Effects of Dark Chocolate on Physiological and Anaerobic Performance Among Healthy Female and Male Adults †
Abstract
1. Introduction
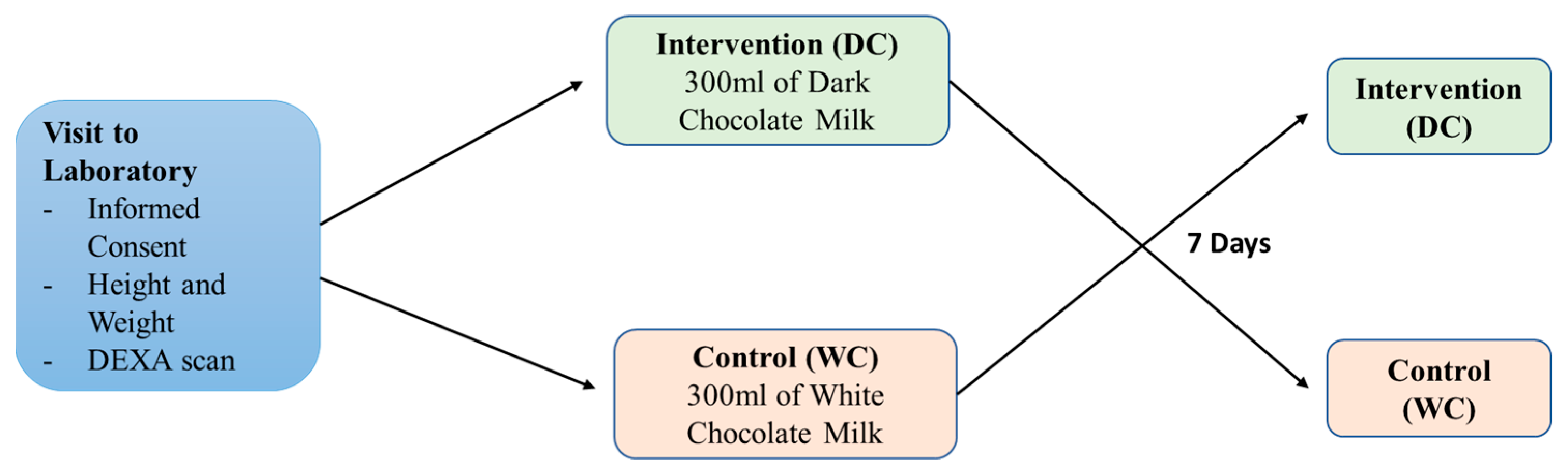
2. Materials and Methods
3. Results
3.1. Physiological Measures
3.1.1. Descriptive Statistics of Participants
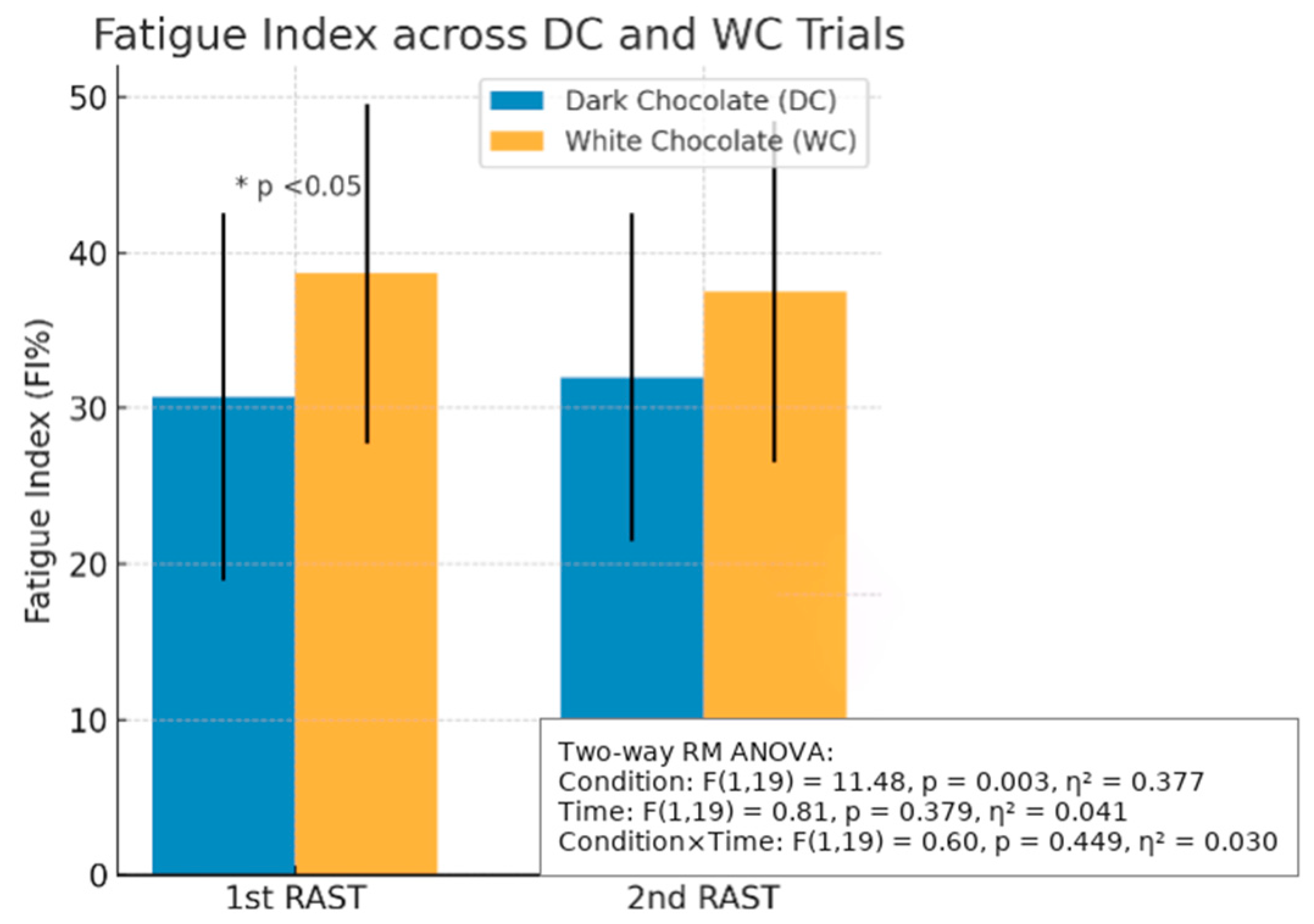
3.1.2. Total Effort Time, Fatigue Index (FI%), and Mean Power Between Trials
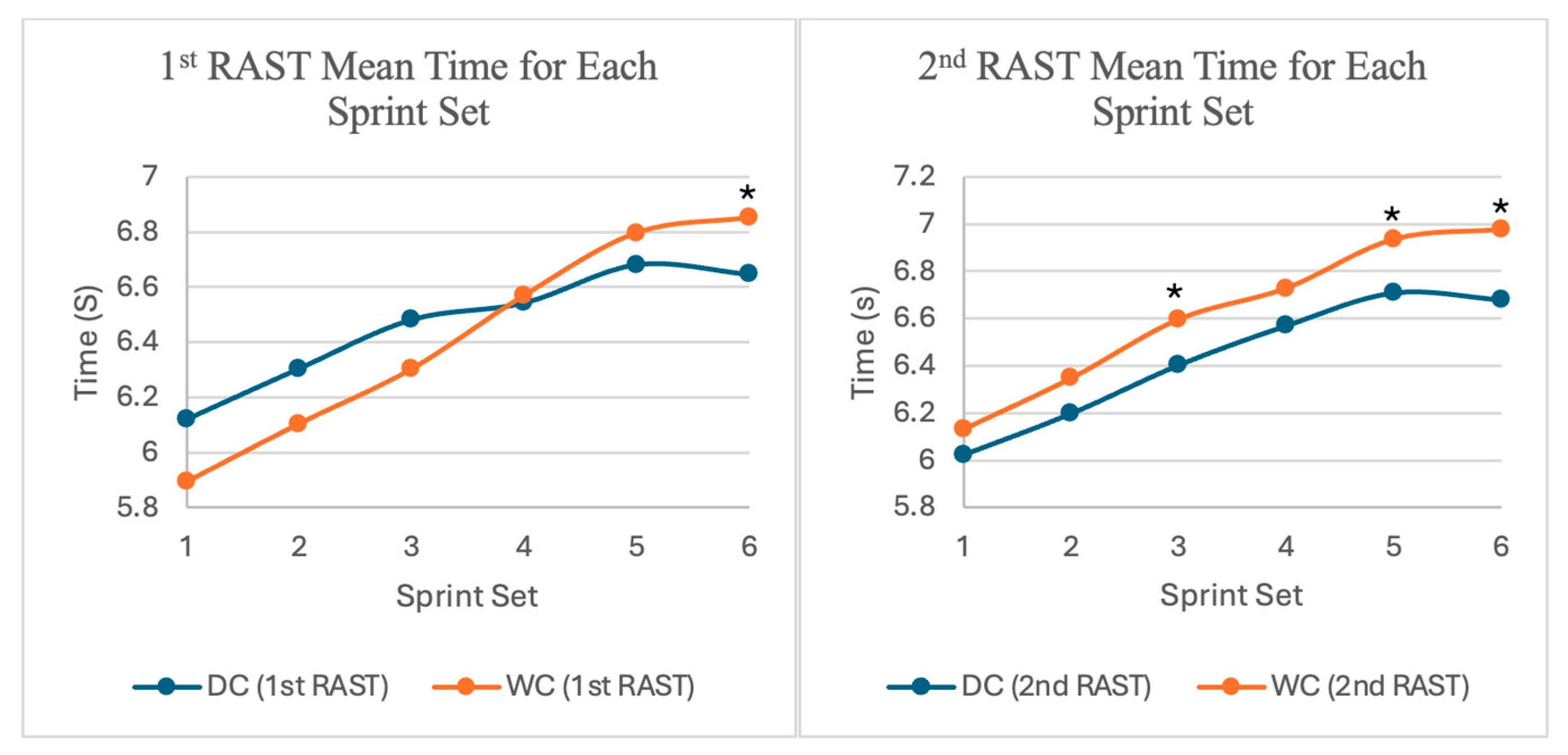
3.1.3. Average Sprint Time Between Sets, Trials, and Gender
3.1.4. Average Power Output Between Trials
3.1.5. Average Heart Rate and Rate of Perceived Exertion Between Trials
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in ultra-marathon running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in ultra-endurance: State of the art. Nutrients 2018, 10, 1990. [Google Scholar] [CrossRef]
- Garthe, I.; Maughan, R.J. Athletes and supplements: Prevalence and perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef]
- Ng, Y.C.; Balasekaran, G.; Foong, S.; Govindaswamy, V.V. Effects Of Dark Chocolate On Physiological And Anaerobic Performance On Healthy Females And Males: 2556. Med. Sci. Sports Exerc. 2024, 56, 748. [Google Scholar] [CrossRef]
- Balasekaran, G.; Foong, K.M.S.; Ng, Y.C. The Effects of Dark Chocolate Supplementation on Rate of Perceived Exertion and Fatigue Index during Anaerobic Sprint Test. In 28th Annual Congress of the European College of Sport Science; Guilhem, G., Rabita, G., Brocherie, F., Tsolakidis, E., Ferrauti, A., Helge, J.W., Piacentini, M.F., Eds.; National Institute of Education, Nanyang Technological University (NIE NTU): Singapore, 2023; Volume 1, p. 1240. [Google Scholar]
- Balasekaran, G.; Scott, F.K.M.; Ng, Y.C.; Govindaswamy, V.V. Effects Of Dark Chocolate Supplementation on Performance and other Physiological Measures In Running-based Anaerobic Sprint Test. Med. Sci. Sports Exerc. 2023, 1, 40–41. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef]
- Steffen, Y.; Schewe, T.; Sies, H. (–)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007, 359, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Williamson, G. The cardiovascular benefits of dark chocolate. Vasc. Pharmacol. 2015, 71, 11–15. [Google Scholar] [CrossRef]
- Martin, F.-P.J.; Rezzi, S.; Peré-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Fay, L.B.; Kochhar, S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Vollaard, N.B.; Choueiri, T.; Wilson, M.T. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Balasekaran, G.; Govindaswamy, V.V.; Kay Peggy, B.P.; Cheo, N.Y. Applied Physiology of Exercise; World Scientific: Singapore, 2021. [Google Scholar]
- Foss, M.L.; Keteyian, S.J. Physiological Basis for Exercise and Sport; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Fredholm, B.B.; Ashihara, H.; Kato, M.; Crozier, A. Distribution, biosynthesis and catabolism of methylxanthines in plants. In Methylxanthines; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 11–31. [Google Scholar]
- Blinks, J.R.; Olson, C.B.; Jewell, B.R.; Bravený, P.A.V.E.L. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle: Evidence for a dual mechanism of action. Circ. Res. 1972, 30, 367–392. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef]
- Nemoto, K.; Kokubun, K.; Ogata, Y.; Koike, Y.; Arai, T.; Yamakawa, Y. Dark chocolate intake may reduce fatigue and mediate cognitive function and gray matter volume in healthy middle-aged adults. Behav. Neurol. 2022, 2022, 6021811. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.-K.; Milbury, P.; Paul, S.M.; Mietus-Snyder, M.L.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Rull, G.; Mohd-Zain, Z.N.; Shiel, J.; Lundberg, M.H.; Collier, D.J.; Johnston, A.; Warner, T.D.; Corder, R. Effects of high flavanol dark chocolate on cardiovascular function and platelet aggregation. Vasc. Pharmacol. 2015, 71, 70–78. [Google Scholar] [CrossRef]
- Robertson, R.J. Perceived Exertion for Practitioners: Rating Effort with the OMNI Picture System; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Serafini, M.; Crozier, A. Milk and absorption of dietary flavanols. Nature 2003, 426, 788. [Google Scholar] [CrossRef]
- Andrade, V.L.; Zagatto, A.M.; Kalva-Filho, C.A.; Mendes, O.C.; Gobatto, C.A.; Campos, E.Z.; Papoti, M. Running-based anaerobic sprint test as a procedure to evaluate anaerobic power. Int. J. Sports Med. 2015, 36, 1156–1162. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, S.; Chatterjee, S. Validity and reliability study of the running-based anaerobic sprint test for evaluating anaerobic power performance as compared to Wingate test in Indian male track and field sprinters. Eur. J. Phys. Educ. Sport Sci. 2022, 8, 39. [Google Scholar] [CrossRef]
- Selmi, M.A.; Sassi, R.H.; Yahmed, M.H.; Moalla, W.; Elloumi, M. Effect of between-set recovery durations on repeated sprint ability in young soccer players. Biol. Sport 2016, 33, 165–172. [Google Scholar] [CrossRef]
- Zagatto, A.M.; Beck, W.R.; Gobatto, C.A. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J. Strength Cond. Res. 2009, 23, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Balasekaran, G.; Govindaswamy, V.V.; Kay Peggy, B.P.; Cheo, N.Y. Applied Physiology of Exercise Laboratory Manual; World Scientific: Singapore, 2021. [Google Scholar]
- Balasekaran, G.; Govindaswamy, V.V.; Loh, R.M.K.; Ng, Y.C.; Thor, D.; Boey, P.; Lim, J. Curricular Guide to Health-Fitness Applications in Physical Education Using the OMNI Perceived Exertion Scale; Pearson Education South Asia Pte Ltd.: Singapore, 2019. [Google Scholar]
- Utter, A.C.; Robertson, R.J.; Nieman, D.C.; Kang, J.I.E. Children’s OMNI scale of perceived exertion: Walking/running evaluation. Med. Sci. Sports Exerc. 2002, 34, 139–144. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Boer, N.F.; Peoples, J.A.; Foreman, A.J.; Dabayebeh, I.M.; Millich, N.B.; Balasekaran, G.; Riechman, S.E.; Gallagher, J.D.; et al. Validation of the Omni Perceived Exertion Scale for Children Using a Mixed Gender/Race Cohort. Med. Sci. Sport Exerc. 2000, 32, 452–458. [Google Scholar] [CrossRef]
- Daugherty, H.J.; Weiss, L.W.; Paquette, M.R.; Powell, D.W.; Allison, L.E. Potential predictors of vertical jump performance: Lower extremity dimensions and alignment, relative body fat, and kinetic variables. J. Strength Cond. Res. 2021, 35, 616–625. [Google Scholar] [CrossRef]
- Pérez-López, A.; Sinovas, M.C.; Álvarez-Valverde, I.; Valades, D. Relationship between body composition and vertical jump performance in young spanish soccer players. J. Sport Hum. Perform. 2015, 3. [Google Scholar] [CrossRef]
- Alemdaroğlu, U. The relationship between muscle strength, anaerobic performance, agility, sprint ability and vertical jump performance in professional basketball players. J. Hum. Kinet. 2012, 31, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Samsonienė, L.; Stukas, R. Dietary acid-base balance in high-performance athletes. Int. J. Environ. Res. Public Health 2020, 17, 5332. [Google Scholar] [CrossRef]
- Clifford, J.; Maloney, K. Nutrition for Athletes; Colorado State: University Extension: Fort Collins, CO, USA, 2015. [Google Scholar]
- Behzadi, M.; Bideshki, M.V.; Ahmadi-Khorram, M.; Zarezadeh, M.; Hatami, A. Effect of dark chocolate/cocoa consumption on oxidative stress and inflammation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of controlled trials. Complement. Ther. Med. 2024, 84, 103061. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, S.; Arab, A.; Mohammadi, H.; Amani, R. The effect of cocoa consumption on markers of oxidative stress: A systematic review and meta-analysis of interventional studies. Complement. Ther. Med. 2020, 48, 102240. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Cohort (n = 20) (Mean ± SD) | Males (n = 10) (Mean ± SD) | Females (n = 10) (Mean ± SD) | p Value |
|---|---|---|---|---|
| Age (years) | 25.07 ± 3.74 | 23.8 ± 1.21 | 26.33 ± 4.95 | 0.134 |
| Height (cm) | 167.60 ± 8.98 | 174.51 ± 5.78 | 160.69 ± 5.52 | 0.000 * |
| Weight (kg) | 64.82 ± 12.26 | 73.91 ± 9.18 | 55.72 ± 7.03 | 0.000 * |
| BMI (kg·m−2) | 22.85 ± 2.48 | 24.18 ± 2.21 | 21.51 ± 2.02 | 0.011 * |
| BF% (%) | 23.21 ± 6.46 | 19.18 ± 6.17 | 27.24 ± 3.74 | 0.002 * |
| Lean Muscle Mass (g) | 48,460.16 ± 13,409.05 | 59,445.22 ± 10,705.85 | 38,573.60 ± 5333.75 | 0.000 * |
| Lean Muscle Mass (%) | 73.58 ± 6.68 | 77.95 ± 6.16 | 69.20 ± 3.70 | 0.001 * |
| BMD (g·cm2) | 1.23 ± 0.08 | 1.27 ± 0.08 | 1.19 ± 0.07 | 0.027 * |
| BMC (g) | 2888.95 ± 601.67 | 3304.60 ± 535.09 | 2473.30 ± 306.60 | 0.000 * |
| DC | WC | p Value | |
|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | ||
| Total Effort Time (s) | 38.78 ± 5.86 | 38.52 ± 4.62 | 0.592 |
| Average Time (s) | 6.46 ± 0.98 | 6.42 ± 0.77 | 0.592 |
| Peak Power (W) | 432.04 ± 216.24 | 440.03 ± 196.63 | 0.615 |
| Relative Power (W·kg−1) | 6.38 ± 2.47 | 6.59 ± 2.27 | 0.373 |
| Mean Power (W) | 345.93 ± 166.96 | 341.86 ± 147.22 | 0.754 |
| Relative Mean Power (W·kg−1) | 5.13 ± 1.89 | 5.11 ± 1.63 | 0.907 |
| FI (%) | 30.71 ± 11.82 | 38.67 ± 10.91 | 0.006 * |
| Resting HR (beats·min−1) | 89.40 ± 13.30 | 95.30 ± 14.95 | 0.019 * |
| Mean HR (beats·min−1) | 157.77 ± 8.24 | 159.70 ± 8.66 | 0.236 |
| HR 6th Set (beats·min−1) | 168.75 ± 7.23 | 170.15 ± 9.18 | 0.250 |
| RPE 6th Set | 7.85 ± 1.31 | 7.90 ± 1.29 | 0.841 |
| DC | WC | p Value | |
|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | ||
| Total Effort Time (s) | 38.58 ± 5.82 | 39.72 ± 6.28 | 0.012 * |
| Average Time (s) | 6.43 ± 0.97 | 6.62 ± 1.05 | 0.012 * |
| Peak Power (W) | 443.76 ± 234.33 | 421.08 ± 209.56 | 0.191 |
| Relative Power (W·kg−1) | 6.53 ± 2.71 | 6.24 ± 2.44 | 0.207 |
| Mean Power (W) | 354.09 ± 173.88 | 323.81 ± 149.32 | 0.009 * |
| Relative Mean Power (W·kg−1) | 5.24 ± 1.99 | 4.81 ± 1.70 | 0.007 * |
| FI (%) | 30.50 ± 11.82 | 35.47 ± 13.85 | 0.099 |
| Resting HR (beats·min−1) | 114.50 ± 16.65 | 114.35 ± 19.44 | 0.951 |
| Mean HR (beats·min−1) | 167.32 ± 7.97 | 165.50 ± 8.23 | 0.321 |
| HR 6th Set (beats·min−1) | 176.60 ± 7.72 | 173.50 ± 7.92 | 0.048 |
| RPE 6th Set | 8.90 ± 0.97 | 8.90 ± 1.17 | 1.000 |
| Male | p Value | Female | p Value | |||
|---|---|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | |||||
| DC | WC | DC | WC | |||
| Total Effort Time (s) | 34.35 ± 1.50 | 34.89 ± 1.17 | 0.307 | 43.21 ± 5.16 | 42.15 ± 3.78 | 0.201 |
| Average Time (s) | 5.73 ± 0.25 | 5.82 ± 0.20 | 0.307 | 7.20 ± 0.86 | 7.03 ± 0.63 | 0.201 |
| Peak Power (W) | 627.34 ± 82.55 | 619.49 ± 67.03 | 0.785 | 236.75 ± 84.54 | 260.57 ± 74.59 | 0.120 |
| Relative Power (W·kg−1) | 8.52 ± 0.76 | 8.47 ± 1.10 | 0.896 | 4.25 ± 1.49 | 4.71 ± 1.34 | 0.111 |
| Mean Power (W) | 492.84 ± 78.80 | 472.44 ± 56.51 | 0.408 | 199.03 ± 68.42 | 211.28 ± 68.32 | 0.205 |
| Relative Mean Power (W·kg−1) | 6.69 ± 0.87 | 6.42 ± 0.68 | 0.386 | 3.56 ± 1.15 | 3.79 ± 1.14 | 0.199 |
| FI (%) | 32.47 ± 8.93 | 39.60 ± 9.14 | 0.020 * | 28.94 ± 10.50 | 37.74 ± 12.88 | 0.089 |
| Resting HR (beats·min−1) | 91.60 ± 9.91 | 93.90 ± 10.60 | 0.235 | 87.20 ± 16.26 | 96.70 ± 18.85 | 0.042 * |
| Mean HR (beats·min−1) | 157.40 ± 10.52 | 159.87 ± 9.61 | 0.373 | 158.13 ± 5.68 | 159.53 ± 8.12 | 0.477 |
| HR 6th Set (beats·min−1) | 170.60 ± 9.14 | 172.50 ± 10.35 | 0.353 | 166.90 ± 4.38 | 167.80 ± 7.66 | 0.546 |
| RPE 6th Set | 7.80 ± 1.40 | 8.40 ± 0.97 | 0.081 | 7.90 ± 1.29 | 7.40 ± 1.43 | 0.138 |
| Male | p Value | Female | p Value | |||
|---|---|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | |||||
| DC | WC | DC | WC | |||
| Total Effort Time (s) | 33.99 ± 1.38 | 35.41 ± 1.50 | 0.004 * | 43.18 ± 4.76 | 44.03 ± 6.30 | 0.281 |
| Average Time (s) | 5.66 ± 0.23 | 5.90 ± 0.26 | 0.004 * | 7.20 ± 0.79 | 7.34 ± 1.05 | 0.281 |
| Peak Power (W) | 656.14 ± 97.43 | 609.11 ± 82.15 | 0.159 | 231.39 ± 78.73 | 233.05 ± 85.44 | 0.876 |
| Relative Power (W·kg−1) | 8.91 ± 1.04 | 8.29 ± 0.98 | 0.148 | 4.15 ± 1.35 | 4.18 ± 1.47 | 0.876 |
| Mean Power (W) | 509.80 ± 69.36 | 454.44 ± 63.37 | 0.010 * | 198.39 ± 71.68 | 193.19 ± 71.69 | 0.353 |
| Relative Mean Power (W·kg−1) | 6.93 ± 0.76 | 6.17 ± 0.74 | 0.009 * | 3.55 ± 1.19 | 3.45 ± 1.19 | 0.337 |
| FI (%) | 35.99 ± 8.93 | 40.58 ± 11.92 | 0.143 | 25.01 ± 11.75 | 30.35 ± 14.30 | 0.326 |
| Resting HR (beats·min−1) | 111.00 ± 13.75 | 108.00 ± 11.37 | 0.318 | 118.00 ± 19.20 | 120.70 ± 24.06 | 0.501 |
| Mean HR (beats·min−1) | 166.63 ± 10.56 | 164.97 ± 8.53 | 0.600 | 168.00 ± 4.63 | 166.03 ± 8.34 | 0.352 |
| HR 6th Set (beats·min−1) | 177.50 ± 9.77 | 173.80 ± 8.04 | 0.174 | 175.70 ± 5.36 | 173.20 ± 8.22 | 0.164 |
| RPE 6th Set | 8.90 ± 1.10 | 9.30 ± 0.68 | 0.168 | 8.90 ± 0.88 | 8.50 ± 1.43 | 0.104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasekaran, G.; Ng, Y.C.; Foong, S.; Ong, X.R.R.; Boey, P. Effects of Dark Chocolate on Physiological and Anaerobic Performance Among Healthy Female and Male Adults. Nutrients 2025, 17, 3317. https://doi.org/10.3390/nu17213317
Balasekaran G, Ng YC, Foong S, Ong XRR, Boey P. Effects of Dark Chocolate on Physiological and Anaerobic Performance Among Healthy Female and Male Adults. Nutrients. 2025; 17(21):3317. https://doi.org/10.3390/nu17213317
Chicago/Turabian StyleBalasekaran, Govindasamy, Yew Cheo Ng, Scott Foong, Xin Rui Rachael Ong, and Peggy Boey. 2025. "Effects of Dark Chocolate on Physiological and Anaerobic Performance Among Healthy Female and Male Adults" Nutrients 17, no. 21: 3317. https://doi.org/10.3390/nu17213317
APA StyleBalasekaran, G., Ng, Y. C., Foong, S., Ong, X. R. R., & Boey, P. (2025). Effects of Dark Chocolate on Physiological and Anaerobic Performance Among Healthy Female and Male Adults. Nutrients, 17(21), 3317. https://doi.org/10.3390/nu17213317





