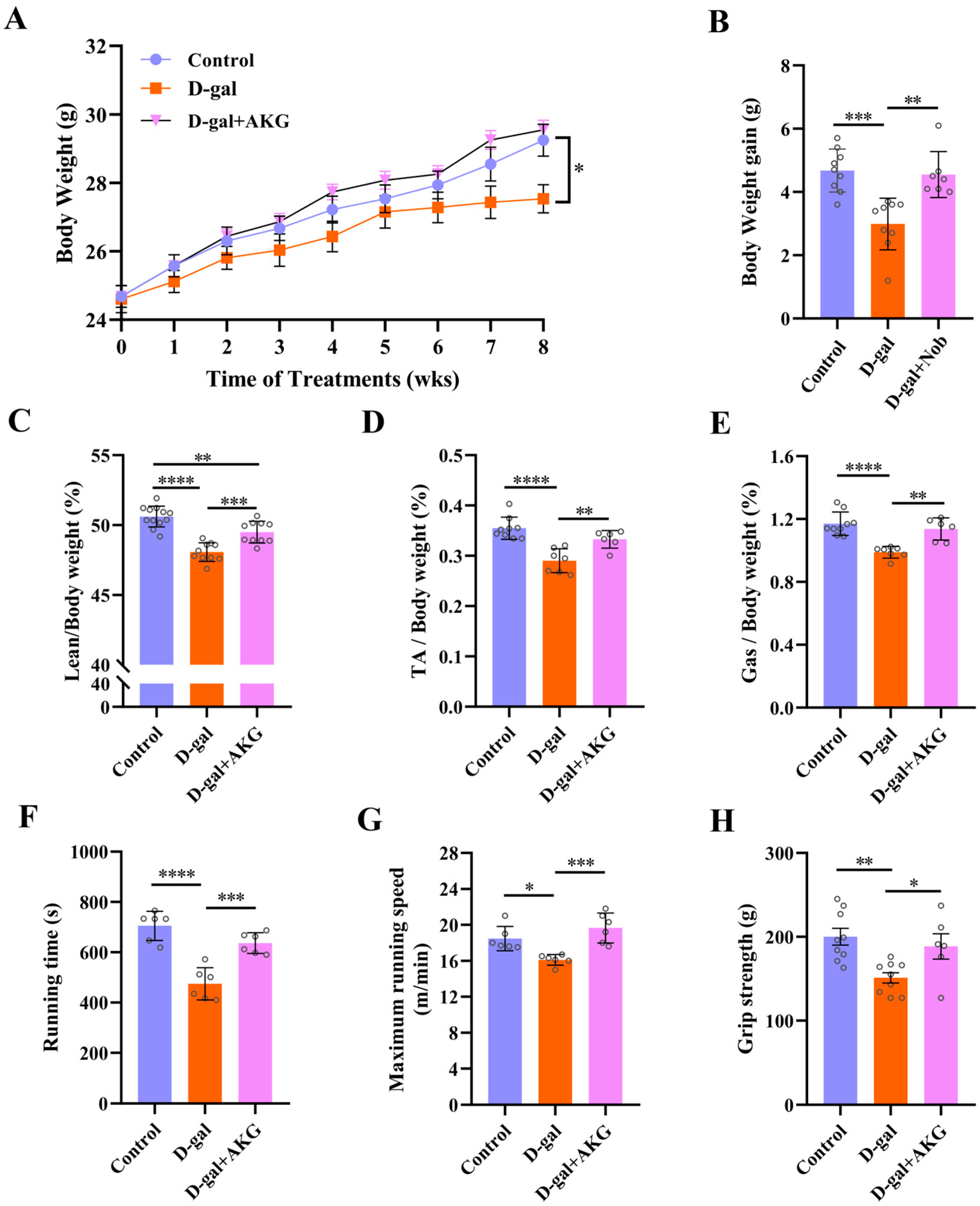
3.1. Effect of AKG on Skeletal Muscle Mass and Function in D-Gal-Induced Aging Mice
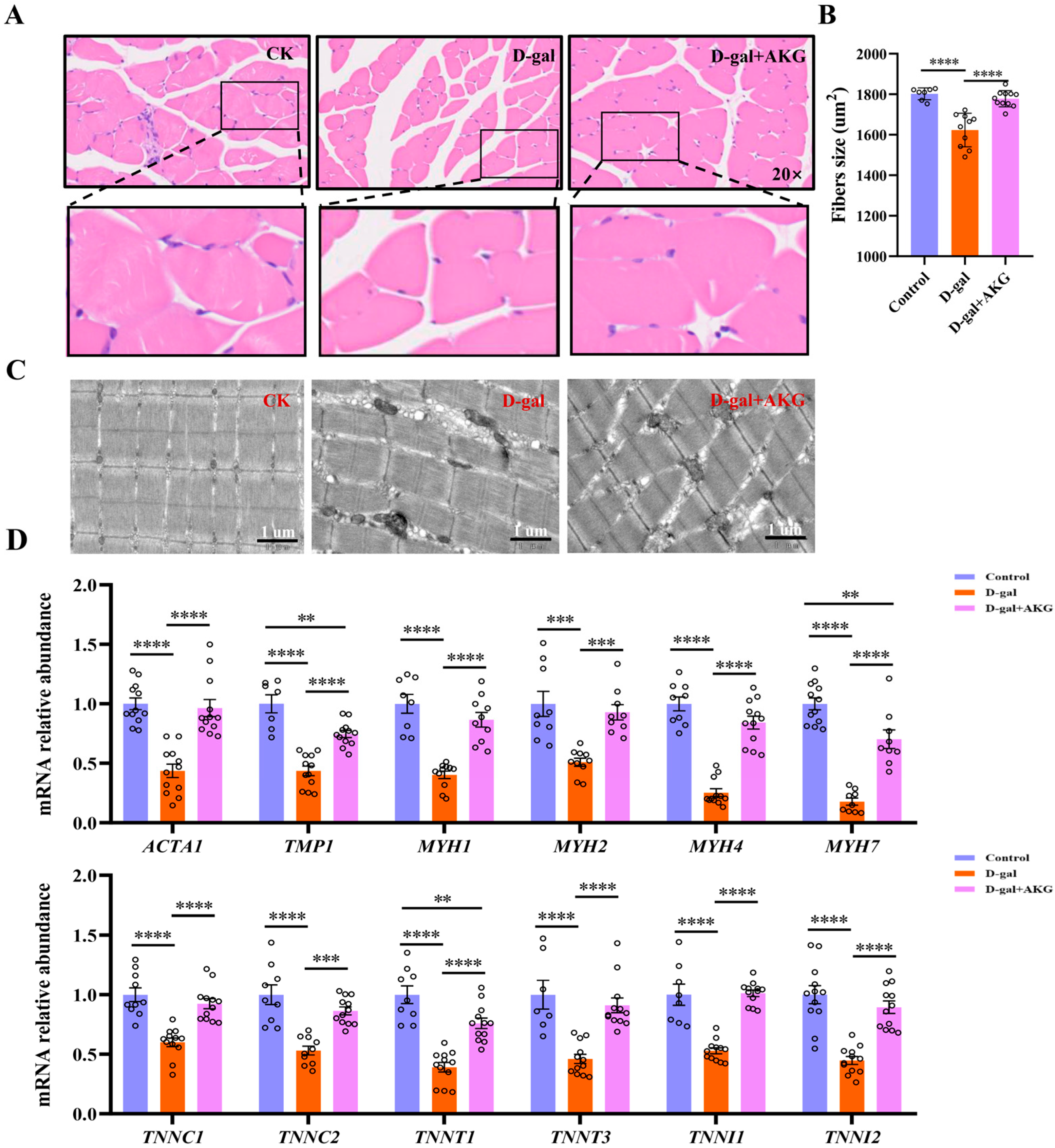
3.2. Effect of AKG on Structure and Composition of Myofiber in D-Gal-Induced Aging Mice
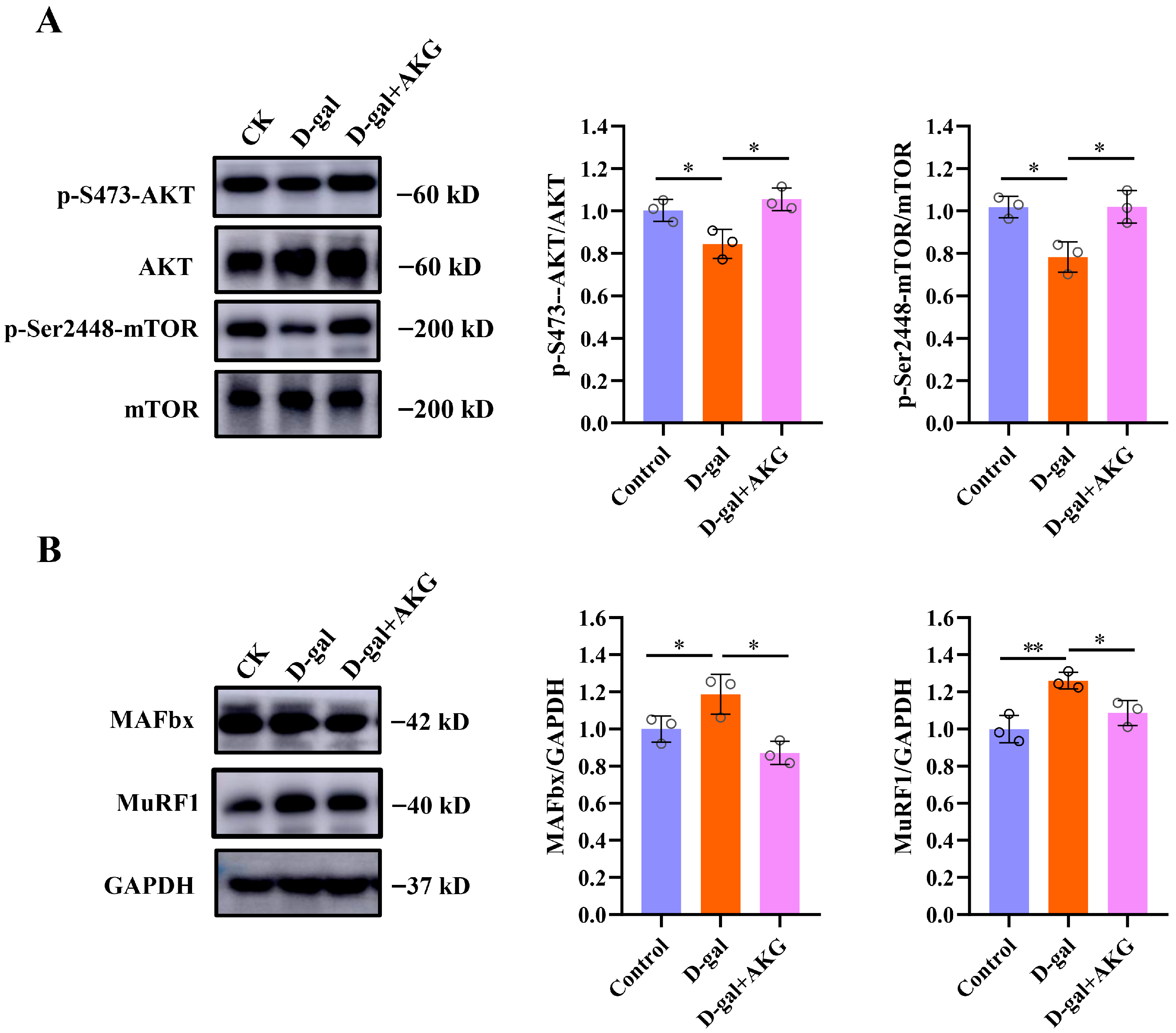
3.3. Effect of AKG on Protein Synthesis and Degradation Signaling Pathways of Myofibers in D-Gal-Induced Aging Mice
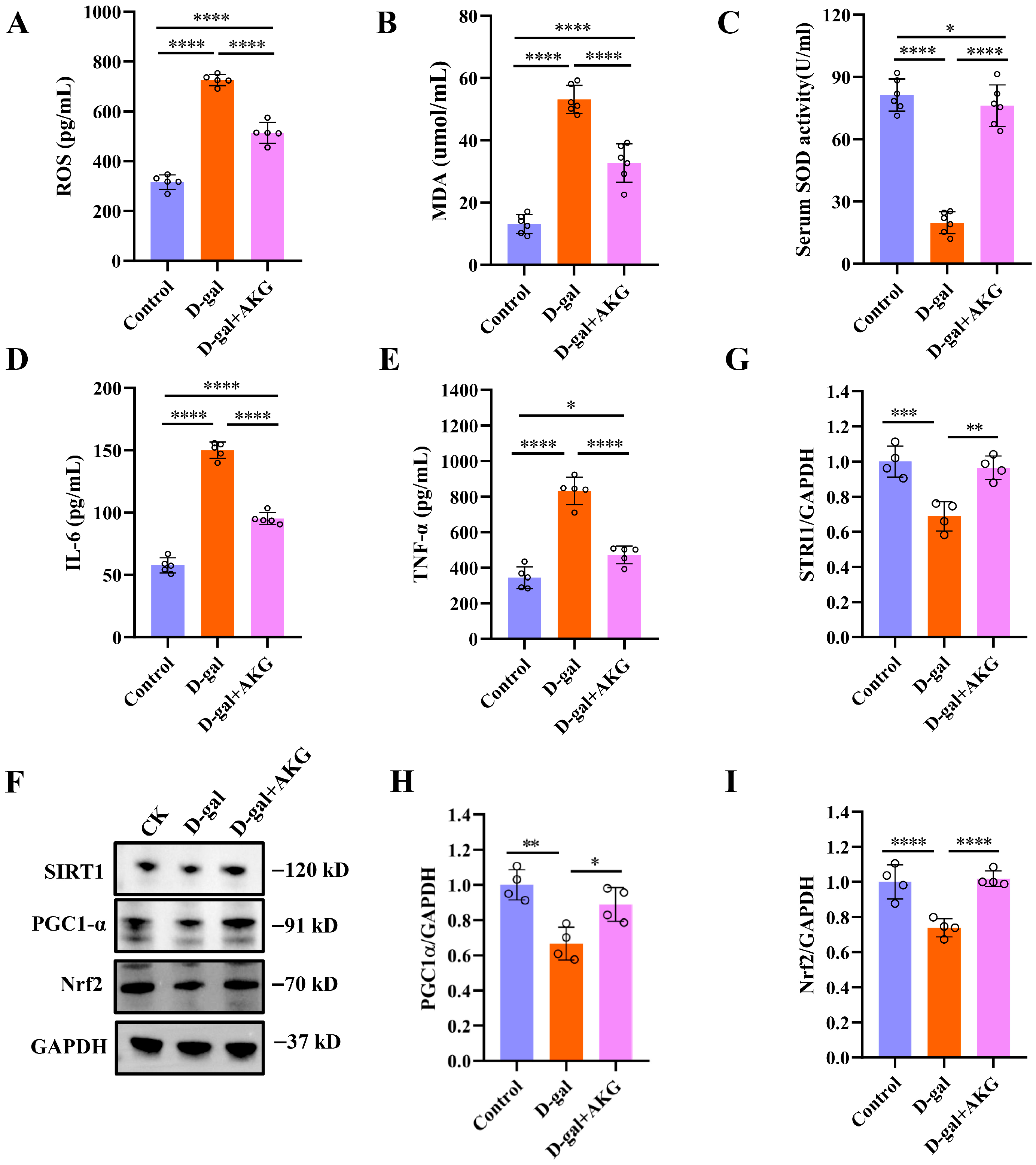
3.4. Effect of AKG on Thermogenesis and Mitochondrial Functionin D-Gal-Induced Aging Mice
3.5. Effect of AKG on Antioxidant and Anti-Inflammatory Abilities in D-Gal-Induced Aging Mice
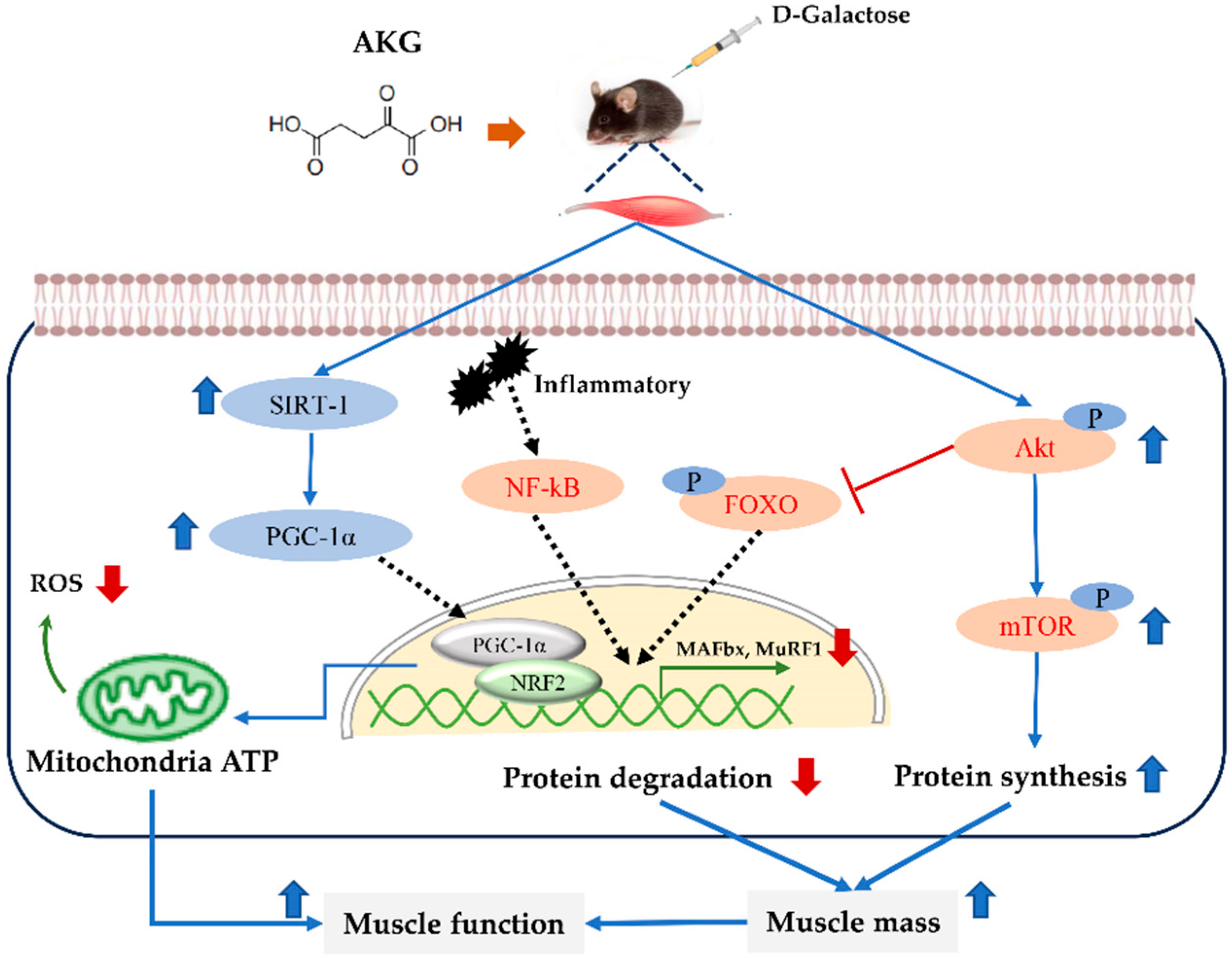
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKG | α-ketoglutarate |
| AKT | Protein kinase B |
| D-gal | D-galactose |
| FoxO | Foxhead box O |
| GAS | Gastrocnemius |
| IL-6 | interleukin-6 |
| MAFbx | Muscle atrophy F-box |
| MDA | Malondialdehyde |
| MuRF1 | Muscle RING Finger 1 |
| mTOR | Rapamycin |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PGC-1α | Proliferator-activated Receptor Gamma Co-activator 1-alpha |
| ROS | Reactive oxygen species |
| SIRT1 | Silent Information Regulator Transcript 1 |
| SOD | Superoxide dismutase D |
| TA | Tibialis anterior |
| TEMs | Transmission electron micrographs |
| TNF-α | tumor necrosis factor-alpha |
References
- Jing, Y.; Zuo, Y.; Yu, Y.; Sun, L.; Yu, Z.; Ma, S.; Zhao, Q.; Sun, G.; Hu, H.; Li, J.; et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell 2023, 14, 499–514. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef]
- Lv, Z.; Meng, J.; Yao, S.; Xiao, F.; Li, S.; Shi, H.; Cui, C.; Chen, K.; Luo, X.; Ye, Y.; et al. Naringenin improves muscle endurance via activation of the Sp1-ERRγ transcriptional axis. Cell Rep. 2023, 42, 113288. [Google Scholar] [CrossRef]
- Grech, A.; Breck, J.; Heidelbaugh, J. Adverse effects of testosterone replacement therapy: An update on the evidence and controversy. Ther. Adv. Drug Saf. 2014, 5, 190–200. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- He, L.; Xu, Z.; Yao, K.; Wu, G.; Yin, Y.; Nyachoti, C.M.; Kim, S.W. The Physiological Basis and Nutritional Function of Alpha-ketoglutarate. Curr. Protein Pept. Sci. 2015, 16, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Q.; Liu, X.; Lu, L.; Li, Z. Impact of Alpha-Ketoglutarate on Skeletal Muscle Health and Exercise Performance: A Narrative Review. Nutrients 2024, 16, 3968. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. Bodybuilding supplement promotes healthy aging and extends life span, at least in mice. Science 2020, 493, 524. [Google Scholar] [CrossRef]
- Ciuffoli, V.; Feng, X.; Jiang, K.; Acevedo-Luna, N.; Ko, K.D.; Wang, A.H.J.; Riparini, G.; Khateb, M.; Glancy, B.; Dell’Orso, S.; et al. Psat1-generated α-ketoglutarate and glutamine promote muscle stem cell activation and regeneration. Genes Dev. 2024, 38, 151–167. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, Y.; Liao, Z.; Xing, K.; Zhu, C.; Xu, Y.; Yu, L.; Wang, L.; Wang, S.; Zhu, X.; et al. α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway. FASEB J. 2018, 32, 488–499. [Google Scholar] [CrossRef]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456.e6. [Google Scholar] [CrossRef]
- Wang, H.H.; Sun, Y.N.; Qu, T.Q.; Sang, X.Q.; Zhou, L.M.; Li, Y.X.; Ren, F.Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.Q.; Sun, Y.X.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Chen, L.-L.; Huang, J.-Q.; Wu, Y.-Y.; Chen, L.-B.; Li, S.-P.; Zhang, X.; Wu, S.; Ren, F.-Z.; Lei, X.-G. Loss of Selenov predisposes mice to extra fat accumulation and attenuated energy expenditure. Redox Biol. 2021, 45, 102048. [Google Scholar] [CrossRef]
- Tsuda, M.; Fukushima, A.; Matsumoto, J.; Takada, S.; Kakutani, N.; Nambu, H.; Yamanashi, K.; Furihata, T.; Yokota, T.; Okita, K.; et al. Protein acetylation in skeletal muscle mitochondria is involved in impaired fatty acid oxidation and exercise intolerance in heart failure. J. Cachexia Sarcopeni 2018, 9, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Yang, W.; Liao, Z.-Y.; Wu, Y.-X.; Fan, Z.; Guo, A.; Yu, J.; Chen, Q.-N.; Wu, J.-H.; Zhou, J.; et al. MICU3 regulates mitochondrial Ca2+-dependent antioxidant response in skeletal muscle aging. Cell Death Dis. 2021, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, J.; Liu, X.; Yang, X.; Fan, J.; Chen, N. Ampelopsin attenuates the atrophy of skeletal muscle from d-gal-induced aging rats through activating AMPK/SIRT1/PGC-1α signaling cascade. Biomed. Pharmacother. 2017, 90, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Ang, S.-T.J.; Tsai, S.-Y. Sarcopenia: Tilting the Balance of Protein Homeostasis. Proteomics 2020, 20, 1800411. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Picard, M.; St-Jean Pelletier, F.; Sgarioto, N.; Auger, M.J.; Vallee, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. Febs Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R. Molecular Mechanism and Pathogenesis of Sarcopenia: An Overview. Int. J. Mol. Sci. 2021, 22, 3032. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.Y.; Kim, C.Y. A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia. Nutrients 2023, 15, 2625. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. d-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Zhou, B.; Zhou, Z.; Xu, N.; Wang, Y. A ROS-mediated mitochondrial pathway and Nrf2 pathway activation are involved in BDE-47 induced apoptosis in Neuro-2a cells. Chemosphere 2017, 184, 679–686. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.; Fontana, F.; Marzagalli, M.; Audano, M.; Beretta, G.; Procacci, P.; Sartori, P.; Mitro, N.; Limonta, P. Ca2+ overload- and ROS-associated mitochondrial dysfunction contributes to delta-tocotrienol-mediated paraptosis in melanoma cells. Apoptosis 2021, 26, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, D.; Sorianello, E.; Segales, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Munoz, J.P.; Sanchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and Fall Injuries Among Adults Aged ≥65 Years—United States, 2014. Morb. Mortal. Wkly. Rep. (MMWR) 2016, 65, 993–998. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1alpha Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef]
- Ma, C.S.; Lv, Q.M.; Zhang, K.R.; Tang, Y.B.; Zhang, Y.F.; Shen, Y.; Lei, H.M.; Zhu, L. Author Correction: NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2023, 44, 2346. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Gospodaryov, D.V.; Lushchak, V.I. Mimicking caloric restriction for anti-aging effects: The pro-oxidant role of alpha-ketoglutarate. Curr. Opin. Toxicol. 2022, 30, 100339. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequence 5′→3′ (Forward) | Primer Sequence 5′→3′ (Reverse) |
|---|---|---|
| ACTA1 | TACCACCGGCATCGTGTTG | GCGCACAATCTCACGTTCAG |
| TMP1 | TTGAAAGCCGAGCCCAAAAAG | TCATACTTCCGGTCAGCATCTT |
| TNNC1 | GCGGTAGAACAGTTGACAGAG | GACAGAAACTCATCGAAGTCCA |
| TNNC2 | GAGGCCAGGTCCTACCTCAG | GGTGCCCAACTCTTTAACGCT |
| TNNT1 | ACTAAAAGACCGCATTGGAGG | AGCTCCCATGTTGGACAGAAC |
| TNNT3 | ACTGCTCCTAAGATCCCGGAA | ATGAGGTCCTTGTTTTGACGC |
| TNNI1 | ATGCCGGAAGTTGAGAGGAAA | TCCGAGAGGTAACGCACCTT |
| TNNI2 | CGGAGGGTGCGTATGTCTG | CAGGTCCCGTTCCTTCTCA |
| MYH1 | CTCTTCCCGCTTTGGTAAGTT | CAGGACATTTCGATTAGATCCG |
| MYH2 | TGGAGGGTGAGGTAGAGAGTG | TTGGATAGATTTGTGTTGGATTG |
| MYH4 | CCGCATCTGTAGGAAGGGG | GTGACCGAATTTGTACTGATGT |
| MYH7 | CCTGCGGAAGTCTGAGAAGG | CTCGGGACACGATCTTGGC |
| COX1 | GAGATGAACAGGGGCACCAA | ATCAGAACGAGCGCAGTGAA |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGGCTGTGAAGTCGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, H.; Zhang, Y.; Wang, X.; Qiao, Z.; Wang, J.; Li, Y.; Sun, Y. α-Ketoglutarate Ameliorates Sarcopenia in D-Galactose-Induced Aging Mice by Modulating Protein Homeostasis and Optimizing Mitochondrial Function. Nutrients 2025, 17, 3336. https://doi.org/10.3390/nu17213336
Zhang Y, Wang H, Zhang Y, Wang X, Qiao Z, Wang J, Li Y, Sun Y. α-Ketoglutarate Ameliorates Sarcopenia in D-Galactose-Induced Aging Mice by Modulating Protein Homeostasis and Optimizing Mitochondrial Function. Nutrients. 2025; 17(21):3336. https://doi.org/10.3390/nu17213336
Chicago/Turabian StyleZhang, Yangguang, Huihui Wang, Yijia Zhang, Xintong Wang, Ziyu Qiao, Jiayu Wang, Yixuan Li, and Yanan Sun. 2025. "α-Ketoglutarate Ameliorates Sarcopenia in D-Galactose-Induced Aging Mice by Modulating Protein Homeostasis and Optimizing Mitochondrial Function" Nutrients 17, no. 21: 3336. https://doi.org/10.3390/nu17213336
APA StyleZhang, Y., Wang, H., Zhang, Y., Wang, X., Qiao, Z., Wang, J., Li, Y., & Sun, Y. (2025). α-Ketoglutarate Ameliorates Sarcopenia in D-Galactose-Induced Aging Mice by Modulating Protein Homeostasis and Optimizing Mitochondrial Function. Nutrients, 17(21), 3336. https://doi.org/10.3390/nu17213336




