Effect of Phenolic Compounds and Terpenes on the Flavour and Functionality of Plant-Based Foods
Abstract
1. Introduction
2. Methods and Scope of the Review
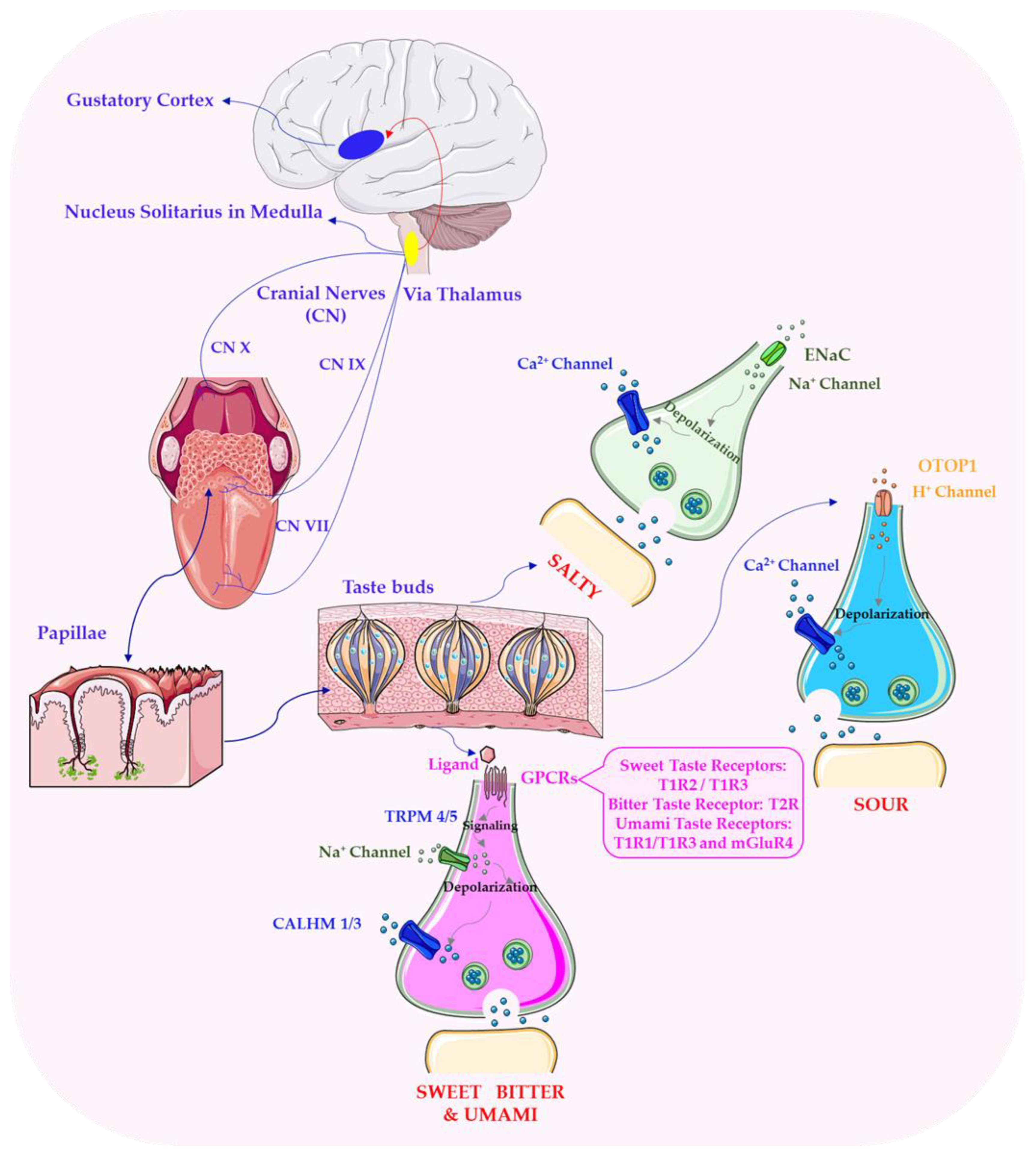
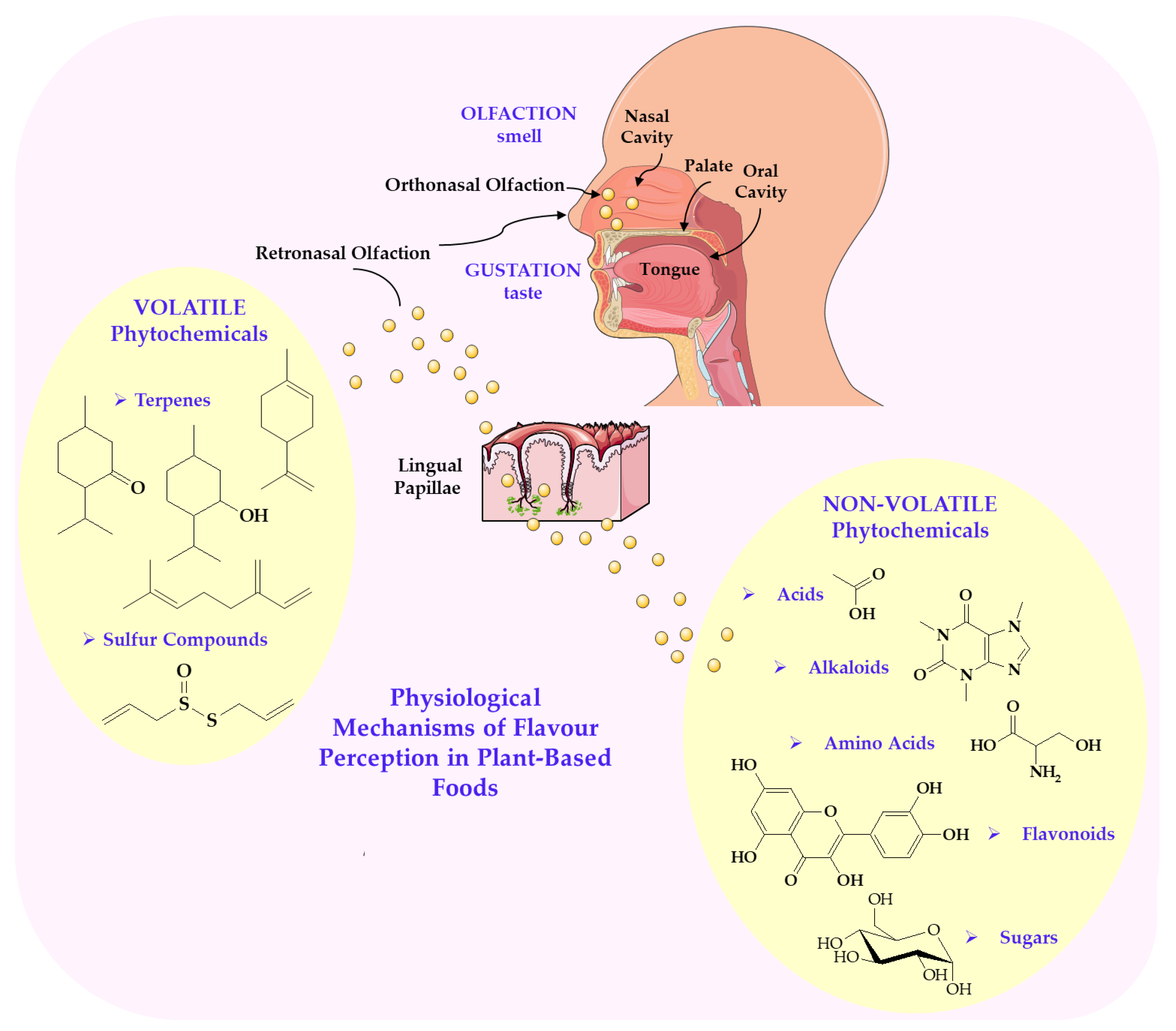
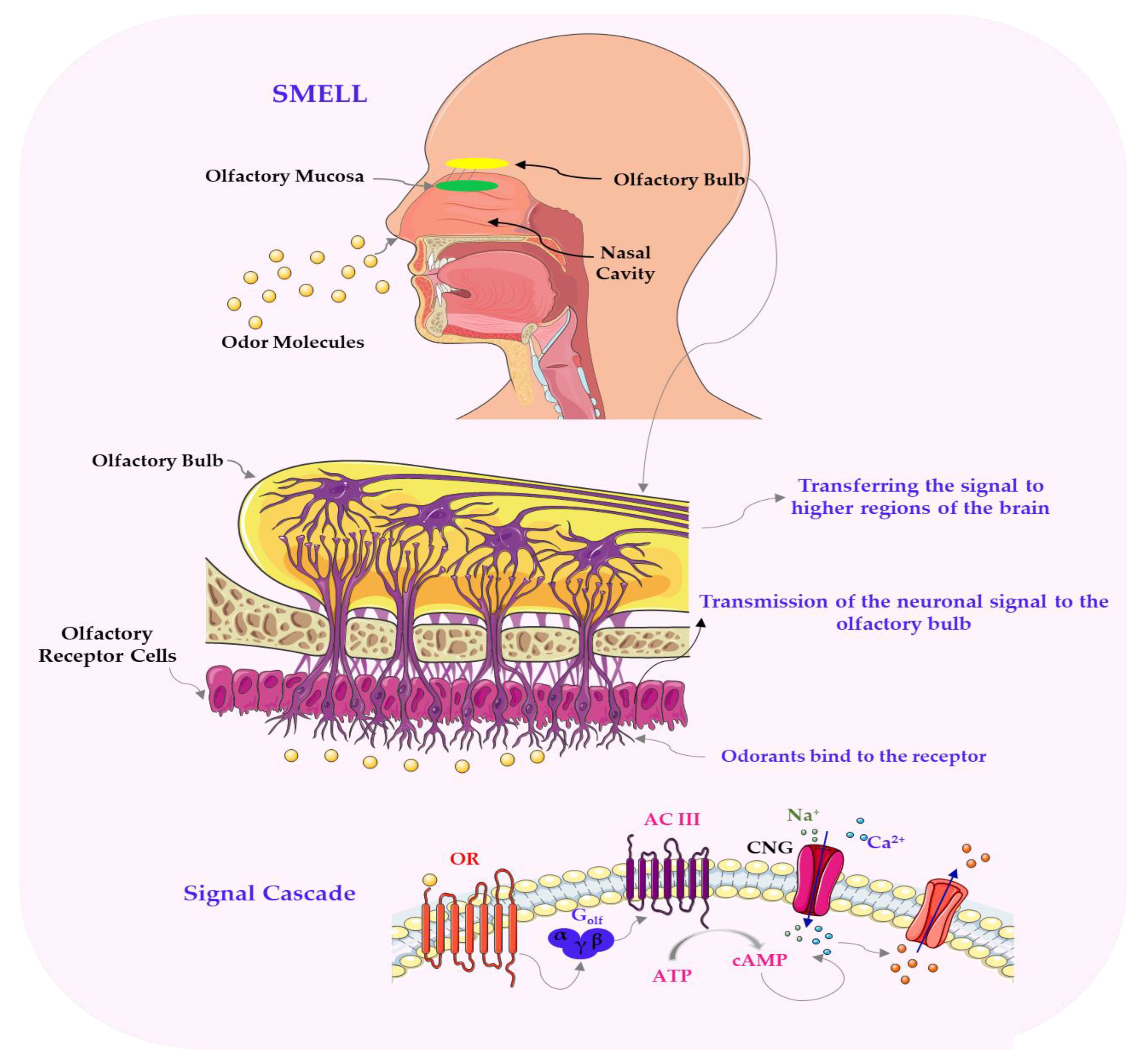
3. Molecular and Physiological Mechanisms of Flavour Perception in Plant-Based Foods
4. Functional Attributes in Food as Quality Indicators
5. Identification and Quantification of Flavour Compounds in Plant Foods
6. Phytochemical Classes and Molecular Interactions Underlying Flavour Perception in Plant-Based Foods
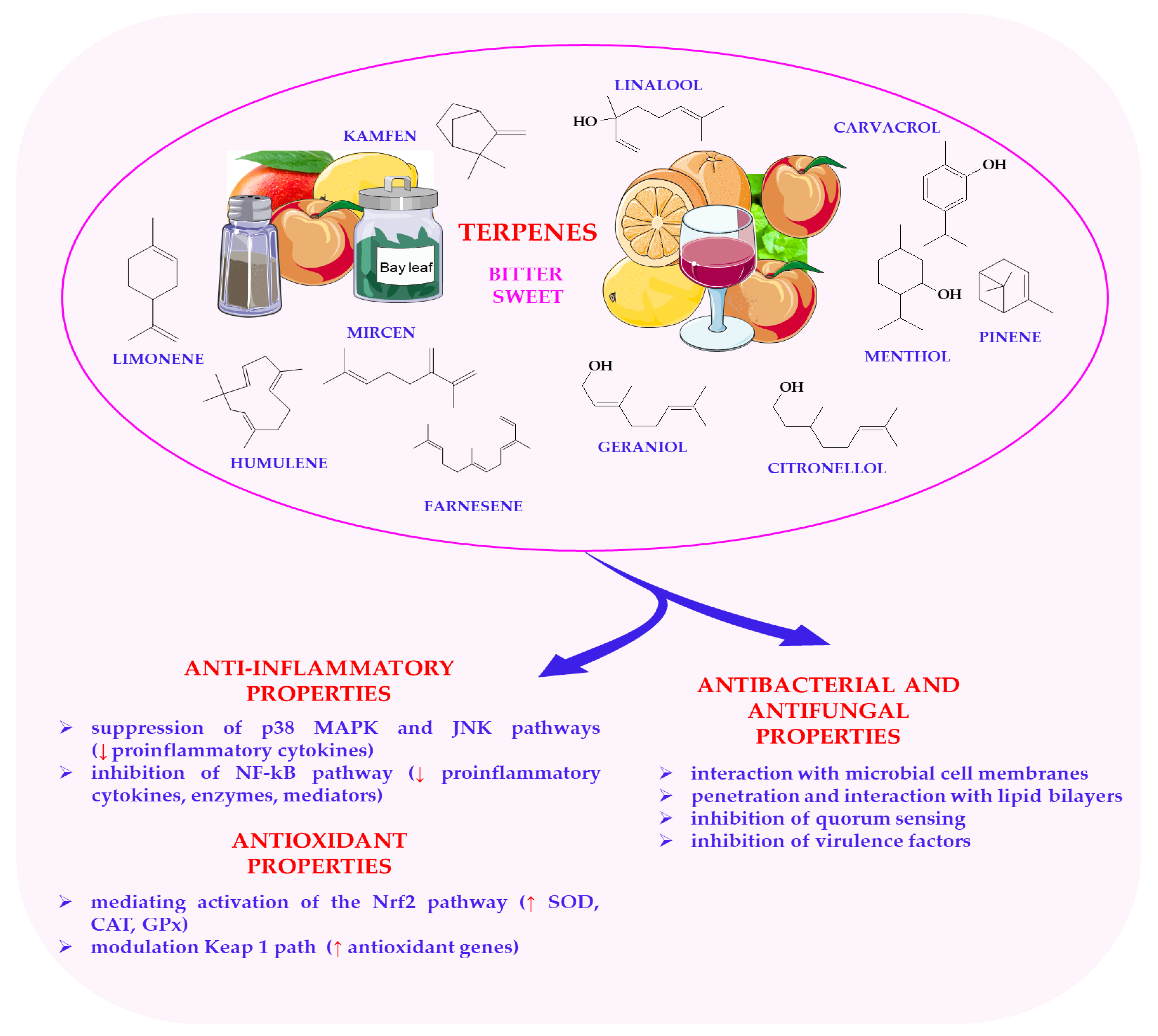
6.1. Terpenes in Plant-Based Foods: Aromatic Complexity and Functional Properties
6.2. Phenolic Compounds in Plant Foods
6.3. Flavonoids in Health-Promoting Effects of Plant-Based Foods
7. Phytochemicals: Linking Flavour Development, Antioxidant and Anti-Inflammatory Effects in Plant-Based Foods
8. Optimising Phytochemical Stability and Bioavailability in Plant-Based Foods Through Processing
9. Study Limitations
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sultanbawa, Y.; Sivakumar, D. Enhanced nutritional and phytochemical profiles of selected underutilized fruits, vegetables, and legumes. Curr. Opin. Food Sci. 2022, 46, 100853. [Google Scholar] [CrossRef]
- Harriden, B.; D’Cunha, N.M.; Kellett, J.; Isbel, S.; Panagiotakos, D.B.; Naumovski, N. Are dietary patterns becoming more processed? The effects of different dietary patterns on cognition: A review. Nutr. Health 2022, 28, 341–356. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez-Zamora, L.; Peñalver, R.; Marín-Iniesta, F.; Taboada-Rodríguez, A.; López-Gómez, A.; Martínez-Hernández, G.B. Applications of Plant Bioactive Compounds as Replacers of Synthetic Additives in the Food Industry. Foods 2023, 13, 47. [Google Scholar] [CrossRef]
- Fabela-Morón, M.F. Bioactive compounds, sensory attributes, and flavor perceptions involved in taste-active molecules in fruits and vegetables. Front. Nutr. 2024, 11, 1427857. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Tachie, C.; Nwachukwu, I.D.; Aryee, A.N.A. Trends and innovations in the formulation of plant-based foods. Food Prod. Proc. Nutr. 2023, 5, 16. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Venter de Villiers, M.; Cheng, J.; Truter, L. The Shift Towards Plant-Based Lifestyles: Factors Driving Young Consumers’ Decisions to Choose Plant-Based Food Products. Sustainability 2024, 16, 9022. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; González-Barrio, R.; Periago, M.J. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants 2023, 12, 976. [Google Scholar] [CrossRef]
- Park, K. The Role of Dietary Phytochemicals: Evidence from Epidemiological Studies. Nutrients 2023, 15, 1371. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Rodríguez-Negrete, E.V.; Morales-González, Á.; Madrigal-Santillán, E.O.; Sánchez-Reyes, K.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Valadez-Vega, C.; Chamorro-Cevallos, G.; Garcia-Melo, L.F.; Morales-González, J.A. Phytochemicals and Their Usefulness in the Maintenance of Health. Plants 2024, 13, 523. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why is it important to understand the nature and chemistry of tannins to exploit their potential as nutraceuticals? Food Res. Int. 2023, 173 Pt 2, 113329. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Brás, N.F.; García-Estévez, I.; Mateus, N.; Rivas-Gonzalo, J.C.; de Freitas, V.; Escribano-Bailón, M.T. Effect of flavonols on wine astringency and their interaction with human saliva. Food Chem. 2016, 209, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules 2024, 14, 234. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, J. The application of polyphenols in food preservation. Adv. Food Nutr. Res. 2021, 98, 35–99. [Google Scholar] [CrossRef]
- Raudone, L.; Savickiene, N. Phytochemical Profiles of Plant Materials: From Extracts to Added-Value Ingredients. Plants 2024, 13, 964. [Google Scholar] [CrossRef]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Novel Time- and Location-Independent Postharvest Treatment of Cocoa Beans: Investigations on the Aroma Formation during “Moist Incubation” of Unfermented and Dried Cocoa Nibs and Comparison to Traditional Fermentation. J. Agric. Food Chem. 2020, 68, 10336–10344. [Google Scholar] [CrossRef] [PubMed]
- Bickel Haase, T.; Schweiggert-Weisz, U.; Ortner, E.; Zorn, H.; Naumann, S. Aroma Properties of Cocoa Fruit Pulp from Different Origins. Molecules 2021, 26, 7618. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.; André, A.; Hühn, T.; Rohn, S.; Chetschik, I. Influence of Aerobic and Anaerobic Moist Incubation on Selected Nonvolatile Constituents–Comparison to Traditionally Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 16335–16346. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef]
- Pedrosa, M.C.; Lima, L.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food Metabolites as Tools for Authentication, Processing, and Nutritive Value Assessment. Foods 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Swarup, S. Metabolomics for Evaluating Flavor-Associated Metabolites in Plant-Based Products. Metabolites 2020, 10, 197. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Mehdizadeh, M.; Bahmid, N.A.; Adli, D.N.; Walker, T.R.; Perestrelo, R.; Câmara, J.S. Phytochemicals and bioactive constituents in food packaging—A systematic review. Heliyon 2023, 9, e21196. [Google Scholar] [CrossRef]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Spence, C. What Is the Relationship between the Presence of Volatile Organic Compounds in Food and Drink Products and Multisensory Flavour Perception? Foods 2021, 10, 1570. [Google Scholar] [CrossRef]
- Scott, K. The sweet and the bitter of mammalian taste. Curr. Opin. Neurobiol. 2004, 14, 423–427. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, M.L.; Grigorova, M.; Katz, D.B.; Maier, J.X. Retronasal Odor Perception Requires Taste Cortex, but Orthonasal Does Not. Curr. Biol. 2019, 29, 62–69.e3. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Sun, Z.; Yan, X.; Gao, X.; Lv, Q.; Wei, Y. Differences in olfactory habituation between orthonasal and retronasal pathways. J. Physiol. Sci. 2021, 71, 36. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Munger, S.D.; Sclafani, A.; de Araujo, I.E.; Roberts, A.; Molinary, S. Mechanisms for sweetness. J. Nutr. 2012, 142, 1134S–1141S. [Google Scholar] [CrossRef]
- Gutierrez, R.; Fonseca, E.; Simon, S.A. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Pydi, S.P.; Sobotkiewicz, T.; Billakanti, R.; Bhullar, R.P.; Loewen, M.C.; Chelikani, P. Amino acid derivatives as bitter taste receptor (T2R) blockers. J. Biol. Chem. 2014, 289, 25054–25066. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Diepeveen, J.; Moerdijk-Poortvliet, T.C.W.; van der Leij, F.R. Molecular insights into human taste perception and umami tastants: A review. J. Food Sci. 2022, 87, 1449–1465. [Google Scholar] [CrossRef]
- Kinnamon, S.C.; Finger, T.E. Recent advances in taste transduction and signaling. F1000Research 2019, 8, 2117. [Google Scholar] [CrossRef]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.; Lomvardas, S. Monoallelic expression of olfactory receptors. Annu. Rev. Cell Dev. Biol. 2015, 31, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Maßberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Rolls, E.T. Chemosensory learning in the cortex. Front. Syst. Neurosci. 2011, 5, 78. [Google Scholar] [CrossRef]
- Samuelsen, C.L.; Vincis, R. Cortical Hub for Flavor Sensation in Rodents. Front. Syst. Neurosci. 2021, 15, 772286. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavor perception. Cell 2015, 161, 24–35. [Google Scholar] [CrossRef]
- Cornelio, P.; Velasco, C.; Obrist, M. Multisensory Integration as per Technological Advances: A Review. Front. Neurosci. 2021, 15, 652611. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Gil, A. Nutrimetabolomics: An Update on Analytical Approaches to Investigate the Role of Plant-Based Foods and Their Bioactive Compounds in Non-Communicable Chronic Diseases. Int. J. Mol. Sci. 2016, 17, 2072. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Geng, L.; Liu, K.; Zhang, H. Lipid oxidation in foods and its implications on proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Nogueira, I.B.R.; Ribeiro, A.M. Flavor Engineering: A comprehensive review of biological foundations, AI integration, industrial development, and socio-cultural dynamics. Food Res. Int. 2024, 196, 115100. [Google Scholar] [CrossRef] [PubMed]
- Vincis, R.; Fontanini, A. Central taste anatomy and physiology. Handb. Clin. Neurol. 2019, 164, 187–204. [Google Scholar] [CrossRef]
- Dobon, B.; Rossell, C.; Walsh, S.; Bertranpetit, J. Is there adaptation in the human genome for taste perception and phase I biotransformation? BMC Evol. Biol. 2019, 19, 39. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef]
- Strauch, C.; Hoang, T.H.; Angenstein, F.; Manahan-Vaughan, D. Olfactory Information Storage Engages Subcortical and Cortical Brain Regions That Support Valence Determination. Cereb. Cortex 2022, 32, 689–708. [Google Scholar] [CrossRef]
- de Araujo, I.E.; Geha, P.; Small, D.M. Orosensory and Homeostatic Functions of the Insular Taste Cortex. Chemosens Percept. 2012, 5, 64–79. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Simon, S.A. The gustatory cortex and multisensory integration. Int. J. Obes. 2009, 33 (Suppl. S2), S34-43. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, H.; Xiao, X. The Insular Cortex: An Interface Between Sensation, Emotion and Cognition. Neurosci. Bull. 2024, 40, 1763–1773. [Google Scholar] [CrossRef]
- Ohla, K.; Toepel, U.; le Coutre, J.; Hudry, J. Visual-gustatory interaction: Orbitofrontal and insular cortices mediate the effect of high-calorie visual food cues on taste pleasantness. PLoS ONE 2012, 7, e32434. [Google Scholar] [CrossRef] [PubMed]
- Seubert, J.; Ohla, K.; Yokomukai, Y.; Kellermann, T.; Lundström, J.N. Superadditive opercular activation to food flavor is mediated by enhanced temporal and limbic coupling. Hum. Brain Mapp. 2015, 36, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015, 127–128, 64–90. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste and smell processing in the brain. Handb. Clin. Neurol. 2019, 164, 97–118. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Wilson, D.A.; Ravel, N.; Mouly, A.M. Olfactory memory networks: From emotional learning to social behaviors. Front. Behav. Neurosci. 2015, 9, 36. [Google Scholar] [CrossRef]
- Talavera, K.; Ninomiya, Y.; Winkel, C.; Voets, T.; Nilius, B. Influence of temperature on taste perception. Cell. Mol. Life Sci. 2007, 64, 377–381. [Google Scholar] [CrossRef]
- Liu, D.; Deng, Y.; Sha, L.; Abul Hashem, M.; Gai, S. Impact of oral processing on texture attributes and taste perception. J. Food Sci. Technol. 2017, 54, 2585–2593. [Google Scholar] [CrossRef]
- Motoki, K.; Spence, C.; Velasco, C. When visual cues influence taste/flavour perception: A systematic review. Food Qual. Pref. 2023, 111, 104996. [Google Scholar] [CrossRef]
- Reed, D.R.; Tanaka, T.; McDaniel, A.H. Diverse tastes: Genetics of sweet and bitter perception. Physiol. Behav. 2006, 88, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Scudine, K.G.O.; Castelo, P.M.; Hoppe, J.P.M.; Portella, A.K.; Silveira, P.P. Early Influences on Development of Sensory Perception and Eating Habits. Adv. Nutr. 2024, 15, 100325. [Google Scholar] [CrossRef]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Steinhaus, M.; Chetschik, I. Decoding the Fine Flavor Properties of Dark Chocolates. J. Agric. Food Chem. 2022, 70, 13730–13740. [Google Scholar] [CrossRef]
- Goya, L.; Kongor, J.E.; de Pascual-Teresa, S. From Cocoa to Chocolate: Effect of Processing on Flavanols and Methylxanthines and Their Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef]
- Dahiana Becerra, L.; Yolanda Ruiz-Pardo, R.; Vaillant, F.; Viviana Zuluaga, M.; Boulanger, R.; Santander, M.; Escobar, S. Modulating fine flavor cocoa attributes: Impact of seed-to-bean transformation under controlled conditions on metabolite, volatile and sensory profiles. Food Res. Int. 2024, 196, 115109. [Google Scholar] [CrossRef]
- Lima, L.J.; Almeida, M.H.; Nout, M.J.; Zwietering, M.H. Theobroma cacao L., “The food of the Gods”: Quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, C.; Granvogl, M. Characterization of the Key Aroma Compounds in Two Commercial Dark Chocolates with High Cocoa Contents by Means of the Sensomics Approach. J. Agric. Food Chem. 2019, 67, 5827–5837. [Google Scholar] [CrossRef]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Comparison of the Aroma Composition and Sensory Properties of Dark Chocolates Made with Moist Incubated and Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 4057–4065. [Google Scholar] [CrossRef]
- Ac-Pangan, M.F.; Engeseth, N.J.; Cadwallader, K.R. Identification of Important Aroma Components and Sensory Profiles of Minimally Processed (Unroasted) and Conventionally Roasted Dark Chocolates. J. Agric. Food Chem. 2023, 71, 9856–9867. [Google Scholar] [CrossRef]
- De Santis, D. Food Flavor Chemistry and Sensory Evaluation. Foods 2024, 13, 634. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical Strategies for Fingerprinting of Antioxidants, Nutritional Substances, and Bioactive Compounds in Foodstuffs Based on High Performance Liquid Chromatography-Mass Spectrometry: An Overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Begnaud, F.; Chaintreau, A. Good quantification practices of flavours and fragrances by mass spectrometry. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150365. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Perez-Hurtado, P.; Palmer, E.; Owen, T.; Aldcroft, C.; Allen, M.H.; Jones, J.; Creaser, C.S.; Lindley, M.R.; Turner, M.A.; Reynolds, J.C. Direct analysis of volatile organic compounds in foods by headspace extraction atmospheric pressure chemical ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1947–1956. [Google Scholar] [CrossRef]
- Epping, R.; Koch, M. On-Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Hu, C.; Yu, B.; Wan, C.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. The flavor substances changes in Fuliang green tea during storage monitoring by GC-MS and GC-IMS. Food Chem. X 2023, 21, 101047. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Biasioli, F.; Sani, G.; Cantini, C.; Märk, T.D.; Gasperi, F. Proton transfer reaction-mass spectrometry (PTR-MS) headspace analysis for rapid detection of oxidative alteration of olive oil. J. Agric. Food Chem. 2006, 54, 7635–7640. [Google Scholar] [CrossRef]
- Cappellin, L.; Loreto, F.; Aprea, E.; Romano, A.; del Pulgar, J.S.; Gasperi, F.; Biasioli, F. PTR-MS in Italy: A multipurpose sensor with applications in environmental, agri-food and health science. Sensors 2013, 13, 11923–11955. [Google Scholar] [CrossRef] [PubMed]
- Acierno, V.; Fasciani, G.; Kiani, S.; Caligiani, A.; van Ruth, S. PTR-QiToF-MS and HSI for the characterization of fermented cocoa beans from different origins. Food Chem. 2019, 289, 591–602. [Google Scholar] [CrossRef]
- Acierno, V.; de Jonge, L.; van Ruth, S. Sniffing out cocoa bean traits that persist in chocolates by PTR-MS, ICP-MS and IR-MS. Food Res. Int. 2020, 133, 109212. [Google Scholar] [CrossRef]
- Pleil, J.D.; Hansel, A.; Beauchamp, J. Advances in proton transfer reaction mass spectrometry (PTR-MS): Applications in exhaled breath analysis, food science, and atmospheric chemistry. J. Breath Res. 2019, 13, 039002. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High Resolution NMR Spectroscopy as a Structural and Analytical Tool for Unsaturated Lipids in Solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Valentino, G.; Graziani, V.; D’Abrosca, B.; Pacifico, S.; Fiorentino, A.; Scognamiglio, M. NMR-Based Plant Metabolomics in Nutraceutical Research: An Overview. Molecules 2020, 25, 1444. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Lee, Y. Instrumental Analysis or Human Evaluation to Measure the Appearance, Smell, Flavor, and Physical Properties of Food. Foods 2023, 12, 3453. [Google Scholar] [CrossRef]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi)Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography-Tandem Mass Spectrometry Technique (LC-MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Oliva, E.; Fanti, F.; Palmieri, S.; Viteritti, E.; Eugelio, F.; Pepe, A.; Compagnone, D.; Sergi, M. Predictive Multi Experiment Approach for the Determination of Conjugated Phenolic Compounds in Vegetal Matrices by Means of LC-MS/MS. Molecules 2022, 27, 3089. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Recent trends of machine learning applied to multi-source data of medicinal plants. J. Pharm. Anal. 2023, 13, 1388–1407. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Wu, M.J.; Zhong, K.J.; Sun, X.J.; Liang, Y.Z.; Dai, Y.H.; Huang, K.L.; Guo, F.Q. Fingerprint developing of coffee flavor by gas chromatography-mass spectrometry and combined chemometrics methods. Anal. Chim. Acta 2007, 588, 216–223. [Google Scholar] [CrossRef]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis Sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef]
- Franzin, M.; Ruoso, R.; Del Savio, R.; Niaki, E.A.; Pettinelli, A.; Decorti, G.; Stocco, G.; Addobbati, R. Quantification of 7 cannabinoids in cannabis oil using GC-MS: Method development, validation and application to therapeutic preparations in Friuli Venezia Giulia region, Italy. Heliyon 2023, 9, e15479. [Google Scholar] [CrossRef]
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods 2023, 12, 3222. [Google Scholar] [CrossRef]
- Wagner, J.; Wilkin, J.D.; Szymkowiak, A.; Grigor, J. Sensory and affective response to chocolate differing in cocoa content: A TDS and facial electromyography approach. Physiol. Behav. 2023, 270, 114308. [Google Scholar] [CrossRef]
- Hossain, M.J.; Alam, A.N.; Kim, S.H.; Kim, C.J.; Joo, S.T.; Hwang, Y.H. Techniques and Emerging Trends in Flavor and Taste Development in Meat. Food Sci. Anim. Resour. 2025, 45, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Singh, A.; Paul, D.; Dasgupta, D.; Urajová, P.; Ghosh, S.; Singh, R.; Sahoo, G.; Ewe, D.; Saurav, K. Recent Advances in the Detection of Food Toxins Using Mass Spectrometry. Chem. Res. Toxicol. 2023, 36, 1834–1863. [Google Scholar] [CrossRef]
- Bharti, A.; Jain, U.; Chauhan, N. Progressive analytical techniques utilized for the detection of contaminants attributed to food safety and security. Talanta Open 2024, 10, 100368. [Google Scholar] [CrossRef]
- Mischler, S.; André, A.; Chetschik, I.; Miescher Schwenninger, S. Potential for the Bio-Detoxification of the Mycotoxins Enniatin B and Deoxynivalenol by Lactic Acid Bacteria and Bacillus spp. Microorganisms 2024, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Mycotoxins in Food: Cancer Risks and Strategies for Control. Foods 2024, 13, 3502. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Abe Cunha, D.H.; Berciano, S. Bioactive compounds for human and planetary health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular mechanisms of taste recognition: Considerations about the role of saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bennick, A. Interaction of tannin with human salivary proline-rich proteins. Arch. Oral Biol. 1998, 43, 717–728. [Google Scholar] [CrossRef]
- McRae, J.M.; Kennedy, J.A. Wine and grape tannin interactions with salivary proteins and their impact on astringency: A review of current research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, X.; Zeng, F. Biological Functions and Health Benefits of Flavonoids in Fruits and Vegetables: A Contemporary Review. Foods 2025, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Genva, M.; Kenne Kemene, T.; Deleu, M.; Lins, L.; Fauconnier, M.L. Is It Possible to Predict the Odor of a Molecule on the Basis of its Structure? Int. J. Mol. Sci. 2019, 20, 3018. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.C.; Xiao, Y.; Liu, H.W.; Liu, P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Koyama, S.; Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef]
- Jabalpurwala, F.A.; Smoot, J.M.; Rouseff, R.L. A comparison of citrus blossom volatiles. Phytochemistry 2009, 70, 1428–1434. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 108151. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Custodio-Mendoza, J.A.; Pokorski, P.; Aktaş, H.; Napiórkowska, A.; Kurek, M.A. Advances in Chromatographic Analysis of Phenolic Phytochemicals in Foods: Bridging Gaps and Exploring New Horizons. Foods 2024, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Kazemi, A.; Iraji, A.; Esmaealzadeh, N.; Salehi, M.; Hashempur, M.H. Peppermint and menthol: A review on their biochemistry, pharmacological activities, clinical applications, and safety considerations. Crit. Rev. Food Sci. Nutr. 2024, 3, 1–26. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Styczyńska, S.; Synowiec, A.; Gniewosz, M.; Bączek, K. Activity of Common Thyme (Thymus vulgaris L.), Greek Oregano (Origanum vulgare L. ssp. hirtum), and Common Oregano (Origanum vulgare L. ssp. vulgare) Essential Oils against Selected Phytopathogens. Molecules 2024, 29, 4617. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.; Nes, W.D.; Gershenzon, J. Both methylerythritol phosphate and mevalonate pathways contribute to biosynthesis of each of the major isoprenoid classes in young cotton seedlings. Phytochemistry 2014, 98, 110–119. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Sommer, S.; Lang, L.M.; Drummond, L.; Buchhaupt, M.; Fraatz, M.A.; Zorn, H. Odor Characteristics of Novel Non-Canonical Terpenes. Molecules 2022, 27, 3827. [Google Scholar] [CrossRef]
- Auffarth, B. Understanding smell—The olfactory stimulus problem. Neurosci. Biobehav. Rev. 2013, 37, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Satou, T.; Takemoto, H.; Koike, K. Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology. Molecules 2021, 26, 2571. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. (Eds.) Neuroscience, 2nd ed.; Taste Receptors and the Transduction of Taste Signals; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11148/ (accessed on 20 May 2025).
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary Phenolic Compounds–Wellbeing and Perspective Applications. Int. J. Mol. Sci. 2024, 25, 4769. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Commisso, M.; Bianconi, M.; Poletti, S.; Negri, S.; Munari, F.; Ceoldo, S.; Guzzo, F. Metabolomic Profiling and Antioxidant Activity of Fruits Representing Diverse Apple and Pear Cultivars. Biology 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Fujimoto, H.; Narita, Y.; Iwai, K.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; Wu, X.; Miyake, K.; Tahara, Y.; et al. Bitterness compounds in coffee brew measured by analytical instruments and taste sensing system. Food Chem. 2021, 342, 128228. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Paloukopoulou, C.; Karioti, A.; Maloupa, E.; Grigoriadou, K. Rosmarinic Acid Production from Origanum dictamnus L. Root Liquid Cultures In Vitro. Plants 2023, 12, 299. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Samanta, S.; Sarkar, T.; Chakraborty, R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Rengasamy, K.R.R. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr. Res. Food Sci. 2022, 5, 1916–1943. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, R.; Rathee, P.; Akkol, E.K.; Khatkar, S.; Lather, A.; Redhu, N.; Khatkar, A. Phenolic Acids–Versatile Natural Moiety with Numerous Biological Applications. Curr. Top. Med. Chem. 2022, 22, 1472–1484. [Google Scholar] [CrossRef]
- Qader, M.; Xu, J.; Yang, Y.; Liu, Y.; Cao, S. Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa. Beverages 2020, 6, 68. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Moon, D.O. Plant-Derived Flavonoids as AMPK Activators: Unveiling Their Potential in Type 2 Diabetes Management through Mechanistic Insights, Docking Studies, and Pharmacokinetics. Appl. Sci. 2024, 14, 8607. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Sair, A.T.; Liu, R.H. Molecular regulation of phenolic compounds on IGF-1 signaling cascade in breast cancer. Food Funct. 2022, 13, 3170–3184. [Google Scholar] [CrossRef]
- Li, S.; Yin, S.; Ding, H.; Shao, Y.; Zhou, S.; Pu, W.; Han, L.; Wang, T.; Yu, H. Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention. Cell Prolif. 2023, 56, e13346. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. Daru 2019, 27, 433–449. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Ríos, J.L.; Schinella, G.R.; Moragrega, I. Phenolics as GABAA Receptor Ligands: An Updated Review. Molecules 2022, 27, 1770. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, A.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.L.; Arráez-Román, D.; Segura-Carretero, A. Neuroprotective Effects of Agri-Food By-Products Rich in Phenolic Compounds. Nutrients 2023, 15, 449. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Suhag, R. Impact of Fining Agents on Color, Phenolics, Aroma, and Sensory Properties of Wine: A Review. Beverages 2024, 10, 71. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Q.; Zhao, S.; Xia, X.; Yan, X.; An, Y.; Mi, X.; Guo, L.; Samarina, L.; Wei, C. Comprehensive co-expression analysis provides novel insights into temporal variation of flavonoids in fresh leaves of the tea plant (Camellia sinensis). Plant Sci. 2020, 290, 110306. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Tzen, J.T.C. Exploring the Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies. Molecules 2022, 27, 5679. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, A Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Zhao, X.; Ai, Y.; Hu, Y.; Wang, Y.; Zhao, L.; Yang, D.; Chen, F.; Wu, X.; Li, Y.; Liao, X. Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation. Foods 2020, 9, 756. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, W.; Niu, Y.; Li, W.; Lu, W.; Yu, L.L. Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling. Foods 2024, 13, 4016. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef]
- Chen, X.Q.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.B.; Zhang, Y.T.; Du, Y.; Jiang, Y.W. Preventive Effects of Catechins on Cardiovascular Disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Angel Prieto, M.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-inflammatory activity of flavonols via inhibiting MAPK and NF-κB signaling pathways in RAW264.7 macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, R. Flavonoids and asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.S.; Rahman, S.; Rupasinghe, H.P.V.; Vazhappilly, C.G. Dietary Flavonoids in p53-Mediated Immune Dysfunctions Linking to Cancer Prevention. Biomedicines 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, A.; Hamid, L.; Nisar, N.; Malik, J.A.; Ali, T.; Bader, G.N. Flavonoids as promising molecules in the cancer therapy: An insight. Curr. Res. Pharmacol. Drug Discov. 2023, 6, 100167. [Google Scholar] [CrossRef] [PubMed]
- de Luna, F.C.F.; Ferreira, W.A.S.; Casseb, S.M.M.; de Oliveira, E.H.C. Anticancer Potential of Flavonoids: An Overview with an Emphasis on Tangeretin. Pharmaceuticals 2023, 16, 1229. [Google Scholar] [CrossRef]
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971. [Google Scholar] [CrossRef]
- Alexander, C.; Parsaee, A.; Vasefi, M. Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease. Biology 2023, 12, 1453. [Google Scholar] [CrossRef]
- Wasowski, C.; Marder, M. Flavonoids as GABAA receptor ligands: The whole story? J. Exp. Pharmacol. 2012, 4, 9–24. [Google Scholar] [CrossRef]
- Shen, M.L.; Wang, C.H.; Chen, R.Y.; Zhou, N.; Kao, S.T.; Wu, D.C. Luteolin inhibits GABAA receptors in HEK cells and brain slices. Sci. Rep. 2016, 6, 27695. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Liu, Z.; Danzengquzhen; Cisangzhuoma; Ma, J.; Li, X.; Huang, X.; Li, B. The neuromodulatory effects of flavonoids and gut Microbiota through the gut-brain axis. Front. Cell. Infect. Microbiol. 2023, 13, 1197646. [Google Scholar] [CrossRef]
- Pimentel, F.A.; Nitzke, J.A.; Klipel, C.B.; Vogt de Jong, E. Chocolate and red wine—A comparison between flavonoids content. Food Chem. 2010, 120, 109–112. [Google Scholar] [CrossRef]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef] [PubMed]
- Ndao, A.; Adjallé, K. Overview of the Biotransformation of Limonene and α-Pinene from Wood and Citrus Residues by Microorganisms. Waste 2023, 1, 841–859. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008, 59, 299–326. [Google Scholar] [CrossRef]
- Pyo, Y.; Kwon, K.H.; Jung, Y.J. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods 2024, 13, 2253. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y. The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: A narrative review. Neural Regen. Res. 2023, 18, 2582–2591. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Gupta, S. Extraction, Characterization, Stability and Biological Activity of Flavonoids Isolated from Chamomile Flowers. Mol. Cell. Pharmacol. 2009, 1, 138. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics 2025, 17, 134. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedić, N.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Polyphenol profile of buckwheat honey, nectar and pollen. R. Soc. Open Sci. 2020, 7, 201576. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Vieira, S.F.; Reis, R.L.; Ferreira, H.; Neves, N.M. Plant-derived bioactive compounds as key players in the modulation of immune-related conditions. Phytochem. Rev. 2024, 24, 343–460. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Huang, C.; Gao, Y.; Li, M.; Zhang, C.; Li, M. Gut microbiota axis: Potential target of phytochemicals from plant-based foods. Food Sci. Hum. Wellness 2023, 12, 1409–1426. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Ysrafil, Y.; Sapiun, Z.; Slamet, N.S.; Mohamad, F.; Hartati, H.; Damiti, S.A.; Alexandra, F.D.; Rahman, S.; Masyeni, S.; Harapan, H.; et al. Anti-inflammatory activities of flavonoid derivates. ADMET DMPK 2023, 11, 331–359. [Google Scholar] [CrossRef]
- He, W.J.; Lv, C.H.; Chen, Z.; Shi, M.; Zeng, C.X.; Hou, D.X.; Qin, S. The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Seidel, V.; Izabela, M.; Monserrat-Mequida, M.; Sureda, A.; Ormazabal, V.; Zuniga, F.A.; Mangalpady, S.S.; Pezzani, R.; Ydyrys, A.; et al. Phenolic compounds as Nrf2 inhibitors: Potential applications in cancer therapy. Cell Commun. Signal. 2023, 21, 89. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.-X. The Biofunctions of Phytochemicals and Their Applications in Farm Animals: The Nrf2/Keap1 System as a Target. Engineering 2017, 3, 738–752. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69 Pt B, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Cheng, C.; Zhang, M.; Su, B.; Hou, Y.; Bai, G. Resveratrol targeting NRF2 disrupts the binding between KEAP1 and NRF2-DLG motif to ameliorate oxidative stress damage in mice pulmonary infection. J. Ethnopharmacol. 2024, 332, 118353. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Zardak, M.; Keshavarz, F.; Mahyaei, A.; Gholami, M.; Moosavi, F.S.; Abbasloo, E.; Abdollahi, F.; Hossein Rezaei, M.; Madadizadeh, E.; Soltani, N.; et al. Quercetin as a therapeutic agent activate the Nrf2/Keap1 pathway to alleviate lung ischemia-reperfusion injury. Sci. Rep. 2024, 14, 23074. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Hine, C.M.; Mitchell, J.R. NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction. J. Clin. Exp. Pathol. 2012, S4, 7329. [Google Scholar] [CrossRef]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Tilmisany, A.K. Cancer and phase II drug-metabolizing enzymes. Curr. Drug Metab. 2003, 4, 45–58. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using plant-based compounds as preservatives for meat products: A review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in health and food processing: Antibacterial, anti-inflammatory, and antioxidant insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.J.; Kim, C.Y. The Development of New Functional Foods and Ingredients. Foods 2024, 13, 3038. [Google Scholar] [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of food processing on antioxidants, their bioavailability and potential relevance to human health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef]
- Conte, A.; Martini, S.; Tagliazucchi, D. Influence of Processing and Digestion on the Stability, Bioac-Cessibility and Bioactivity of Food Polyphenols. Foods 2023, 12, 851. [Google Scholar] [CrossRef]
- DeBenedictis, J.N.; de Kok, T.M.; van Breda, S.G. Impact of Processing Method and Storage Time on Phytochemical Concentrations in an Antioxidant-Rich Food Mixture. Antioxidants 2023, 12, 1252. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197 Pt A, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Dhaouefi, Z.; Maatouk, M.; Sassi, A.; Boudhiba, N.; Ioannou, I.; Ghedira, K.; Chekir-Ghedira, L.; Kilani-Jaziri, S. Heat treatment improves the immunomodulatory and cellular antioxidant behavior of a natural flavanone: Eriodictyol. Int. Immunopharmacol. 2018, 61, 317–324. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Sarıtaş, S.; Portocarrero, A.C.M.; Miranda López, J.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Park, H.-Y.; Sim, E.-Y.; Kim, H.-S.; Choi, H.-S. Microbial Fermentation in Food: Impact on Functional Properties and Nutritional Enhancement—A Review of Recent Developments. Fermentation 2025, 11, 15. [Google Scholar] [CrossRef]
- Luo, X.; Dong, M.; Liu, J.; Guo, N.; Li, J.; Shi, Y.; Yang, Y. Fermentation: Improvement of pharmacological effects and applications of botanical drugs. Front. Pharmacol. 2024, 15, 1430238. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Proc. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; van der Kooi, C.J. Coloration of Flowers by Flavonoids and Consequences of pH Dependent Absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef]
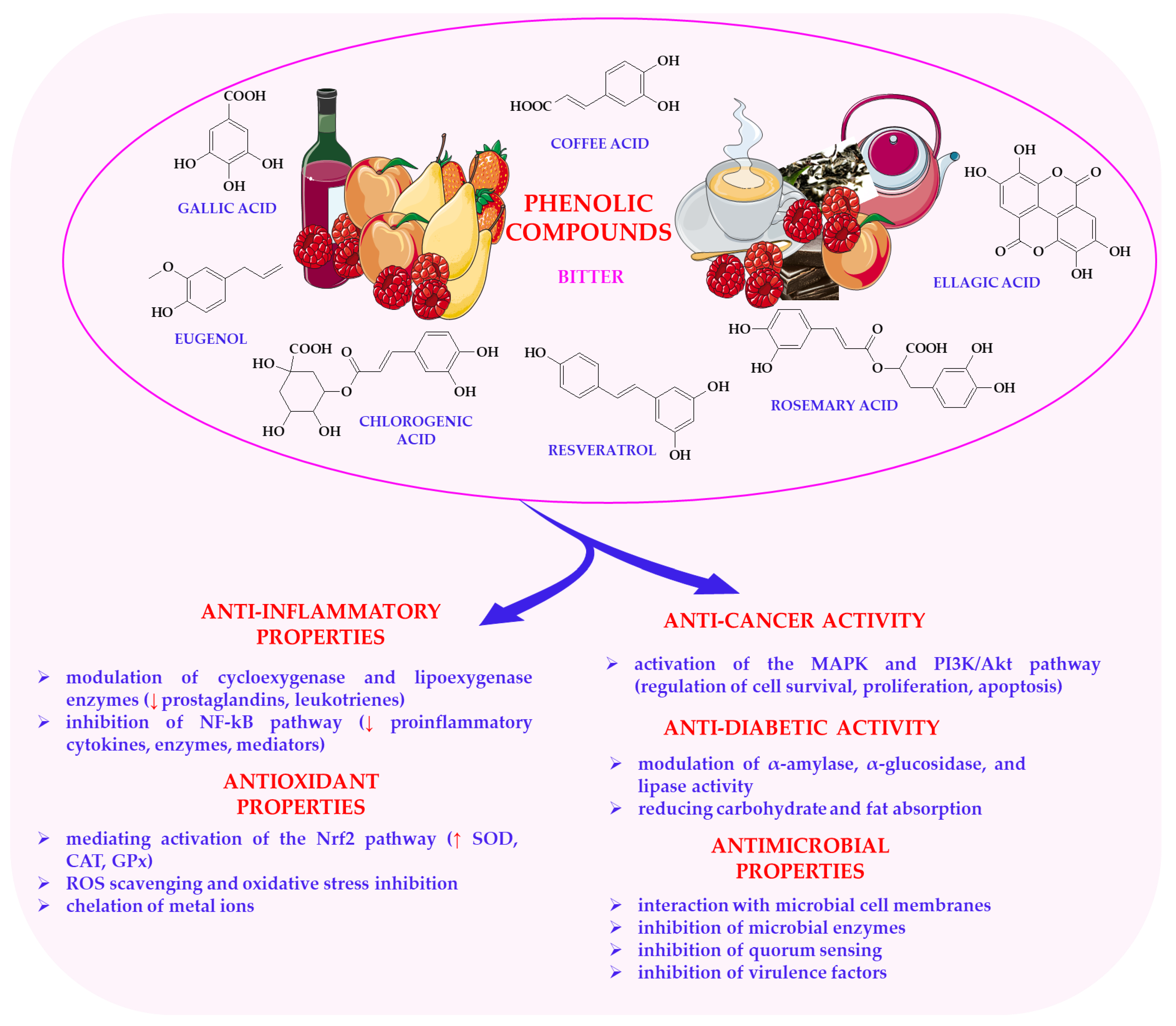
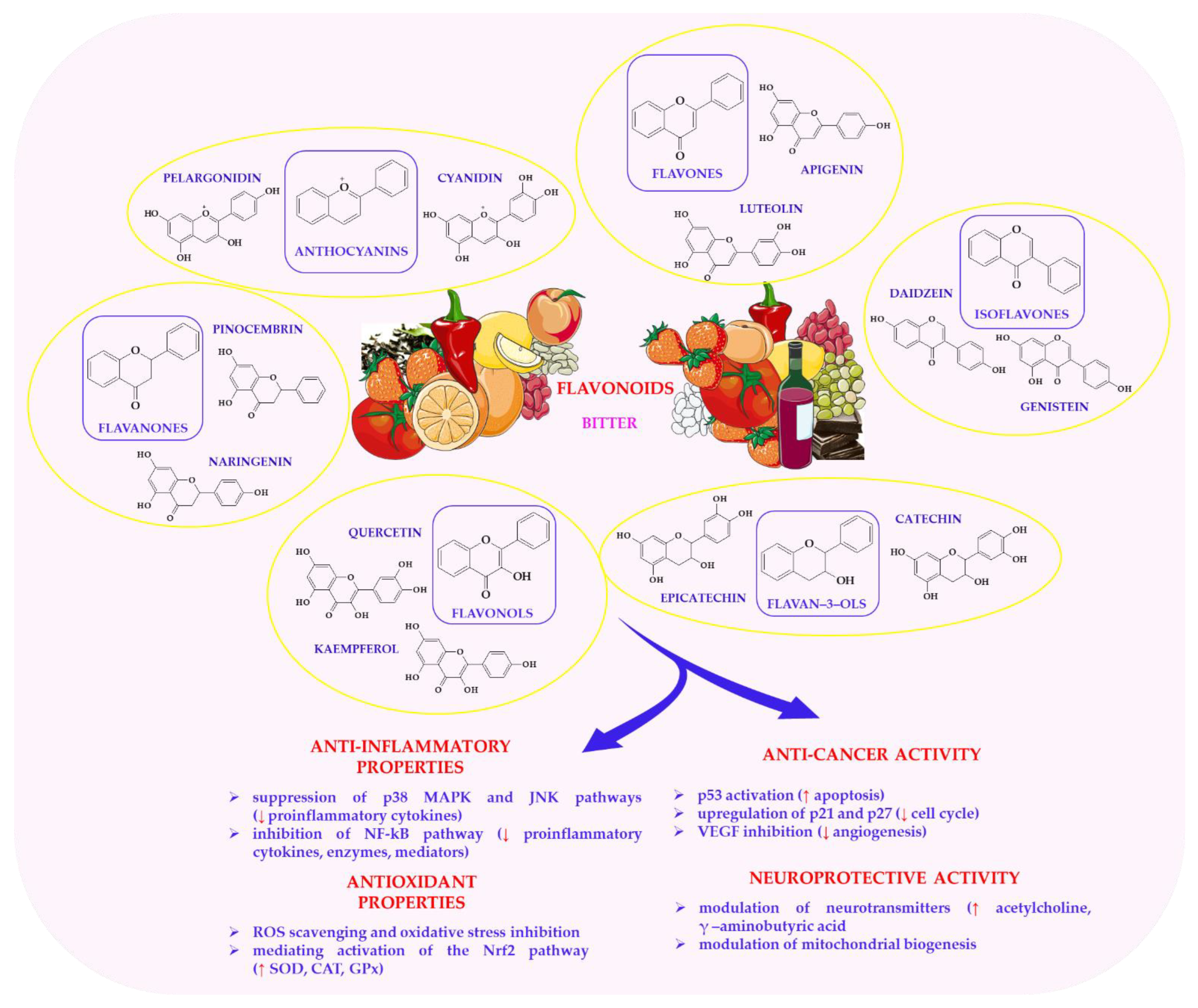
| Compound | Plant Source | Sensory Characteristics | Bioactive Effect | References |
|---|---|---|---|---|
| Quercetin | Apples, onions, tea, berries | Bitter, astringent | Antioxidant, anti-inflammatory, anticancer, neuroprotective, anti-diabetic | [135,220,227,251] |
| Kaempferol | Kale, spinach, tea | Slightly bitter | Antioxidant, anti-inflammatory, neuroprotective, anticancer | [220,252,253] |
| Catechins (e.g., epicatechin) | Green tea, dark chocolate | Astringent, slightly bitter | Antioxidant, cardioprotective, neuroprotective, anticancer | [218,223] |
| Anthocyanins | Berries, hibiscus | Tart, fruity | Antioxidant, anti-inflammatory, neuroprotective | [135,254] |
| Apigenin | Chamomile | Sweet, floral | Neuroprotective, anti-inflammatory, antioxidant | [228,255] |
| Resveratrol | Red wine, grapes | Slightly bitter, fruity | Antioxidant, anti-inflammatory, cardioprotective, anticancer | [245,256] |
| Pinocembrin | Buckwheat honey | Slightly bitter, robust | Antioxidant, neuroprotective | [257] |
| Limonene | Citrus fruits | Citrus, fresh | Antioxidant, anti-inflammatory, antimicrobial | [20,154] |
| Pinene | Pine needles, rosemary | Pine, fresh, resinous | Anti-inflammatory, antimicrobial, antioxidant | [20,160] |
| Myrcene | Hops, mango, lemongrass | Earthy, herbaceous, tropical | Anti-inflammatory, analgesic | [20,156] |
| Linalool | Lavender, mint | Floral, sweet | Sedative, anti-anxiety, antioxidant | [20,161] |
| Terpinene | Thyme, oregano | Citrus, herbaceous | Antioxidant, antimicrobial | [20,162] |
| Caryophyllene | Black pepper, cloves | Spicy, peppery | Anti-inflammatory, analgesic, antimicrobial | [20,163] |
| Geraniol | Geranium, rose | Sweet, floral | Antioxidant, anti-inflammatory | [20,164] |
| Citronellol | Rose, geranium | Sweet, floral | Antioxidant, antimicrobial | [20,164] |
| Eugenol | Cloves | Spicy, clove-like | Antimicrobial, antioxidant, analgesic | [159,180] |
| Vanillin | Vanilla pods | Sweet, creamy | Antioxidant, antimicrobial | [20,248] |
| Chlorogenic acid | Coffee, apples | Slightly bitter, earthy | Antioxidant, anti-diabetic | [179,193] |
| Ellagic acid | Berries | Tart, earthy | Antioxidant, anticancer | [176,177] |
| Gallic acid | Dark honey, dark chocolate | Astringent, slightly bitter | Antioxidant, anticancer | [182,213] |
| Major Compounds | Plant Sources | Processing Technique | Effect on Stability | Effect on Bioavailability | Effect on Sensory Profile | Key References |
|---|---|---|---|---|---|---|
| Quercetin, kaempferol, catechins, anthocyanins | Apples, onions, tea, red wine, dark chocolate | Thermal (blanching, pasteurization, cooking) | Partial degradation; anthocyanins sensitive; catechins moderately stable | Slight increase due to breakdown into smaller metabolites, but bioactivity may change | Color fading; slight bitterness remains | [283,286,287] |
| Quercetin, kaempferol | Berries, citrus | Fermentation | Generally stable; some microbial transformation | Enhanced due to conversion of glycosides to aglycones | Improved flavor complexity | [293,296] |
| Quercetin, catechins | Dark chocolate, tea | Encapsulation/Nanoformulation | Stabilized against heat, light, and oxidation | Significantly improved solubility and absorption | Minimal impact on taste; reduced astringency | [289,290,292] |
| Quercetin, kaempferol | Berries, Citrus | pH adjustment | Acidic or alkaline conditions can alter stability | Changes in solubility and ionization may enhance or reduce bioavailability | Altered color, flavor, aroma | [300,301] |
| Limonene, pinene, myrcene, linalool | Citrus, herbs, mango, hops | Thermal | Highly volatile; significant losses | Reduced due to degradation | Loss of aroma and aromatic intensity | [20,285] |
| Linalool, geraniol | Lavender, mint, rose | Fermentation | Slight biotransformation; relatively stable | Slightly improved due to release from matrix | Enhanced floral notes; new aroma compounds | [164,294] |
| Limonene, pinene | Citrus, pine | Encapsulation/Nanoformulation | High stability; protected from heat and oxidation | Improved controlled release and absorption | Preserved aroma; prolonged sensory perception | [290,291] |
| Chlorogenic acid, ellagic acid, tannins, resveratrol | Coffee, berries, tea, red wine | Thermal | Partial degradation; tannins moderately stable | Extractability may increase but degradation reduces bioactivity | Bitterness and astringency may increase | [187,288] |
| Catechins, flavonoids | Tea, coffee, cocoa | Fermentation | Microbial transformation can produce more bioactive metabolites | Increased bioavailability | Enhanced flavor complexity; production of SCFAs | [193,298] |
| Flavonoids, tannins | Berries, chocolate | Encapsulation/Nanoformulation | Protected from oxidation | Increased absorption; targeted release | Minimal sensory change | [125,184] |
| Flavonoids, phenolic acids | Various fruits and vegetables | Oxidative stress/Storage conditions | Degradation due to reactive oxygen species | Reduced if not stabilized | Color and flavor changes | [30,288] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurhaluk, N.; Buyun, L.; Kołodziejska, R.; Kamiński, P.; Tkaczenko, H. Effect of Phenolic Compounds and Terpenes on the Flavour and Functionality of Plant-Based Foods. Nutrients 2025, 17, 3319. https://doi.org/10.3390/nu17213319
Kurhaluk N, Buyun L, Kołodziejska R, Kamiński P, Tkaczenko H. Effect of Phenolic Compounds and Terpenes on the Flavour and Functionality of Plant-Based Foods. Nutrients. 2025; 17(21):3319. https://doi.org/10.3390/nu17213319
Chicago/Turabian StyleKurhaluk, Natalia, Lyudmyla Buyun, Renata Kołodziejska, Piotr Kamiński, and Halina Tkaczenko. 2025. "Effect of Phenolic Compounds and Terpenes on the Flavour and Functionality of Plant-Based Foods" Nutrients 17, no. 21: 3319. https://doi.org/10.3390/nu17213319
APA StyleKurhaluk, N., Buyun, L., Kołodziejska, R., Kamiński, P., & Tkaczenko, H. (2025). Effect of Phenolic Compounds and Terpenes on the Flavour and Functionality of Plant-Based Foods. Nutrients, 17(21), 3319. https://doi.org/10.3390/nu17213319








