Monoterpenoids from the Roots of Liquidambar formosana (Formosan Sweet Gum) Exhibit Senomorphic Activity Against Cellular Senescence
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. HR-ESI-MS/MS and Molecular Networking
2.4. Extraction and Isolation
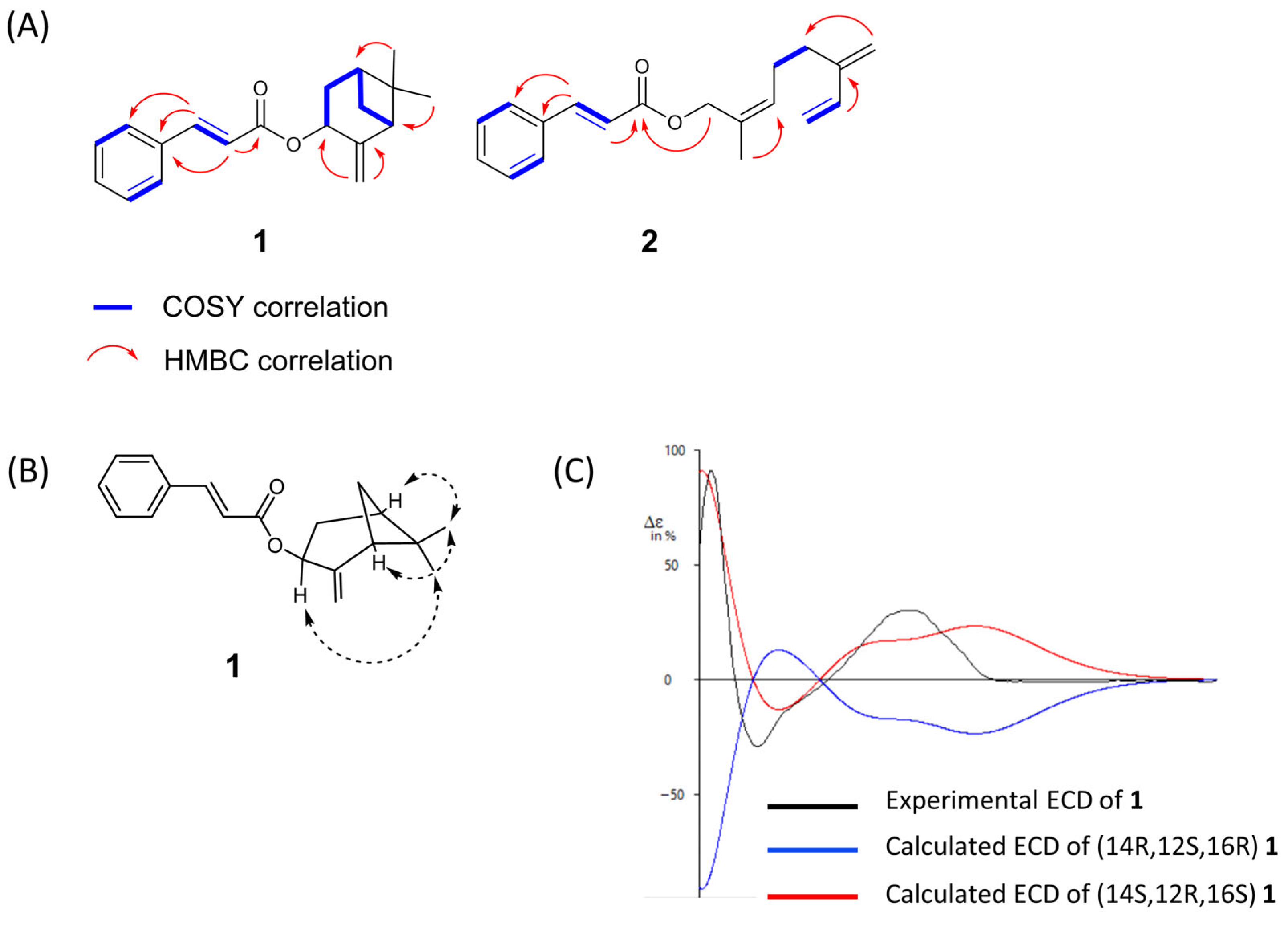
2.5. ECD Calculation
2.6. Cell Culture
2.7. p16INK4A Promoter Activity-Related Luciferase Reporter Assay
2.8. MTT Assay
2.9. Senescence-Associated β-Galactosidase Staining
2.10. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.11. Statistical Analyses
3. Results
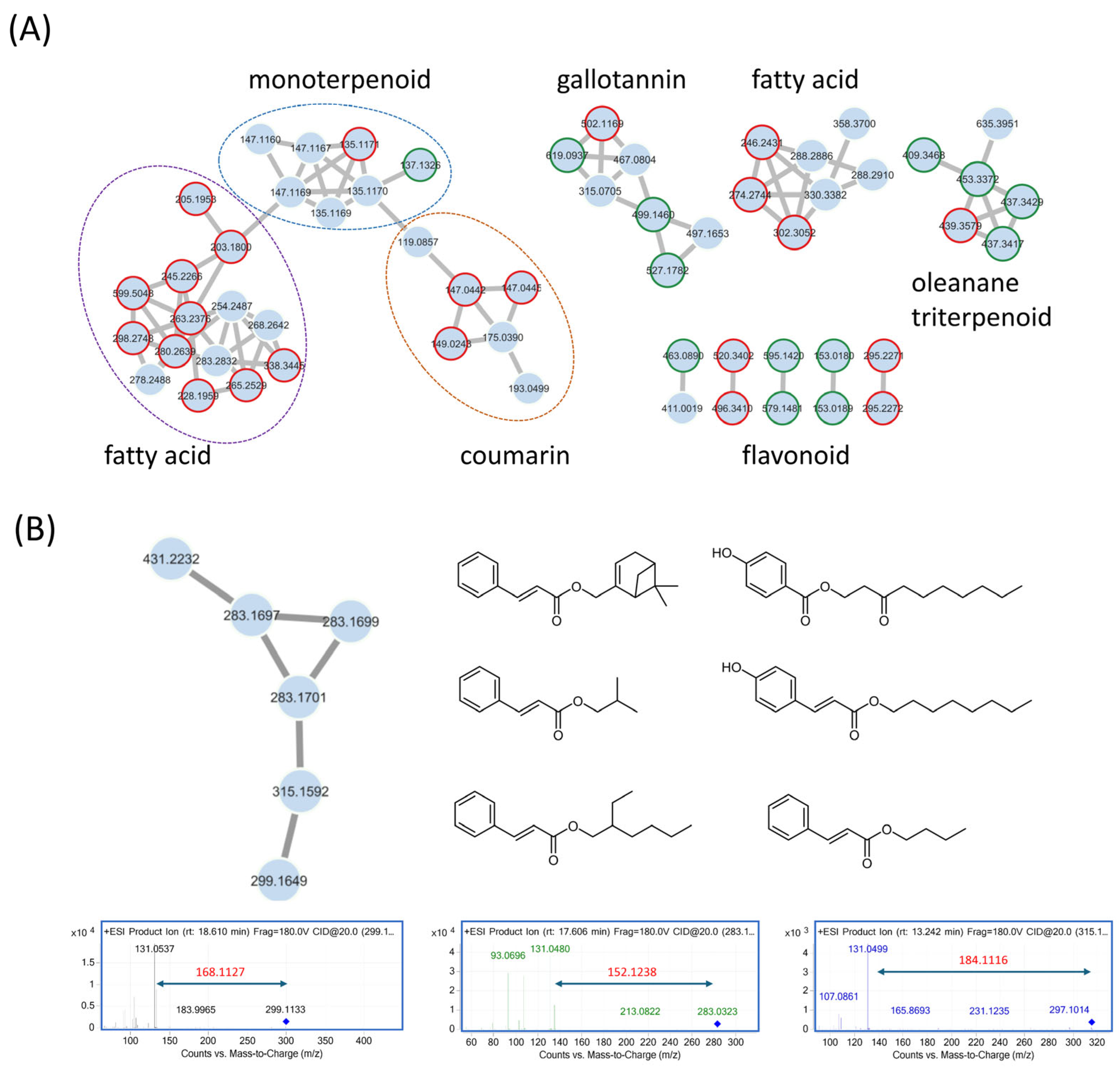
3.1. Feature-Based Molecular Networking of the Liquidambar formosana Root Extract
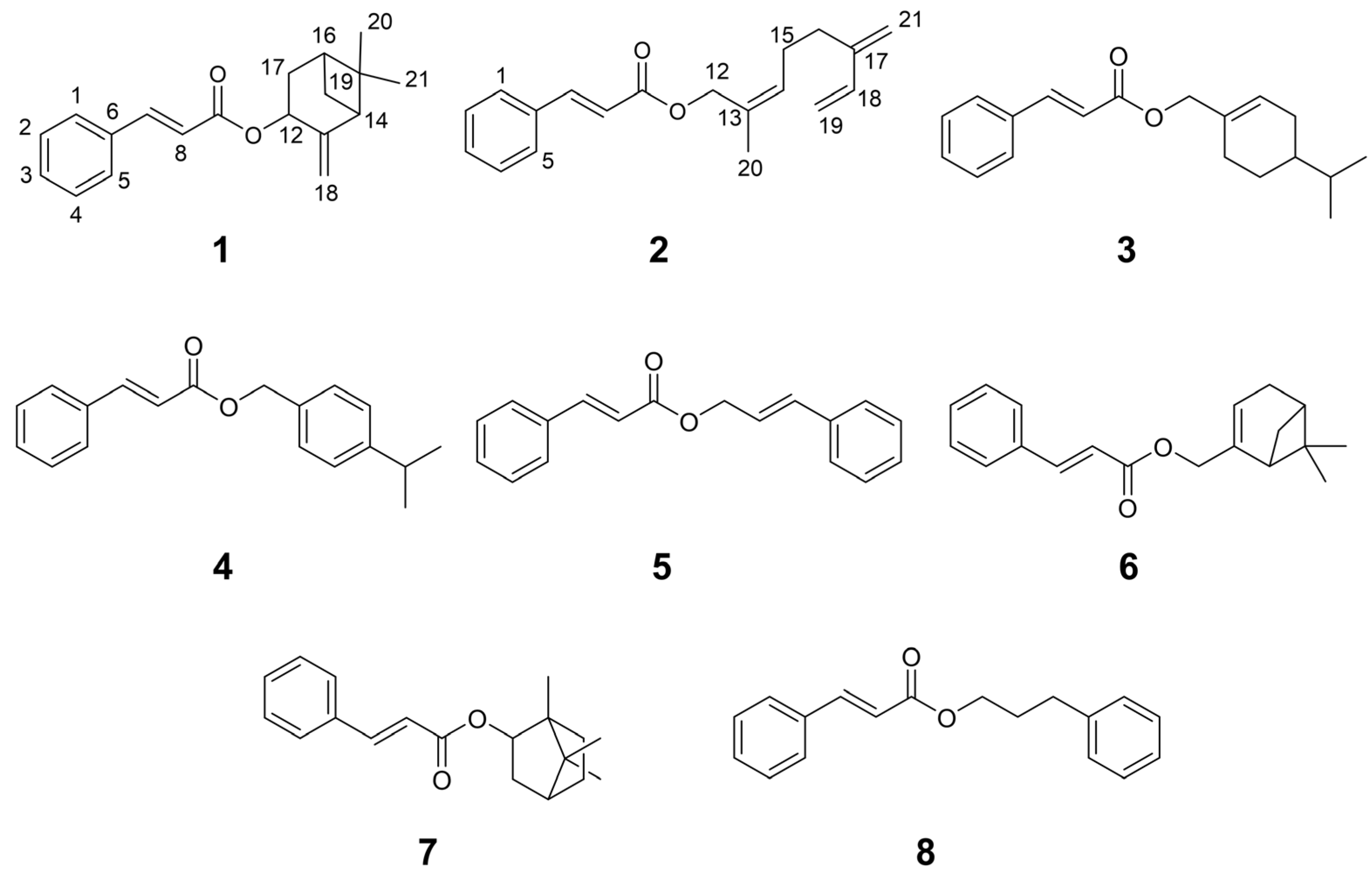
3.2. Isolation and Structural Elucidation of New Compounds 1 and 2
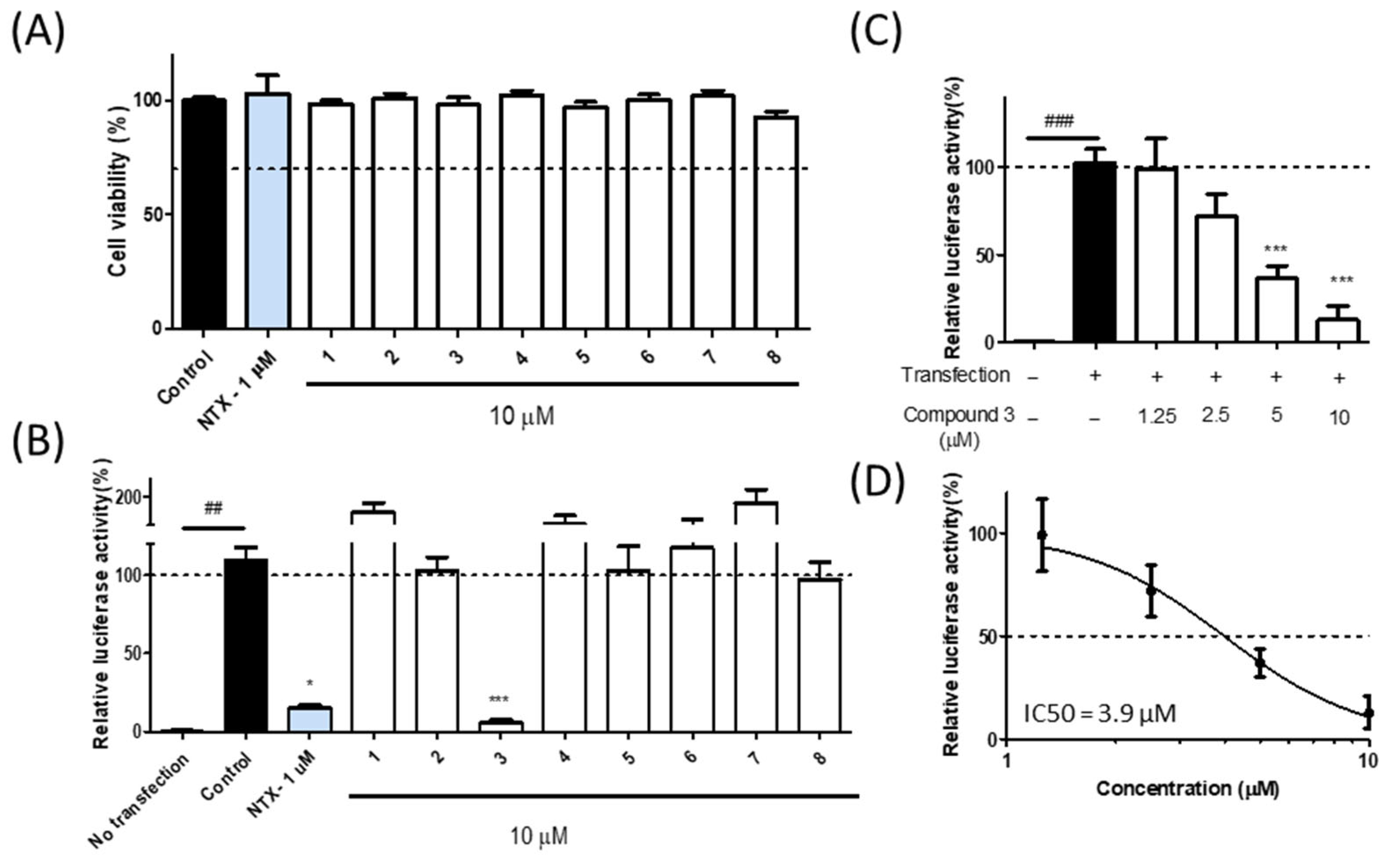
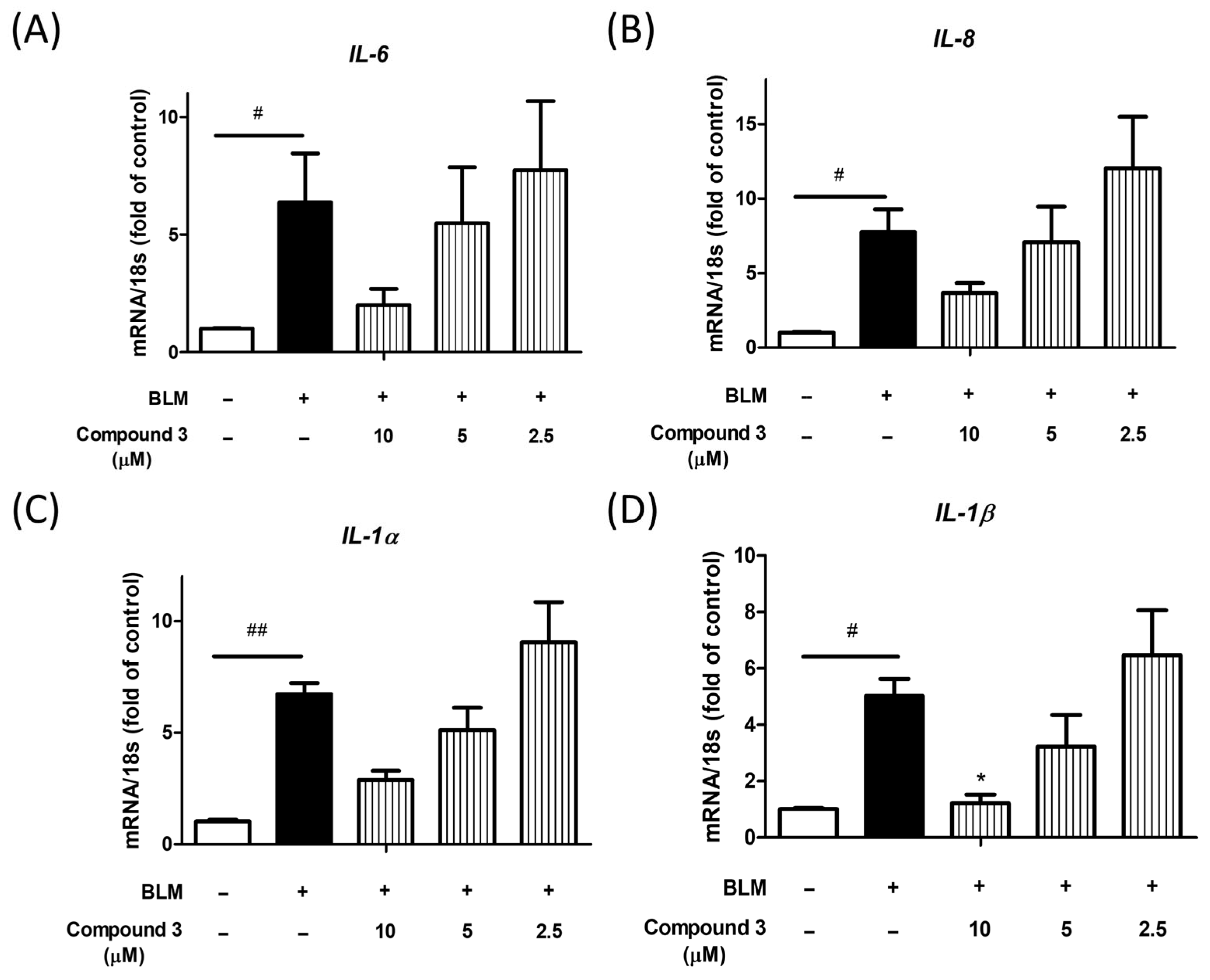
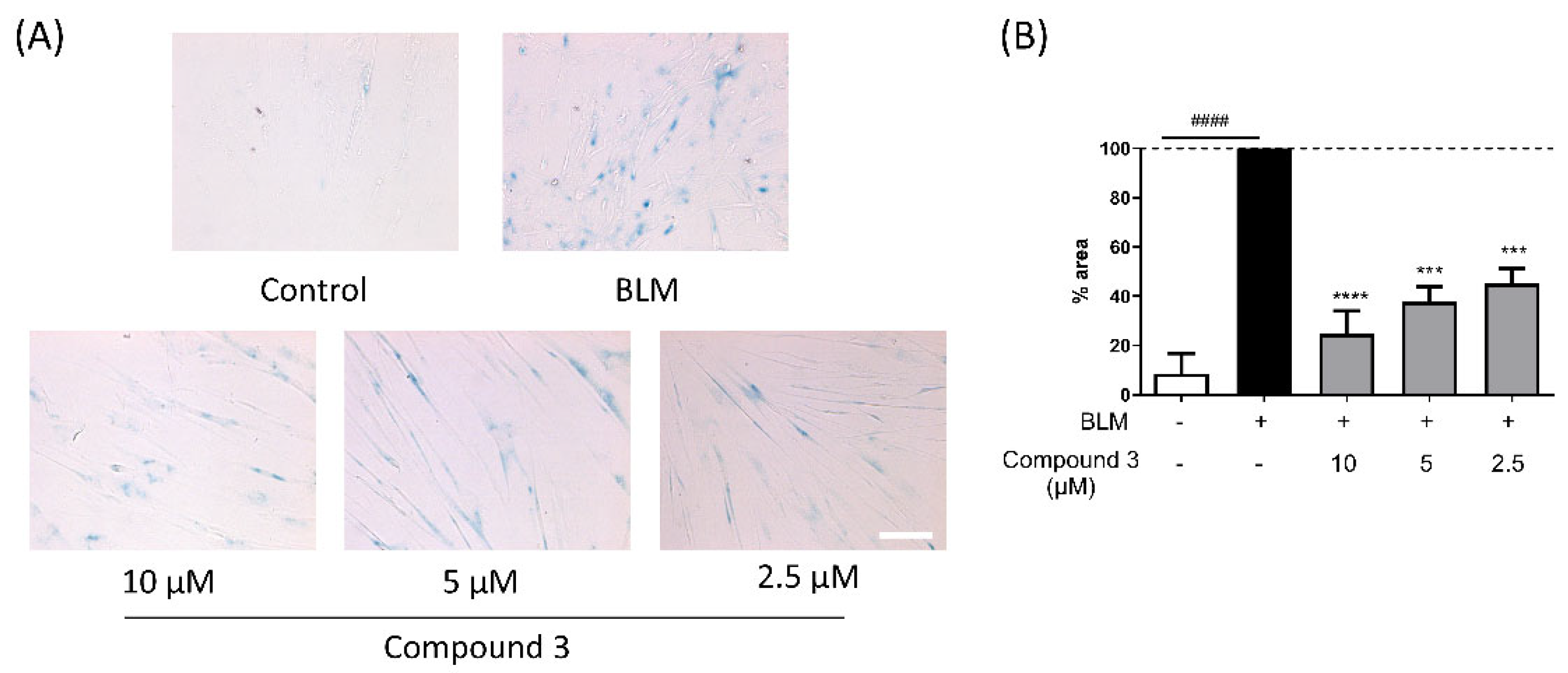
3.3. Bioactivity of Isolated Compounds from Liquidambar formosana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCFs | Cytoplasmic chromatin fragments |
| ECD | Electronic circular dichroism |
| GNPS | Global natural products social molecular networking |
| HMBC | Heteronuclear multi-bond correlation |
| HPLC | High-performance liquid chromatography |
| HRESIMS | High-resolution electrospray ionization mass spectrometry |
| HSQC | Heteronuclear single quantum coherence |
| LC-MS | Liquid chromatography–mass spectrometry |
| NMR | Nuclear magnetic resonance |
| SASP | Senescence-associated secretory phenotype |
| STAT3 | Signal transducer and activator of transcription 3 |
References
- Ma, Y.; Ding, S.; Qiu, Y. Chromosome-level genome assembly of American sweetgum (Liquidambar styraciflua, Altingiaceae). Sci. Data 2024, 11, 1078. [Google Scholar] [CrossRef]
- Ouyang, X.L.; Yi, S.; Lu, H.; Wu, S.; Zhao, H. Liquidambar formosana Hance: A mini-review of chemical constituents and pharmacology. Eur. J. Med. Plants 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Chien, S.-C.; Xiao, J.-H.; Tseng, Y.-H.; Kuo, Y.-H.; Wang, S.-Y. Composition and antifungal activity of balsam from Liquidambar formosana Hance. Holzforschung 2013, 67, 345–351. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.F.; Tu, Z.C.; Zhao, Y.; Wang, H.; Li, G.J.; Sha, X.M. a-Glucosidase inhibition, anti-glycation and antioxidant activities of Liquidambar formosana Hance leaf, and identification of phytochemical profile. S. Afr. J. Bot. 2017, 113, 239–247. [Google Scholar] [CrossRef]
- Zhu, Y.; Guan, Y.-J.; Chen, Q.-Z.; Yuan, L.-H.; Xu, Q.-Q.; Zhou, M.-L.; Liu, H.; Lin, W.; Zhang, Z.-D.; Zhou, Z.-L. Pentacyclic Triterpenes from the resin of Liquidambar formosana have anti-angiogenic properties. Phytochemistry 2021, 184, 112676. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Enders, G.H.; David Dynlacht, B.; Harlow, E.D. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature 1995, 375, 506–510. [Google Scholar] [CrossRef]
- McConnell, B.B.; Gregory, F.J.; Stott, F.J.; Hara, E.; Peters, G. Induced expression of p16 INK4a inhibits both CDK4-and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol. Cell. Biol. 1999, 19, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Wu, X.; Ji, T.; Xu, B.; Han, Y.; Sun, M.; Jiang, S.; Li, T.; Hu, W.; Deng, C.; et al. Bakuchiol: A newly discovered warrior against organ damage. Pharmacol. Res. 2019, 141, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, N.J.; Clark, T.N.; McMann, E.J.; van Santen, J.A.; Haeckl, F.J.; Gray, C.A.; Linington, R.G. Annotation of natural product compound families using molecular networking topology and structural similarity fingerprinting. Nat. Commun. 2023, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.Y.; Tian, K.; Sun, J.X.; Wang, W.; Liu, H.C.; Yang, J.; Jiang, M.Y.; Huang, X.Z. Anti-inflammatory monoterpene esters from the stems of Illigera aromatica. Nat. Prod. Res. 2021, 35, 960–966. [Google Scholar] [CrossRef]
- do Vale, J.A.; Rodrigues, M.P.; Lima, Â.M.A.; Santiago, S.S.; de Almeida Lima, G.D.; Almeida, A.A.; de Oliveira, L.L.; Bressan, G.C.; Teixeira, R.R.; Machado-Neves, M. Synthesis of cinnamic acid ester derivatives with antiproliferative and antimetastatic activities on murine melanoma cells. Biomed. Pharmacother. 2022, 148, 112689. [Google Scholar] [CrossRef]
- Shang, X.F.; Xiao, L.; Su, J.T.; Wei, S.Y.; Wang, Y.S.; Yang, J.H. Chemical constituents of Litsea euosma. Chem. Nat. Compd. 2019, 55, 1138–1140. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Stevisalicinone (X), a new diterpene type, and other constituents from Stevia species. Chem. Inform. 1986, 17, 1974–1983. [Google Scholar]
- Hayashi, H.; Yasukochi, S.; Sakamoto, T.; Hatano, M.; Ishihara, K. Insight into the mechanism of the acylation of alcohols with acid anhydrides catalyzed by phosphoric acid derivatives. J. Org. Chem. 2021, 86, 5197–5212. [Google Scholar] [CrossRef]
- Funasaka, S.; Mukaiyama, T. A versatile, practical, and inexpensive reagent, pyridine-3-carboxylic anhydride (3-PCA), for condensation reactions. Bull. Chem. Soc. Jpn. 2008, 81, 148–159. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Khongkow, M.; Iempridee, T.; Lourith, N. Food hydroxycinnamic acids alleviate ageing in dermal cells. Food Prod. Process. Nutr. 2024, 6, 86. [Google Scholar] [CrossRef]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.N.; Menezes, A.M.S.; Viana, G.S.B. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990, 42, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kohno, T.; Baeg, G.; Akiyama, T.; Yokota, J. Growth suppression of non-small cell lung carcinoma cells by the introduction of the p16 (INK4A) gene. Int. J. Oncol. 1997, 10, 33–39. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Kojima, H.; Inoue, T.; Kunimoto, H.; Nakajima, K. IL-6-STAT3 signaling and premature senescence. Jak-Stat 2013, 2, e25763. [Google Scholar] [CrossRef]
- Wang, T.; Notta, F.; Navab, R.; Joseph, J.; Ibrahimov, E.; Xu, J.; Zhu, C.-Q.; Borgida, A.; Gallinger, S.; Tsao, M.-S. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes. Mol. Cancer Res. 2017, 15, 3–14. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Li, W.-c.; Tang, B.-x.; Li, J.; Zang, Y. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol. Sin. 2021, 42, 2058–2068. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Aoshiba, K.; Tsuji, T.; Kameyama, S.; Itoh, M.; Semba, S.; Yamaguchi, K.; Nakamura, H. Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp. Toxicol. Pathol. 2013, 65, 1053–1062. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Z.; Bin, Y.; Wang, J.; Rao, X.; Wu, G.; Dong, X.; Tong, F. Ionizing radiation induces vascular smooth muscle cell senescence through activating NF-κB/CTCF/p16 pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 166994. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zheng, Y.Z.; Li, Y.-Q.; Wong, C.S. Cellular senescence in mouse hippocampus after irradiation and the role of p53 and p21. J. Neuropathol. Exp. Neurol. 2017, 76, 260–269. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Ivanov, A.; Pawlikowski, J.; Manoharan, I.; van Tuyn, J.; Nelson, D.M.; Rai, T.S.; Shah, P.P.; Hewitt, G.; Korolchuk, V.I.; Passos, J.F. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013, 202, 129–143. [Google Scholar] [CrossRef]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017, 550, 402–406. [Google Scholar] [CrossRef]
- Fraga, M.F.; Agrelo, R.; Esteller, M. Cross-talk between aging and cancer: The epigenetic language. Ann. N. Y. Acad. Sci. 2007, 1100, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, 13921. [Google Scholar] [CrossRef]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Rolt, A.; Nair, A.; Cox, L.S. Optimisation of a screening platform for determining IL-6 inflammatory signalling in the senescence-associated secretory phenotype (SASP). Biogerontology 2019, 20, 359–371. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Weber, A.; Roxlau, T.; Gaestel, M.; Kracht, M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 2165–2175. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, H.S.; Park, S.C.; Park, J.T.; Kim, H.S.; Oh, W.K. Identification of a novel senomorphic agent, avenanthramide C, via the suppression of the senescence-associated secretory phenotype. Mech. Ageing Dev. 2020, 192, 111355. [Google Scholar] [CrossRef]
- Imb, M.; Véghelyi, Z.; Maurer, M.; Kühnel, H. Exploring senolytic and senomorphic properties of medicinal plants for anti-aging therapies. Int. J. Mol. Sci. 2024, 25, 10419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, X.; Huang, J.; Wu, M.; Zhang, S.; Wang, D.; Liu, S.; Fan, H. Fisetin alleviated bleomycin-induced pulmonary fibrosis partly by rescuing alveolar epithelial cells from senescence. Front. Pharmacol. 2020, 11, 553690. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 b | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 7.37 (m) | 129.0 | 7.37 (m) | 129.0 |
| 2 | 7.38 (m) | 130.3 | 7.38 (m) | 130.4 |
| 3 | 7.37 (m) | 129.0 | 7.37 (m) | 129.0 |
| 4 | 7.52 (m) | 128.2 | 7.53 (m) | 128.2 |
| 5 | 134.6 | 134.5 | ||
| 6 | 7.52 (m) | 128.2 | 7.53 (m) | 128.2 |
| 7 | 7.68 (d, 16.0) | 144.6 | 7.70, (d, 16.0) | 144.9 |
| 8 | 6.45 (d, 16.0) | 119.0 | 6.47, (d, 16.0) | 118.2 |
| 9 | 166.7 | 167.0 | ||
| 12 | 5.70 (d, 8.0) | 68.8 | 4.60 (2H, s) | 70.3 |
| 13 | 150.5 | 129.3 | ||
| 14 | 2.56 (t, 5.45) | 50.9 | 7.38 (m) | 130.7 |
| 15 | 40.7 | 2.26 (2H, br s) | 26.5 | |
| 16 | 2.02 (m) | 39.7 | 2.28 (2H, br s) | 30.9 |
| 17 | 1.88 (m), 2.45 (m) | 33.5 | 145.8 | |
| 18 | 4.93 (br s), 5.11 (br s) | 114.4 | 6.38, (dd, 17.6, 10.8) | 138.9 |
| 19 | 1.58 (m), 1.69 (m) | 28.1 | 5.07 (d, 10.8), 5.24 (d, 17.6) | 116.1 |
| 20 | 0.73 (3H, s) | 22.1 | 1.71 (3H, s) | 14.2 |
| 21 | 1.31 (3H, s) | 26.0 | 5.02 (d, 12.3) | 113.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, M.T.T.; Vu, Q.H.; Mai, V.-H.; Ponce-Zea, J.E.; Choi, S.; An, J.-P.; Oh, W.-K. Monoterpenoids from the Roots of Liquidambar formosana (Formosan Sweet Gum) Exhibit Senomorphic Activity Against Cellular Senescence. Nutrients 2025, 17, 3321. https://doi.org/10.3390/nu17213321
Le MTT, Vu QH, Mai V-H, Ponce-Zea JE, Choi S, An J-P, Oh W-K. Monoterpenoids from the Roots of Liquidambar formosana (Formosan Sweet Gum) Exhibit Senomorphic Activity Against Cellular Senescence. Nutrients. 2025; 17(21):3321. https://doi.org/10.3390/nu17213321
Chicago/Turabian StyleLe, Minh Thi Tuyet, Quang Huy Vu, Van-Hieu Mai, Jorge Eduardo Ponce-Zea, Seri Choi, Jin-Pyo An, and Won-Keun Oh. 2025. "Monoterpenoids from the Roots of Liquidambar formosana (Formosan Sweet Gum) Exhibit Senomorphic Activity Against Cellular Senescence" Nutrients 17, no. 21: 3321. https://doi.org/10.3390/nu17213321
APA StyleLe, M. T. T., Vu, Q. H., Mai, V.-H., Ponce-Zea, J. E., Choi, S., An, J.-P., & Oh, W.-K. (2025). Monoterpenoids from the Roots of Liquidambar formosana (Formosan Sweet Gum) Exhibit Senomorphic Activity Against Cellular Senescence. Nutrients, 17(21), 3321. https://doi.org/10.3390/nu17213321






