Natural Compounds Targeting SIRT1 and Beyond: Promising Nutraceutical Strategies Against Atherosclerosis
Abstract
1. Introduction
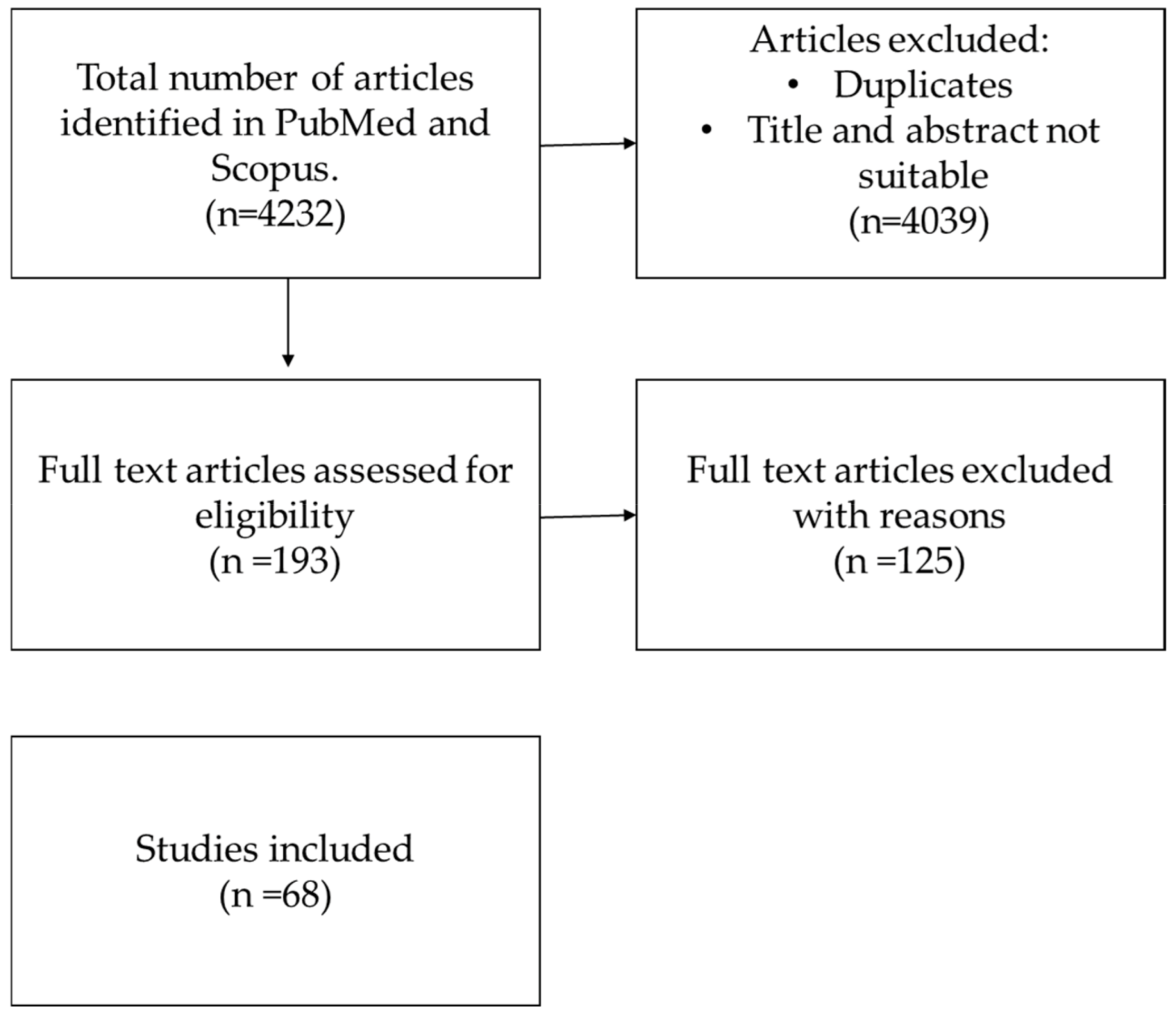
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Elegibility Criteria
2.3. Study Selection
2.4. Data Extraction
3. Results
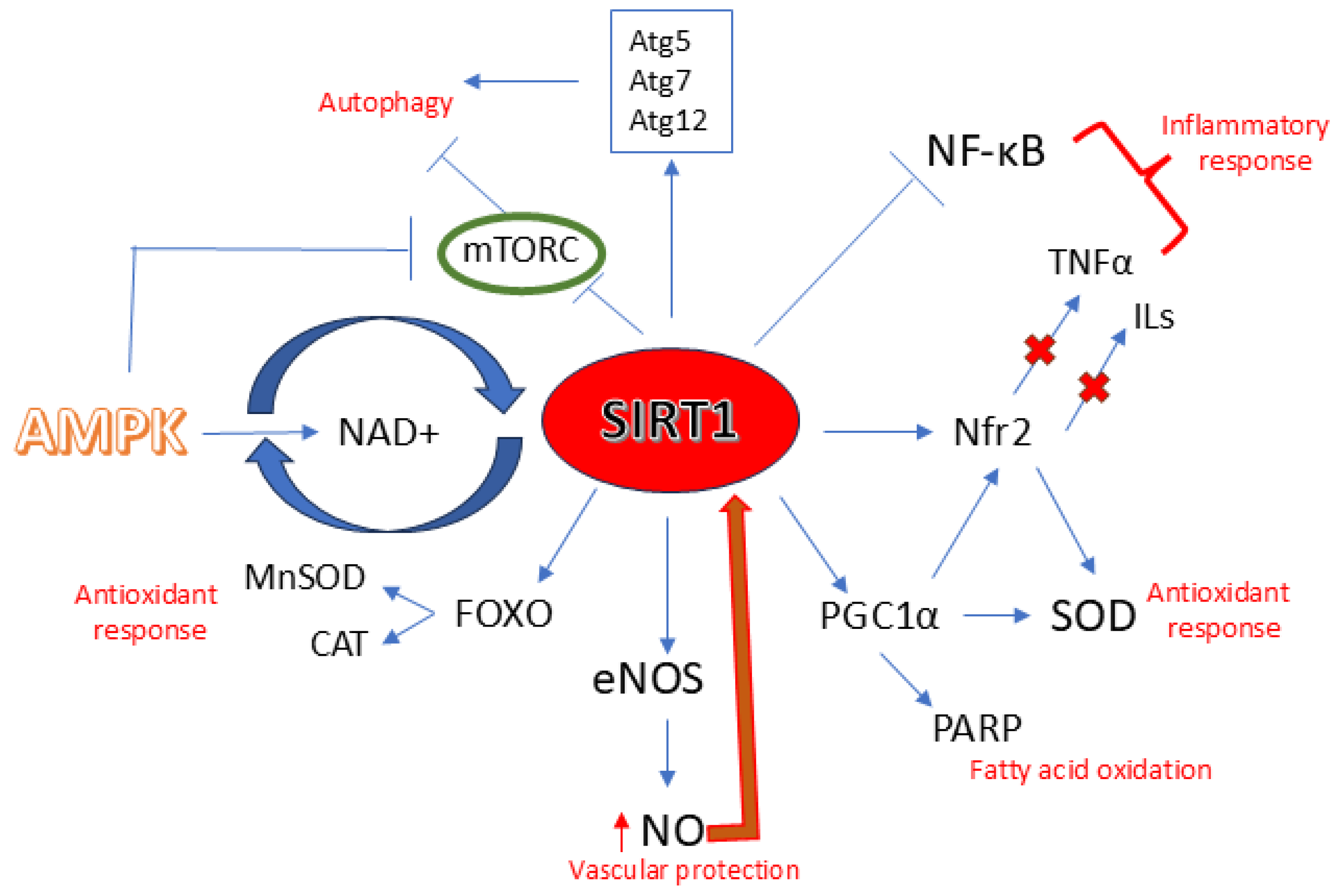
3.1. SIRT1 and Atherosclerosis
3.1.1. SIRT1 and Aging
3.1.2. SIRT1 and eNOS/NO
3.1.3. SIRT1 and Inflammation
3.1.4. SIRT1 and Oxidative Stress
3.1.5. SIRT1 and Autophagy
3.2. Natural Activators and Atherosclerosis
3.2.1. Resveratrol
3.2.2. Quercetin
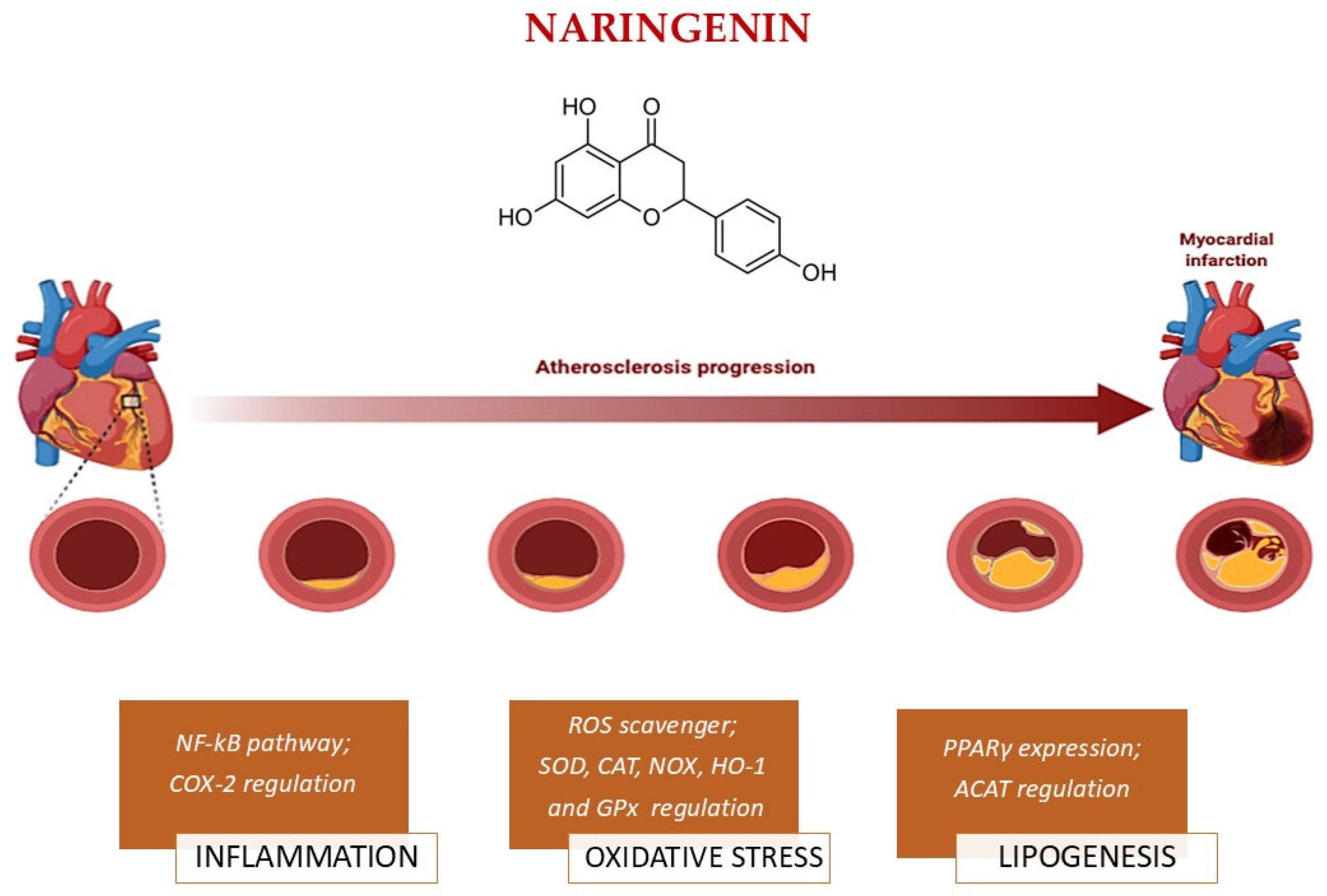
3.2.3. Naringenin
3.2.4. Curcumin
3.2.5. Berberine
3.2.6. Fisetin
3.2.7. Piceatannol
3.2.8. Honokiol
3.2.9. Epigallocatechin-3-Gallate
3.2.10. Hydroxytyrosol
3.3. Safety Considerations
3.3.1. Resveratrol
3.3.2. Quercetin
3.3.3. Naringenin
3.3.4. Curcumin
3.3.5. Berberine
3.3.6. Fisetin
3.3.7. Piceatannol
3.3.8. Honokiol
3.3.9. Epigallocatechin-3-Gallate
3.3.10. Hydroxytyrosol
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ACAT | Hepatic cholesterol acyltransferase |
| ACC | Acetyl-CoA carboxylase |
| Akt | Protein kinase B |
| AMPK | Adenosine monophosphate-activated kinase |
| ApoB | ApolipoproteinB |
| ARE | Antioxidant response element |
| ATGL | Adipose triglyceride lipase |
| BBR | Berberine |
| BKCa | Calcium-activated potassium channel |
| cAMP | Cyclic adenosine monophosphate |
| CAT | Catalase |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CGI-58 | Comparative gene identification-58 |
| COX-2 | Cyclooxygenase-2 |
| CRC | Curcumin |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 |
| EGCG | Epigallocatechin-3-gallate |
| eNOS | Endothelial nitric oxide synthase |
| EP300 | E1A binding protein p300 acetyltransferase |
| Erk | Extracellular signal-regulated kinases |
| FAS | Fatty acid synthase |
| FIS | Fisetin |
| FOXO | Forkhead box-O |
| Gal-3-NLRP3 | Galectin-3 NLR family pyrin domain containing 3 |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HCAEC | Human coronary artery endothelial cell |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HMGB1 | High Mobility Group Box 1 |
| HNK | Honokiol |
| HO-1 | Heme oxygenase-1 |
| HOCl | Hypochlorous acid |
| HT | Hydroxytyrosol |
| ICAM-1 | Intercellular adhesion molecule-1 |
| Il-1β | Interleukin-1β |
| Il-6 | Interleukin-6 |
| Il-8 | Interleukin-8 |
| iNOS | Inducible nitric oxide synthase |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha |
| JNK | c-Jun N-terminal kinases |
| KL | Klotho |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| LKB1 | Liver kinase B1 |
| LOX | Lipoxygenases |
| LXRα | Calmodulin-liver X receptor α |
| MAP1LC3 | Microtubule-associated protein 1 light chain 3 |
| MAPK | Mitogen activated protein kinase |
| MCP-1 | Monocyte chemo attractant protein-1 |
| MDA | Malondialdehyde |
| MMP-9 | Metalloproteinase-9 |
| Mn-SOD | Manganese superoxide dismutase |
| MPO | Myeloperoxidase |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NAD | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAR | Naringenin |
| NF-κB | Nuclear factor kappa B |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor protein 3 |
| NO | Nitric oxide |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| oxLDL | Oxidized low-density lipoprotein |
| PCT | Piceatannol |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PKC-δ | Protein kinase C-delta |
| PLIN1 | Perilipin 1 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| Prx | Peroxiredoxin |
| QRC | Quercetin |
| RASMC | Rat aortic smooth muscle cells |
| RCT | Reverse cholesterol transport |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| SR-A1 | Scavenger receptor class A |
| SREBP-1 | Sterol regulatory element-binding transcription factor 1 |
| TC | Total cholesterol |
| TF | Tissue factor |
| TFEB | Transcription factor EB |
| TG | Triglycerides |
| TLR4 | Toll-like receptor 4 |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| TNFα | Tumor necrosis factor α |
| TR2 | Thioredoxin reductase 2 |
| Trx2 | Thioredoxin 2 |
| TSC2 | Tuberous Sclerosis Complex 2 |
| TXA2 | Thromboxane A2 |
| uPa | Urokinase plasminogen activator |
| UVEC | Umbilical vein endothelial cells |
| VCAM-1 | Vascular adhesion molecule-1 |
| VSMC | Vascular smooth muscle cells |
| XO | Xanthine oxidases |
References
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 10, 2290–2307. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Łanoszka, K.; Vlčková, N. Natural Sirtuin1 Activators and Atherosclerosis: An Overview. Curr. Atheroscler. Rep. 2023, 25, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, R.; Hua, Y.; Ling, S.; Xu, X. Naringenin ameliorates vascular senescence and atherosclerosis involving SIRT1 activation. J. Pharm. Pharmacol. 2023, 75, 1021–1033. [Google Scholar] [CrossRef]
- Domi, E.; Hoxha, M.; Prendi, E.; Zappacosta, B. A Systematic Review on the Role of SIRT1 in Duchenne Muscular Dystrophy. Cells 2021, 10, 1380. [Google Scholar] [CrossRef]
- Ciccone, L.; Piragine, E.; Brogi, S.; Camodeca, C.; Fucci, R.; Calderone, V.; Nencetti, S.; Martelli, A.; Orlandini, E. Resveratrol-like Compounds as SIRT1 Activators. Int. J. Mol. Sci. 2022, 23, 15105. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes. Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Zu, Y.; Liu, L.; Lee, M.Y.; Xu, C.; Liang, Y.; Man, R.Y.; Vanhoutte, P.M.; Wang, Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 2010, 106, 1384–1393. [Google Scholar] [CrossRef]
- Thompson, A.M.; Wagner, R.; Rzucidlo, E.M. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H533–H541. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Burba, I.; Ruggeri, C.; Zeni, F.; Bertolotti, M.; Scopece, A.; Pompilio, G.; Raucci, A. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated proinflammatory secretory factors. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1304–1311. [Google Scholar] [CrossRef]
- Sazdova, I.; Hadzi-Petrushev, N.; Keremidarska-Markova, M.; Stojchevski, R.; Sopi, R.; Shileiko, S.; Mitrokhin, V.; Gagov, H.; Avtanski, D.; Lubomirov, L.T.; et al. SIRT-associated attenuation of cellular senescence in vascular wall. Mech. Ageing Dev. 2024, 220, 111943. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Magerko, K.A.; Lawson, B.R.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011, 589, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Eto, M.; Kano, M.R.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1634–1639. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef]
- Stein, S.; Schäfer, N.; Breitenstein, A.; Besler, C.; Winnik, S.; Lohmann, C.; Heinrich, K.; Brokopp, C.E.; Handschin, C.; Landmesser, U.; et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging 2010, 2, 353–360. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Breitenstein, A.; Wyss, C.A.; Spescha, R.D.; Franzeck, F.C.; Hof, D.; Riwanto, M.; Hasun, M.; Akhmedov, A.; von Eckardstein, A.; Maier, W.; et al. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLoS ONE 2013, 8, e53106. [Google Scholar] [CrossRef]
- Li, Y.; Ni, J.; Guo, R.; Li, W. In patients with coronary artery disease and type 2 diabetes, SIRT1 expression in circulating mononuclear cells is associated with levels of inflammatory cytokines but not with coronary lesions. BioMed Res. Int. 2016, 2016, 8734827. [Google Scholar] [CrossRef]
- de Kreutzenberg, S.V.; Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Semplicini, A.; Dalla Man, C.; Cobelli, C.; Fadini, G.P.; Avogaro, A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: Potential biochemical mechanisms. Diabetes 2010, 59, 1006–1015. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Tsuda, S.; Kanasaki, K.; Koya, D. Calorie restriction in overweight males ameliorates obesity-related metabolic alterations and cellular adaptations through anti-aging effects, possibly including AMPK and SIRT1 activation. Biochim. Biophys. Acta 2013, 1830, 4820–4827. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Gorenne, I.; Kumar, S.; Gray, K.; Figg, N.; Yu, H.; Mercer, J.; Bennett, M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 2013, 127, 386–396. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef]
- Kaufmann, A.; Beier, V.; Franquelim, H.G.; Wollert, T. Molecular mechanism of autophagic membrane scaffold assembly and disassembly. Cell 2014, 156, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; McBurney, M.; Robbins, P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE 2010, 5, e9199. [Google Scholar] [CrossRef]
- Banreti, A.; Sass, M.; Graba, Y. The emerging role of acetylation in the regulation of autophagy. Autophagy 2013, 9, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 503–510. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B. Bioactive compounds as inhibitors of inflammation, oxidative stress and metabolic dysfunctions via regulation of cellular redox balance and histone acetylation state. Foods 2023, 12, 925. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 activation by natural phytochemicals: An overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Sahebkar, A.; Zabetian-Targhi, F.; Maleki, V. The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review. Biofactors 2019, 45, 651–665. [Google Scholar] [CrossRef]
- Nallasamy, P.; Kang, Z.Y.; Sun, X.; Anandh Babu, P.V.; Liu, D.; Jia, Z. Natural compound resveratrol attenuates TNF-α-induced vascular dysfunction in mice and human endothelial cells: The involvement of the NF-κB signaling pathway. Int. J. Mol. Sci. 2021, 22, 12486. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK–SIRT1–FOXO pathway: In-depth analysis and breakthrough prospects of oxidative stress-induced diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, J.; Huang, Y.; Cao, K.; Xu, Y.; Wu, J.M. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin. Med. J. 2002, 115, 378–380. [Google Scholar]
- Dobrydneva, Y.; Williams, R.L.; Blackmore, P.F. Trans-resveratrol inhibits calcium influx in thrombin-stimulated human platelets. Br. J. Pharmacol. 1999, 128, 149–157. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Meng, F.; Mackman, N.; Rao, L.V. Mechanism of resveratrol-mediated suppression of tissue factor gene expression. Thromb. Haemost. 2002, 87, 155–162. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and vascular function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, J.; Hu, Z.; Sun, B. Resveratrol protects against atherosclerosis by downregulating the PI3K/AKT/mTOR signaling pathway in atherosclerosis model mice. Exp. Ther. Med. 2022, 23, 414. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L.; Qi, L.; Yang, X.; Jin, Y.; Yin, H.; Huang, Y.; Sheng, J.; Wang, X. Resveratrol improved atherosclerosis by increasing LDLR levels via the EGFR–ERK1/2 signaling pathway. Lipids Health Dis. 2025, 24, 167. [Google Scholar] [CrossRef]
- Brito, P.; Almeida, L.M.; Dinis, T.C. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite: Protection against LDL oxidation. Free Radic. Res. 2002, 36, 621–631. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Son, D.J.; Kim, Y.S.; Kim, M.-K.; Yoon, B.-E.; Pyee, J.; Hong, J.T.; Go, Y.-M.; et al. Antiatherogenic effect of resveratrol attributed to decreased expression of ICAM-1. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: A mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Huang, H.M.; Hsieh, S.J.; Jeng, K.C.G.; Kuo, J.S. Resveratrol inhibits in terleukin-6 production in cortical mixed glial cells under hy poxia/hypoglycemia followed by reoxygenation. J. Neuroimmunol. 2001, 112, 28–34. [Google Scholar] [CrossRef]
- Culpitt, S.V.; Rogers, D.F.; Fenwick, P.S.; Shah, P.; De Matos, C.; Russell, R.E.K.; Barnes, P.J.; Donnelly, L.E. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax 2003, 58, 942–946. [Google Scholar] [CrossRef]
- Bo, B.; Ratliff, H.C.; Park, M.C.; Kuo, Z.; Ungvari, A.; Csiszar, B.; Krasnikov, K.; Sodhi, F.; Zhang, A.; Nasjletti, M.S.; et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: Proteomic approach. Am. J. Pathol. 2009, 174, 34–43. [Google Scholar]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Cardiovasc. Res. 2017, 113, 498–510. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lu, L.; Liu, Y.; Ma, J.; Yang, L.; Li, L.; Guo, H.; Yu, S.; Ren, J.; Bai, H.; et al. Quercetin improves ischemia/reperfusion-induced cardiomyocyte apoptosis via SIRT1/PGC-1α signaling. J. Cell. Biochem. 2019, 120, 9747–9757. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Pharmacol. 2022, 13, 956213. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.Y.; Tang, F.; Liu, D.; Zhao, X.L.; Zhang, J.N.; Xia, J.; Wu, J.J.; Yang, Y.; Peng, C.; et al. New perspectives on the therapeutic potential of quercetin in non-communicable diseases. Nutrients 2024, 16, 1452. [Google Scholar]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-κB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Tian, R.; Lu, N. Quercetin attenuates vascular endothelial dysfunction in atherosclerotic mice by inhibiting myeloperoxidase and NADPH oxidase function. Chem. Res. Toxicol. 2023, 36, 260–269. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory and oxidative stress responses in diabetic high-fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jiang, L.Y.; Wang, Y.C.; Ma, D.F.; Li, X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [PubMed]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin attenuates atherosclerotic inflammation by inhibiting galectin-3-NLRP3 signaling pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Li, S.; Cao, H.; Shen, D.; Jia, Q.; Chen, C.; Xing, S.L. Quercetin protects against ox-LDL-induced injury via regulation of ABCA1, LXR-α and PCSK9 in RAW264.7 macrophages. Mol. Med. Rep. 2018, 18, 799–806. [Google Scholar]
- Jia, X.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Douet, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Front. Pharmacol. 2019, 10, 1653. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic potential of quercetin as an antiatherosclerotic agent in cardiovascular disease: A review. Evid. Based Complement. Alternat. Med. 2020, 2020, 5926381. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, F. Quercetin inhibits the formation of atherosclerotic plaque by protecting vascular endothelial cells. Life Sci. 2019, 232, 116612. [Google Scholar]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity and interact with aspirin to enhance antiplatelet effects. TH Open 2019, 3, e244–e258. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ros-regulated Pi3k/Akt signaling pathway. BioMed Pharmacother. 2017, 85, 658. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and the prevention of atherosclerosis. Nutrients 2012, 4, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Adetunji, J.A.; Fasae, K.D.; Awe, A.I.; Paimo, O.K.; Adegoke, A.M.; Akintunde, J.K.; Sekhoacha, M.P. The protective roles of citrus flavonoids, naringenin, and naringin on endothelial cell dysfunction in diseases. Heliyon 2023, 9, e17166. [Google Scholar] [CrossRef]
- Jomova, K.; Kuca, K.; Alomar, S.Y.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Int. J. Mol. Sci. 2023, 24, 12345. [Google Scholar] [CrossRef]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, D.I.; Kim, W.J.; Moon, S.K. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-alpha-induced vascular smooth muscle cells. Mol. Nutr. Food Res. 2009, 53, 1582–1591. [Google Scholar] [CrossRef]
- Herath, H.M.; Takano-Ishikawa, Y.; Yamaki, K. Inhibitory effect of some flavonoids on tumor necrosis factor-alpha production in lipopolysaccharide-stimulated mouse macrophage cell line J774.1. J. Med. Food 2003, 6, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, S.; Wang, H.; Liu, S.; Liu, G.; Chen, H.; Kang, J.; Wang, H. Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and vitro. Food Sci. Hum. 2023, 12, 1174–1183. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Kim, J.R.; Han, J.I.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Saad, B. Prevention and treatment of obesity-related inflammatory diseases by edible and medicinal plants and their active com pounds. Immuno 2022, 2, 609–629. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Peng, B.; Zou, H.; Li, S.; Wang, J.; Cao, J. Curcumin improves necrotising microscopic colitis and cell pyroptosis by activating SIRT1/NRF2 and inhibiting the TLR4 signalling path way in newborn rats. Innate Immun. 2020, 26, 609–617. [Google Scholar] [CrossRef]
- Soetikno, V.; Watanabe, K.; Sari, F.R.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Arozal, W.; Sukumaran, V.; Lakshmanan, A.P.; Arumugam, S.; et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-alpha and PKC-beta1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011, 55, 1655–1665. [Google Scholar] [CrossRef]
- Matacchione, G.; Gurău, F.; Silvestrini, A.; Tiboni, M.; Mancini, L.; Valli, D.; Rippo, M.R.; Recchioni, R.; Marcheselli, F.; Carnevali, O.; et al. Anti-SASP and anti-inflammatory activ ity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology 2021, 22, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.L.; Jia, Y.; Wan, Z.; An, Z.L.; Yang, S.; Han, F.F.; Gong, L.L.; Xuan, L.L.; Ren, L.L.; Zhang, W.; et al. Curcumin inhibits the formation of atherosclerosis in ApoE−/− mice by sup pressing cytomegalovirus activity in endothelial cells. Life Sci. 2020, 257, 117658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Ching, L.C.; Huang, J.C.; Chen, C.Y.; Chiang, A.N.; Kou, Y.R.; Shyue, S.K.; Lee, T.S. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol. Nutr. Food Res. 2012, 56, 691–701. [Google Scholar] [CrossRef]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- Tan, W.; Wang, Y.; Wang, K.; Wang, S.; Liu, J.; Qin, X.; Dai, Y.; Wang, X.; Gao, X. Improvement of endothelial dysfunction of berberine in atherosclerotic mice and mechanism exploring through TMT-based proteomics. Oxidative Med. Cell Longev. 2020, 31, 8683404. [Google Scholar] [CrossRef]
- Li, C.; Jiang, S.; Wang, H.; Wang, Y.; Han, Y.; Jiang, J. Berberine exerts protective effects on cardiac senescence by regulating the Klotho/SIRT1 signaling pathway. Biomed. Pharmacother. 2022, 151, 113097. [Google Scholar] [CrossRef]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kałużna-Oleksy, M.; Bil-Lula, I. The biological role of Klotho protein in the development of cardiovascular diseases. BioMed Res. 2018, 24, 5171945. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, Q.; Yu, Y.; Yang, F.; Bai, R.; Fan, X. Efficacy and underlying mechanisms of berberine against lipid metabolic diseases: A review. Front. Pharmacol. 2023, 15, 1283784. [Google Scholar] [CrossRef]
- Wu, M.; Yang, S.; Wang, S.; Cao, Y.; Zhao, R.; Li, X.; Xing, Y.; Liu, L. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE−/− mice. Front. Pharmacol. 2020, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Bonaventura, A.; Bianchi, L.; Romano, D.; Maffioli, P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert. Opin. Biol. Ther. 2013, 13, 475–482. [Google Scholar] [CrossRef]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, X.; Ghanam, K.; Zhang, S.; Zhao, T.; Zhu, X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism 2014, 63, 1167–1177. [Google Scholar] [CrossRef]
- Ma, S.-R.; Tong, Q.; Lin, Y.; Pan, L.-B.; Fu, J.; Peng, R.; Zhang, X.-F.; Zhao, Z.-X.; Li, Y.; Yu, J.-B.; et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Krishnakumar, I.M.; Jaja-Chimedza, A.; Joseph, A.; Balakrishnan, A.; Maliakel, B.; Swick, A. Enhanced bioavailability and pharmacokinetics of a novel hybrid-hydrogel formulation of fisetin orally administered in healthy individuals: A randomised double-blinded comparative crossover study. J. Nutr. Sci. 2022, 11, e74. [Google Scholar] [CrossRef]
- Shia, C.-S.; Tsai, S.-Y.; Kuo, S.-C.; Hou, Y.-C.; Chao, P.-D.L. Metabolism and Pharmacokinetics of 3,3,4,7-Tetrahydroxyflavone (Fisetin), 5-Hydroxyflavone, and 7-Hydroxyflavone and Antihemolysis Effects of Fisetin and Its Serum Metabolites. J. Agric. Food Chem. 2009, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Jia, Q.; Cao, H.; Chen, C.; Xing, S.; Huang, Y.; Shen, D. Fisetin ameliorates atherosclerosis by regulating PCSK9 and LOX-1 in apoE-/- mice. Exp. Ther. Med. 2021, 21, 25. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, Y.; Ilyas, I.; Wang, L.; Little, P.J.; Xu, S. The natural polyphenol fisetin in atherosclerosis prevention: A mechanistic review. J. Pharm. Pharmacol. 2025, 77, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.; Choo, S.; Kim, J.; Bae, M.C.; Baek, J.S. Fisetin suppresses pulmonary inflammatory responses through heme oxygenase-1 mediated downregulation of inducible nitric oxide synthase. J. Med. Food 2020, 23, 1163–1168. [Google Scholar] [CrossRef]
- Lian, T.W.; Wang, L.; Lo, Y.H.; Huang, I.J.; Wu, M.J. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim. Biophys. Acta 2008, 1781, 601–609. [Google Scholar] [CrossRef]
- Yoo, H.; Ku, S.K.; Han, M.S.; Kim, K.M.; Bae, J.S. Anti-septic effects of fsetin in vitro and in vivo. Infammation 2014, 37, 1560–1574. [Google Scholar] [CrossRef]
- Tahanian, E.; Sanchez, L.A.; Shiao, T.C.; Roy, R.; Annabi, B. Flavonoids targeting of IκB phosphorylation abrogates carcinogen-induced MMP-9 and COX-2 expression in human brain endothelial cells. Drug Des. Devel Ther. 2011, 5, 299–309. [Google Scholar]
- Hada, Y.; Uchida, H.A.; Wada, J. Fisetin attenuates lipopolysaccharide-induced inflammatory responses in macrophage. Biomed. Res. Int. 2021, 13, 5570885. [Google Scholar] [CrossRef]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Prieto, M.A.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-infammatory activity of flavonols via inhibiting MAPK and NF-κB signaling pathways in RAW264.7 macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.H.; Ko, W.C.; Ko, F.N.; Teng, C.M. Inhibition of platelet aggregation by some flavonoids. Thromb. Res. 1991, 64, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.N.; Colman, R.W. Thrombin- and cathepsin G-induced platelet aggregation: Effect of protein kinase C inhibitors. Anal. Biochem. 1993, 210, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef]
- Dai, Y.; Lim, J.X.; Yeo, S.C.M.; Xiang, X.; Tan, K.S.; Fu, J.H.; Huang, J.H.; Lin, H.S. Biotransformation of piceatannol, a dietary resveratrol derivative: Promises to human health. Mol. Nutr. Food Res. 2020, 2, 64. [Google Scholar]
- Nguyen, M.C.; Ryoo, S. Intravenous administration of piceatannol, an arginase inhibitor, improves endothelial dysfunction in aged mice. Korean J. Physiol. Pharmacol. 2016, 21, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ashikawa, K.; Majumdar, S.; Banerjee, S.; Bharti, A.C.; Shishodia, S.; Aggarwal, B.B. Piceatannol Inhibits TNF-Induced NF-κB Activation and NF-κB-Mediated Gene Expression Through Suppression of IκBα Kinase and p65 Phosphorylation. J. Immunol. 2002, 169, 6490–6497. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem. Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef]
- Lee, B.; Lee, E.J.; Kim, D.I.; Park, S.K.; Kim, W.J.; Moon, S.K. Inhibition of proliferation and migration by piceatannol in vascular smooth muscle cells. Toxicol. Vitr. 2009, 23, 1284–1291. [Google Scholar] [CrossRef]
- Yang, J.S.; Tongson, J.; Kim, K.H.; Park, Y. Piceatannol attenuates fat accumulation and oxidative stress in steatosis-induced HepG2 cells. Curr. Res. Food Sci. 2020, 8, 392–399. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kershaw, J.; Chen, C.Y.; Komanetsky, S.M.; Zhu, Y.; Guo, X.; Myer, P.R.; Applegate, B.; Kim, K.H. Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J. Nutr. Biochem. 2022, 105, 108998. [Google Scholar] [CrossRef]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, P.; Wu, A.H. Honokiol inhibits carotid artery atherosclerotic plaque formation by suppressing inflammation and oxidative stress. Aging 2020, 12, 8016–8028. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, R.; Wang, S.; Li, S.; Sheng, H.; Wu, J.; Qu, Y. Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IkappaB/NF-kappaB, in atherosclerotic cell model. Exp. Mol. Med. 2015, 47, e171. [Google Scholar] [CrossRef]
- Ye, J.S.; Chen, L.; Lu, Y.Y.; Lei, S.Q.; Peng, M.; Xia, Z.Y. SIRT3 activator honokiol ameliorates surgery/anesthesia-induced cognitive decline in mice through anti-oxidative stress and anti-inflamma tory in hippocampus. CNS Neurosci. Ther. 2019, 25, 355–366. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Hu, C.; Li, Z.; Hu, J. Honokiol suppresses TNF-α-induced migration and matrix metalloproteinase expression by blocking NF-κB activation via the ERK signaling pathway in rat aortic smooth muscle cells. Acta Histochem. 2014, 116, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kawata, A.; Seki, Y.; Koh, T.; Yuhara, K.; Maruyama, T.; Machino, M.; Ito, S.; Kadoma, Y.; Fujisawa, S. Comparative inhibitory effects of magnolol, honokiol, eugenol and bis-eugenol on cyclooxygenase-2 expression and nuclear factor-kappa B activation in RAW264.7 macrophage-like cells stimulated with fimbriae of Porphyromonas gingivalis. Vivo 2012, 26, 941–950. [Google Scholar]
- Liu, J.; Zhang, T.; Zhu, J.; Ruan, S.; Li, R.; Guo, B.; Lin, L. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy. Chin. Med. 2021, 16, 115. [Google Scholar] [CrossRef]
- Ye, H.; Meng, Y. Honokiol regulates endoplasmic reticulum stress by promoting the activation of the sirtuin 1-mediated protein kinase B pathway and ameliorates high glucose/high fat-induced dysfunction in human umbilical vein endothelial cells. Endocr. J. 2021, 68, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.Y.; Chou, W.C.; Chan, S.H.; Wu, S.Y.; Chen, H.I.; Li, C.W.; Hsieh, P.L.; Chu, P.M.; Chen, Y.A.; Ou, H.C.; et al. Epigallocatechin Gallate Reduces Homocysteine-Caused Oxidative Damages through Modulation SIRT1/AMPK Pathway in Endothelial Cells. Am. J. Chin. Med. 2021, 49, 113–129. [Google Scholar] [CrossRef]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Wang, Z.M.; Gao, W.; Wang, H.; Zhao, D.; Nie, Z.L.; Shi, J.Q.; Zhao, S.; Lu, X.; Wang, L.S.; Yang, Z.J. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF-α-induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells. Cell Physiol. Biochem. 2014, 33, 1349–1358. [Google Scholar] [CrossRef]
- Chae, Y.J.; Kim, C.H.; Ha, T.S.; Hescheler, J.; Ahn, H.Y.; Sachinidis, A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell Physiol. Biochem. 2007, 20, 859–866. [Google Scholar] [CrossRef]
- HongByun, E.; Fujimura, Y.; Yamada, K.; Tachibana, H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J. Immunol. 2010, 185, 33–45. [Google Scholar] [CrossRef]
- Wang, T.; Xiang, Z.; Wang, Y.; Li, X.; Fang, C.; Song, S.; Li, C.; Yu, H.; Wang, H.; Yan, L.; et al. (-)-Epigallocatechin Gallate Targets Notch to Attenuate the Inflammatory Response in the Immediate Early Stage in Human Macrophages. Front. Immunol. 2017, 8, 433. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Zhang, X.; Liao, Z.; Gu, L.; Liu, Z.; Zhong, Q.; Wei, H.; Fang, X. The effect of green tea polyphenols on gut microbial diversity and fat deposition in C57BL/6J HFA mice. Food Funct. 2016, 7, 4956–4966. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.Z.; Wu, Y.; Wang, R.Q.; Chen, J.W.; Chen, J.; Zhang, Y.; Chen, Y.J.; Geng, M.; Xu, Z.D.; et al. (–)-Epigallocatechin-3-Gallate Ameliorates Atherosclerosis and Modulates Hepatic Lipid Metabolic Gene Expression in Apolipoprotein E Knockout Mice: Involvement of TTC39B. Front. Pharmacol. 2018, 9, 195. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell Biochem. 2018, 448, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, C.; Pan, Q. Mechanisms Underlying the Anti-Atherosclerotic Effects of EGCG. Curr. Mol. Med. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharm. 2018, 96, 88–96. [Google Scholar] [CrossRef]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-1 Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf2 De Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; López de las Hazas, M.-C.; Piñol, C.; Rubió, L.; Motilva, M.-J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291. [Google Scholar] [CrossRef]
- Muñoz-Marín, J.; De la Cruz, J.P.; Reyes, J.J.; López-Villodres, J.A.; Guerrero, A.; López-Leiva, I.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Hydroxytyrosyl alkyl ether derivatives inhibit platelet activation after oral administration to rats. Food Chem. Toxicol. 2013, 58, 295–300. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de Las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed. Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Gadecka, A.; Nowak, N.; Bulanda, E.; Janiszewska, D.; Dudkowska, M.; Sikora, E.; Bielak-Zmijewska, A. The senolytic cocktail, dasatinib and quercetin, impacts the chromatin structure of both young and senescent vascular smooth muscle cells. Geroscience 2025, 47, 3907–3925. [Google Scholar] [CrossRef]
- Millar, C.L.; Iloputaife, I.; Baldyga, K.; Norling, A.M.; Boulougoura, A.; Vichos, T.; Tchkonia, T.; Deisinger, A.; Pirtskhalava, T.; Kirkland, J.L.; et al. TA pilot study of senolytics to improve cognition and mobility in older adults at risk for Alzheimer’s disease. EBioMedicine 2025, 113, 105612. [Google Scholar] [CrossRef]
- Adams, J.A.; Uryash, A.; Mijares, A.; Eltit, J.M.; Lopez, J.R. Endothelial and Cardiovascular Effects of Naringin: A Systematic Review. Nutrients 2025, 17, 2658. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef] [PubMed]
- Curcumin’s Effect on Diabetic Patients with Atherosclerotic Cardiovascular Risk. ClinicalTrials.gov ID NCT05753436. Available online: https://clinicaltrials.gov/study/NCT05753436?intr=curcumin&cond=Diabetes%20Mellitus%20Type%202&rank=4 (accessed on 1 August 2025).
- Ji, L.; Ma, J.; Ma, Y.; Cheng, Z.; Gan, S.; Yuan, G.; Liu, D.; Li, S.; Liu, Y.; Xue, X.; et al. Berberine Ursodeoxycholate for the Treatment of Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2462185. [Google Scholar] [CrossRef] [PubMed]
- Evaluating the Tolerability and Effects of Berberine on Major Metabolic Biomarkers: A Pilot Study. ClinicalTrials.gov ID NCT03976336. Available online: https://clinicaltrials.gov/study/NCT03976336 (accessed on 1 August 2025).
- Fisetin to Improve Vascular Function in Older Adults. ClinicalTrials.gov ID NCT06133634. Available online: https://clinicaltrials.gov/study/NCT06133634 (accessed on 1 August 2025).
- Pilot Trial of Fisetin in Healthy Volunteers and Older Patients with Multimorbidity. ClinicalTrials.gov ID NCT06431932. Available online: https://clinicaltrials.gov/study/NCT06431932 (accessed on 1 August 2025).
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef]
- Zhuang, Q.; Pan, R.; Liu, X.; Xu, W.; Wang, H.; Zhang, X.; Lai, X.; Wang, H.; Zhang, L.; Jiang, J. A validated ultra-HPLC-MS/MS method for determination of honokiol in human plasma and its application to a clinical pharmacokinetic study. Bioanalysis 2019, 11, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Wilasrusmee, K.T.; Sitticharoon, C.; Keadkraichaiwat, I.; Maikaew, P.; Pongwattanapakin, K.; Chatree, S.; Sririwichitchai, R.; Churintaraphan, M. Epigallocatechin gallate enhances sympathetic heart rate variability and decreases blood pressure in obese subjects: A randomized control trial. Sci. Rep. 2024, 14, 21628. [Google Scholar] [CrossRef]
- Johnson, J.J.; Siblini, H.; Al-Hendy, A.; Segars, J.H.; González, F.; Taylor, H.S.; Singh, B.; Carson, S.A.; Christman, G.M.; Huang, H.; et al. Evaluating the Effect of Epigallocatechin Gallate (EGCG) in Reducing Folate Levels in Reproductive Aged Women by MTHFR and DHFR Genotype in Combination with Letrozole or Clomiphene. Clin. Transl. Sci. 2025, 18, e70189. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Alami, M.; Zoubdane, N.; Sidibé, H.; Berrougui, H.; Fülöp, T.; Nguyen, M.; Khalil, A. High-Tyrosol/Hydroxytyrosol Extra Virgin Olive Oil Enhances Antioxidant Activity in Elderly Post-Myocardial Infarction Patients. Antioxidants 2025, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Moratilla-Rivera, I.; Pérez-Jiménez, J.; Ramos, S.; Portillo, M.P.; Martín, M.Á.; Mateos, R. Hydroxytyrosol supplementation improves antioxidant and anti-inflammatory status in individuals with overweight and prediabetes: A randomized, double-blind, placebo-controlled parallel trial. Clin. Nutr. 2025, 52, 17–26. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 1, 91–98. [Google Scholar] [CrossRef]
- Shrestha, A.; Elliott, S.; Abasszade, J.H.; Wu, K.; Worland, T.; Simpson, I.; Dev, A. Drug-Induced Liver Injury Associated with Turmeric and Piperine: A Case and Review. Case Rep. Gastroenterol. 2025, 1, 96–106. [Google Scholar] [CrossRef]
- Guofei, L.; Mingming, Z.; Limei, Z. The drug interaction potential of berberine hydrochloride when co-administered with simvastatin, fenofibrate, gemfibrozil, metformin, glimepiride, nateglinide, pioglitazone and sitagliptin in beagles. Arab. J. Chem. 2022, 2, 103562. [Google Scholar]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Prieto, A.I.; Puerto, M.; Pichardo, S. Toxicological Evaluation of Piceatannol, Pterostilbene, and ε-Viniferin for Their Potential Use in the Food Industry: A Review. Foods 2021, 10, 592. [Google Scholar] [CrossRef]
- Grajecki, D.; Ogica, A.; Boenisch, O.; Hübener, P.; Kluge, S. Green tea extract-associated acute liver injury: Case report and review. Clin. Liver Dis. 2022, 6, 181–187. [Google Scholar] [CrossRef]
- European Food Safety Authority. Safety of Hydroxytyrosol as a Novel Food Pursuant to Regulation (EC) No 258/97. 2017. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4728 (accessed on 1 August 2025).
| Compound | SIRT1 Activation | Oxidative Stress | Inflammation | Vascular Protection | Lipid Metabolism |
|---|---|---|---|---|---|
| Resveratrol | Directly activates SIRT1 via allosteric modulation; Indirectly activates SIRT1 via AMPK activation, and NAD+ increase. | ROS scavenger; Limits LDL oxidation; Enhances antioxidant enzymes (SOD, GPx, CAT, HO-1). | Inhibits NF-κB; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-8); Inhibits PGE2 and COX2 expression. | Improves endothelial function; Increases eNOS; Reduces adhesion molecules and chemokines (CCL2, CXCL1/KC, ICAM-1, VCAM-1). | Reduces TC, TG, LDL-C; increases HDL-C; upregulates LDLR expression. |
| Quercetin | Indirect activation of SIRT1. It promotes SIRT1 activation increasing NAD+/NADH ratio and increasing SIRT1 mRNA. | ROS scavenger; Limits LDL oxidation; Inhibits oxidative enzymes (NOX, XO); Enhances antioxidant enzymes (SOD, CAT, GPx). | Inhibits NF-κB; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-10, lkBα); Inhibits COX and LOX. | Improves endothelial function; Increases eNOS; Reduces adhesion molecules (ICAM-1, VCAM-1); Inhibits platelet aggregation (TXA2 reduction, MAPK and PI3K/Akt modulation); Inhibits NLRP3 activation. | Reduces TC, TG; Enhances cholesterol efflux via ABCA1 and through the upregulation of PPARγ and LXRα. |
| Naringenin | Indirect activation of SIRT1. It promotes SIRT1 activation through AMPK activation, increased NAD+ levels. | ROS scavenger; Limits LDL oxidation; Inhibits oxidative enzymes (NOX); Activates Nrf2; Enhances antioxidant enzymes (SOD, CAT, GPx, HO-1). | Inhibits NF-κB; Suppresses MAPK; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-8, IL-1β); Reduces MMP9 expression; Inhibits PGE2 and COX2 expression. | Protects endothelium; Reduces adhesion molecules (ICAM-1, VCAM-1). | Reduces LDL, VLDL, LDL-C, TG; Increases HDL-C; Decreases ACAT activity; Inhibits the hepatic synthesis of (ApoB)-containing lipoproteins; Enhances PPARγ. |
| Curcumin | Indirectly activates SIRT1 via AMPK activation and upregulating SIRT1 transcription. | Activates Nrf2/ARE axis; Enhances antioxidant enzymes (SOD, CAT, GPx, HO-1); Reduces ROS and inhibits LDL oxidation. | Inhibits NF-κB; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); Inhibits TLR4 expression. | Protects endothelium; Reduces adhesion molecules (ICAM-1, VCAM-1). | Reduces LDL-C, TC, TG; Increases HDL-C; Enhances cholesterol efflux via ABCA1 and through the upregulation of PPARγ and LXRα. |
| Berberine | Indirectly activates SIRT1 via AMPK activation, mitochondrial modulation and NAD+ enhancement. | Reduces ROS; Inhibits oxidative enzymes (NOX); Enhances antioxidant enzymes (SOD, GPx). | Inhibits NF-κB; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); Increases IL-10 and adiponectin. | Protects endothelium; Increases eNOS; Reduces LOX-1 expression; Reduces adhesion molecules (ICAM-1, VCAM-1). | Reduces LDL-C, TC, TG; Increases HDL-C; Promotes the leptin-to-adiponectin ratio. |
| Fisetin | Weakly directly activates SIRT1 and Indirectly activates SIRT1 via AMPK activation and increasing SIRT1 expression. | Reduces ROS; Enhances antioxidant enzymes (SOD, GPx, CAT, HO-1); Inhibits oxidative enzymes (NOX); Modulates PKC-δ, p38, and Nrf2-ARE signaling pathways; Inhibits LDL oxidation. | Inhibits Akt, NF-κB and Erk½; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); Inhibits pro-inflammatory genes (MCP-1, iNOS, uPA); Inhibits COX2 and MMP9 expression. | Inhibits platelets aggregation; Inhibits PKC activity; Reduces adhesion molecules (ICAM-1). | Reduces TC, LDL-C, VLDL-C. |
| Piceatannol | Directly activates SIRT1 and also indirectly through upregulating SIRT1 expression. | Reduces ROS; Enhances antioxidant enzymes (HO-1); Promotes Nrf2 activation. | Inhibits NF-κB; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, lkBα); Inhibits COX2 and MMP9 expression. | Protects endothelium; Blocks Erk½, JNK, PI3K/Akt pathways; Inhibits VSMC migration. | Inhibits lipogenesis; Reduces TG; Blocks SREBP-1, ACC, PPARγ; Lowers circulating free fatty acids: downregulates CD36 and induces degradation of ATGL, CGI-58 and PLIN1. |
| Honokiol | Directly activates SIRT1 and indirectly by enhancing SIRT1 expression. | Reduced ROS; Inhibits Fe(III) ADP/NADH; Enhances antioxidant enzymes (SOD, CAT); Enhances Keap1/Nrf2/ARE and GSH; Inhibits LDL oxidation. | Inhibits NF-κB and MAPK; Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); Inhibits pro-inflammatory enzymes (COX2, NOS, iNOS); | Protects endothelium; Inhibits TXA2 formation; Promotes NO releasing; Reduces adhesion molecules (ICAM-1, VCAM-1). | Reduces LDL-C, TC, TG; Increases HDL-C; Promotes lipophagy through SIRT3/AMPK pathway. |
| Epigallocatechin-3-gallate | Weakly directly activates SIRT1 and Indirectly activates SIRT1 via AMPK activation and enhancing SIRT1 expression. | Reduced ROS; Inhibits oxidative enzymes (NOX); Enhances antioxidant enzymes (SOD, HO-1); Promotes Nrf2 activation. | Inhibits NF-κB and MAPK; Inhibits pro-inflammatory cytokines (TNF-α, CRP); Inhibits TLR4 expression; Binds notch receptors, blocking notch inflammatory cascade. | Protects endothelium; Reduces adhesion molecules (ICAM-1, VCAM-1). | Reduces TG, TC and LDL-C; Increases HDL-C; Downregulates PPARγ and FAS expression; Blocks SREBP-1; Activates the X receptor-mediated cholesterol efflux genes (ABCA1, ABCG5/8 and LXRα/β). |
| Hydroxytyrosol | Indirectly activates SIRT1 by promoting SIRT1 expression. | Reduced ROS; Reduces malondialdehyde (MDA); Activates AKT1; Enhances antioxidant enzymes (SOD), HO-1); Promotes Nrf2 activation; Enhances GSH. | Inhibits pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); Inhibits COX2 expression. | Improves endothelial function; Increases eNOS; Reduces adhesion molecules (ICAM-1, VCAM-1); decreases E-selectin, P-selectin; Inhibits TX and prostacyclin synthesis. | Regulates reverse cholesterol efflux by increasing ABCA1 expression and activating AMPK pathway and phosphorylation of p38. |
| Compound | Sources | Design | Type/Dose | Population | Primary Outcomes | Limitations |
|---|---|---|---|---|---|---|
| Resveratrol | Reviews/meta-analysis and ongoing pilot RCTs (2023–2025). | Multiple RCTs and pilot trials. | Oral resveratrol supplements; High-dose resveratrol (ranging from ~0.5–1.5 g/day). | Elderly or high-risk cardiovascular subjects, metabolic disease cohorts. | Mixed and inconsistent effects on endothelial/vascular biomarkers; some trials show anti-inflammatory or modest metabolic effect [162]. | Contradictory results between studies; need larger standardized trials. |
| Quercetin | Trials of quercetin alone and dasatinib + quercetin pilot human trials; recent 2024–2025 trials target senescence and vascular endpoints. | Small RCTs/pilot studies. | Quercetin dosing varies (intermittent high dose in senolytic protocols; or daily supplement doses). | Older adults, patients with CVD. | Early evidence that quercetin reduces senescent cell markers and may improve physical/cognitive function or vascular biomarkers in pilot studies [163,164]. | Small pilot trials; combined drug effects dasatinib + quercetin make complicate to attribute the effects; long term outcomes and safety need larger trials. |
| Naringenin | Systematic reviews and recent pilot clinical studies through 2024–2025; preclinical to pilot human data on blood pressure and metabolic effects. | Mostly pilot human studies and many preclinical studies; a few small RCTs/pilot interventions reported. | Oral supplementation; doses variable depending on extract form short durations in pilot. | Hypertensive models, metabolic syndrome models, human pilot cohorts. | Preclinical and pilot data show antihypertensive and cardioprotective signals; some human pilot studies report improved vascular biomarkers [165]. | Human data still limited vs. strong preclinical literature; small sample sizes and short follow-up; dosing/formulation heterogeneity. |
| Curcumin | Multiple 2023–2025 meta-analyses and RCTs. | RCTs and pooled analyses. | Different formulations (standard and enhanced bioavailability). | Obese patients with type 2 diabetes; other at-risk groups. | Improvement in atherogenic risk markers, lipids and inflammatory biomarkers [166,167]. | High heterogeneity of formulations/doses; many trials are short; variable quality across trials. |
| Berberine | Multiple meta-analyses and pilot trial (2023–2025). | Randomized, double-blind trials and many small RCTs. | Oral formulations, generally 0.5–1.5 g/day. | Adults with type 2 diabetes/metabolic disorders. | Improvements in cardiometabolic parameters; improvement in lipid profile [168,169]. | Trial often targets metabolic endpoints rather than direct atherosclerosis hallmarks; trial formulations vary limiting comparability. |
| Fisetin | Pilot/translational clinical trials (2023–2025); intermittent fisetin vascular trials. | Small pilot RCTs/early human trials. | Intermittent high-dose regimens used in pilot senolytic protocols (days of dosing repeated monthly). | Older adults/healthy volunteers/vascular aging cohorts. | Signals of reduced markers of cellular senescence, improved vascular function or surrogate measures in early trials [107,170,171]. | Mostly pilots are in early phase; small number of participants; surrogate endpoints rather than hard CV outcomes. |
| Piceatannol | Limited cardiovascular RCTs: randomized, placebo-controlled study. | 39 subjects, 19 (BMI ≥ 25), 20 (BMI < 25). | Early PK studies. | 20 mg/day or placebo capsules for 8 weeks. | CV endpoints not relevant [172]. | Small sample size; effect only in overweight men; short duration; surrogate markers. |
| Honokiol | Very limited controlled human data. | Small and heterogenous cohorts. | Magnolia extracts (variable honokiol content). | Short pilot exposures/traditional use. | Human CV efficacy data lacking; human safety data limited [173]. | Limited data on safety/tolerability in humans. |
| EGCG | Randomized, double-blind, placebo-controlled trials; meta-analyses (2023–2025). | Small to moderate sample sizes (60–200 per trial). | Dose ranging widely (up to 800 mg/day). | Obese subjects, metabolic disease cohorts. | Modest reduction in ratio LDL/TC, reduction in TG; modest improvements in blood pressure and metabolic markers [174,175]. | Heterogeneity of doses; some trials are small and short; variable formulations. |
| Hydroxytyrosol | Pilot RCTs and randomized nutritional intervention studies (2023–2025). | Pilot randomized trials (parallel or crossover). | EVOO enriched with hydroxytyrosol or purified hydroxytyrosol. | Healthy older adults, overweight/prediabetes; post myocardial infarction in elderly. | Improvements in antioxidant markers and HDL functions, improved endothelial markers in some studies. Safe at typical intake levels [176,177]. | Often small, short trials’ dietary background (other olive oil components); surrogate endpoints. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domi, E.; Hoxha, M. Natural Compounds Targeting SIRT1 and Beyond: Promising Nutraceutical Strategies Against Atherosclerosis. Nutrients 2025, 17, 3316. https://doi.org/10.3390/nu17213316
Domi E, Hoxha M. Natural Compounds Targeting SIRT1 and Beyond: Promising Nutraceutical Strategies Against Atherosclerosis. Nutrients. 2025; 17(21):3316. https://doi.org/10.3390/nu17213316
Chicago/Turabian StyleDomi, Elisa, and Malvina Hoxha. 2025. "Natural Compounds Targeting SIRT1 and Beyond: Promising Nutraceutical Strategies Against Atherosclerosis" Nutrients 17, no. 21: 3316. https://doi.org/10.3390/nu17213316
APA StyleDomi, E., & Hoxha, M. (2025). Natural Compounds Targeting SIRT1 and Beyond: Promising Nutraceutical Strategies Against Atherosclerosis. Nutrients, 17(21), 3316. https://doi.org/10.3390/nu17213316






