The Impact of Diet on the Fecal Microbiota Transplantation Success in Patients with Gastrointestinal Diseases—A Literature Review
Abstract
1. Introduction
2. Methods
2.1. The Impact of Microbiota on Health and the Impact of Diet on Microbiota
2.2. Requirements for Stool Donors
2.3. Indications, Risks, and Procedure for Conducting FMT
- Relative contraindications: recent gastrointestinal surgery, severe acute illness, pregnancy and lactation, pediatric patients, elderly patients;
- Absolute contraindications: severe immunocompromise, gastrointestinal obstruction, toxic megacolon, recent major surgery, gastrointestinal tract perforation [3].
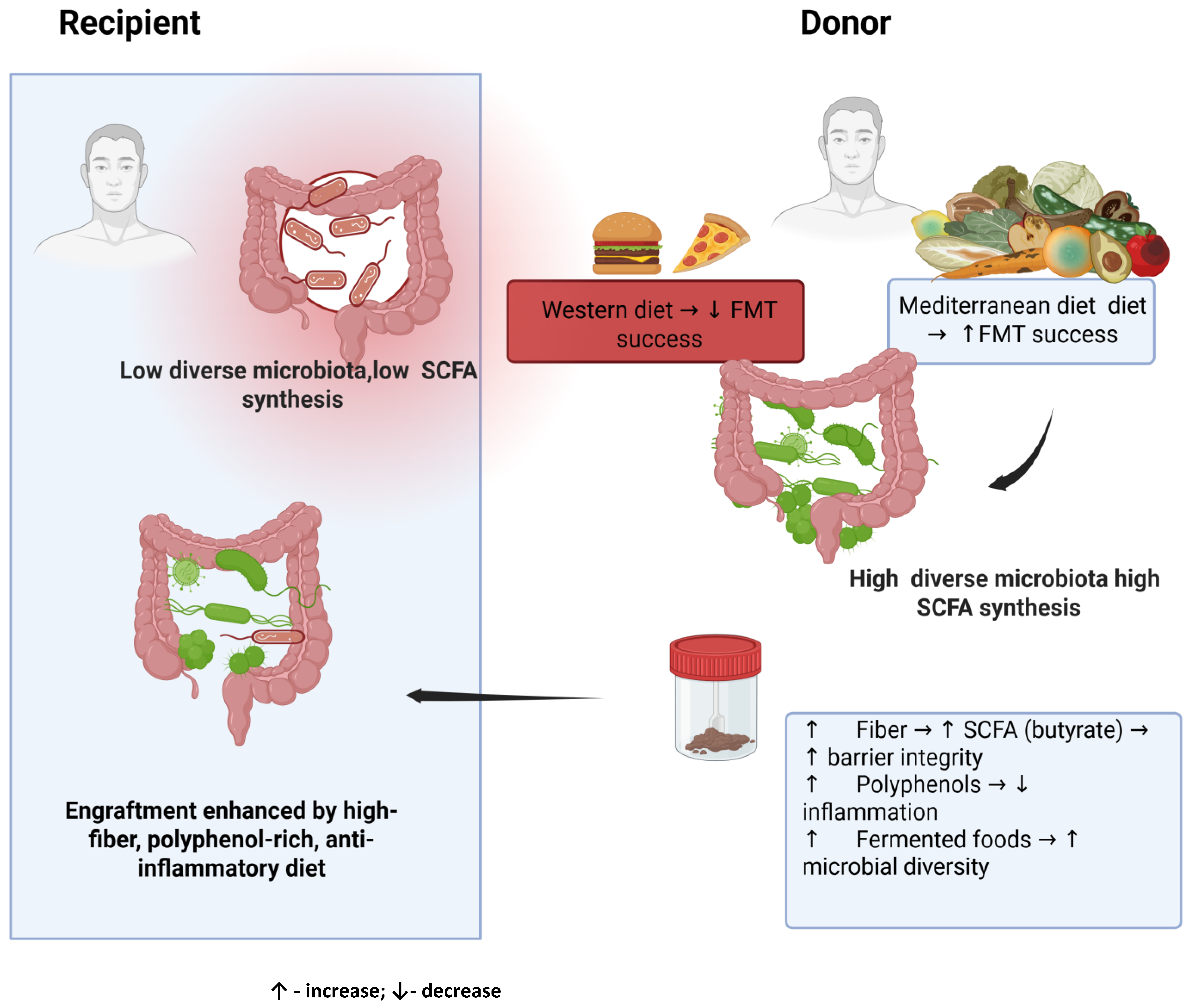
2.4. Scientific Research in the Context of Diet and the Effectiveness of FMT in Gastrointestinal Problems
2.5. Dietary Recommendations for Donors and Recipients
- Fiber fermentable to SCFA—resistant starch, inulin, arabinoxylans—provide substrates for saccharolytic fermentation, increasing butyrate levels and supporting the integrity of the mucosal membrane and immune barrier. Sources of these fibers include whole grains, legumes, bananas, onions, leeks, and chicory root [70].
- Fermented foods such as yogurt, kefir, sauerkraut, kimchi, and miso provide live microorganisms and bioactive metabolites. They increase the diversity of microorganisms and enrich Lactobacillus and Bifidobacterium, while reducing the number of pathogens [61].
- Chrono-nutrition—regular meals with higher energy intake in the early part of the day improve metabolic homeostasis and microbial stability in the circadian cycle [65].
- A Western-style diet rich in saturated and trans fats, refined sugars, and highly processed foods. These foods reduce microbial diversity, inhibit efficient SCFA synthesis, promote the growth of pro-inflammatory taxa (Enterobacetriaceae), and damage the intestinal barrier, exacerbating endotoxemia [66,67].
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.-W.; Fischer, M. Fecal Microbiota Transplantation. Clin. Colon Rectal Surg. 2023, 36, 151–156. [Google Scholar] [CrossRef]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Salehi Omran, H.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and Efficacy of Fecal Microbiota Transplantation (FMT) as a Modern Adjuvant Therapy in Various Diseases and Disorders: A Comprehensive Literature Review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef]
- Ray, R.; Hack, S.A.; Vij, A.K.; Gbenla, K.I.; Khatri, S.; Aravind Rongali, D.; Khalid, A.; Anjum, A.; Fancy, R.S.; Mirza, M.S.S. Efficacy of Fecal Microbiota Transplantation (FMT) Versus Standard Antibiotic Therapy in Recurrent Clostridioides Difficile (CDI/rCDI) Infection: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e90614. [Google Scholar] [CrossRef]
- Weerakoon, S.; Avula, S.; Mandefro, B.T.; Sundara, S.V.; Lu, X.; Busmail, H.; Malasevskaia, I.A. Microbiota-Based Therapies for Recurrent Clostridium Difficile Infection: A Systematic Review of Their Efficacy and Safety. Cureus 2025, 17, e90737. [Google Scholar] [CrossRef]
- Nagayama, M.; Gogokhia, L.; Longman, R.S. Precision Microbiota Therapy for IBD: Premise and Promise. Gut Microbes 2025, 17, 2489067. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Naqvi, S.A.A.; Shadali, A.H.; Khan, H.; Christof, M.; Niu, C.; Schwartz, D.A.; Adler, D.G. Fecal Microbiota Transplantation (FMT) and Clinical Outcomes Among Inflammatory Bowel Disease (IBD) Patients: An Umbrella Review. Dig. Dis. Sci. 2025, 70, 1873–1896. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key Determinants of Success in Fecal Microbiota Transplantation: From Microbiome to Clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Clancy, A.K.; Gunaratne, A.W.; Borody, T.J. Dietary Management for Faecal Microbiota Transplant: An International Survey of Clinical and Research Practice, Knowledge and Attitudes. Front. Nutr. 2021, 8, 653653. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.M.; Hoeg, A.; Zehra, H.; Shah, P.; Johnson, R.; Hutchison, K.; Kocher, M.; Lin, A.W.; Johnson, A.J.; Vaughn, B.P. Nutritional Optimization of Fecal Microbiota Transplantation in Humans: A Scoping Review. Gut Microbes 2025, 17, 2446378. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Chittim, C.L.; Irwin, S.M.; Balskus, E.P. Deciphering Human Gut Microbiota-Nutrient Interactions: A Role for Biochemistry. Biochemistry 2018, 57, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut Microbiota and Obesity. Clin. Nutr. Exp. 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.; González, A.; Vázquez-Baeza, Y.; Weiss, S.; Humphry, G.; Berg-Lyons, D.; Knights, D.; Unno, T.; Bobr, A.; Kang, J.; et al. Dynamic Changes in Short- and Long-Term Bacterial Composition Following Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. Microbiome 2015, 3, 10. [Google Scholar] [CrossRef]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Högenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Mégraud, F.; et al. A Standardised Model for Stool Banking for Faecal Microbiota Transplantation: A Consensus Report from a Multidisciplinary UEG Working Group. United Eur. Gastroenterol. J. 2021, 9, 229–247. [Google Scholar] [CrossRef]
- Mullish, B.H.; Merrick, B.; Quraishi, M.N.; Bak, A.; Green, C.A.; Moore, D.J.; Porter, R.J.; Elumogo, N.T.; Segal, J.P.; Sharma, N.; et al. The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridioides Difficile Infection and Other Potential Indications: Second Edition of Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. J. Hosp. Infect. 2024, 148, 189–219. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Gill, S.K.; Tasnim, N.; Ahmadi-Vand, Z.; Jay, M.; Gibson, D.L. Methods for Improving Human Gut Microbiome Data by Reducing Variability through Sample Processing and Storage of Stool. PLoS ONE 2015, 10, e0134802. [Google Scholar] [CrossRef]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; Imdad, A.; Altayar, O.; AGA Clinical Guidelines Committee. Electronic address: Clinicalpractice@gastro.org AGA Clinical Practice Guideline on Fecal Microbiota-Based Therapies for Select Gastrointestinal Diseases. Gastroenterology 2024, 166, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Gefen, R.; Dourado, J.; Emile, S.H.; Wignakumar, A.; Rogers, P.; Aeschbacher, P.; Garoufalia, Z.; Horesh, N.; Wexner, S.D. Fecal Microbiota Transplantation for Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Control Trials. Technol. Coloproctol. 2025, 29, 103. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-J.; Dong, H.-B.; Ren, Y.-T.; Jiang, B. Efficacy and Safety of Fecal Microbiota Transplantation for the Induction of Remission in Active Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Transl. Med. 2022, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, Y.; Zhang, Y.; Zhao, T.; Cong, J. Fecal Microbiota Transplantation for Induction of Remission in Crohn’s Disease: A Systematic Review and Meta-Analysis. Int. J. Colorectal. Dis. 2023, 38, 62. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Xu, F.; Li, N.; Wang, C.; Xing, H.; Chen, D.; Wei, Y. Clinical Efficacy of Fecal Microbiota Transplantation for Patients with Small Intestinal Bacterial Overgrowth: A Randomized, Placebo-Controlled Clinic Study. BMC Gastroenterol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides Difficile Infection: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Tauxe, W.M.; Dhere, T.; Ward, A.; Racsa, L.D.; Varkey, J.B.; Kraft, C.S. Fecal Microbiota Transplant Protocol for Clostridium Difficile Infection. Lab. Med. 2015, 46, e19–e23. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium Difficile Infection with Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Cold, F.; Svensson, C.K.; Petersen, A.M.; Hansen, L.H.; Helms, M. Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides Difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study. Cells 2022, 11, 435. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, Oral FMT for Long-Term Maintenance Therapy in Ulcerative Colitis: Results of a Single-Center, Prospective, Randomized Pilot Study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef] [PubMed]
- Murgiano, M.; Bartocci, B.; Puca, P.; di Vincenzo, F.; Del Gaudio, A.; Papa, A.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. Int. J. Mol. Sci. 2025, 26, 3059. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.A.; Baig, M.; Puli, S.R. Adverse Events in Fecal Microbiota Transplantation: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2022, 35, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cao, J.; Deng, Z.; Ma, Y.; Liu, J.; Wang, H. Effect of Fiber and Fecal Microbiota Transplantation Donor on Recipient Mice Gut Microbiota. Front. Microbiol. 2021, 12, 757372. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin Enhances the Effect of Fecal Microbiota Transplantation in Ulcerative Colitis by Delaying the Loss of Diversity of Gut Flora. BMC Microbiol. 2016, 16, 255. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, H.; Gu, L.; Nie, Y.; Ding, C.; Ge, X.; Yang, B.; Gong, J.; Li, N. Long-Term Follow-up of the Effects of Fecal Microbiota Transplantation in Combination with Soluble Dietary Fiber as a Therapeutic Regimen in Slow Transit Constipation. Sci. China Life Sci. 2018, 61, 779–786. [Google Scholar] [CrossRef]
- Kedia, S.; Virmani, S.; Vuyyuru, S.K.; Kumar, P.; Kante, B.; Sahu, P.; Kaushal, K.; Farooqui, M.; Singh, M.; Verma, M.; et al. Faecal Microbiota Transplantation with Anti-Inflammatory Diet (FMT-AID) Followed by Anti-Inflammatory Diet Alone Is Effective in Inducing and Maintaining Remission over 1 Year in Mild to Moderate Ulcerative Colitis: A Randomised Controlled Trial. Gut 2022, 71, 2401–2413. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Shabat, C.S.; Hirsch, A.; Zittan, E.; Mentella, M.C.; Petito, V.; Cohen, N.A.; Ron, Y.; Isakov, N.F.; Pfeffer, J.; et al. Faecal Transplantation for Ulcerative Colitis From Diet Conditioned Donors Followed by Dietary Intervention Results in Favourable Gut Microbial Profile Compared to Faecal Transplantation Alone. J. Crohn’s Colitis 2024, 18, 1606–1614. [Google Scholar] [CrossRef]
- Bertin, L.; Zanconato, M.; Crepaldi, M.; Marasco, G.; Cremon, C.; Barbara, G.; Barberio, B.; Zingone, F.; Savarino, E.V. The Role of the FODMAP Diet in IBS. Nutrients 2024, 16, 370. [Google Scholar] [CrossRef]
- Huang, H.-L.; Zhu, J.-Q.; Yang, L.-S.; Wu, Q.; Shou, D.-W.; Chen, H.-T.; Ma, J.; Li, Y.-Q.; Xu, H.-M.; Zhou, Y.-J. Fecal Microbiota Transplantation Combined with a Low FODMAP Diet for the Treatment of Irritable Bowel Syndrome with Predominant Diarrhea. Oxidative Med. Cell. Longev. 2022, 2022, 5121496. [Google Scholar] [CrossRef]
- Gogokhia, L.; Tran, N.; Grier, A.; Nagayama, M.; Xiang, G.; Funez-dePagnier, G.; Lavergne, A.; Ericsson, C.; Ben Maamar, S.; Zhang, M.; et al. Donor Composition and Fiber Promote Strain Engraftment in a Randomized Controlled Trial of Fecal Microbiota Transplant for Ulcerative Colitis. Med 2025, 6, 100707. [Google Scholar] [CrossRef]
- Xiang, L.; Yu, Y.; Ding, X.; Zhang, H.; Wen, Q.; Cui, B.; Zhang, F. Exclusive Enteral Nutrition Plus Immediate vs. Delayed Washed Microbiota Transplantation in Crohn’s Disease with Malnutrition: A Randomized Pilot Study. Front. Med. 2021, 8, 666062. [Google Scholar] [CrossRef] [PubMed]
- The OpenBiome Foundation. Available online: https://openbiome.org/clinical-considerations-for-donor-screening/ (accessed on 10 September 2025).
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Peluzio, M.D.C.G.; Dias, M.d.M.e.; Martinez, J.A.; Milagro, F.I. Kefir and Intestinal Microbiota Modulation: Implications in Human Health. Front. Nutr. 2021, 8, 638740. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef]
- Anghel, A.C.; Țăranu, I.; Orțan, A.; Marcu Spinu, S.; Dragoi Cudalbeanu, M.; Rosu, P.M.; Băbeanu, N.E. Polyphenols and Microbiota Modulation: Insights from Swine and Other Animal Models for Human Therapeutic Strategies. Molecules 2024, 29, 6026. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. The Ancestral and Industrialized Gut Microbiota and Implications for Human Health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar] [CrossRef] [PubMed]
| Author | n | Study Design | Condition | Intervention | Outcomes | Study Limitations |
|---|---|---|---|---|---|---|
| [57] | 27 | Randomized 4-arm trial | UC (mild to moderate) | A single FMT or placebo with or without psyllium fiber supplementation for 8 weeks (recipient) | Single-dose FMT demonstrated clinical efficacy for UC compared to placebo but revealed no benefit of fiber supplementation. |
|
| [53] | 66 | Open-label RCT | UC (mild to moderate) | FMT + AID vs. SMT | FMT + AID had better induction (65.7 % vs. 35.5 %) and maintained deep remission at 48 weeks (25% vs. 0%) than SMT. |
|
| [51] | 20 | RCT | UC (mild to moderate) | FMT vs. FMT + pectin | Pectin decreased the Mayo score by preserving the diversity of the gut flora following FMT for UC and enhanced the effect of FMT. |
|
| [52] | 29 | Clinical Trial | Slow transit constipation | FMT + pectin | FMT in combination with soluble dietary fiber (pectin) had both short-term (4 week) and long-term (1 year) efficacy in treating constipation |
|
| [58] | 19 | Randomized pilot study | CD with malnutrition | EEN + timing of WMT (WMT-Day1 = 8, WMT-DAY8 = 11) | EEN + immediate WMT improved the nutritional status and induced clinical remission in malnourished CD patients. |
|
| [56] | 80 | A retrospective analysis of single-arm open-label prospective study | IBS with predominant diarrhea | FMT alone vs. FMT + LFD | LFD enhanced the efficacy of FMT, increased the gut microbial diversity after FMT, and strengthened the inhibitory effect of FMT on conditional pathogens. |
|
| [54] | 21 | Comparative Study | UC | Group 1: FMT alone Group 2: FMT with donors’ dietary pre-conditioning and UCED for the patients | FMT from diet conditioned donors followed by the UCED led to microbial alterations associated with favorable microbial profiles which correlated with decreased fecal calprotectin |
|
| [40] | 165 | Randomized, double-blind, placebo-controlled study | IBS | Group 1: placebo Group 2: 30 g FMT Group 3: 60 g FMT Super donor with healthy diet and dietary supplements rich in proteins, vitamins, fiber and minerals) | FMT is more successful than placebo in curing IBS |
|
| [10] | 18 | Observational Pilot Study | IBS, IBD undergoing FMT | Basic dietary education (by nurse) + high fiber diet (minimum 30 g/day) + inulin and pectin supplements + FMT | Higher diet quality associated with better outcomes post-FMT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorniak, N.; Gaweł, K.; Deskur, A.; Pawlus, J.; Stachowska, E. The Impact of Diet on the Fecal Microbiota Transplantation Success in Patients with Gastrointestinal Diseases—A Literature Review. Nutrients 2025, 17, 3314. https://doi.org/10.3390/nu17203314
Komorniak N, Gaweł K, Deskur A, Pawlus J, Stachowska E. The Impact of Diet on the Fecal Microbiota Transplantation Success in Patients with Gastrointestinal Diseases—A Literature Review. Nutrients. 2025; 17(20):3314. https://doi.org/10.3390/nu17203314
Chicago/Turabian StyleKomorniak, Natalia, Katarzyna Gaweł, Anna Deskur, Jan Pawlus, and Ewa Stachowska. 2025. "The Impact of Diet on the Fecal Microbiota Transplantation Success in Patients with Gastrointestinal Diseases—A Literature Review" Nutrients 17, no. 20: 3314. https://doi.org/10.3390/nu17203314
APA StyleKomorniak, N., Gaweł, K., Deskur, A., Pawlus, J., & Stachowska, E. (2025). The Impact of Diet on the Fecal Microbiota Transplantation Success in Patients with Gastrointestinal Diseases—A Literature Review. Nutrients, 17(20), 3314. https://doi.org/10.3390/nu17203314






