Assessing Dietary Patterns, Lifestyle Practices, and Forest Foods with Bioactive Potential to Address Micronutrient Deficiencies and Noncommunicable Diseases in Northeast India
Abstract
1. Introduction
2. Materials and Methods
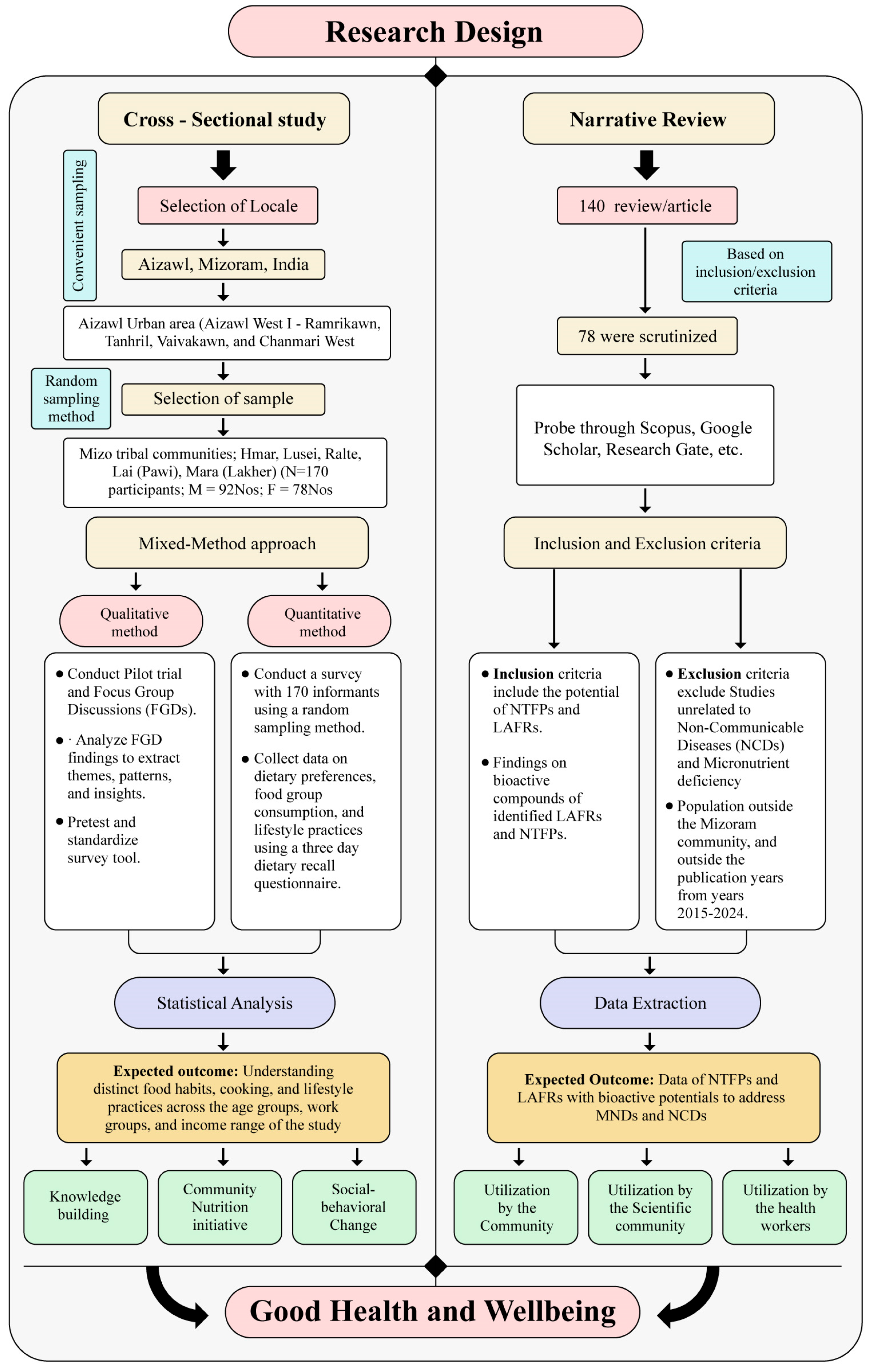
2.1. Study Design
2.2. Phase 1: Cross-Sectional Study
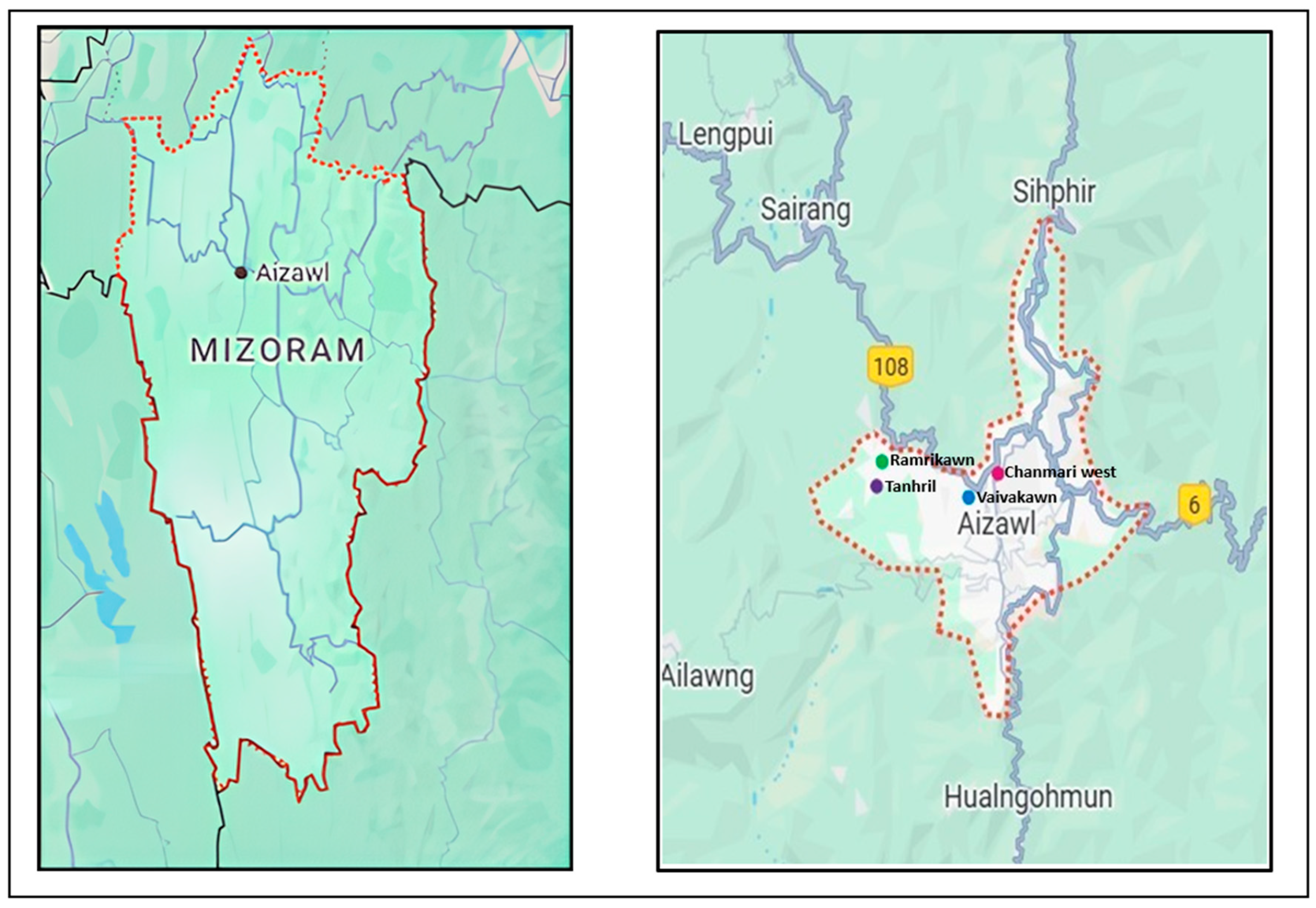
2.2.1. Study Locale
2.2.2. Participants and Sampling
2.2.3. Data Collection and Analysis
2.3. Phase 2: Narrative Literature Review
3. Results and Discussion
3.1. Impact on Dietary Preferences
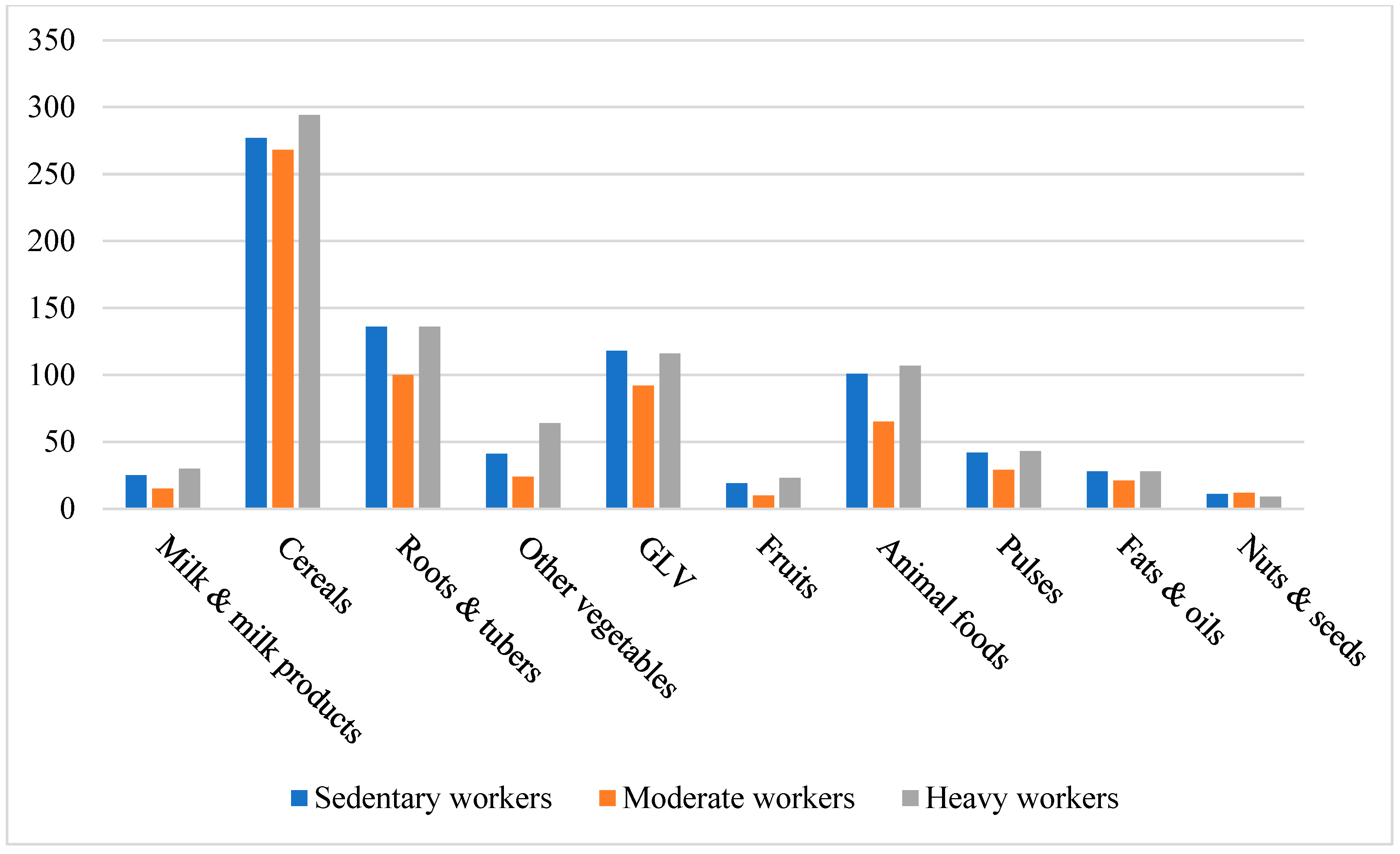
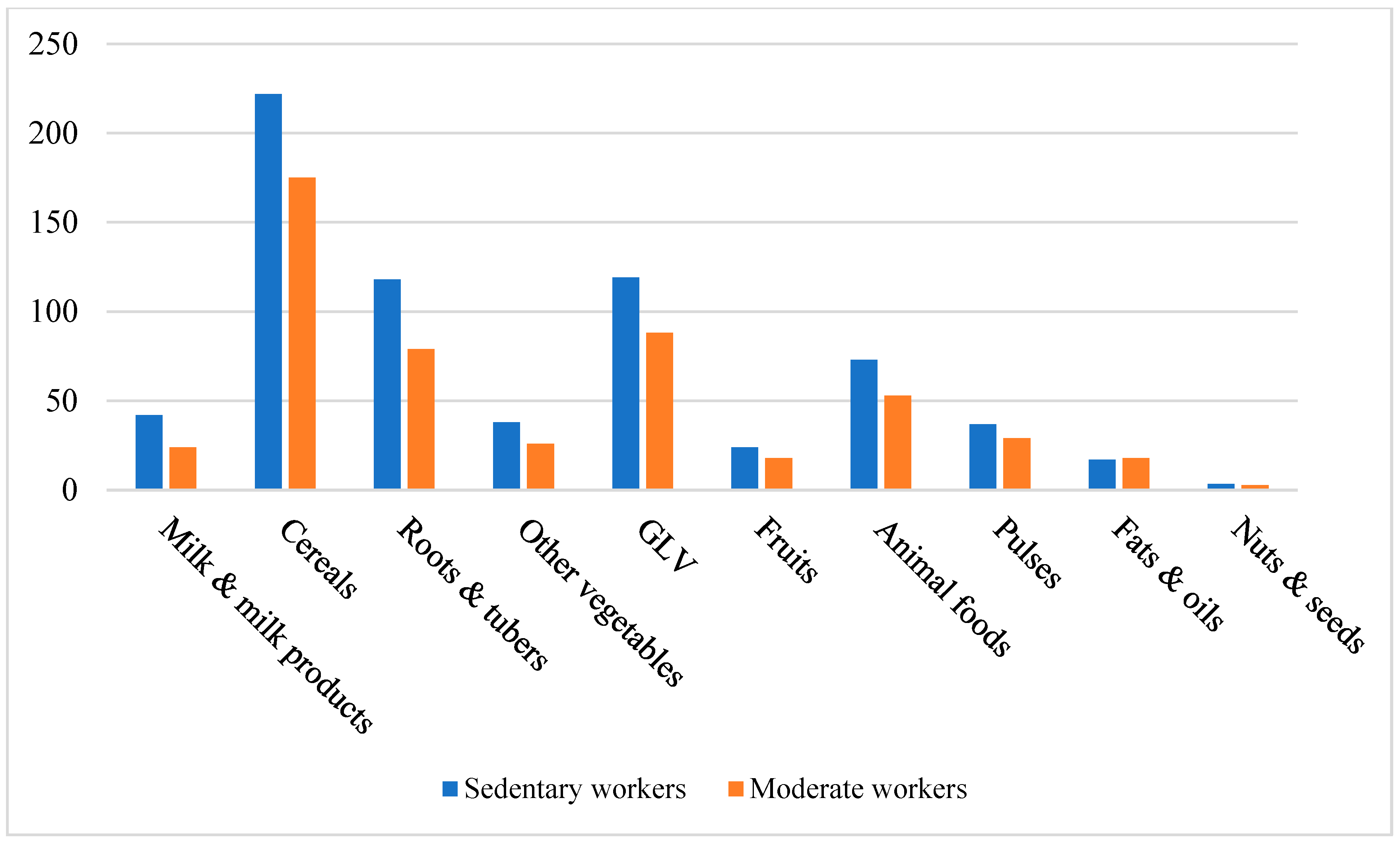
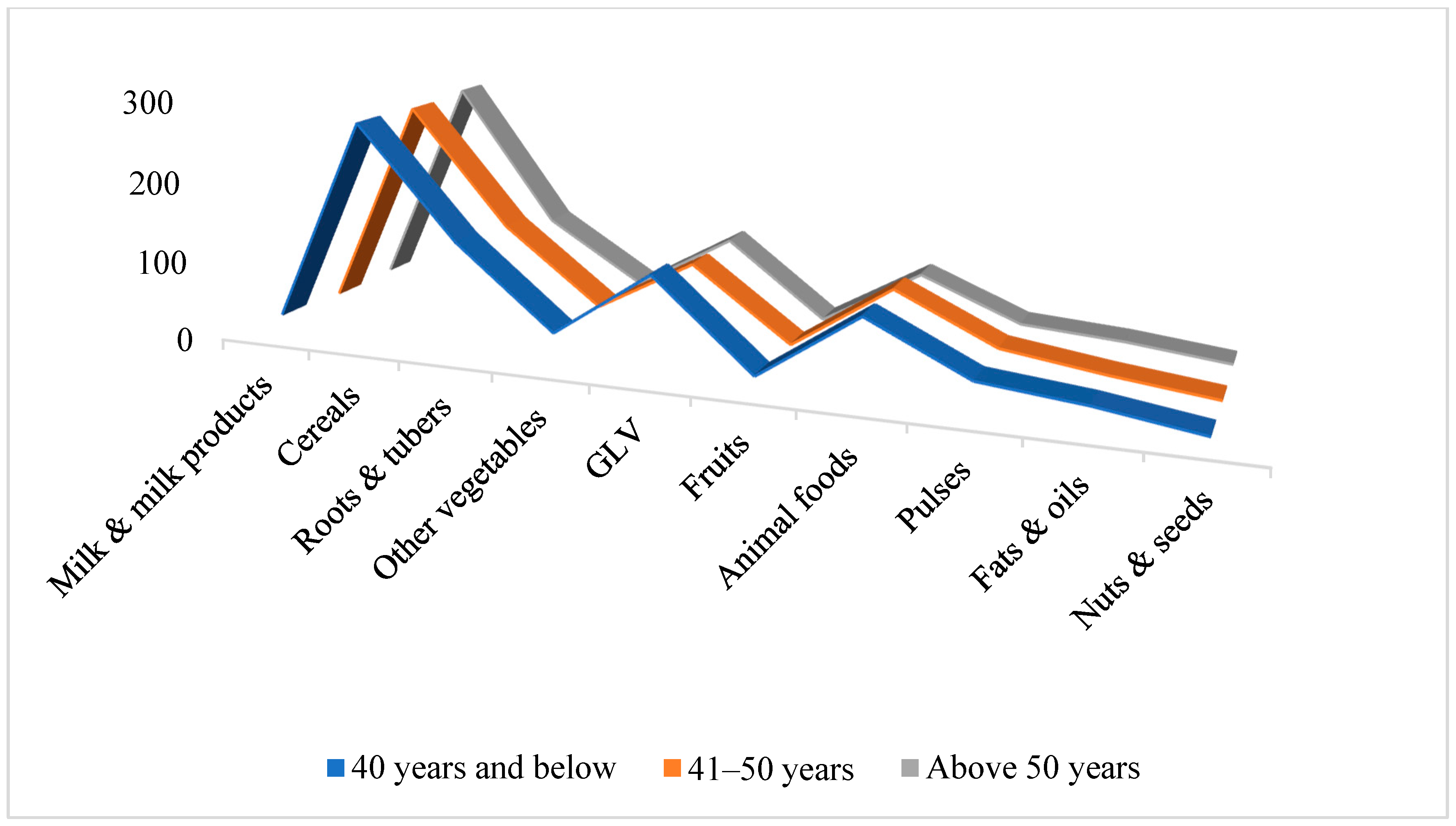
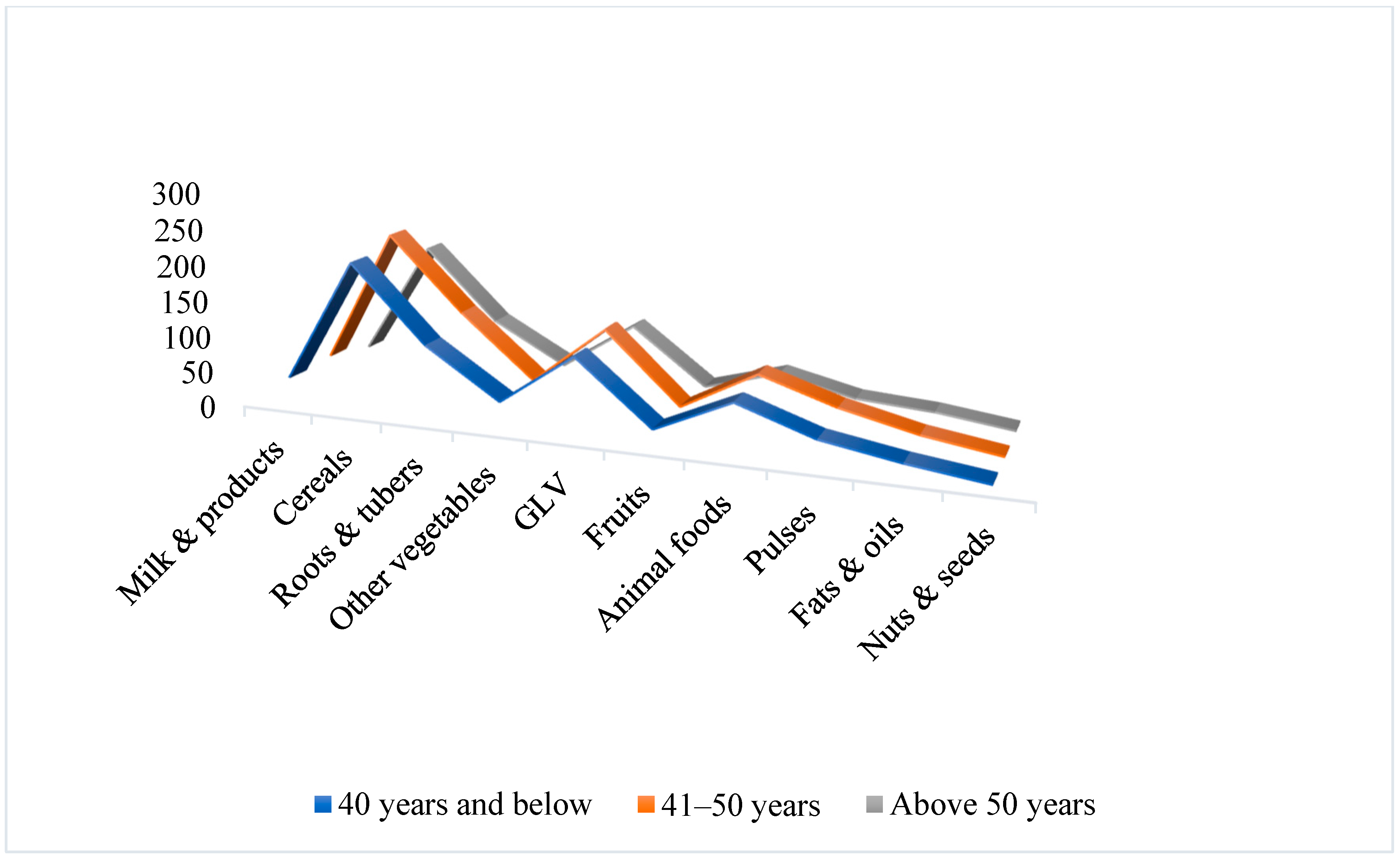
3.2. Impact on Food Group Consumption Among Mizo Tribal Communities
Dietary Diversity Score Assessment
3.3. Impact on the Prevailing Lifestyle Practices of the Mizo Tribes
3.4. Bioactive Potential of NTFPs and LAFRS
4. Challenges and Gaps
5. Conclusions
6. Policy Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Sociodemographic Profile of the Respondents
- Participant name ……………………
- Age ……….
- Gender ☐ Male ☐ Female ☐ Other
- Tribe/Subtribe (please specify) …………….
- Marital Status:
- 6.
- Education Level:
- 7.
- Occupation …………………
- 8.
- Monthly household income (Indian Rupees, INR)
- 9.
- Household Size ……………
- 10.
- Area of Residence ☐ Urban ☐ Peri-urban ☐ Rural
Appendix A.2. Self-Structured 3-Day Dietary Recall Questionnaire
| Day | Time of Meal | Cereals and Millet | Pulses | Animal Foods | Green Leafy Vegetables | Other Vegetables | Roots and Tubers (Excluding Potato) | Fruits | Milk and Milk Products | Fats and Oils | Nuts and Seeds |
| Day 1 | Breakfast | ||||||||||
| Lunch | |||||||||||
| Dinner | |||||||||||
| Day 2 | Breakfast | ||||||||||
| Lunch | |||||||||||
| Dinner | |||||||||||
| Day 3 | Breakfast | ||||||||||
| Lunch | |||||||||||
| Dinner | |||||||||||
| Mean Actual nutrient Intake | |||||||||||
Appendix A.3. Top 5 Priority Food Preferences Questionnaire
| Priority Options | Breakfast/ Local Name | Lunch/ Local Name | Dinner/ Local Name | Snacks/ Local Name |
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 |
Appendix A.4. Questions to Elicit Daily Food-Related Habits, Cooking Methods, Lifestyle Practices, and Any Traditional or Cultural Practices You Follow While Preparing or Consuming Food
- How do you consume/prepare animal foods?
- Are there any ingredients or preparations unique to your culture or household?
- How do you usually preserve food? Do you use fermentation, drying, or smoking?
- What kinds of fats/oil do you use while cooking?
- How often do you or your family consume processed foods?
- Any food additives or flavoring agents added while cooking?
- Are commercial products (such as baking soda or packaged foods) commonly used?
- Do you or your community still follow foraging or gardening practices?
- What cooking methods are common in your home? (steaming, boiling, grilling, etc.)
- Are there any known or diagnosed NCDs or micronutrient deficiencies?
- Has your cooking or eating habits changed over the years?
- Do you skip meals? If so, why? Will it be replaced with any food?
- How active are people in your community? Are smoking or alcohol habits, or tobacco, common?
- Do you or your family participate in any physical exercise?
- How often do you include homegrown or local fresh foods?
References
- Mipun, P.; Bhat, N.A.; Borah, D.; Kumar, Y. Nontimber forest products and their contribution to healthcare and livelihood security among the Karbi tribe in Northeast India. Ecol. Process. 2019, 8, 41. [Google Scholar] [CrossRef]
- Magry, M.A.; Cahill, D.; Rookes, J.; Narula, S.A. Nontimber forest products: Evolution, development and research. Biodiversity 2024, 25, 120–141. [Google Scholar] [CrossRef]
- Kirthika, P.; Janci Rani, P.R. Identification of functional properties of nontimber forest produce and locally available food resources in promoting food security among Irula tribes of South India. J. Public Health 2020, 28, 503–515. [Google Scholar] [CrossRef]
- Saitluanga, B.L.; Hmangaihzela, L.; Lalfakzuala, J.K. Social well-being, ethnicity, and regional development in Mizoram, northeast India. Geojournal 2021, 87, 3277–3289. [Google Scholar] [CrossRef]
- Zomawia, E.; Zami, Z.; Vanlallawma, A.; Kumar, N.S.; Zothanzama, J.; Tlau, L.; Chhakchhuak, L.; Pachuau, L.; Pautu, J.L.; Hmangaihzuali, E.V. Cancer awareness, diagnosis, and treatment needs in Mizoram, India: Evidence from 18 years trends (2003–2020). Lancet Reg. Health Southeast Asia 2023, 17, 100281. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S.; Roselind, F.S.; et al. Cancer statistics 2020: Report from National Cancer Registry Programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef]
- Anjana, R.M.; Pradeepa, R.; Deepa, M.; Datta, M.; Sudha, V.; Unnikrishnan, R.; Nath, L.M.; Das, A.K.; Madhu, V.; Rao, P.V.; et al. The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study: Methodological details. J. Diabetes Sci. Technol. 2011, 5, 906–914. [Google Scholar] [CrossRef]
- Anjana, R.M.; Unnikrishnan, R.; Deepa, M.; Pradeepa, R.; Tandon, N.; Das, A.K.; Joshi, S.; Bajaj, S.; Jabbar, P.K.; Das, H.K.; et al. Metabolic noncommunicable disease health report of India: The ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023, 11, 474–489. [Google Scholar] [CrossRef]
- Bisai, S.; Dutta, S.; Mohapatra, P.K.D. Traditional food consumption pattern and nutritional status of Oraons: An Asian Indian indigenous community. Front. Sustain. Food Syst. 2023, 7, 969264. [Google Scholar] [CrossRef]
- Yilmaz, H.; Yilmaz, A. Hidden Hunger in the Age of Abundance: The Nutritional Pitfalls of Modern Staple Crops. Food Sci. Nutr. 2025, 13, e4610. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Family Welfare (MoHFW), Government of India; UNICEF; Population Council. Comprehensive National Nutrition Survey (CNNS) National Report; Ministry of Health and Family Welfare (MoHFW), Government of India: New Delhi, India, 2019.
- Vanlalhlua, C. Profile of serum vitamin D levels among individuals in Mizoram: A retrospective study. Prog. Med. Sci. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.L. Vitamin D receptor (VDR) gene polymorphisms modify the response to vitamin D supplementation: A systematic review and meta-analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Ashok, S.; Nguyen, P.H.; Singh, S.K.; Sarwal, R.; Bhatia, N.; Johnston, R.; Joe, W.; Menon, P. State Nutrition Profile: Mizoram. In POSHAN Data Note 77; International Food Policy Research Institute: New Delhi, India, 2022. [Google Scholar]
- International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS-5), 2019–2021; IIPS: Mizoram, Mumbai, 2021; Available online: https://ruralindiaonline.org/en/library/resource/national-family-health-survey-nfhs-5-2019-21-mizoram/ (accessed on 25 March 2024).
- Lalrohlui, F.; Ghatak, S.; Zohmingthanga, J.; Hruaii, V.; Kumar, N.S. Fermented pork fat (Sa-um) and lifestyle risk factors as potential indicators for type 2 diabetes among the Mizo population, Northeast India. J. Health Popul. Nutr. 2021, 40, 32. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Byrne, N.M.; Hills, A.P. Cultural influences on dietary choices. Prog. Cardiovasc. Dis. 2025, 90, 22–26. [Google Scholar] [CrossRef]
- Khanna, S.K. Cultural Influences on Food: Dietary and Health Implications. Ecol. Food Nutr. 2021, 60, 633–635. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- Trickett, E.J.; Beehler, S.; Deutsch, C.; Green, L.W.; Hawe, P.; McLeroy, K.; Miller, R.L.; Rapkin, B.D.; Schensul, J.J.; Schulz, A.J.; et al. Advancing the science of community-level interventions. Am. J. Public Health 2011, 101, 1410–1419. [Google Scholar] [CrossRef]
- Vispute, S.; Mandlik, R.; Sanwalka, N.; Gondhalekar, K.; Khadilkar, A. Dietary diversity and food variety scores and their association with nutrition and health status of Indian children and adolescents: A multicenter study. Nutrition 2023, 111, 112039. [Google Scholar] [CrossRef] [PubMed]
- Paira, K.; Ao, M. Exploring the determinants of dietary nutrients intake among the rural households: A cross-sectional study in Jhargram district of West Bengal, India. Discov. Public Health 2024, 21, 224. [Google Scholar] [CrossRef]
- ICMR-NIN Expert Group. Nutrient Requirements for Indians, Recommended Dietary Allowances (RDA) and Estimated Average Requirements (EAR); Indian Council of Medical Research-National Institute of Nutrition: Hyderabad, India, 2020. [Google Scholar]
- Weerasekara, P.C.; Withanachchi, C.R.; Ginigaddara, G.A.S.; Ploeger, A. Understanding Dietary Diversity, Dietary Practices, and Changes in Food Patterns in Marginalized Societies in Sri Lanka. Foods 2020, 9, 1659. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Excessive consumption of fats and cardiovascular disease. In Nutrients and Oxidative Stress: Biochemistry Aspects and Pharmacological Insights; SpringerBriefs in Food, Health and Nutrition; Springer: Cham, Switzerland, 2024; pp. 59–68, Chapter 7. [Google Scholar] [CrossRef]
- Alamnia, T.T.; Sargent, G.M.; Kelly, M. Dietary patterns and associations with metabolic risk factors for noncommunicable disease. Sci. Rep. 2023, 13, 21028. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system—Working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Kadyan, S.; Sharma, A.; Arjmandi, B.H.; Singh, P.; Nagpal, R. Prebiotic potential of dietary beans and pulses and their resistant starch for aging-associated gut and metabolic health. Nutrients 2022, 14, 1726. [Google Scholar] [CrossRef]
- French, S.A.; Wall, M.; Mitchell, N.R. Household income differences in food sources and food items purchased. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Ma, W.; Wang, X.; Li, C.; Liang, T. Income-based environmental effects of family food consumption and the affordability toward healthy diets. Sustain. Prod. Consum. 2024, 51, 371–384. [Google Scholar] [CrossRef]
- Asadi-Ghalhari, M.; Mohammadbeigi, A.; Salehi, A.; Izanloo, H.; Ghorbani, Z.; Vanaki, V.; Ramazani, R. Prevalence of using baking soda in different types of most commonly consumed breads by Iranian people. Adv. Hum. Biol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Taeger, M.; Thiele, S. Replacement of milk and dairy products with soy-based alternatives to avoid nutrient deficiencies in a milk-free diet? J. Nutr. 2024, 154, 163–173. [Google Scholar] [CrossRef]
- Kennedy, G.; Wang, Z.; Maundu, P.; Hunter, D. The role of traditional knowledge and food biodiversity to transform modern food systems. Trends Food Sci. Technol. 2022, 130, 32–41. [Google Scholar] [CrossRef]
- Chang, Y.B.; Ahn, Y.; Seo, D.; Bae, S.; Suh, H.J.; Hong, Y.H.; Jung, E.Y. Centella asiatica lowers body fat accumulation via regulating cholesterol homeo-stasis- and lipid metabolism-related genes in mice with high-fat, high-sugar diet-induced obesity. Appl. Biol. Chem. 2023, 66, 88. [Google Scholar] [CrossRef]
- Jahan, F.; Islam, M.B.; Moulick, S.P.; Bashera, M.A.; Hasan, M.S.; Tasnim, N.; Saha, T.; Boby, F.; Waliullah, M.; Saha, A.K.; et al. Nutritional characterization and antioxidant properties of various edible portions of Cucurbita maxima: A potential source of nutraceuticals. Heliyon 2023, 9, e16628. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, N.; Elzaawely, A.A.; Srisuwan, S.; Sripanidkulchai, B.; Müller, C.P. Phytomedicine potential of Oroxylum indicum root and its constituents: Targeting Alzheimer’s disease. Plants 2025, 14, 223. [Google Scholar] [CrossRef]
- Bonilla Ocampo, D.A.; Paipilla, A.F.; Marín, E.; Vargas-Molina, S.; Petro, J.L.; Pérez-Idárraga, A. Die-tary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134. [Google Scholar] [CrossRef]
- Mangal, P.; Khare, P.; Jagtap, S.; Bishnoi, M.; Kondepudi, K.K.; Bhutani, K.K. Screening of six Ayurvedic medicinal plants for anti-obesity potential: An investigation on bioactive constituents from Oroxylum indicum (L.) Kurz bark. J. Ethnopharmacol. 2017, 197, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, N.R.; Choudhury, P.; Barbhuiya, R.A.; Singh, B. Importance of non-timber forest products (NTFPs) in rural livelihood: A study in Patharia Hills Reserve Forest, northeast India. Trees For. People 2020, 3, 100042. [Google Scholar] [CrossRef]
- Ghosh-Jerath, S.; Kapoor, R.; Singh, A.; Downs, S.; Barman, S.; Fanzo, J. Leveraging traditional ecological knowledge and access to nutrient-rich indigenous foods to help achieve SDG 2: An analysis of the indigenous foods of Sauria Paharias, a vulnerable tribal community in Jharkhand, India. Front. Nutr. 2020, 7, 61. [Google Scholar] [CrossRef]
- Nedungadi, P.; Jayakumar, A.; Raman, R. Personalized health monitoring system for managing well-being in rural areas. J. Med. Syst. 2017, 42, 22. [Google Scholar] [CrossRef]
- Laldingliani, T.B.C.; Thangjam, N.M.; Zomuanawma, R.; Lalramnghinglova, H. Ethnomedicinal study of medicinal plants used by Mizo tribes in Champhai district of Mizoram, India. J. Ethnobiol. Ethnomed. 2022, 18, 22. [Google Scholar] [CrossRef]
- Zhang, T.; Yi, Q.; Han, Y.; Zhao, L.; Gui, S.; Liu, W.; Wang, H. Chemical constituents from the leaves of Callicarpa arborea Roxb. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2023, 110, 104683. [Google Scholar] [CrossRef]
- Nghakliana, F.; Fanai, J.; Tochhawng, L.; Balachandar, V.; Zothansiama, N. Anticancer activity of Callicarpa arborea Roxb. extracts against Type-II human lung adenocarcinoma cell line, A549. J. Environ. Biol. 2020, 41 (Suppl. S4), 901–907. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Phytochemicals derived from Catharanthus roseus and their health benefits. Technologies 2020, 8, 80. [Google Scholar] [CrossRef]
- Hira, F.A.; Islam, A.; Mitra, K.; Bithi, U.H.; Ahmed, K.S.; Islam, S.; Abdullah, S.M.; Uddin, N. Comparative analysis of phytochemicals and antioxidant characterization among different parts of Catharanthus roseus: In vitro and in silico investigation. Biochem. Res. Int. 2024, 2024, 1904029. [Google Scholar] [CrossRef]
- Deb, P.K.; Khound, P.; Bhattacharjee, S.; Choudhury, P.; Sarma, H.; Devi, R.; Sarkar, B. Variation in chemical constituents, in vitro bioactivity and toxicity profile among different parts of Clerodendrum glandulosum Lindl. (C. colebrookianum Walp.). South Afr. J. Bot. 2021, 140, 50–61. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Guo, S.; Ge, Y.; Na Jom, K. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Inhibitory effects on clinical isolated bacteria and simultaneous HPLC quantitative analysis of flavone contents in extracts from Oroxylum indicum. Molecules 2019, 24, 1937. [Google Scholar] [CrossRef]
- Singh, L.S.; Singh, W.S. Centella asiatica and its bioactive compounds: A comprehensive approach to managing hyperglycemia and associated disorders. Discov. Plants 2024, 1, 54. [Google Scholar] [CrossRef]
- Idris, F.N.; Mohd Nadzir, M. Comparative studies on different extraction methods of Centella asiatica and extracts bioactive compounds effects on antimicrobial activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, O.A.; Singh, V.; Singh, G. Chemical composition, traditional uses and biological activities of Artemisia species. J. Pharmacogn. Phytochem. 2020, 9, 1124–1140. [Google Scholar]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules. 2020, 25, 4415. [Google Scholar] [CrossRef] [PubMed]
- Jatav, R.; Kumar, S.; Mety, S.; Netravati, H.; Vidya, P. Qualitative phytochemical analysis of Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen: A medicinal plant of India. Medico Biowealth India 2024, XVI, 1. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Udenigwe, C.C. Zanthoxylum species: A comprehensive review of traditional uses, phytochemistry, pharmacological, and nutraceutical applications. Molecules 2021, 26, 4023. [Google Scholar] [CrossRef]
- Dong, L.-M.; Xu, Q.-L.; Liu, S.-B.; Zhang, S.-X.; Liu, M.-F.; Duan, J.-L.; Ouyang, J.-K.; Hu, J.-T.; Fu, F.-Y.; Tan, J.-W. Germacrane sesquiterpene dilactones from Mikania micrantha and their antibacterial and cytotoxic activity. Molecules 2023, 28, 2119. [Google Scholar] [CrossRef] [PubMed]
- Shak, A.H.; Shafie, N.H.; Mohd Esa, N.; Bahari, H. Nutritional, phytochemical and pharmacological properties of Mikania micrantha Kunth. Pertanika J. Sch. Res. Rev. 2016, 2, 123–132. [Google Scholar]
- Prakash, D.; Bisht, D.; Shakya, A.K. Medicinal properties and phytoconstituents of Alstonia scholaris: A review. VIVECHAN Int. J. Res. 2020, 11, 1–7. [Google Scholar]
- Pandey, K.; Shevkar, C.; Bairwa, K.; Kate, A.S. Pharmaceutical perspective on bioactives from Alstonia scholaris: Ethnomedicinal knowledge, phytochemistry, clinical status, patent space, and future directions. Phytochem. Rev. 2020, 19, 191–233. [Google Scholar] [CrossRef]
- Tanase Apetroaei, V.; Istrati, D.I.; Vizireanu, C. Plant-Derived Compounds in Hemp Seeds (Cannabis sativa L.): Extraction, Identification and Bioactivity—A Review. Molecules 2024, 30, 124. [Google Scholar] [CrossRef]
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods—A Review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef]
- Hasan, M.; Islam, E.; Hossain, S.; Akter, M.; Rahman, A.A.; Kazi, M.; Khan, S.; Parvin, M.S. Unveiling the therapeutic potential: Evaluation of anti-inflammatory and antineoplastic activity of Magnolia champaca Linn’s stem bark isolate through molecular docking insights. Heliyon 2024, 10, e22972. [Google Scholar] [CrossRef]
- Ruwali, P.; Adhikari, M.; Sharma, S. Phytochemical and antioxidant properties of various extracts of Michelia champaca leaves. Int. J. Pharm. Pharm. Sci. 2019, 11, 56–61. [Google Scholar] [CrossRef]
- Karn, A.; Quasim, M.; Hmar, E.B.; Paul, S.; Sharma, H. An updated review of Rubus ellipticus (an edible shrub), its bioactive constituents and functional properties. Sci. Phytochem. 2022, 1, 22–33. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Naik, B.; Kumar, V.; Gupta, A.K.; Kumar, S.; Preet, M.S.; Sharma, N.; Rustagi, S. Untapped potential of nonconventional Rubus species: Bioactivity, nutrition, and livelihood opportunities. Plant Methods 2023, 19, 114. [Google Scholar] [CrossRef]
- Lalrinawmi, H.; Vabeikhokhei, J.M.; Zothanzama, J.; Zohmangaiha, N. Edible mushrooms of Mizoram. Sci. Vis. 2017, 17, 172–181. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite Mushrooms (Termitomyces), a potential source of nutrients and bioactive compounds exhibiting human health benefits: A review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Farhana, S.S.; Usharani, B.; Chandrasekar, S.; Durairaj, R. Qualitative and quantitative analysis of Cinnamomum tamala leaf extract. J. Adv. Zool. 2023, 44, 3071–3081. [Google Scholar] [CrossRef]
- Tiwari, S.; Talreja, S. Importance of Cinnamomum tamala in the treatment of various diseases. Pharmacogn. J. 2020, 12, 1792–1796. [Google Scholar] [CrossRef]
- Kumar, A.; Thangjam, N.M.; Rani, P.; Lalmuankima, H.T. The potential of medicinal and aromatic plants cultivation to develop the agri-business in Mizoram. Environ. Ecol. 2023, 41, 1651–1661. [Google Scholar] [CrossRef]
- Ralte, L.; Sailo, H.; Singh, Y.T. Ethnobotanical study of medicinal plants used by the indigenous community of the western region of Mizoram, India. J. Ethnobiol. Ethnomed. 2024, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Abedimanesh, N.; Somi, M.-H.; Khosroushahi, A.Y.; Moradi, S. Anticancer properties of red beetroot hydroalcoholic extract and its main constituent; betanin on colorectal cancer cell lines. BMC Complement. Med. Ther. 2023, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, H.S.; Kim, J.-H.; Kim, S.-L.; Kim, K.C.; Perera, R.M.T.D.; Kim, S.-C.; Lee, D.-S. Bioactive constituents from Carica papaya fruit: Implications for drug discovery and pharmacological applications. Appl. Biol. Chem. 2024, 67, 103. [Google Scholar] [CrossRef]
- Sapkota, B.; Devkota, H.P.; Poudel, P. Citrus maxima (Brum.) Merr. (Rutaceae): Bioactive Chemical Constituents and Pharmacological Activities. Evid. Based Complement. Altern. Med. 2022, 2022, 8741669. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives—Health-Promoting Phytobiotic Against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Eanyika, L.U.; Anosike, C.A.; Oji, C.N.; Chibuogwu, C.C. Phytochemistry, micronutrient composition, and antioxidant potentials of Citrus maxima (Shaddock) fruit juice. J. Pharmacogn. Phytochem. 2022, 11, 20–23. [Google Scholar] [CrossRef]
- Abel, A.; Mustapha, A.B. Phytochemical and Elemental Composition of Lemon (Citrus limon) and Mistletoe (Viscum album): Investigating the Medicinal Potential of These Plants for Therapeutic Applications. Newport Int. J. Sci. Exp. Sci. 2024, 5, 103–107. [Google Scholar] [CrossRef]
- Qurban, F.; Hussain, S.; Waqas, M.; Shahzad, H.H.; Rukhsar, A.; Javed, A. Phytochemistry, nutritional, and pharmacological potential of citrus limonum. Sci. Inq. Rev. 2024, 8, 1–23. [Google Scholar] [CrossRef]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef]
- Gurung, S.; Poudel, P.; Adhikari, N.; Lamichhane, G.; Thapa, R. Crassocephalum crepidioides (Benth.) S. Moore: Traditional Uses, Chemical Constituents, and Biological Activities. In Medicinal Plants of the Asteraceae Family; Devkota, H.P., Aftab, T., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Can, N.M.; Thao, D.T.P. Wound Healing Activity of Crassocephalum crepidioides (Benth.) S. Moore. Leaf Hydroethanolic Extract. Oxidative Med. Cell. Longev. 2020, 2020, 2483187. [Google Scholar] [CrossRef]
- Thoudam, S.; Sharma, D. A Review Study on Nutraceutical and Nutritional Efficacy of Crassocephalum crepidioides. 2020. Available online: https://www.researchgate.net/publication/347259630 (accessed on 23 January 2024).
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; et al. Nutritional Value, Phytochemical Potential, and Therapeutic Benefits of Pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Sivakumar, D. Pumpkin Leaves (Cucurbita maxima, Cucurbita moschata and Cucurbita pepo). In CABI eBooks; CABI: Long Beach, CA, USA, 2022; pp. 83–96. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; De Almeida, M.J.; Sousa, T.L.; Santos, D.C.D.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Uthpala, T.G.; Marapana, R.A.U.; Lakmini, K.P.; Wettimuny, D.C. Nutritional Bioactive Compounds and Health Benefits of Fresh and Processed Cucumber (Cucumis sativus L.). Sumerianz J. Biotechnol. 2020, 3, 75–82. [Google Scholar] [CrossRef]
- Sattar, S.; Ali, F.; Iftikhar, M.; Saleem, A.; Maha, M.; Wajid, M.; Nisar, M.F. Biological components in cucumbers (Cucumis sativus L.): Implications for pickle manufacturing and health benefits in fresh and processed varieties. Qeios 2024. [Google Scholar] [CrossRef]
- Saxena, H.O.; Das, A.; Parihar, S. Dillenia pentagyna Roxb.: A Review on Phytochemistry and Pharmacology. J. Phytopharmacol. 2022, 11, 295–299. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A critical review of Ginger’s (Zingiber officinale) antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health-beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Anjali, C.; Kuruvila, B.; Maneesha, P.K.; Joseph, M. Phytochemical constitution and antioxidant activity of functional herbal drink from Indian gooseberry (Emblica officinalis Gaertn.) fruits containing spices and condiments. Food Prod. Process. Nutr. 2023, 5, 12. [Google Scholar] [CrossRef]
- Avinash, P.G.; Hamid Shams, R.; Dash, K.K.; Shaikh, A.M.; Ungai, D.; Harsányi, E.; Suthar, T.; Kovács, B. Recent insights into the morphological, nutritional and phytochemical properties of Indian gooseberry (Phyllanthus emblica) for the development of functional foods. Plants 2024, 13, 574. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xu, C.Y.; Mazhar, M.S.; Naiker, M. Nutritional value and therapeutic benefits of dragon fruit: A comprehensive review with implications for establishing Australian industry standards. Molecules 2024, 29, 5676. [Google Scholar] [CrossRef]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.M.M.B.; Direito, R.; Goulart, R.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-inflammatory, antioxidant, and other health effects of dragon fruit and potential delivery systems for its bioactive compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Thakur, D.; Singh, S.; Bakshi, M.; Singh, S.K. Dragon fruit: Exploring bioactive compounds and their promising role in functional food innovation and value-added products. J. Food Compos. Anal. 2025, 141, 107370. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Azizan, A.; Lee, A.X.; Hamid, N.A.A.; Maulidiani, M.; Mediani, A.; Ghafar, S.Z.A.; Zolkeflee, N.K.Z.; Abas, F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by 1H NMR. Foods 2020, 9, 173. [Google Scholar] [CrossRef]
- De Oliveira Barretto, L.C.; De Jesus Da Silveira Moreira, J.; Santos, J.A.B.; Narendra, N.; Santos, R.A.R. Characterization and extraction of volatile compounds from pineapple (Ananas comosus L. Merril) processing residues. Food Sci. Technol. 2013, 33, 638–645. [Google Scholar] [CrossRef]
- Bajwa, H.K.; Santosh, O.; Chongtham, N. Bioactive compounds in bamboo shoot. In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Switzerland, 2021; pp. 419–440. [Google Scholar] [CrossRef]
- Bajwa, H.K.; Santosh, O.; Nirmala, C. Bamboo shoot for food and nutritional security. J. Pharmacogn. Phytochem. 2021, 10, 24–30. [Google Scholar]
- Ma, T.; Mo, W.; Lv, B.; Wang, W.; He, H.; Jian, C.; Liu, X.; Li, S.; Guo, Y. A review of the nutritional composition, storage challenges, processing technology, and widespread use of bamboo shoots. Foods 2024, 13, 3539. [Google Scholar] [CrossRef] [PubMed]
- Gorrepati, K.; Krishna, R.; Singh, S.; Shirsat, D.V.; Soumia, P.S.; Mahajan, V. Harnessing the nutraceutical and therapeutic potential of Allium spp.: Current insights and future directions. Front. Nutr. 2024, 11, 1497953. [Google Scholar] [CrossRef]
- Iwar, K.; Ochar, K.; Seo, Y.A.; Ha, B.K.; Kim, S.H. Alliums as potential antioxidants and anticancer agents. Int. J. Mol. Sci. 2024, 25, 8079. [Google Scholar] [CrossRef]
- Barbu, I.A.; Ciorîță, A.; Carpa, R.; Moț, A.C.; Butiuc-Keul, A.; Pârvu, M. Phytochemical characterization and antimicrobial activity of several Allium extracts. Molecules 2023, 28, 3980. [Google Scholar] [CrossRef]
- Pant, Y.; Lingwan, M.; Masakapalli, S.K. Metabolic, biochemical, mineral and fatty acid profiles of edible Brassicaceae microgreens establish them as promising functional food. Food Chem. Adv. 2023, 3, 100461. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. CyTA J. Food 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Castaldo, L.; Sessa, R.; Ricci, L.; Vardaro, E.; Izzo, L.; Grosso, M.; Ritieni, A.; Laneri, S. Chemical profile and promising applications of Cucurbita pepo L. flowers. Antioxidants 2024, 13, 1476. [Google Scholar] [CrossRef]
- Iñiguez-Luna, M.I.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Morales-Flores, F.J.; Cortes-Cruz, M.; Watanabe, K.N. Natural bioactive compounds of Sechium spp. for therapeutic and nutraceutical supplements. Front. Plant Sci. 2021, 12, 772389. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Suwalsky, M.; Colina, J.R.; Castillo, I.; Rosado-Pérez, J.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Phytochemical analysis and antioxidant and anti-inflammatory capacity of the extracts of fruits of the Sechium hybrid. Molecules 2020, 25, 4637. [Google Scholar] [CrossRef] [PubMed]
- Veigas, G.J.; Bhattacharjee, A.; Hegde, K.; Shabaraya, A.R. A brief review on Sechium edule. Int. J. Pharm. Sci. Rev. Res. 2020, 65, 165–168. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Dharavath, S.B.; Taduri, S. GC–MS profiling and antibacterial activity of Solanum khasianum leaf and root extracts. Bull. Natl. Res. Cent. 2022, 46, 127. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Shasthree, T. Qualitative screening and quantitative determination of secondary metabolites from different plant extracts of Solanum khasianum Clarke. Res. J. Chem. Environ. 2022, 26, 113–123. [Google Scholar] [CrossRef]
| Priority Options | Breakfast/ Local Name | Lunch/ Local Name | Dinner/ Local Name | Snacks Local Name | Dominant Nutritional Attributes |
|---|---|---|---|---|---|
| 1 | Sawhchiar (Mizo fried rice) | Tea and bread | Rice and Bai (Green leafy vegetable soup) | Mizo sticky rice cakes |  |
| 2 | Chowmein (Stir-fried noodles) | Noodles | Rice and Bawngchawl (Mizo-style curry) | Pancakes |  |
| 3 | Rice and Vawksa rep (Smoked pork) | Tea and biscuits | Rice and Chamthong (Mizo veggie stew) | Panchu pui (Deep-fried snack made with rice and seasonings) |  |
| 4 | Rice, potato, and Dal | Tea and chips | Arsa Buhchiar (Rice cooked with chicken—porridge) | Nghapui (Fried/roasted peanuts seasoned with spices) |  |
| 5 | Bawmchawlini (Mizo-style rice with meat porridge) | Bread and Jam | Rice and sausage (pork) | Thums (Fermented bamboo shoots) |  |
 Rich in CHO;
Rich in CHO;  Rich in protein and healthy fats;
Rich in protein and healthy fats;  Highly refined carbs and added sugars;
Highly refined carbs and added sugars;  Unhealthy fat and high calories;
Unhealthy fat and high calories;  Rich in vitamins and minerals.
Rich in vitamins and minerals.| Food Groups | Male n = 92 Nos (27–54 Years) | Female n = 78 Nos (27–54 Years) | ||||
|---|---|---|---|---|---|---|
| ICMR RDA (g/day) | Mean Actual Intake (g) | %Excess /Deficit | ICMR RDA (g/day) | Mean Actual Intake (g) | %Excess/ Deficit | |
| Cereals | 275 | 278 ± 45 | +1.09 | 200 | 208 ± 62 | +4 |
| Pulses | 80 | 40 ± 16 | −50 | 60 | 34 ± 17 | −43 |
| Animal foods | 80 | 96 ± 44 | +20 | 60 | 67 ± 32 | +11.66 |
| Green leafy vegetables | 100 | 114 ± 32 | +14 | 100 | 109 ± 36 | +9 |
| Other Vegetables | 200 | 130 ± 53 | −35 | 200 | 105 ± 43 | −47.5 |
| Roots and tubers (excluding potato) | 100 | 41 ± 23 | −58.33 | 100 | 35 ± 13 | −65 |
| Fruits | 150 | 18 ± 9 | −88 | 150 | 22 ± 14 | −85.33 |
| Milk & milk products | 300 | 24 ± 14 | −92 | 300 | 36 ± 19 | −88 |
| Fats and oils | 25 | 27 ± 11 | +8 | 15 | 17 ± 4 | +13.33 |
| Nuts and seeds | 30 | 11 ± 11 | −63.33 | 30 | 3 ± 7 | −97 |
| DDS Parameter | Males (n = 78) | Female (n = 92) | Total (n = 170) |
|---|---|---|---|
| Low diversity (≤4), n (%) | 13 (16.7%) | 25 (27.2%) | 38 (22.4%) |
| Medium diversity (5–6), n (%) | 42 (53.8%) | 36 (39.1%) | 78 (45.8%) |
| High diversity (≥7), n (%) | 23 (29.5%) | 31 (33.7%) | 54 (31.8%) |
| Mean DDS ± SD | 5.8 ± 1.2 | 5.4 ± 1.4 | 5.6 ± 1.3 |
| Food Groups | p-Values from ANOVA (Males) | p-Values from t-Tests (Females) |
|---|---|---|
| Milk & milk products | 0.0068 | 0 |
| Cereals | 0.4659 | 0.0156 |
| Roots & tubers | 0 | 0.0002 |
| Other vegetables | 0.0011 | 0.0001 |
| Green leafy vegetables | 0.0011 | 0.0003 |
| Fruits | 0.0005 | 0.0132 |
| Animal foods | 0.006 | 0.0009 |
| Pulses | 0.1826 | 0.0019 |
| Fats & oils | 0.0039 | 0.0936 |
| Nuts & seeds | 0.8495 | 0.0196 |
| Food Groups | p-Values (Males) | p-Values (Females) |
|---|---|---|
| Milk & milk products | 0.218 | 0.0363 |
| Cereals | 0.9467 | 0.1018 |
| Roots & tubers | 0.7196 | 0.0856 |
| Other vegetables | 0.0854 | 0.0002 |
| Green leafy vegetables | 0.0262 | 0.1238 |
| Fruits | 0.2105 | 0.5822 |
| Animal foods | 0.265 | 0.0066 |
| Pulses | 0.1552 | 0.0009 |
| Fats & oils | 0.1403 | 0.347 |
| Nuts & seeds | 0.5449 | 0.1103 |
| Category | Favorable Practices | Unfavorable Practices |
|---|---|---|
| Eating Habits | Inclusion of Local Foods | High Salt Intake |
| Consumption of green leafy vegetables | Excessive Sugar Consumption | |
| Consumption of Bai (Boiled Vegetables) | Processed Foods | |
| Consumption of fermented foods | Skipping Meals | |
| Moderate use of spices | Unbalanced Snacking | |
| Preparation and usage of ash filtrate from the plant’s stems and leaves | Use of commercial baking soda | |
| Low oil consumption | Increased use of saum (fermented pork fat) | |
| Utilization of seasonal resources | Smoked meat consumption | |
| Intake of common vegetables | Less intake of milk, fruits, roots, and tubers | |
| Lifestyle Factors | Utilization of traditional food preservation techniques | Sedentary Lifestyle |
| Promotion of Home gardening | Alcohol and Substance Abuse | |
| Purchase of Local produce to support local farmers | Stress | |
| Living close to rich natural resources | Smoking | |
| Practice of sustainable resource management | Less physical activity | |
| Cooking Practices | Utilization of fresh forest foods | Declining traditional cooking practices such as foraging of wild ingredients, traditional preservation methods, steaming of bamboo, etc. |
| Boiling, steaming, grilling, and stewing methods | Emerging Reliance on Packaged Foods |
| S. No | Species Name; Local Name; Common Name | Parts Used/ Preparation & Consumption Methods | Addressing the Type of NCDs/Micronutrient Deficiency Specific to the Plant | Major Bioactive Compounds | References |
|---|---|---|---|---|---|
| 1. | Callicarpa arborea Roxb; Beauty berry; Hnah kiah | Leaf, Bark/Decoction | Diabetes, Cancers | clerodane, diterpenoid, flavonoids, tannins | [42,43,44] |
| 2. | Catharanthus roseus (L.); Kumtluang; Bright eyes | Leaf, whole plant, Root/Juice, decoction | Diabetes, Hypertension, CVDs, Cancers | vincristine, vinblastine, vindolidine | [42,45,46] |
| 3. | Clerodendrum glandulosum Lindl; Phuihnam; Hill glory bower | Leaf, stem, root/Decoction | Hypertension, Diabetes, Obesity, CVDs | caffeic acid, luteolin, apigenin | [42,47] |
| 4. | Helianthus annus; Sunflower; Ni-hawi | Seeds, stem, roots, leaf, flower/Raw, Oil, Meal | Cancer, obesity, diabetes, hypertension, CVDs/Bone health | apigenin, quercetin, caffeic acid | [42,48,49] |
| 5. | Oroxylum indicum; Broken bones; Ar-chang-kawm | Bark/Decoction | CVDs, Cancer, Diabetes, Asthma | baicalein, oroxylin A, chrysin, scutellarin, ellagic acid | [42,50] |
| 6. | Centella asiatica; Indian Pennywort; Darbengbur/lambak | Leaf, Stem/Decoction, Powder | Diabetes, Cancers | quercetin, madecassoside, kaempferol, asiaticoside, flavonoids | [42,51,52] |
| 7. | Artemisia vulgaris; Mugwort; Sai | Plant/Decoction | Cancers, Diabetes, Hypertension, Obesity | artemisinin, apigenin, quercetin | [42,53,54] |
| 8. | Zanthoxylum asiaticum; Orange climber; Ching-it | Stem, bark, leaves, roots/Decoction | Cancers/Sickle cell anemia | alkaloids, phenols, tannins, flavonoids | [42,55,56] |
| 9. | Mikania micrantha; Bitter vine; Japan-hlo | Leaves, Whole plant/Decoction | Stroke, Hypertension, Diabetes, Cancers | saponin, phenols, flavonoids | [42,57,58] |
| 10. | Alstonia scholaris (L.) R.; Devil’s tree; Thuamriat | Bark, stem, leaves, and roots/Decoction | Cancers, Diabetes, Heart disorders, Hypertension | alkaloid, flavonoids, triterpenoids | [42,59,60] |
| 11. | Cannabis sativa L.; Hemp; Trip Kanza | Seeds | Cancers, CVDs, Diabetes, Hypertension/Bone health | cannabinoids, terpenoids, polyphenols | [42,61,62] |
| 12. | Magnolia champaca L.; Champak; Ngiau | Stem bark, Leaf/Maceration | Hypertension, Cancers | flavonoids, phenols, alkaloids | [42,63,64] |
| 13. | Rubus ellipticus; Yellow Himalayan raspberry; Zawngṭa | Leaves, fruits, shoots, root bark/Decoction, Juice | Cancer, Diabetes | quercetin, ellagic acid, catechin | [65,66] |
| 14. | Termitomyces heimii; Wild mushroom; Pasawntlung | Edible portion/Cooked | Hyperlipidemia, Cancers | quercetin, saponins, tannins | [67,68] |
| 15. | Cinnamomum tamala; Indian bay leaf; Theipui | Leaves | Hypercholesterolemia, Cancer, CVDs, Diabetes | terpenoids, flavonoids, phenolic acids | [69,70,71] |
| S. No | Species Name; Local Name; Common Name | Parts Used/ Preparation & Consumption Methods | Addressing the Type of NCDs/Micronutrient Deficiency Specific to the Plant | Major Bioactive Compounds | References |
|---|---|---|---|---|---|
| 1. | Beta vulgaris L.; Beetroot; Beetroot | Rhizome/Raw, Juice | Cancers/Anemia | β-carotene, lutein, lycopene | [42,72,73] |
| 2. | Carica papaya L.; Thingfanghma; Papaya | Seed, Fruit, Leaf, Sap/Paste, Raw | Cancers, Diabetes, Obesity, CVDs, and Asthma | quercetin, caffeic acid, lycopene | [72,74] |
| 3. | Citrus maxima; Sertawk; Pomelo | Whole plant, whole fruit, albedo, Peel, Leaf, Pulp, and Seed/Raw | Hypertension, Cancer, Obesity/Osteoporosis | naringin, hesperidin, quercetin | [72,75,76,77,78] |
| 4. | Citrus limon (L.); Nimbu; Lemon | Fruit, Stem, Leaf, Peel/Juice, Decoction | Neurodegenerative diseases, CVDs, Hypertension, diabetes, cancer/Scurvy, osteoporosis | ascorbic acid, polyphenols, flavonoids | [72,79,80] |
| 5. | Colocasia esculenta (L.); Dawl; Elephant ear | Stem, shoots, flowers, sap, bulbs, leaves/juice, boiled, stewed | Cancer, Diabetes/Anemia | terpenoids, alkaloids, flavonoids | [42,72,81] |
| 6. | Crassocephalum crepidioides; Buar thau; Fireweed ragleaf | Leaf/Paste | Diabetes, Cancer, Obesity, Hypertension/Anemia | gallic acids, catechin, rutin | [42,72,82,83,84] |
| 7. | Cucurbita maxima Duchesne; Mai; Pumpkin | Fruit, flesh, seed, leaf, and peel/raw, boiled, steamed | Diabetes, Obesity, Cancers, Hypertension, CVDs/Anemia | gallic acid, kaempferol, quercetin | [35,42,72,85,86] |
| 8. | Curcuma longa L.; Aieng; Turmeric | Rhizome/Powder, Juice | Cancers, Diabetes, CVDS | curcumin, ar-turmerone | [42,72,87] |
| 9. | Cucumis sativus L.; Fanghma; Cucumber | Leaf, flesh, seeds, Skin/Raw, decoction | Hypertension, Diabetes, Cancers | cucurbitacin B | [42,72,88,89] |
| 10. | Dillenia pentagyna Roxb; Kaihzawl; Elephant Apple | Leaf, Bark, Fruits/Decoction | Cancer, Diabetes, CVDs | lupeol, naringenin, β-sitosterol, gallic acid | [42,72,90] |
| 11. | Zingiber officinale; Ginger; Sawhthing | Rhizome/Raw, Decoction | Diabetes, CVDs, Cancers/Anemia, Osteoporosis | gingerol, shogaol, zingerone, β-sitosterol, quercetin, rutin | [42,72,91,92] |
| 12. | Phyllanthus emblica L.; Indian Gooseberry; Sunhlu | Fruit/Raw, Juice | Diabetes, Cancers/Anemia, Scurvy | ascorbic acid, ellagic acid, gallic acid | [42,72,93,94] |
| 13. | Hylocereus undatus; Dragon fruit; Dragon fruit | Flesh, seeds, and peels | Obesity, Diabetes, Cancers, CVDs/Anemia | quercetin, ascorbic acid, β-carotene | [42,95,96,97] |
| 14. | Ananas comosus L.; Pineapple; Lakhuih | Pulp, Leaf, Root, Skin, core, stem/Raw, Juice, Paste | CVDs, Diabetes, Cancers/Bone and Oral health | bromelain, vitamin C, phenols | [42,98,99,100] |
| 15. | Phyllostachys edulis; Bamboo shoot; Mautuai | Edible portion/Boiled | Coronary heart disease, Cancers, Diabetes, obesity/Micronutrient deficiencies | vitamin E, caffeic acid, iron, vitamin C | [42,101,102,103] |
| 16. | Allium schoenoprasum; Chives; Purun-hnah | Leaves | Hypertension, Cancers, Diabetes, Asthma, Hyperlipidemia | allicin, S-allyl cysteine, quercetin, polyphenols, flavonoids | [42,104,105,106] |
| 17. | Brassica juncea; Mustard; Tampui | Leaves, Stem/Decoction | Cancers, Obesity, Diabetes | sinapate, naringin, rutin, catechins, polyphenols | [42,107,108] |
| 18. | Cucurbita pepo L.; Pumpkin; Maian | Leaves, seeds, pulp, plant, flowers/Paste, Cooked, Decoction, Oil | Hypertension, CVDs/Cataract | quercetin, glucosides, kaempferol, tocopherols, phenols | [42,109,110] |
| 19. | Sechium edule; Chayote; Skut hnah | Leaves, Edible portion, roots, shoots/Extract | Cancers, Hypertension, Obesity, Diabetes, Hyperlipidemia, CVDs | gallic acid, chlorogenic acid, quercetin, rutin, flavonoids | [42,111,112,113] |
| 20. | Solanum khasianum; Nightshade; Tawkte | Berries, Leaf, Root, stem, petiole/Extract | Cancers, Diabetes, CVDs, Hypertension | saponins, steroids, alkaloids, flavonoids, phenols | [42,114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrick, D.; Ramaswamy, J.; Palanisamy, T.; Raman, R.; Nedungadi, P. Assessing Dietary Patterns, Lifestyle Practices, and Forest Foods with Bioactive Potential to Address Micronutrient Deficiencies and Noncommunicable Diseases in Northeast India. Nutrients 2025, 17, 3311. https://doi.org/10.3390/nu17203311
Patrick D, Ramaswamy J, Palanisamy T, Raman R, Nedungadi P. Assessing Dietary Patterns, Lifestyle Practices, and Forest Foods with Bioactive Potential to Address Micronutrient Deficiencies and Noncommunicable Diseases in Northeast India. Nutrients. 2025; 17(20):3311. https://doi.org/10.3390/nu17203311
Chicago/Turabian StylePatrick, Devaprasanna, Jancirani Ramaswamy, Thangavel Palanisamy, Raghu Raman, and Prema Nedungadi. 2025. "Assessing Dietary Patterns, Lifestyle Practices, and Forest Foods with Bioactive Potential to Address Micronutrient Deficiencies and Noncommunicable Diseases in Northeast India" Nutrients 17, no. 20: 3311. https://doi.org/10.3390/nu17203311
APA StylePatrick, D., Ramaswamy, J., Palanisamy, T., Raman, R., & Nedungadi, P. (2025). Assessing Dietary Patterns, Lifestyle Practices, and Forest Foods with Bioactive Potential to Address Micronutrient Deficiencies and Noncommunicable Diseases in Northeast India. Nutrients, 17(20), 3311. https://doi.org/10.3390/nu17203311







