Abstract
Background: Food allergies are increasingly recognized as a global health concern, influenced by early-life nutrition and the gut microbiome. This systematic review examined randomized controlled trials from 2005 to 2025 assessing the effects of probiotics, prebiotics, and synbiotics in preventing food allergies. Methods: Fourteen studies involving 5685 participants, including pregnant women, infants, and children with or without diagnosed food allergies, were analyzed. While several interventions demonstrated modulation of gut microbiota and immune responses, most trials reported no statistically significant reduction in IgE-mediated food allergy compared with placebo. Results: Some evidence suggested benefits from early exposure to allergenic foods and specific probiotic strains, such as Lactobacillus rhamnosus GG, particularly in cow’s milk allergy. However, heterogeneity in study designs, strains, dosages, and diagnostic criteria limited generalizability. Conclusions: Overall, microbiome-targeted nutritional interventions show biological plausibility but inconsistent clinical efficacy. Future large-scale, standardized, and mechanistic studies integrating microbiome, genetic, and environmental data are warranted to define optimal strategies for allergy prevention.
1. Introduction
A food allergy (FA) is a pathogenic immunological response triggered by consuming an antigen found in food proteins. Minimal exposure to allergic foods can cause clinical symptoms, such as urticaria, lung irritation, and gastrointestinal problems, that range in intensity from moderate to life-threatening [1]. Food allergies (FA) can be classified as either immunoglobulin E (IgE)-mediated or non-IgE-mediated, depending on the role of IgE in their development [2]. According to recent reviews of studies published in the past few years, food allergies affect approximately 8% of children and 10% of adults in developed countries, representing a significant public health issue. While the global prevalence of food allergies differs across regions, environmental factors potentially interacting with genetic susceptibility are considered the main contributors to their rising incidence [3]. In recent decades, both the prevalence of food allergies and the rate of hospitalizations due to food-induced anaphylaxis have risen significantly. This trend is often described as the “second wave of the allergy epidemic,” succeeding in the earlier surge in respiratory allergies and asthma over the past several decades [4,5,6]. Food allergens were found to be responsible for 79% of recurrent anaphylaxis cases and 37% of intensive care unit (ICU) admissions related to anaphylaxis [7]. IgE-mediated food allergies are most prevalent during infancy and early childhood, largely due to the relatively high occurrence of egg and cow’s milk allergies, which often resolve as children grow older. On the other hand, allergies to peanuts and tree nuts, which also usually appear in infancy, are less likely to go away and hence become more common in later life [8]. The most frequent allergies are fish and shellfish for adults, peanuts and tree nuts for youngsters, and milk and eggs for babies. Since milk or egg allergies in young children are likely to go away, it is critical to monitor them and reintroduce the food whenever feasible [9]. There are various ways to classify food reactions, but the two most prevalent ones are toxic and nontoxic. Nontoxic reactions can be further separated into immunological and nonimmunological reactions. The type of reaction determines the further division of immunologic responses [10].
Early life nutrition and its interaction with the gut microbiome are considered pivotal in shaping the likelihood of developing a food allergy [11,12]. The gut microbiota plays a crucial role in establishing oral tolerance, and disruptions caused by factors like Cesarean delivery, diet, or antibiotic use may impact the development of disease [13]. The gut microbiome generates metabolites, especially short-chain fatty acids (SCFAs), that connect nutrition, microbiome, and the immune system [11].
A class of beneficial, active bacteria known as probiotics can colonize the host’s gut to enhance the balance of intestinal microbes and provide positive effects [14]. Probiotics are anticipated to be used in the clinical treatment of allergic illnesses since they can improve intestinal mucus’s immunological function and preserve intestinal flora’s dynamic balance [15]. It has also been demonstrated that probiotics can lessen the allergic reactions to several food allergies. By preserving the stability and balance of intestinal flora, fortifying the intestinal barrier, and boosting the body’s metabolism, probiotics have a major impact on the capacity to increase the human body’s immune response and suppress allergies [16]. Another study indicates that probiotics can influence the immune response by enhancing pathogen elimination and modulating the adaptive immune system. When given during the perinatal period, they may help prevent allergies, although their effectiveness in allergy treatment is still uncertain [17].
Prebiotics aim to improve health by targeting the microbiota associated with humans and animals. Prebiotics are nonviable substrates that act as nourishment for beneficial bacteria that are carried by the host, such as native (resident) microorganisms, and provide probiotic strains, whereas probiotics use living microorganisms. Prebiotics are therefore distinct from most dietary fibers, including cellulose, pectins, and xylans, which promote the growth of a diverse range of gut microbes. What we mean by this is that a prebiotic should induce a metabolism skewed toward bacteria that promotes health within the native environment rather than being widely metabolized [18]. Prebiotics can indirectly influence the immune system by altering the composition and abundance of gut microbiota [19].
Synbiotics are a combination of probiotics and prebiotics used to enhance the health of humans or animals [20]. Probiotic bacteria use prebiotics as a specific growth substrate in synbiotic food products [21,22]. A group of specialists recently updated the notion of synbiotics by the International Scientific Association for Probiotics and Prebiotics. They distinguish between two kinds of synbiotics: synergistic and complementary. A probiotic and a prebiotic that work together to provide one or more health advantages without requiring co-dependent functions make up a complementary synbiotic. Co-administered microorganisms selectively use a substrate found in a synergistic symbiotic [23]. The use of probiotics and prebiotics together (synbiotics) may provide a synergistic effect in enhancing gut health [24].
This review aims to examine the impact of nutrition and the gut microbiota, including probiotics, prebiotics, and synbiotics on reducing the prevalence of food allergies. Additionally, it also focuses on summarizing the clinical trials found in the scope of the food allergy test study.
2. Methods
2.1. Search Strategy and Study Selection
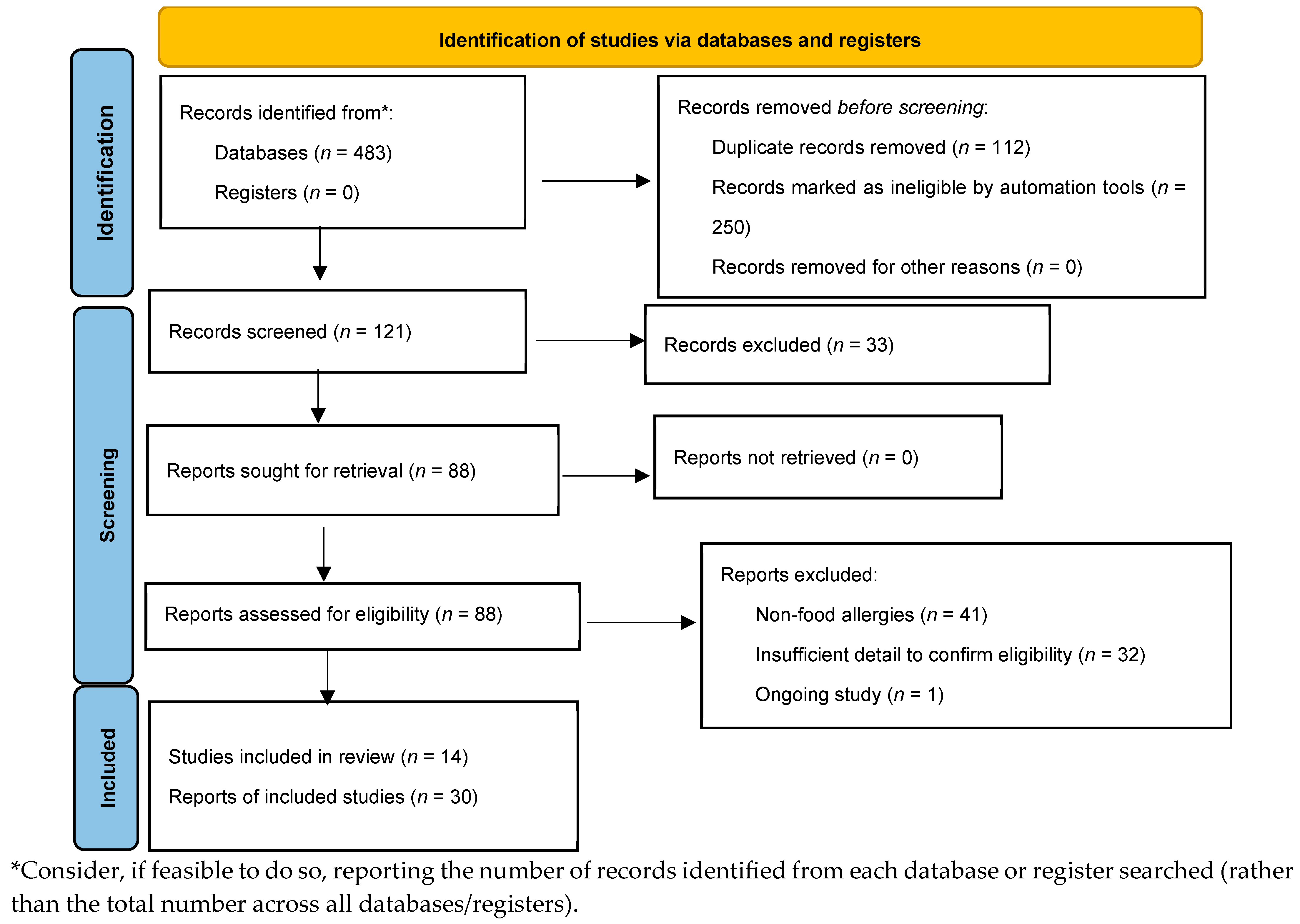
A comprehensive literature search was conducted in PubMed, Scopus, Multidisciplinary Digital Publishing Institute (MDPI), and Web of Science databases from 2005 until 31 March 2025. Detailed search strategies were developed for each database related to the keywords of food allergies, gut microbiome, nutrition, probiotics, prebiotics, diet, immunomodulatory metabolites, and IgE-mediated food allergies (Figure 1). We searched for randomized controlled trials (RCTs) published from 2005 to the present. Reference lists for primary research and review articles were manually reviewed for additional references, and PubMed was examined for errata or retractions related to the included studies. The review process was described using a PRISMA diagram [25].
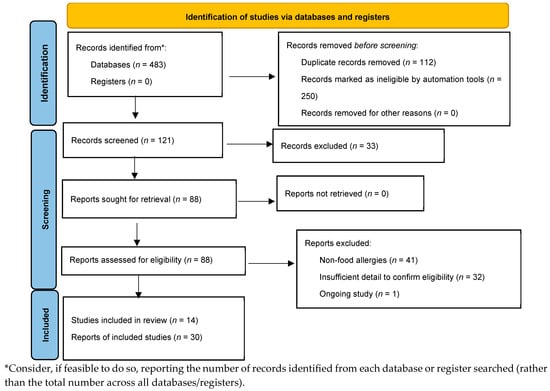
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow.
2.2. Participants
This review considered studies that included participants from the neonatal period up to three years of age, as this is a critical window for immune system maturation, gut microbiome development, and the onset of food allergies. Eligible studies involved newborns, infants, and young children with or without a diagnosed food allergy, as well as studies that recruited pregnant women who consumed probiotic or prebiotic interventions during pregnancy to influence allergy outcomes in their offspring. Both maternal and child populations were therefore included to capture evidence on the role of early-life nutritional and microbiome-related interventions in the prevention or management of food allergies.
2.3. Types of Interventions
This review encompassed research that examined dietary and microbiome-focused strategies to prevent or treat food allergies in young children. Probiotic, prebiotic, synbiotic, or postbiotic treatments were eligible if they were given directly to newborns and young children (0–3 years old) or indirectly through maternal nutrition during pregnancy and/or lactation. If dietary changes were intended to alter immunological tolerance or the composition of the gut microbiota, supplementary feeding techniques, elimination diets, or the introduction of hydrolyzed formulas were also taken into consideration. Any form of intervention, such as capsules, powders, fortified foods, or formula milk, could be used, and it was included regardless of frequency, duration, or dosage. Excluded studies evaluated vaccinations, non-nutritional interventions, or pharmaceutical treatments.
2.4. Risk of Bias
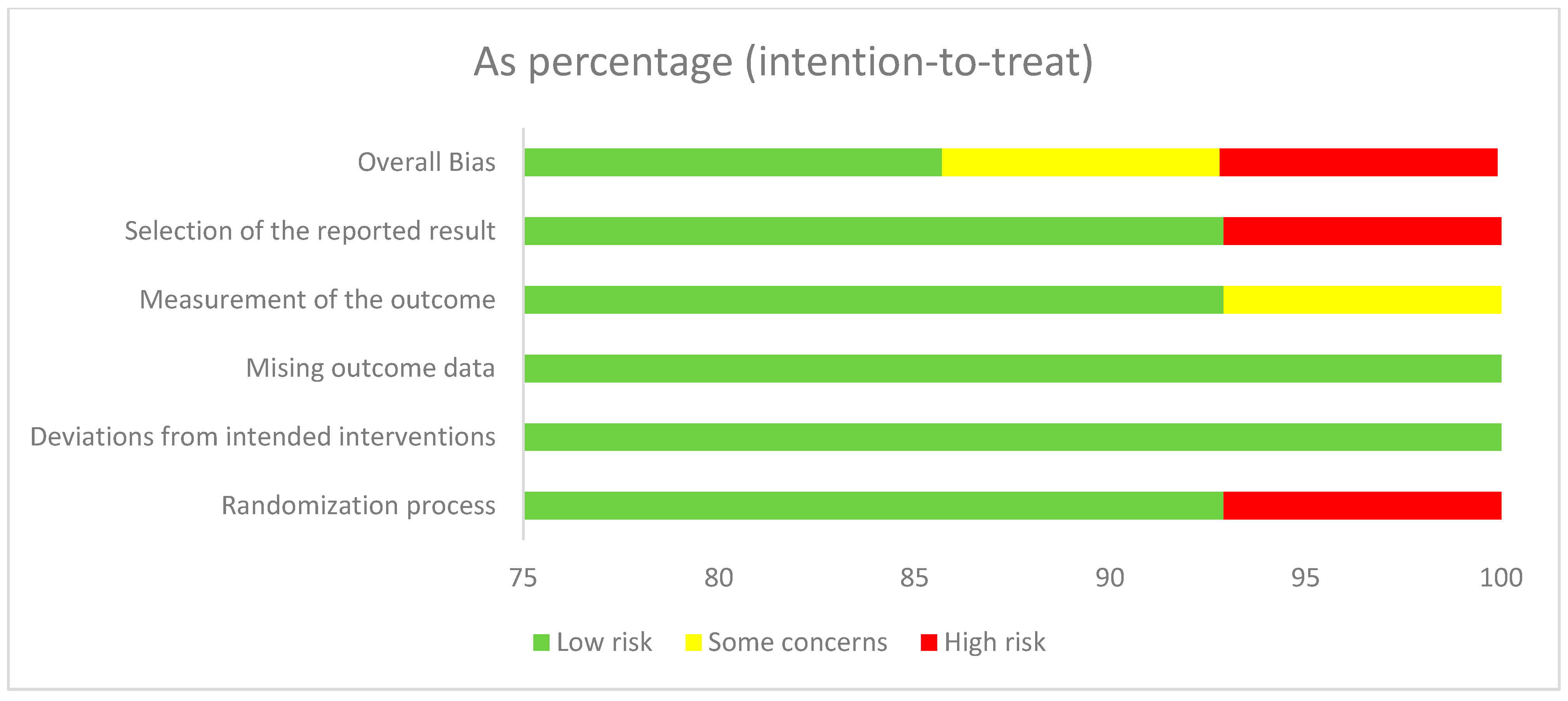
The RoB2 tool summary and graph results are shown in Figure 2. One report shows “high risk” for overall bias and selection of the reported results. Two studies reported “some concerns” for overall bias and measurement of the outcome. Another report on issues of “high risk” with the randomization process involved only one study. The review process involved three independent reviewers, each of whom conducted their evaluations independently to minimize bias.
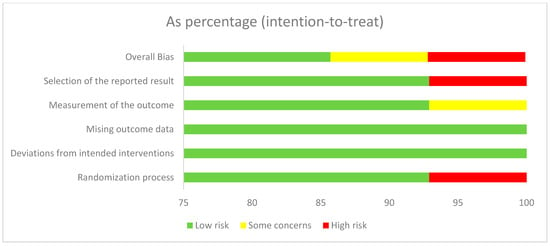
Figure 2.
Risk of bias graph.
3. Prevalence of Food Allergies
Food allergies (FA), chronic immune-mediated illnesses, have been found to affect individuals of different ages, socioeconomic backgrounds, and ethnicities [26,27]. Due to the noticeable increase in incidence and healthcare use over recent decades across many regions, food allergies have emerged as a major global concern [28].
A recent systematic review reported that in Europe, the point prevalence and lifetime prevalence of self-reported food allergies are 13.1% and 19.9%, respectively. By comparison, prevalence based on skin prick tests is 5.7%, oral food challenges (OFC) show 0.8%, and assessments using specific IgE (sIgE) levels indicate 16.6% [29]. Another recent systematic review and meta-analysis found that the prevalence of the eight most common food allergies in Europe did not differ between the periods 2000–2012 and 2012–2021 [30]. A 2018 US population-based cross-sectional survey estimated that about 1 in 13 children had at least one active IgE-mediated food allergy, consistent with previous estimates from a similar-sized US parent-report survey [31,32]. The survey also estimated that 10.8% (95% CI, 10.4–11.1%) of US adults had a confirmed food allergy, although 19.0% (95% CI, 18.5–19.5%) self-reported having one, and 5.1% had both a confirmed food allergy and a corresponding physician diagnosis for at least one allergy [28].
Although it is challenging to make definitive conclusions on trends in Asia because of the scarcity of large population-based epidemiological surveys, these trends seem to differ significantly from those of Western populations [33]. Children from five cities in China (Hong Kong and Guangzhou), Russia (Tomsk), India (Bengaluru and Mysore), and a rural county in Southern China (Shaoguan) took part in a multicenter epidemiological study using the EuroPrevall screening questionnaire. Overall, food allergy rates were low, with Hong Kong showing the highest prevalence of probable food allergy at 1.50%, followed by Russia at 0.87%, Shaoguan at 0.69%, Guangzhou at 0.21%, and India at 0.14% [34]. Both sensitization and food allergy were significantly higher in children born and raised in Hong Kong compared to those who immigrated from mainland China. This finding underscores the crucial role of early-life exposures in shaping the development of food sensitization and allergies later in life. Similarly, compared to other countries, the Indian population shows a notably lower prevalence of food allergies (1.2%) but a higher rate of food sensitization (26.5%) [35].
In Africa, the health burden of food allergies remains poorly characterized, with only a few notable studies [36,37,38]. For example, a prospective observational study at a pediatric university hospital in Cape Town reported a high overall prevalence of food sensitization (66%) and food allergy (40%), most commonly to egg (25%) and peanut (24%) [39]. The study also identified severe eczema, age under 2 years, and early-onset atopic dermatitis (before 6 months) as significant risk factors for food allergy. The unexpectedly high prevalence of food allergies observed prompted the initiation of the South African Food Sensitization and Food Allergy (SAFFA) study, a larger investigation conducted in an unselected pediatric population [40].
4. Intestinal Permeability, Gut Microbiota, and Their Roles in Food Allergies
Due to its possible involvement in the development of FA, leaky guts have attracted a lot of attention. According to the “epithelial barrier hypothesis”, the development of allergy disorders and sensitization can be attributed to intestinal barrier malfunction, which heightens vulnerability to environmental stimuli [41]. Intestinal integrity changes, which are frequently linked to leaky guts, may contribute to the observed increase in FA prevalence, according to this theory [42]. Industrialization and the use of ultra-processed foods (UPFs) are two examples of environmental and lifestyle factors that are thought to contribute to the disruption of intestinal barrier integrity [41]. These outside variables, which make up the external exposure, can affect the gut microbiota and epithelial barriers, which can have a major impact on the emergence of allergy disorders [43]. The integrity of intestinal epithelium is negatively impacted by food emulsifiers found in UPFs, such as polysorbate 20 and 80 [44]. It is believed that lower vitamin and antioxidant levels in UPFs make people more vulnerable to allergic reactions [43]. Additionally, it has been suggested that the higher incidence of FA may be related to heightened exposure to advanced glycation end products (AGEs) [45].
When the epithelial barrier is breached, the immune system releases inflammatory mediators called alarmins [46]. Alarmins, including TSLP, IL-33, and IL-25, are cytokines produced by epithelial cells in response to cellular damage induced by stress or infection [47,48,49]. While these cytokines play important functions in gut epithelial homeostasis, they can also create a pro-allergic microenvironment by activating T helper 2 and type 2 innate lymphocytes [50,51,52,53,54,55,56,57].
Allergic compounds, including food proteins, toxins, and microbial metabolites, can pass through the intestinal epithelium and interact with immune cells, leading to gut-associated lymphoid tissue [58]. An abnormal immunological response triggers the creation of allergen-specific IgE antibodies, which activate mast cells and basophils. When exposed to an allergen again, IgE antibodies attach to mast cells and basophils, causing the release of proteases and inflammatory mediators such as histamine [49,59]. The immune response affects intestinal permeability, leading to increased allergen transit and subsequent hypersensitive reactions. Allergies can compromise the mucosal barrier, leading to increased intestinal permeability [60,61,62].
Interactions between microbiota and hosts are critical for immune system regulation. The gut microbiota may play a role in FA development by modulating food allergy tolerance [63]. The gut microbiome increases tolerance by activating ROR-γt+ regulatory T-cells, which can be assisted by microbial synthesis of short-chain fatty acids like butyrate [64]. The intestinal microbiota can decrease allergy sensitization to food antigens by activating immune cells to produce IL-22. This improves intestinal epithelial integrity and reduces immune system contact with allergens [65].
5. The Effects of the Gut Microbiome
The collection of microorganisms and their genetic components inside a certain habitat is known as the microbiome. The importance of microbiomes to human physiology has become evident. The microbiome is believed to mediate health and disease, much like gene variations in the human genome [66]. Interactions between the microbiota and the host are critical to immune system modulation. While the use of antibiotics can disrupt gut homeostasis and drastically raise the risk of allergic disorders, early life exposure to maternal bacteria through vaginal or natural delivery and breast milk greatly promotes the establishment of a healthy gut microbiota and immune system [67].
The gut microbiota is a key player in the intricate sensitization system. Since cesarean babies do not inherit their mother’s vaginal microbiome, they are known to be more susceptible to allergy disorders. Additionally, low microbial diversity and an increased Enterobacteriaceae/Bacteroidaceae ratio are linked to later food sensitization in children. Furthermore, short-chain fatty acids (SCFAs), which are metabolites of bacteria, are also implicated. SCFAs are byproducts of bacterial fermentation, primarily from Firmicutes, of which butyrate and propionate are the most important. The anti-inflammatory properties of these molecules enhance the integrity of the epithelial barrier and lessen the likelihood of sensitization to dietary allergies [68].
Dysbiosis, a disorder that disrupts the original microbiota composition, causes food allergies [68]. The disruption of the gut microbiota and decreased parasite infection rates brought about by improved hygiene practices have led to a Th2-biased immune response in response to harmless stimuli like food or pollens, as well as a drop in RORγt+ Treg cells of IL10-producing regulatory B cells [69,70,71]. Another reason for the conflicting association between food allergies and the hygiene hypothesis was presented in a recent publication. They discovered that the parasitic flatworm Schistosoma can produce the “blocking antibody” IgG, which can cross-react with allergens like Ara h 1 and prevent allergic hypersensitivity reactions [72]. Another chemical that the parasite secretes, TGF-β, could activate Foxp3+ Treg cells. In allergic reactions, they may work together to inhibit the FcεRI on mast cells and counteract IgE [73].
Recent study on the gut microbiota of persons with food allergies has utilized 16S rRNA gene sequencing, which encodes for a prokaryotic ribosome component. The 16S rRNA gene enables more extensive bacterial identification without the restrictions of culture-based approaches, since it incorporates hypervariable regions and highly conserved primer binding sites, which can produce species-specific signature sequences [74]. Gut dysbiosis may occur before food allergies develop, according to findings from well-defined 16S rRNA sequencing studies of food sensitivity and allergies. Sensitization (sIgE < 0.10 kUA/L) to at least one dietary allergy among milk, egg, peanut, soy, and wheat was connected to lower relative abundances of Haemophilus, Dialister, Dora, and Clostridium in stool samples taken from 225 US children aged 3 to 6 months. Furthermore, in stool samples taken between the ages of three and six months, the researchers discovered decreased relative abundances of Citrobacter, Oscillospira, Lactococcus, and Dorea in children who, by the age of three years, had a food allergy, as indicated by sensitization and perceptible allergic symptoms [75].
The role of microbiomes in food allergy has been better understood by murine models, which have yielded interesting experimental insights. These models indicate that gut microbiota may influence the risk of food allergies. In contrast to wild-type mice, mice with a gain-of-function mutation in the IL-4 receptor-α chain that are prone to food allergies have distinct intestinal abundances of Lachnospiraceae, Lactobacillaceae, Rikenallaceae, and Porphyromononadaceae. It appeared that illness vulnerability was transferred when the gut microbiota of these food allergy-prone mice was transferred to germ-free mice. Reconstituted mice showed OVA-specific IgE responses and symptoms resembling anaphylaxis upon OVA challenge. It is interesting to note that while treating these food allergy-prone mice with Treg cells specific to the immunodominant OVA peptide reduced allergen sensitization and anaphylaxis correlations, the gut microbiota did not return to normal, indicating that immunomodulatory mechanisms other than Treg cells are involved [76].
The gut microbiome has the potential to alter basophil populations, which can therefore influence allergic reactions. Mice given broad-spectrum antibiotics showed increased numbers of steady-state circulating basophil populations, improved Th2 cell responses, and higher IgE concentrations [77]. Basophil removal in these mice lowered Th2 cell responses, implying that basophils had a role in the observed allergic inflammation. Microbe-generated signals have also been identified to regulate the proliferation of bone-marrow resident precursor populations for basophil development [77].
The discovery that the gut microbiota can influence a person’s susceptibility to food allergies opens the prospect of therapeutic benefits from modifying the gut microbiota for the host’s benefit. Given the potential benefits and opportunities to impact the development and treatment of food allergies, this topic is of tremendous interest [78].
6. Effects of Nutrition on Food Allergies
Dietary interventions, along with the use of probiotics, prebiotics, synbiotics, and fecal microbiota transplantation, can effectively alter the gut microbiome [78]. The following table lists the impact of probiotic strains on food allergies (Table 1).

Table 1.
Probiotic effects on food allergies.
Probiotic use is often intended to alter the intestinal microbiota’s composition and activity [15,87]. The discovery that allergic children have a unique microbiota composition supports the case for altering the gut microbiota in the case of allergies [88,89]. When given in sufficient quantities, probiotics are best described as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [90]. The two primary bacterial genera that make up most probiotics are Lactobacillus and Bifidobacterium. These probiotics provide several advantages for the immune system and gastrointestinal tract, such as avoiding autoimmune disorders, allergies, and infections [91].
In a double-blind, randomized, placebo-controlled trial (DBPCRT), Lactobacillus rhamnosus GG (LGG) was administered to mothers who had at least one first-degree relative with an atopic disorder during pregnancy and to their infants for six months following delivery. At two years of age, the probiotic group had half the number of atopic eczema cases as the placebo group. At that time, LGG looked to be successful in preventing early atopic disease in high-risk children [92].
Probiotic supplementation with Lactobacillus casei and Bifidobacterium lactis in children with milk allergies for a year did not improve the remission of their milk allergy, according to clinical investigations [93,94]. After 6 and 12 months, however, a different study of children with milk allergies who were supplemented with Lactobacillus rhamnosus GG and extensively hydrolyzed casein formula showed higher rates of milk allergy resolution than a control group that was given the formula alone [93,94]. The absolute risk difference between the experimental arms in terms of cow’s milk tolerance at 12 months was 0.20 (95% CI 0.05–0.35). Researchers compared stool samples from healthy and cow’s milk-allergic infants both before and after they received extensively hydrolyzed formula, with or without Lactobacillus rhamnosus GG. They suggested that the presence of certain bacterial strains is associated with tolerance, as tolerant infants exhibited greater abundance of Blautia and Roseburia species and higher butyrate concentrations in their gut microbiome [95].
Research has also explored the effects of Lactobacillus rhamnosus GG on peanut allergy, based on mechanisms comparable to its proposed influence on other food allergies. In an experimental study, participants received peanut oral immunotherapy supplemented with Lactobacillus rhamnosus GG for 18 months, resulting in markedly higher desensitization rates than those observed with placebo treatment (82.1% versus 3.6%) [96].
In mice with milk β-lactoglobulin allergy, Lactobacillus paracasei L9 was shown to have a regulatory effect on the Th1/Th2 imbalance of lymphocytes. This effect may have been related to the bacteria’s ability to increase the number of CD4+FOXOP3+Treg cells and encourage the secretion of regulatory cytokines by DCs [97,98,99]. When Lactobacillus triggers a mucosal immune response, it stimulates Th2 cells to generate a significant amount of IL-5. Important IgA-stimulating substances cause B lymphocytes to release IgA, which forms the antibody secretory IgA (sIgA) by covalently attaching to protein receptors made by intestinal mucosal epithelial cells [94]. Pathogenic antigens are bound by the secreted globulin antibody sIgA, which stops them from adhering to the intestinal mucosa [100].
Experiments have been conducted to determine whether Bifidobacterium infantis (BB) and its antioxidant enzyme superoxide dismutase (SOD) have therapeutic effects on FA OVA-sensitized mouse models. It was shown that giving BB orally dramatically decreased the amounts of OVA-specific IgE and IgG1 in the serum and splenocytes of allergic mice, along with the release of IL-4, IL-5, and IL-13. Additionally, BB therapy reduced anaphylactic symptoms. BB enhanced the levels of glutathione (GSH) and SOD in dendritic cells (DCs), lowered reactive oxygen species (ROS) and malondialdehyde (MDA), and decreased oxidative stress in DCs. The explanation is that BB suppressed the production of TIM4, a receptor that facilitates TH2 responses and mediates DC absorption of apoptotic cells. By decreasing its phosphorylation and nuclear translocation, BB also inhibited the activation of STAT6, a transcription factor that controls the production of TIM4 [101].
According to findings from earlier gut microbiota research, probiotic benefits are probably strain-specific, and therefore careful consideration of strain-level effects and interventions is merited [78]. Probiotics have been shown to increase Th1 and regulatory cytokine production in vitro, but their ambiguous and unpredictable immunomodulatory effect in vivo is debatable [102]. According to this viewpoint, the data supporting the use of probiotics to treat FA seems debatable. There are currently no clear guidelines about which strain to use, at what dosage, and for how long, even though cow milk allergy (CMA) seems to be the most researched type of FA [103].
6.1. Mechanism of Probiotics in Gut Modulation
According to Mazziotta et al. [104], probiotic bacteria that are consumed attach themselves to intestinal epithelial cells and use pattern recognition receptors (PRRs) to activate them. Probiotic-stimulated cytokines activate T regulatory (Treg) cells, which preserve immunological homeostasis in the gut mucosa. Tregs are important in reducing the immune response because they are powerful immune response suppressors. Tregs induction results in the release of transforming growth factor (TGF)-β or IL-10. Furthermore, probiotics cause B cells to mature into plasma cells that produce immunoglobulin (Ig)A. Cytokine and chemokine release from intestinal epithelial cells creates a microenvironment in the intestinal lamina propria that enables B cells to proliferate clonally and make IgAs. IgAs regulate bacterial adherence to the host tissue after migrating past the epithelium and into the mucus layer [104].
6.2. Prebiotics
Typically, linked carbohydrates like oligosaccharides and short-chain polysaccharides make up prebiotics. The molecular property of these molecules is that they cannot be broken down by the intestinal tract’s enzymes. As a result, they can either directly interact with the surrounding cells or act as food sources for microbes deemed “beneficial,” such as Bacteroides and Bifidobacterium [105].
Distinct prebiotics encourage the proliferation of particular native gut microbial populations. While the resulting strain- and species-level changes are often unpredictable, prebiotics hold great promise for influencing the gut microbiome. The gut environment, especially factors such as pH, strongly affects the dynamics of interspecies interactions. Consequently, personalized microbial profiles should be carefully considered during the development of prebiotics to maximize effectiveness and minimize safety risks [106]. Lists of relevant studies is added in the table of effects of prebiotics (Table 2).

Table 2.
A summary of studies that have investigated the effects of prebiotics.
Non-digestible food ingredients, known as prebiotics, specifically enhance the proliferation and metabolic activity of the host’s commensal microbiota. Fiber, a common prebiotic, is utilized by gut bacteria to generate short-chain fatty acids, which are believed to contribute to the reduction in allergic inflammation [120,121]. Prebiotics are often added to infant formulas. Those that remain intact through the upper gastrointestinal tract reach the large intestine, where they are specifically metabolized by beneficial microorganisms [122]. Prebiotic nutrition in babies has not been found to have any impact on the onset of food allergies in clinical trials [122,123,124]. However, in certain individual investigations, the risk of eczema and asthma decreased [122,123,124].
An excellent illustration is the prebiotic Bimuno (Clasado Biosciences Ltd., Reading, UK). In terms of GOS, it is a lactose-based mixture made with probiotic Bifidobacterium bifidum NCIMB 41171 enzymes. It can directly interact with the immune system to enhance the gut’s barrier function. In overweight people with metabolic syndrome, its beneficial immunomodulatory effect was further evidenced by a considerable rise in gut immunological parameters that protect against pathogens and a decrease in inflammatory markers in the blood and feces [125].
Prebiotics, known as human milk oligosaccharides (HMOs), are found in human milk. Oligosaccharides (5 g/L to 23 g/L) with a lactose-reducing end extended with fucosylated and/or sialylated N-acetyllactosamine units make up human milk and colostrum. The size, charge, and order of the more than 150 HMO configurations vary [126]. Neutral fucosylated and non-fucosylated oligosaccharides are the most common HMOs [127]. HMOs are minimally absorbed through the intestinal wall and provide no direct nutritional value to the newborn [128]. Rather, it is proposed that HMOs can serve the infant in a variety of additional capacities. They serve as prebiotics, encouraging the development of advantageous intestinal flora and influencing the gut microbiome. They are also favored substrates for several gut bacterial species. The fermentation of HMOs by the gut microbiota produces short-chain fatty acids (SCFA), which are essential for intestinal health [129].
6.3. Synbiotics
Synbiotics function to stimulate the growth of native gut bacterial strains and to increase the survival rate of health-promoting microbes delivered through dietary sources [17]. How synbiotics influence metabolic health is not yet fully understood. Notably, the health effects of synbiotics are probably dependent on the combination of probiotics and prebiotics used [130]. The use of synbiotics to modify the human gut microbiota appears promising given the vast array of potential combinations [131].
Synbiotics, combining probiotics and prebiotics, are under investigation in clinical trials for their role in allergy prevention. In a prospective, randomized, double-blind controlled trial, 110 full-term infants with cow’s milk allergy were given either amino acid–based formula (AAF) alone or AAF with synbiotics. Both groups showed normal growth and a reduction in allergic symptoms. This is the first study to demonstrate that an AAF containing a specific synbiotic blend promotes normal growth in infants with cow’s milk allergy, comparable to that observed with AAF alone [132]. A summary of the relevant research has been listed in the table below that investigated the impact of probiotics, prebiotics, and synbiotics in managing food allergies (Table 3).

Table 3.
Summary of the impact of nutrition on food allergies.
7. Discussion
A review of cumulative research suggests that nutrition interventions such as probiotics, prebiotics, or synbiotics may reduce the likelihood of allergic reactions. However, clinical trials have not demonstrated significant differences when compared to placebo-controlled groups. This lack of strong evidence limits the clinical relevance and practical application of these nutritional approaches in managing food allergies.
Multiple studies have found no significant differences between intervention and placebo groups regarding the prevention of or reduction in cow’s milk protein allergy (CMPA) or other food allergies. For example, research by Palmer et al. [134] indicated that prebiotic or probiotic supplementation during pregnancy and postpartum did not substantially decrease the occurrence of IgE-mediated food allergies in children and Komulainen et al. [142]. Similar findings were made by Chatchatee et al. [139] and Loke et al. [140], who reported no discernible benefit of using probiotics or synbiotics over control formulas in terms of helping allergic youngsters improve their outcomes or acquire tolerance. However, a few studies demonstrated potential benefits. Sakihara et al. [146] found that infants who drank cow’s milk formula from an early age had a much lower incidence of CMA cases than those who did not (0.8% vs. 6.8%, p < 0.001). In a similar vein, Skjerven et al. [145] discovered that early exposure to allergenic foods, especially in a food intervention group, decreased the occurrence of food allergies when compared to non-intervention.
Biological impacts were also demonstrated in animal model experiments. Although it is unclear how this might translate to human outcomes, Xu et al. [136] showed that high-dose probiotic supplementation dramatically decreased OVA-specific IgE levels in mice, indicating a reduction in an allergic response. A notable study by Nocerino et al. [143] revealed that, when compared to other formulas, babies treated with an extensively hydrolyzed casein formula including Lactobacillus rhamnosus GG (EHCF + LGG) had the lowest incidence of atopic symptoms for 36 months.
The findings of this review are consistent with the broader body of evidence showing mixed outcomes regarding the efficacy of nutritional interventions, particularly probiotics, prebiotics, and synbiotics in managing or preventing food allergies. Several studies included in this review, such as those by Cukrowska et al. [133] and Nocerino et al. [143], demonstrated improvements in allergic symptoms and immune modulation following probiotic supplementation, particularly with Lactobacillus rhamnosus GG and related strains. However, other studies (Palmer et al. [134]; Yamamoto-Hanada et al. [138]; Komulainen et al. [142]) found no statistically significant effects, mirroring the inconsistency frequently reported in the wider literature.
Recent meta-analyses have similarly underscored the variability in outcomes. A 2024 systematic review reported that probiotics are likely to have no significant effect on reducing eczema scores among infants with cow’s milk allergy. However, modest improvements may occur in children with other allergic conditions [147]. Another comprehensive review emphasized that combined maternal and infant supplementation produced greater preventive benefits than supplementation during only one life stage, highlighting the importance of timing and developmental context [148]. Conversely, earlier meta-analyses of Tang et al. [149] and Cuello-Garcia et al. [150] concluded that prenatal or postnatal probiotic use did not significantly prevent food allergies overall, though modest risk reduction was observed for atopy or sensitization outcomes [149,150].
In 2012, the World Allergy Organization’s (WAO) Special Committee on Food Allergy and Nutrition conducted a qualitative and narrative assessment of probiotic treatment for allergic diseases. The scientists found that probiotics do not have an established role in allergy prevention or treatment. There is no evidence that a single probiotic pill or class of nutrients can significantly impact allergy symptoms or long-term disease progression [151]. A few years later, the WAO organized a panel of guidelines to create evidence-based suggestions for using probiotics to avoid allergies. Using a GRADE approach, they declared that there is currently no proof that probiotic supplementation lowers a child’s allergy. The WAO guideline panel concluded that there is a probable advantage to utilizing probiotics, primarily from the prevention of eczema, after considering all important outcomes [152].
Since bacterial microbiota has been the focus of most recent studies on microbiome in food allergies, future studies could evaluate the virome and microbiome’s contributions. Analytical tools, reference databases, and sequencing techniques for evaluating the microbiome and virome are less advanced than those for the bacterial microbiome [153]. However, research on microbiomes beyond the bacterial microbiome is becoming possible due to the growing availability of tools for the analysis of data from shotgun metagenomic sequencing, which is whole-genome sequencing done on genomic DNA from a mixed microbial population [154,155]. Studies of microbiomes, as well as data acquired from genome-wide association, have increased our understanding of food allergies [156,157,158,159]. Integrating system-wide data is crucial for constructing predictive models of complex biological interactions and systems, leading to a more comprehensive knowledge of food allergies [88,154,160,161].
Even though most human clinical trials show only slight or no statistically significant improvements in allergy prevention from probiotics, prebiotics, or synbiotics, some research suggests that certain interventions (such as early allergen exposure or specific probiotic strains) may be beneficial in preventing allergies. Administration of Lactobacillus rhamnosus GG is likely to promote tolerance in infants with suspected cow’s milk allergy. Only studies focusing on CMA were included, as no research was identified on the use of probiotics for treating other types of food allergies in children [103]. The only probiotic strain with the most robust and reliable RCT data for avoiding or enhancing some allergic outcomes is Lactobacillus rhamnosus GG (LGG), particularly in early-life allergy prevention trials and in newborns with cow’s milk allergy. This advantage, however, is strain-specific and primarily shown in children rather than adults; there is conflicting data regarding other strains or general allergy prevention. Results vary widely, most likely due to variations in populations, strains utilized, durations, and allergy criteria.
8. Study Limitations
This systematic review has a few limitations that should be noted. First, the included studies had significant variation in terms of participant age groups, interventions (kind, strain, and dose of probiotics, prebiotics, or synbiotics), duration of supplementation, and outcome measures, making direct comparison and pooling of results difficult. This heterogeneity can complicate the synthesis of data and may limit the ability to draw definitive conclusions from the review. Many trials had limited sample sizes and short follow-up periods, making it difficult to determine long-term effects on allergy avoidance. Furthermore, most of the research was conducted in high-income nations, which may limit the findings’ applicability to more diverse populations with varying genetic, environmental, and nutritional exposures. This can result in a limited representation of the available evidence, potentially impacting the generalizability of the review findings on different populations or settings. The use of different diagnostic criteria for food allergy among studies may have resulted in variability in reported outcomes.
9. Conclusions
Nutritional treatments like probiotics, prebiotics, and synbiotics offer molecular plausibility but variable clinical effects, highlighting the gut microbiome’s crucial but complex role in preventing food allergies. When compared to placebo, most randomized controlled trials show modest or non-significant results, despite some studies showing benefits from strains or early allergen exposure. Standardized preventative guidelines are challenging to produce because of these disparities, which most likely reflect variation in study designs, populations, intervention timing, and definitions of allergy outcomes. Therefore, the information now available indicates that while microbiome-modulating techniques have potential, they cannot yet be considered as effective preventive approaches.
Thorough, extensive, and long-term studies that combine microbiome analysis with genetic and environmental factors that influence allergy risk should be given top priority in future research. The identification of strain-specific effects, the best times for interventions, and synergistic dietary strategies should receive special attention. The development of focused, individualized tactics may be guided by a more thorough understanding of host–microbiome–immune interactions made possible by advancements in multi-omics and systems biology. Nutritional control of the gut microbiota should not be the mainstay of allergy prophylaxis until such evidence is consolidated; rather, it should be an auxiliary field of study.
Author Contributions
M.A.N.B. contributed to the study’s conception, design, data collection, analysis, manuscript drafting, J.T.V. contributed to the study’s conception, design, data collection, analysis, manuscript drafting, and approval of the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing does not apply to this article, as no new data were created or analyzed in this study.
Acknowledgments
The manuscript was proofread and edited by a native English speaker through the official editing service of Semmelweis University. All authors meet the 4 ICMJE criteria for authorship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for thought: A comprehensive review of food allergy 2021–2023. J. Allergy Clin. Immunol. 2024, 153, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.; Allen, K.J. Food allergy: Riding the second wave of the allergy epidemic. Pediatr. Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Gowland, M.H.; Sharma, V.; Ierodiakonou, D.; Harper, N.; Garcez, T.; Pumphrey, R.; Boyle, R.J. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992–2012. J. Allergy Clin. Immunol. 2015, 135, 956–963.e1. [Google Scholar] [CrossRef]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy 2016, 46, 1099–1110. [Google Scholar] [CrossRef]
- Pouessel, G.; Beaudouin, E.; Tanno, L.K.; Drouet, M.; Deschildre, A.; Labreuche, J.; Renaudin, J.M.; Allergy Vigilance Network®. Food-related anaphylaxis fatalities: Analysis of the Allergy Vigilance Network® database. Allergy 2019, 74, 1193–1196. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.L.; Tang, M.L.K.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J. Allergy Clin. Immunol. 2017, 140, 145–153.e8. [Google Scholar] [CrossRef]
- Tedner, S.G.; Asarnoj, A.; Thulin, H.; Westman, M.; Konradsen, J.R.; Nilsson, C. Food allergy and hypersensitivity reactions in children and adults: A review. J. Intern. Med. 2022, 291, 283–302. [Google Scholar] [CrossRef]
- Uzzaman, A.; Cho, S.H. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012, 33 (Suppl. 1), 96–99. [Google Scholar] [CrossRef]
- Catassi, G.; Catassi, G.; Gasbarrini, A.; Ianiro, G.; Cammarota, G.; Giorgio, V.; Aloi, M.; Gasbarrini, A.; Cammarota, G. The role of diet and nutritional interventions for the infant gut microbiome. Nutrients 2024, 16, 400. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A link between early life nutrition and gut microbiome in the development of food allergy. Life 2021, 11, 384. [Google Scholar] [CrossRef]
- Rachid, R.; Chatila, T.A. The role of the gut microbiota in food allergy. Curr. Opin. Pediatr. 2016, 28, 748–753. [Google Scholar] [CrossRef]
- Singh, V.P.; Sharma, J.; Babu, S.; Rizwanulla, S.A.; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar]
- Di Costanzo, M.; Carucci, L.; Berni Canani, R.; Biasucci, G. Gut microbiome modulation for preventing and treating pediatric food allergies. Int. J. Mol. Sci. 2020, 21, 5275. [Google Scholar] [CrossRef]
- Steele, L.; Mayer, L.; Berin, M.C. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol. Res. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Navidshad, B.; Faseleh Jahromi, M.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2016, 206, 1–9. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.; Warchol, M.; Grill, J.P.; Schneider, F. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J. Appl. Microbiol. 2001, 90, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Metabiotics: One step ahead of probiotics; an insight into mechanisms involved in anticancerous effect in colorectal cancer. Front. Microbiol. 2016, 7, 1940. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Liong, M.-T. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food allergy across the globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Hultquist, H.; Dyer, A.; Jiang, J.; Gupta, R.; Warren, C. Phenotypic characterization of childhood- and adult-onset food allergy among adults in the United States. J. Allergy Clin. Immunol. Glob. 2022, 1, 257–264. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of food allergy in Europe: An updated systematic review and meta-analysis. Allergy 2023, 78, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.I.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence estimates of eight big food allergies in Europe: Updated systematic review and meta-analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef]
- Warren, C.M.; Sehgal, S.; Sicherer, S.H.; Gupta, R.S. Epidemiology and the growing epidemic of food allergy in children and adults across the globe. Curr. Allergy Asthma Rep. 2024, 24, 95–106. [Google Scholar] [CrossRef]
- Li, J.; Ogorodova, L.M.; Mahesh, P.A.; Wang, M.H.; Fedorova, O.S.; Leung, T.F.; Fernandez-Rivas, M.; Mills, E.N.C.; Potts, J.; Kummeling, I.; et al. Comparative study of food allergies in children from China, India, and Russia: The EuroPrevall-INCO surveys. J. Allergy Clin. Immunol. Pract. 2020, 8, 1349–1358.e16. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, P.A.; Wong, G.W.; Ogorodova, L.; Potts, J.; Leung, T.F.; Fedorova, O.; Holla, A.D.; Fernandez-Rivas, M.; Clare Mills, E.N.; Kummeling, I.; et al. Prevalence of food sensitization and probable food allergy among adults in India: The EuroPrevall INCO study. Allergy 2016, 71, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.J.; Steenhoff, A.P.; Gray, C. Food allergy in Africa: Myth or reality? Clin. Rev. Allergy Immunol. 2014, 46, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.L. Food allergy in South Africa. Curr. Allergy Asthma Rep. 2017, 17, 35. [Google Scholar] [CrossRef]
- Gezmu, A.M.; Kung, S.J.; Shifa, J.Z.; Nakstad, B.; Brooks, M.; Joel, D.; Arscott-Mills, T.; Puerto, E.C.; Šaltytė Benth, J.; Tefera, E. Pediatric spectrum of allergic diseases and asthma in a tertiary level hospital in Botswana: An exploratory retrospective cross-sectional study. J. Asthma Allergy 2020, 13, 213–223. [Google Scholar] [CrossRef]
- Gray, C.L.; Levin, M.E.; Zar, H.J.; Potter, P.C.; Khumalo, N.P.; Volkwyn, L.; Fenemore, B.; du Toit, G. Food allergy in South African children with atopic dermatitis. Pediatr. Allergy Immunol. 2014, 25, 572–579. [Google Scholar] [CrossRef]
- Basera, W.; Botha, M.; Gray, C.L.; Lunjani, N.; Watkins, A.S.; Venter, C.; Allen, K.J.; Hlela, C.; Zar, H.J.; Levin, M.E. The South African food sensitisation and food allergy population-based study of IgE-mediated food allergy: Validity, safety, and acceptability. Ann. Allergy Asthma Immunol. 2015, 115, 113–119. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Schleimer, R.P. The barrier hypothesis and Oncostatin M: Restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease. Tissue Barriers 2017, 5, e1341367. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Akin, B.G.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Ogulur, I.; Yazici, D.; Pat, Y.; Bingöl, E.N.; Babayev, H.; Ardicli, S.; Heider, A.; Rückert, B.; Sampath, V.; Dhir, R.; et al. Mechanisms of gut epithelial barrier impairment caused by food emulsifiers polysorbate 20 and polysorbate 80. Allergy 2023, 78, 2441–2455. [Google Scholar] [CrossRef]
- Paparo, L.; Coppola, S.; Nocerino, R.; Pisapia, L.; Picariello, G.; Cortese, M.; Voto, L.; Maglio, M.; Miele, E.; Carucci, L.; et al. How advanced glycation end products could facilitate the occurrence of food allergy. J. Allergy Clin. Immunol. 2023, 152, 161–173. [Google Scholar] [CrossRef]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Gambardella, A.R.; Marone, G.; Schroeder, J.T.; Mattei, F.; Schiavoni, G.; Varricchi, G. Basophils from allergy to cancer. Front. Immunol. 2022, 13, 1056838. [Google Scholar] [CrossRef]
- Poto, R.; Loffredo, S.; Marone, G.; Di Salvatore, A.; de Paulis, A.; Schroeder, J.T.; Varricchi, G. Basophils beyond allergic and parasitic diseases. Front. Immunol. 2023, 14, 1190034. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.R.; Poto, R.; Tirelli, V.; Schroeder, J.T.; Marone, G.; Mattei, F.; Varricchi, G.; Schiavoni, G. Differential effects of alarmins on human and mouse basophils. Front. Immunol. 2022, 13, 894163. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Sy, C.B.; Siracusa, M.C. The therapeutic potential of targeting cytokine alarmins to treat allergic airway inflammation. Front. Physiol. 2016, 7, 214. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Pan, G.; French, D.; Mao, W.; Maruoka, M.; Risser, P.; Lee, J.; Foster, J.; Aggarwal, S.; Nicholes, K.; Guillet, S.; et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 2001, 167, 6559–6567. [Google Scholar] [CrossRef]
- Lee, J.B.; Chen, C.Y.; Liu, B.; Mugge, L.; Angkasekwinai, P.; Facchinetti, V.; Dong, C.; Liu, Y.; Rothenberg, M.E.; Hogan, S.P.; et al. IL-25 and CD4⁺ Th2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J. Allergy Clin. Immunol. 2016, 137, 1216–1225.e5. [Google Scholar] [CrossRef] [PubMed]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial and epithelial cells in vivo: A novel “alarmin”? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Varricchi, G.; Poto, R.; Ianiro, G.; Punziano, A.; Marone, G.; Gasbarrini, A.; Spadaro, G. Gut microbiome and common variable immunodeficiency: Few certainties and many outstanding questions. Front. Immunol. 2021, 12, 712915. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Poto, R.; Marone, G.; Schroeder, J.T. IL-3 in the development and function of basophils. Semin. Immunol. 2021, 54, 101510. [Google Scholar] [CrossRef] [PubMed]
- Secondulfo, M.; Iafusco, D.; Carratù, R.; Demagistris, L.; Sapone, A.; Generoso, M.; Mezzogiorno, A.; Sasso, F.; Cartenì, M.; De Rosa, R.; et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 2004, 36, 35–45. [Google Scholar] [CrossRef]
- Majamaa, H.; Isolauri, E. Evaluation of the gut mucosal barrier: Evidence for increased antigen transfer in children with atopic eczema. J. Allergy Clin. Immunol. 1996, 97, 985–990. [Google Scholar] [CrossRef]
- Caffarelli, C.; Cavagni, G.; Menzies, I.S.; Bertolini, P.; Atherton, D.J. Elimination diet and intestinal permeability in atopic eczema: A preliminary study. Clin. Exp. Allergy 1993, 23, 28–31. [Google Scholar] [CrossRef]
- Rachid, R.; Stephen-Victor, E.; Chatila, T.A. The microbial origins of food allergy. J. Allergy Clin. Immunol. 2021, 147, 808–813. [Google Scholar] [CrossRef]
- Iweala, O.I.; Nagler, C.R. The microbiome and food allergy. Annu. Rev. Immunol. 2019, 37, 377–403. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and food allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. the CHILD Study Investigators. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; Cahenzli, J.; Lawson, M.A.E.; Ng, D.C.K.; Slack, E.; Hapfelmeier, S.; McCoy, K.D.; Macpherson, A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011, 34, 794–806. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef]
- Igetei, J.E.; el-Faham, M.; Liddell, S.; Doenhoff, M.J. Antigenic cross-reactivity between Schistosoma mansoni and peanut: A role for cross-reactive carbohydrate determinants (CCDs) and implications for the hygiene hypothesis. Immunology 2017, 150, 506–517. [Google Scholar] [CrossRef]
- Johnston, C.J.C.; Smyth, D.J.; Kodali, R.B.; White, M.P.J.; Harcus, Y.; Filbey, K.J.; Hewitson, J.P.; Hinck, C.S.; Ivens, A.; Kemter, A.M.; et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 2017, 8, 1741. [Google Scholar] [CrossRef]
- Weinstock, G.M. Genomic approaches to studying the human microbiota. Nature 2012, 489, 250–256. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.Q.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Hill, D.A.; Siracusa, M.C.; Abt, M.C.; Kim, B.S.; Kobuley, D.; Kubo, M.; Kambayashi, T.; LaRosa, D.F.; Renner, E.D.; Orange, J.S.; et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 2012, 18, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively Hydrolyzed Casein Formula Containing Lactobacillus rhamnosus GG Reduces the Occurrence of Other Allergic Manifestations in Children with Cow’s Milk Allergy: 3-Year Randomized Controlled Trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Min, F.; Bai, T.; Wang, Z.; Liu, Y.; Yang, F.; Li, Z.; Di, C.; Lin, M.; Li, X.; et al. Bifidobacterium breve M-16V Alleviates Cow’s Milk Allergy in a Mouse Model via Gut Microbiota-Derived Indole-3-Propionic Acid–Aryl Hydrocarbon Receptor Signaling Axis. Allergy 2025, in press. [CrossRef]
- Kim, W.G.; Kang, G.D.; Kim, H.I.; Han, M.J.; Kim, D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 Alleviate Allergic Rhinitis in Mice by Restoring Th2/Treg Imbalance and Gut Microbiota Disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef]
- Duan, C.; Ma, L.; Qin, M.; Zhang, L.; Hu, S.; Liu, L.; Sun, Y.; Ma, F.; Li, D. Potential of Lactobacillus plantarum A56 in Relieving Food Allergy through Immunoregulation, Antioxidation, and Reshaping Intestinal Microbiota. J. Nutr. Biochem. 2024, 125, 109560. [Google Scholar] [CrossRef]
- Nakata, J.; Hirota, T.; Umemura, H.; Nakagawa, T.; Kando, N.; Futamura, M.; Nakamura, Y.; Ito, K. Additive Effect of Lactobacillus acidophilus L-92 on Children with Atopic Dermatitis Concomitant with Food Allergy. Asia Pac. Allergy 2019, 9, e18. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Markiewicz, L.H.; Szyc, A.M.; Dietrich, M.A.; Szymkiewicz, A.; Fotschki, J. Lactobacillus casei LcY Decreases Milk Protein Immunoreactivity of Fermented Buttermilk but Also Contains IgE-Reactive Proteins. Food Res. Int. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Yao, M.; Xu, Q.; Luo, Y.; Shi, J.; Li, Z. Study on Reducing Antigenic Response and IgE-Binding Inhibitions of Four Milk Proteins by Lactobacillus casei 1134. J. Sci. Food Agric. 2015, 95, 1303–1312. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Mu, K.; Xue, W. Lactobacillus paracasei AH2 Isolated from Chinese Sourdough Alleviated Gluten-Induced Food Allergy through Modulating Gut Microbiota and Promoting Short-Chain Fatty Acid Accumulation in a BALB/c Mouse Model. J. Sci. Food Agric. 2024, 104, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Isolauri, E.; He, F.; Hashimoto, H.; Benno, Y.; Salminen, S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J. Allergy Clin. Immunol. 2001, 108, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations/World Health Organization. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002; Available online: http://www.fao.org/3/a0512e/a0512e.pdf (accessed on 2 June 2025).
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; de Foy, J.M.P.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582.e5. [Google Scholar] [CrossRef]
- Hol, J.; van Leer, E.H.; Elink Schuurman, B.E.; de Ruiter, L.F.; Samsom, J.N.; Hop, W.; Neijens, H.J.; de Jongste, J.C.; Nieuwenhuis, E.E.; Cow’s Milk Allergy Modified by Elimination and Lactobacilli Study Group. The acquisition of tolerance toward cow’s milk through probiotic supplementation: A randomized, controlled trial. J. Allergy Clin. Immunol. 2008, 121, 1448–1454. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Tang, M.L.; Ponsonby, A.L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744.e8. [Google Scholar] [CrossRef]
- Aoki-Yoshida, A.; Yamada, K.; Hachimura, S.; Sashihara, T.; Ikegami, S.; Shimizu, M.; Totsuka, M. Enhancement of oral tolerance induction in DO11.10 mice by Lactobacillus gasseri OLL2809 via increase of effector regulatory T cells. PLoS ONE 2016, 11, e0158643. [Google Scholar] [CrossRef]
- Fu, L.; Song, J.; Wang, C.; Fu, S.; Wang, Y. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory T cells and alterations in gut microbiota. Front. Immunol. 2017, 8, 1536. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376–385. [Google Scholar] [PubMed]
- Strugnell, R.A.; Wijburg, O.L.C. The role of secretory antibodies in infection immunity. Nat. Rev. Genet. 2010, 8, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Luo, Y.; Liu, Z.; Yang, P.; Gui, Y. Probiotics SOD inhibited food allergy via downregulation of STAT6-TIM4 signaling on DCs. Mol. Immunol. 2018, 103, 71–77. [Google Scholar] [CrossRef]
- Flinterman, A.E.; Knol, E.F.; van Ieperen-van Dijk, A.G.; Timmerman, H.M.; Knulst, A.C.; Bruijnzeel-Koomen, C.A.; Pasmans, S.G.; van Hoffen, E. Probiotics have a different immunomodulatory potential in vitro versus ex vivo upon oral administration in children with food allergy. Int. Arch. Allergy Immunol. 2007, 143, 237–244. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as treatment for food allergies among pediatric patients: A meta-analysis. World Allergy Organ. J. 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Opinion: Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Atopic Dermatitis during the First Six Months of Age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.F.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A Specific Mixture of Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides Induces a Beneficial Immunoglobulin Profile in Infants at High Risk for Allergy. Allergy 2009, 64, 484–487. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early Neutral Prebiotic Oligosaccharide Supplementation Reduces the Incidence of Some Allergic Manifestations in the First 5 Years of Life. J. Biol. Regul. Homeost. Agents 2012, 26 (Suppl. 3), 49–59. [Google Scholar]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef] [PubMed]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- Seppo, A.E.; Bu, K.; Jumabaeva, M.; Thakar, J.; Choudhury, R.A.; Yonemitsu, C.; Bode, L.; Martina, C.A.; Allen, M.; Tamburini, S.; et al. Infant Gut Microbiome Is Enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with Traditional Farming Lifestyle. Allergy 2021, 76, 3489–3503. [Google Scholar] [CrossRef]
- Sprenger, N.; Tytgat, H.L.P.; Binia, A.; Austin, S.; Singhal, A. Biology of Human Milk Oligosaccharides: From Basic Science to Clinical Evidence. J. Hum. Nutr. Diet. 2022, 35, 280–299. [Google Scholar] [CrossRef]
- Rodríguez-Benítez, M.V.; Gámez-Belmonte, R.; Gil-Campos, M.; Hernández-Chirlaque, C.; Bouzas, P.R.; Sánchez de Medina, F.; Martínez-Augustin, O. Premature Birth Infants Present Elevated Inflammatory Markers in the Meconium. Front. Pediatr. 2021, 8, 627475. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Li, W.; Zhao, Y. NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases. Front. Immunol. 2021, 12, 732933. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zheng, B.; He, H.; Chen, L. Effects of Non-Covalent Binding of Lignans with Rice Starch Driven by High-Pressure Homogenization on the Starch Structure and In Vitro Nutritional Characteristics. Food Funct. 2022, 13, 9243–9253. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Chen, X.; Chen, H.; Xu, C.; Yang, Y. Lacticaseibacillus paracasei Alleviates Food Allergy by Regulating Gut Microbiota and Short-Chain Fatty Acids in a Mouse Model. Front. Nutr. 2022, 9, 874321. [Google Scholar]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K. Prebiotics in infants for allergy prevention. Cochrane Database Syst. Rev. 2013, 3, CD006474. [Google Scholar] [CrossRef]
- Grimshaw, K.; Logan, K.; O’Donovan, S.; Kiely, M.; Patient, K.; van Bilsen, J.; Beyer, K.; Campbell, D.E.; Garcia-Larsen, V.; Grabenhenrich, L.; et al. Modifying the infant’s diet to prevent food allergy. Arch. Dis. Child. 2017, 102, 179–186. [Google Scholar] [CrossRef]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2018, 141, 1334–1342.e5. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human Milk Glycobiome and Its Impact on the Infant Gastrointestinal Microbiota. Proc. Natl. Acad. Sci. USA 2010, 108, 4653–4658. [Google Scholar] [CrossRef]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human Milk Oligosaccharides and Associations with Immune-Mediated Disease and Infection in Childhood: A Systematic Review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rudloff, S. Compositional Analysis and Metabolism of Human Milk Oligosaccharides in Infants. Nestlé Nutr. Inst. Workshop Ser. 2017, 88, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef]
- De Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics and Synbiotics. In Food Biotechnology, Advances in Biochemical Engineering/Biotechnology; Stahl, U., Donalies, U.E.B., Nevoigt, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 1–66. [Google Scholar] [CrossRef]
- Scavuzzi, B.M.; Henrique, F.C.; Miglioranza, L.H.S.; Simão, A.N.C.; Dichi, I. Impact of prebiotics, probiotics and synbiotics on components of the metabolic syndrome. Ann. Nutr. Disord. Ther. 2014, 1, 1009. [Google Scholar]
- Burks, A.W.; Harthoorn, L.F.; van Ampting, M.T.; Oude Nijhuis, M.M.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B.; et al. Synbiotics-supplemented amino acid-based formula supports adequate growth in cow’s milk allergic infants. Pediatr. Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The effectiveness of probiotic Lactobacillus rhamnosus and Lactobacillus casei strains in children with atopic dermatitis and cow’s milk protein allergy: A multicenter, randomized, double-blind, placebo-controlled study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Palmer, D.J.; Cuthbert, A.R.; Sullivan, T.R.; Pretorius, R.A.; Garssen, J.; Rueter, K.; Jenmalm, M.C.; Keelan, J.A.; Silva, D.; Prescott, S.L. Effects of pregnancy and lactation prebiotics supplementation on infant allergic disease: A randomized controlled trial. J. Allergy Clin. Immunol. 2025, 155, 144–152. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Czerkies, L.; Reyes, K.; Collins, B.; Heine, R.G. Confirmed hypoallergenicity of a novel whey-based extensively hydrolyzed infant formula containing two human milk oligosaccharides. Nutrients 2019, 11, 1447. [Google Scholar] [CrossRef]
- Xu, H.; Duan, X.; Wang, Y.; Geng, W. Amelioration effect of Lactobacillus kefiranofaciens ZW3 on ovalbumin-induced allergic symptoms in BALB/c mice. Foods 2024, 14, 16. [Google Scholar] [CrossRef]
- Zhu, P.; Savova, M.V.; Kindt, A.; PRESTO Study Team; Wopereis, H.; Belzer, C.; Harms, A.C.; Hankemeier, T. Exploring the fecal metabolome in infants with cow’s milk allergy: The distinct impacts of cow’s milk protein tolerance acquisition and of synbiotic supplementation. Mol. Nutr. Food Res. 2025, 69, e202400583. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Sato, M.; Toyokuni, K.; Irahara, M.; Hiraide-Kotaki, E.; Harima-Mizusawa, N.; Morita, H.; Matsumoto, K.; Ohya, Y. Combination of heat-killed Lactiplantibacillus plantarum YIT 0132 (LP0132) and oral immunotherapy in cow’s milk allergy: A randomised controlled trial. Benef. Microbes 2023, 14, 17–30. [Google Scholar] [CrossRef]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance development in cow’s milk-allergic infants receiving amino acid-based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar] [CrossRef] [PubMed]
- Loke, P.; Orsini, F.; Lozinsky, A.C.; Gold, M.; O’Sullivan, M.D.; Quinn, P.; Lloyd, M.; Ashley, S.E.; Pitkin, S.; Axelrad, C.; et al. Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): A multicentre, randomised, phase 2b trial. Lancet Child Adolesc. Health 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: A 2 × 2 factorial, randomized, non-treatment controlled trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, M.; Saros, L.; Vahlberg, T.; Nermes, M.; Jartti, T.; Laitinen, K. Maternal fish oil and/or probiotics intervention: Allergic diseases in children up to two years old. Pediatr. Allergy Immunol. 2023, 34, e14004. [Google Scholar] [CrossRef]
- Nocerino, R.; Bedogni, G.; Carucci, L.; Cosenza, L.; Cozzolino, T.; Paparo, L.; Palazzo, S.; Riva, L.; Verduci, E.; Berni Canani, R. The impact of formula choice for the management of pediatric cow’s milk allergy on the occurrence of other allergic manifestations: The Atopic March Cohort Study. J. Pediatr. 2021, 232, 183–191.e3. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Lie, A.; Vettukattil, R.; Rehbinder, E.M.; LeBlanc, M.; Asarnoj, A.; Carlsen, K.H.; Despriee, Å.W.; Färdig, M.; Gerdin, S.W.; et al. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2022, 399, 2398–2411. [Google Scholar] [CrossRef]
- Sakihara, T.; Otsuji, K.; Arakaki, Y.; Hamada, K.; Sugiura, S.; Ito, K. Randomized trial of early infant formula introduction to prevent cow’s milk allergy. J. Allergy Clin. Immunol. 2021, 147, 224–232.e8. [Google Scholar] [CrossRef] [PubMed]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Systematic Review and Meta-Analysis on Probiotics as Treatment for Food Allergies among Pediatric Patients: A 2024 Update. Pediatr. Allergy Immunol. 2025, 36, e70028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, L.; Xia, J.; Cheng, L.; Chen, G.; Wang, J.; Raghavan, V. Probiotics Supplementation during Pregnancy or Infancy on Multiple Food Allergies and Gut Microbiota: A Systematic Review and Meta-Analysis. Nutr. Rev. 2025, 83, e25–e41. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Lahtinen, S.J.; Boyle, R.J. Probiotics and prebiotics: Clinical effects in allergic disease. Curr. Opin. Pediatr. 2010, 22, 626–634. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Núñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Fiocchi, A.; Burks, W.; Bahna, S.L.; Bielory, L.; Boyle, R.J.; Cocco, R.; Dreborg, S.; Goodman, R.; Kuitunen, M.; Haahtela, T.; et al. Clinical use of probiotics in pediatric allergy (CUPPA): A World Allergy Organization position paper. World Allergy Organ. J. 2012, 5, 148–167. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Schadt, E.E. Systems biology of asthma and allergic diseases: A multiscale approach. J. Allergy Clin. Immunol. 2015, 135, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tessler, M.; Neumann, J.S.; Afshinnekoo, E.; Pineda, M.; Hersch, R.; Velho, L.F.M.; Segovia, B.T.; Lansac-Toha, F.A.; Lemke, M.; DeSalle, R.; et al. Large-scale differences in microbial biodiversity discovery between 16S amplicon and shotgun sequencing. Sci. Rep. 2017, 7, 6589. [Google Scholar] [CrossRef]
- Marenholz, I.; Grosche, S.; Kalb, B.; Rüschendorf, F.; Blümchen, K.; Schlags, R.; Harandi, N.; Price, M.; Hansen, G.; Seidenberg, J.; et al. Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat. Commun. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Ellis, G.; Ben-Shoshan, M.; Martino, D.; Ferreira, M.A.; Allen, K.; et al. Genome-wide association study and meta-analysis in multiple populations identifies new loci for peanut allergy and establishes C11orf30/EMSY as a genetic risk factor for food allergy. J. Allergy Clin. Immunol. 2018, 141, 991–1001. [Google Scholar] [CrossRef]
- Hirota, T.; Takahashi, A.; Kubo, M.; Tsunoda, T.; Tomita, K.; Sakashita, M.; Yamada, T.; Fujieda, S.; Tanaka, S.; Doi, S.; et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 2012, 44, 1222–1226. [Google Scholar] [CrossRef]
- Hong, X.; Hao, K.; Ladd-Acosta, C.; Hansen, K.D.; Tsai, H.J.; Liu, X.; Xu, X.; Thornton, T.A.; Caruso, D.; Keet, C.A.; et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat. Commun. 2015, 6, 6304. [Google Scholar] [CrossRef]
- Knight, R.; Callewaert, C.; Marotz, C.; Hyde, E.R.; Debelius, J.W.; McDonald, D.; Sogin, M.L. The microbiome and human biology. Annu. Rev. Genomics Hum. Genet. 2017, 18, 65–86. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).