Lacticaseibacillus paracasei L21 and Its Postbiotics Ameliorate Ulcerative Colitis Through Gut Microbiota Modulation, Intestinal Barrier Restoration, and HIF1α/AhR-IL-22 Axis Activation: Combined In Vitro and In Vivo Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
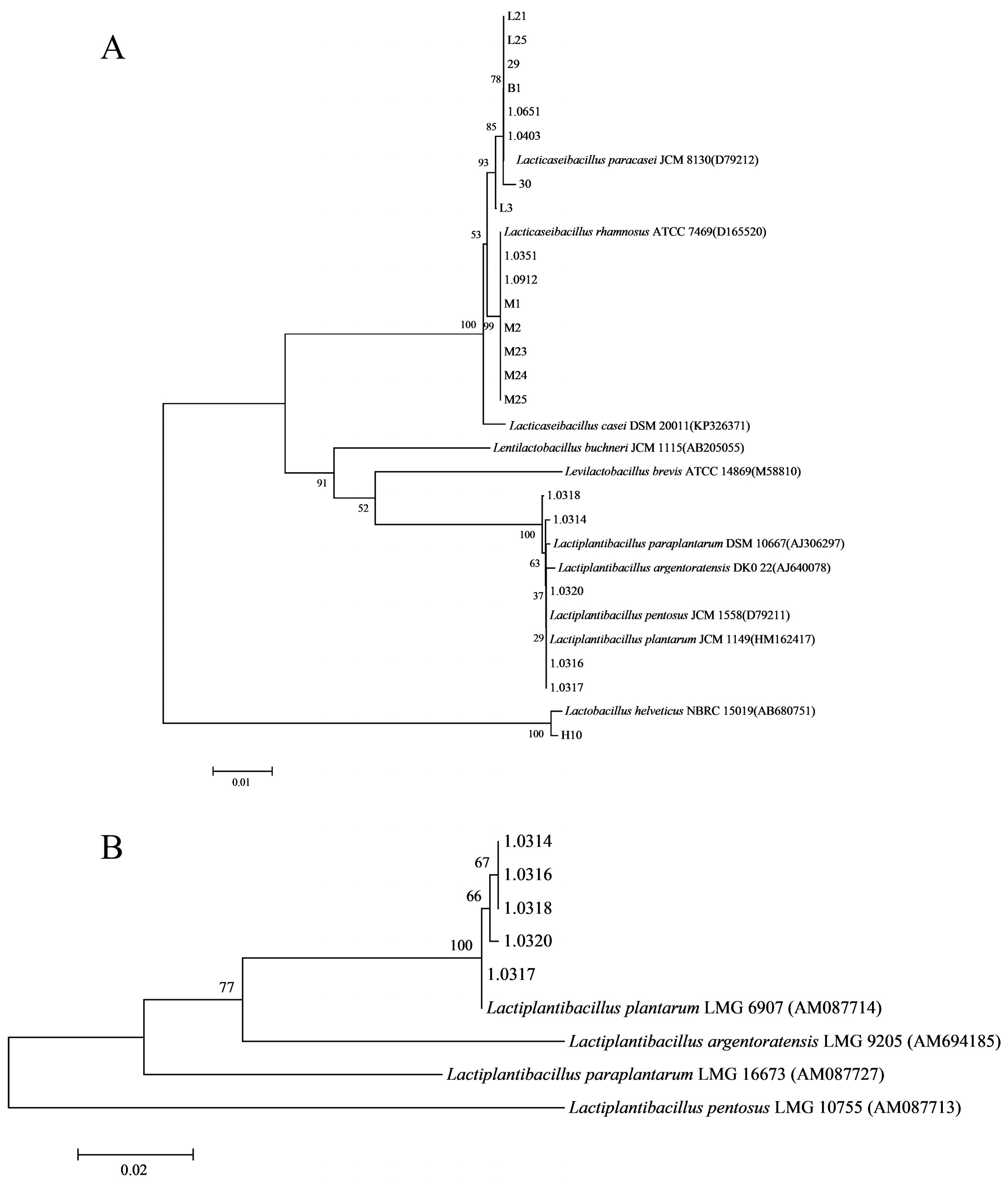
2.2. Isolation, Purification, and Identification of Strains
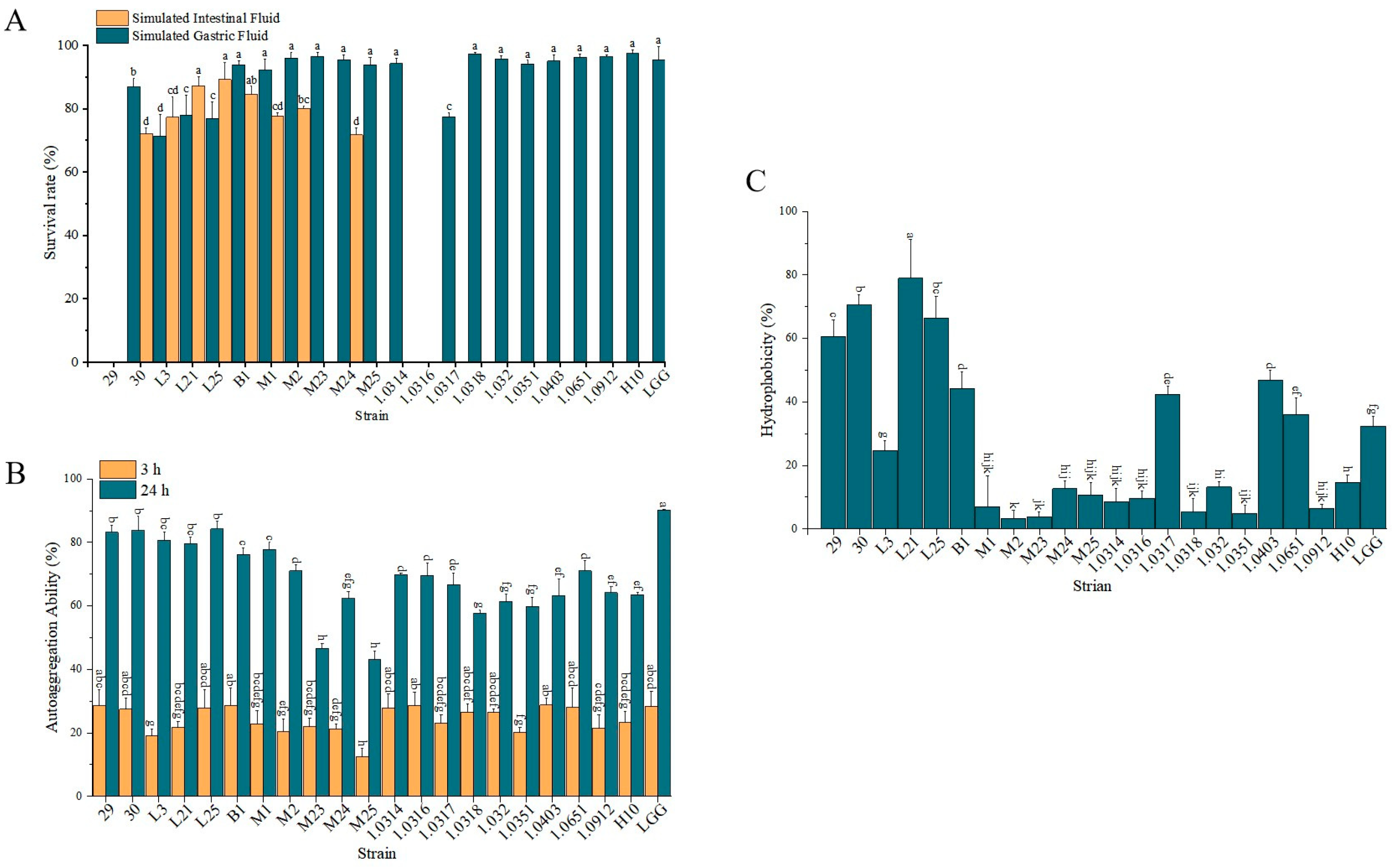
2.3. Determination of Probiotic Properties of Lactobacillus Strains
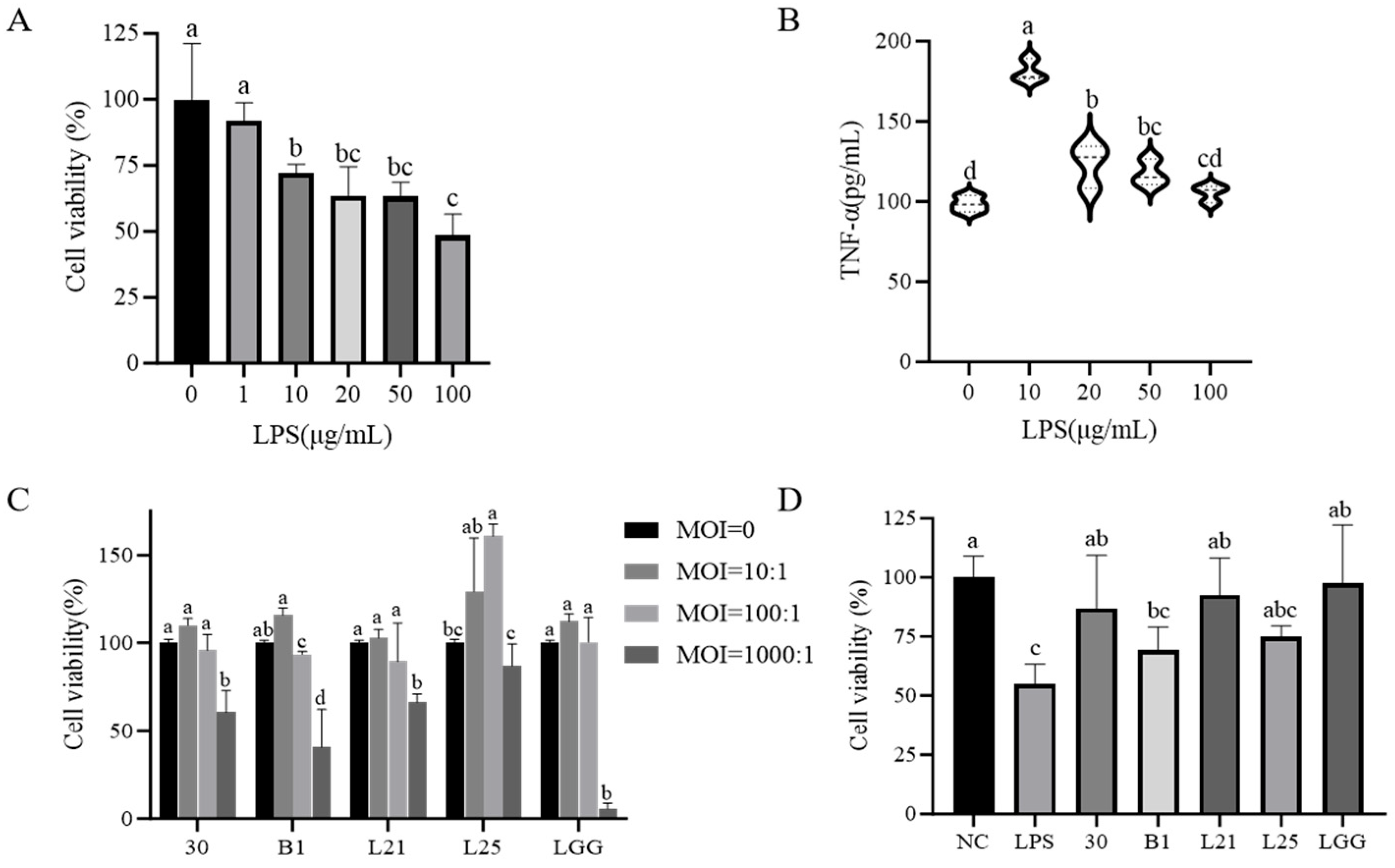
2.4. Cell Culture and Treatment
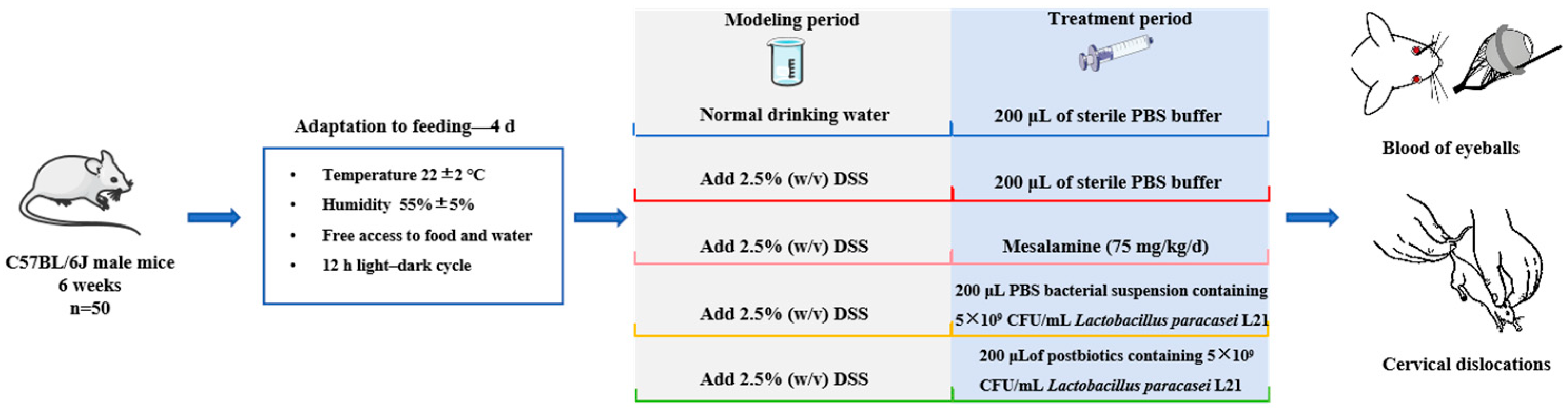
2.5. Animals Experimental Design
2.6. Immunofluorescence
2.7. Immunohistochemistry
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Inflammatory Cytokine
2.10. Morphological Analysis
2.11. Western Blot
2.12. 16S rRNA Sequencing
2.13. Quantification of Short-Chain Fatty Acid (SCFA)
2.14. Statistical Analysis
3. Results
3.1. Biological Characteristics of Experimental Strains
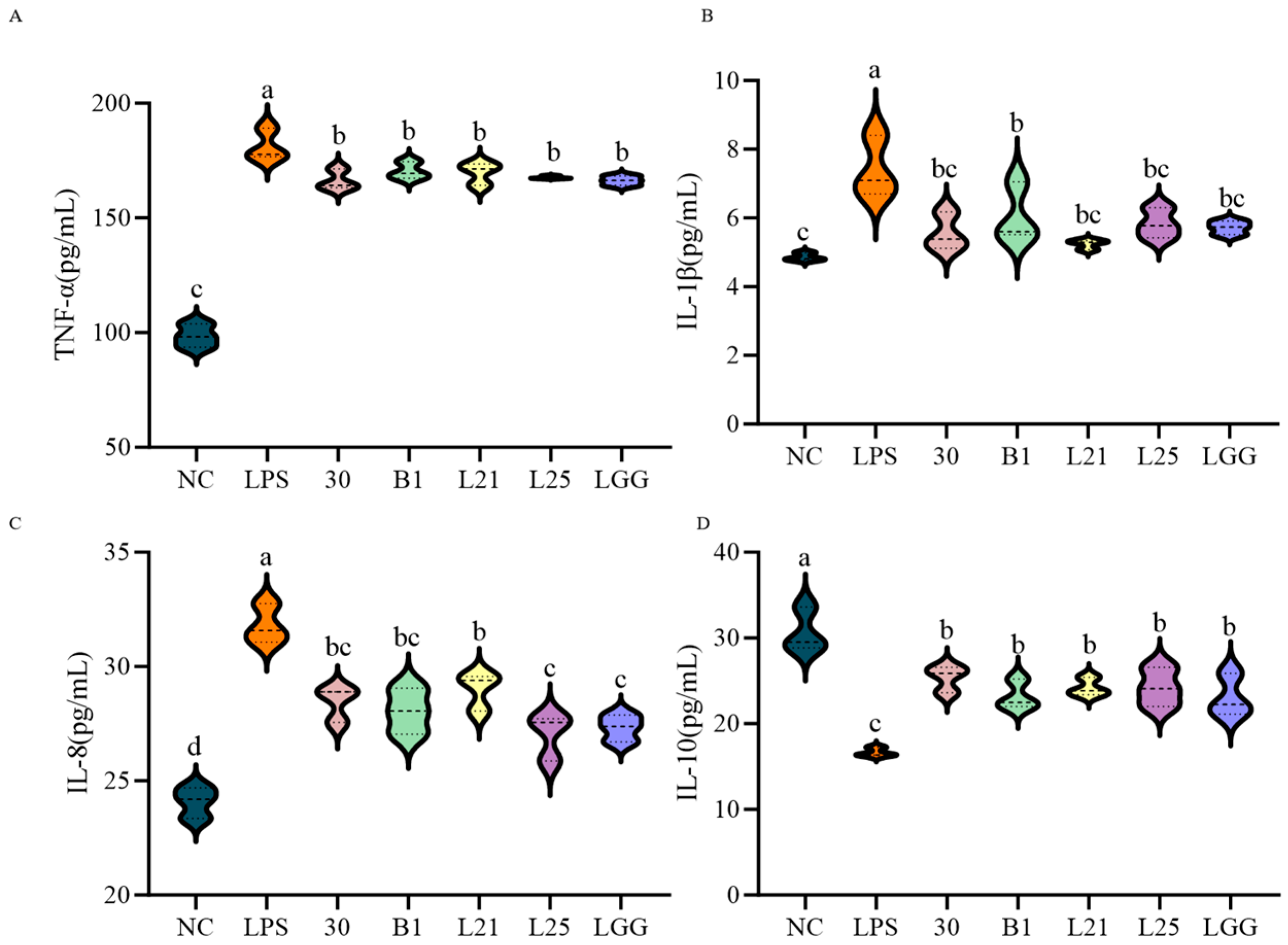
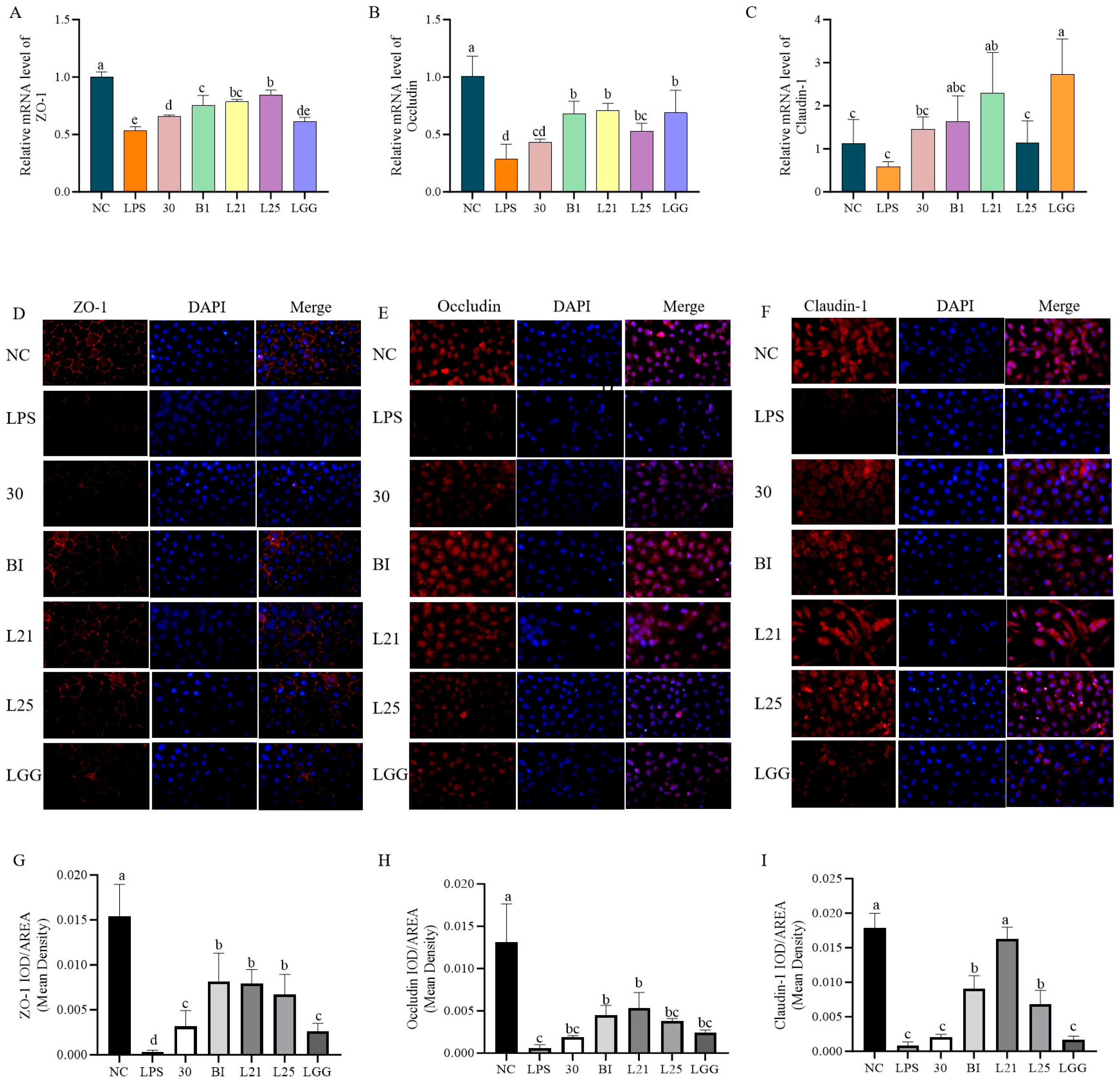
3.2. In Vitro Assessment of the Protective Effect of Probiotics on LPS-Induced Inflammation and Barrier Function in IEC-6 Cells
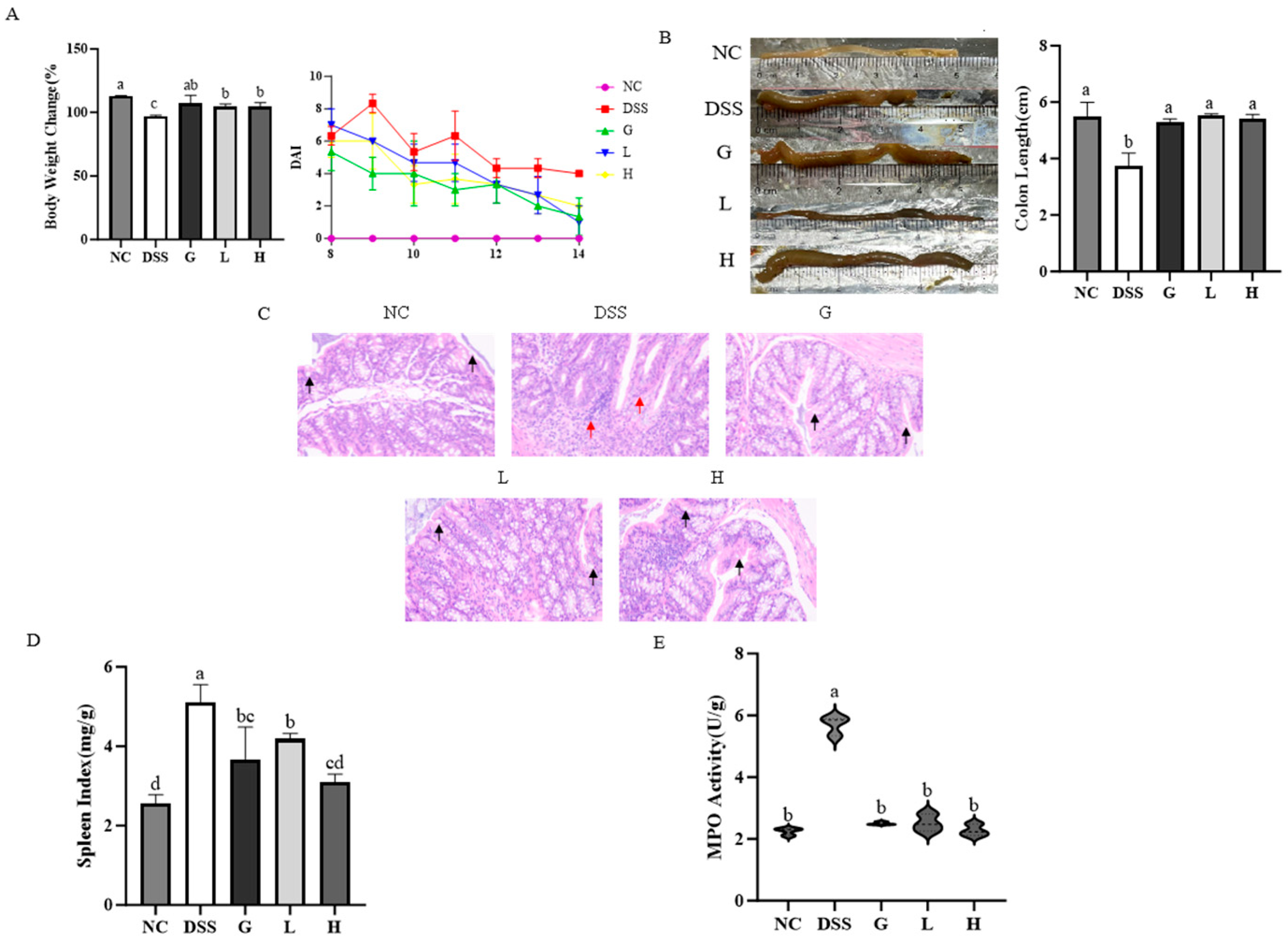
3.3. L. paracasei L21 and H-L21 Mitigate the Histopathology and Basic Indicators of DSS-Induced UC Mice
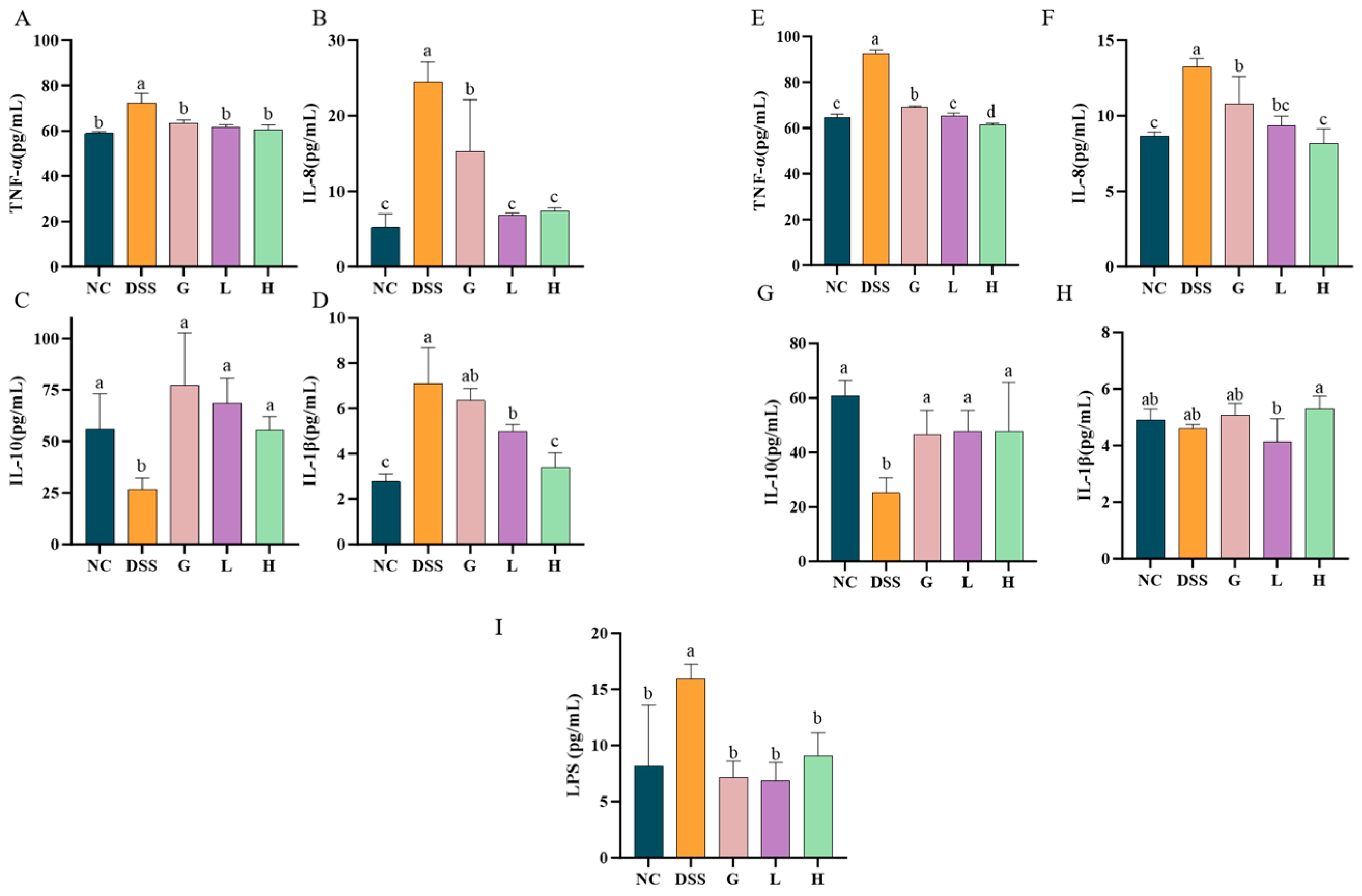
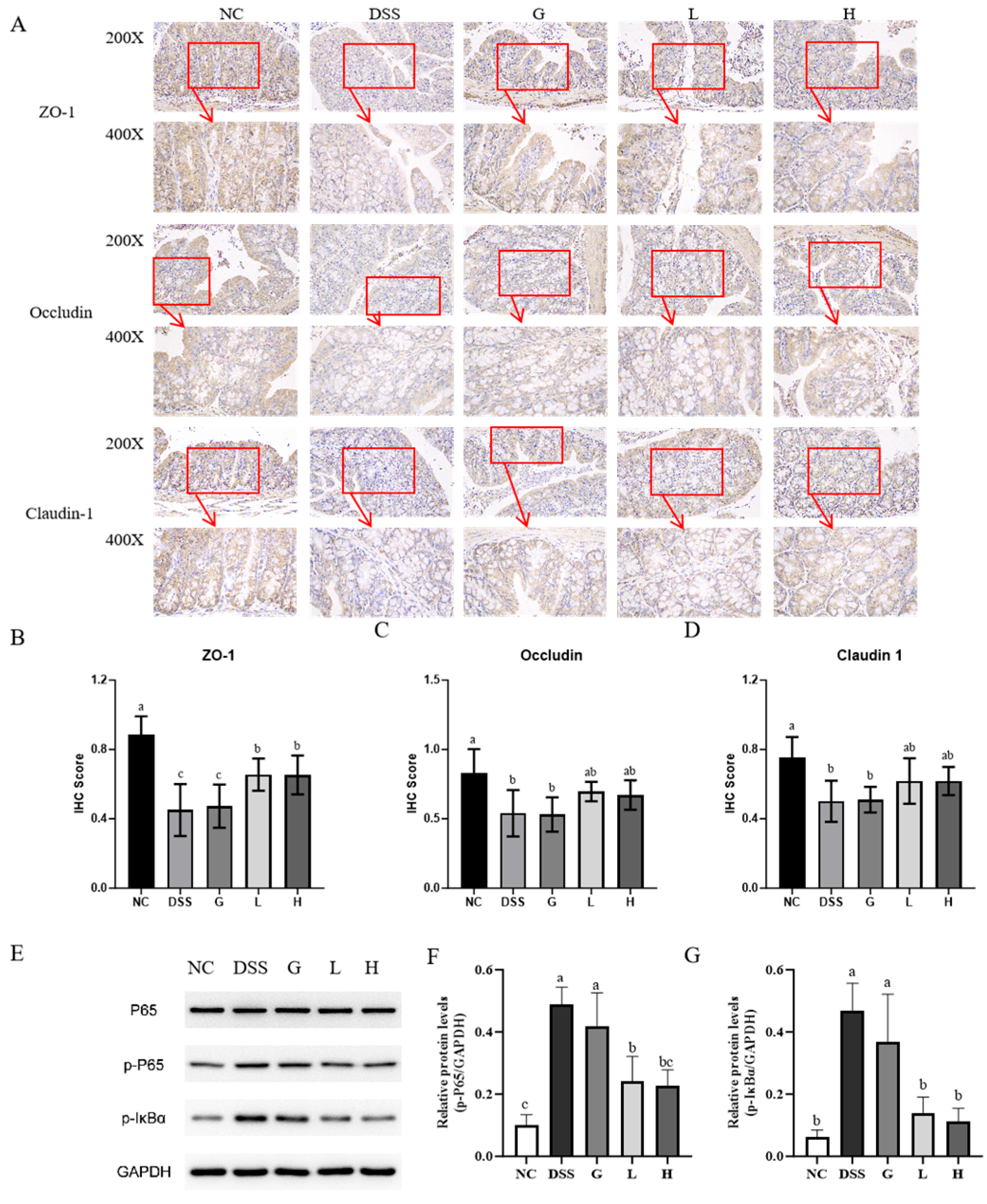
3.4. L. paracasei L21 and H-L21 Interventions Balance Inflammatory Dysregulation and Restore the Intestinal Barrier
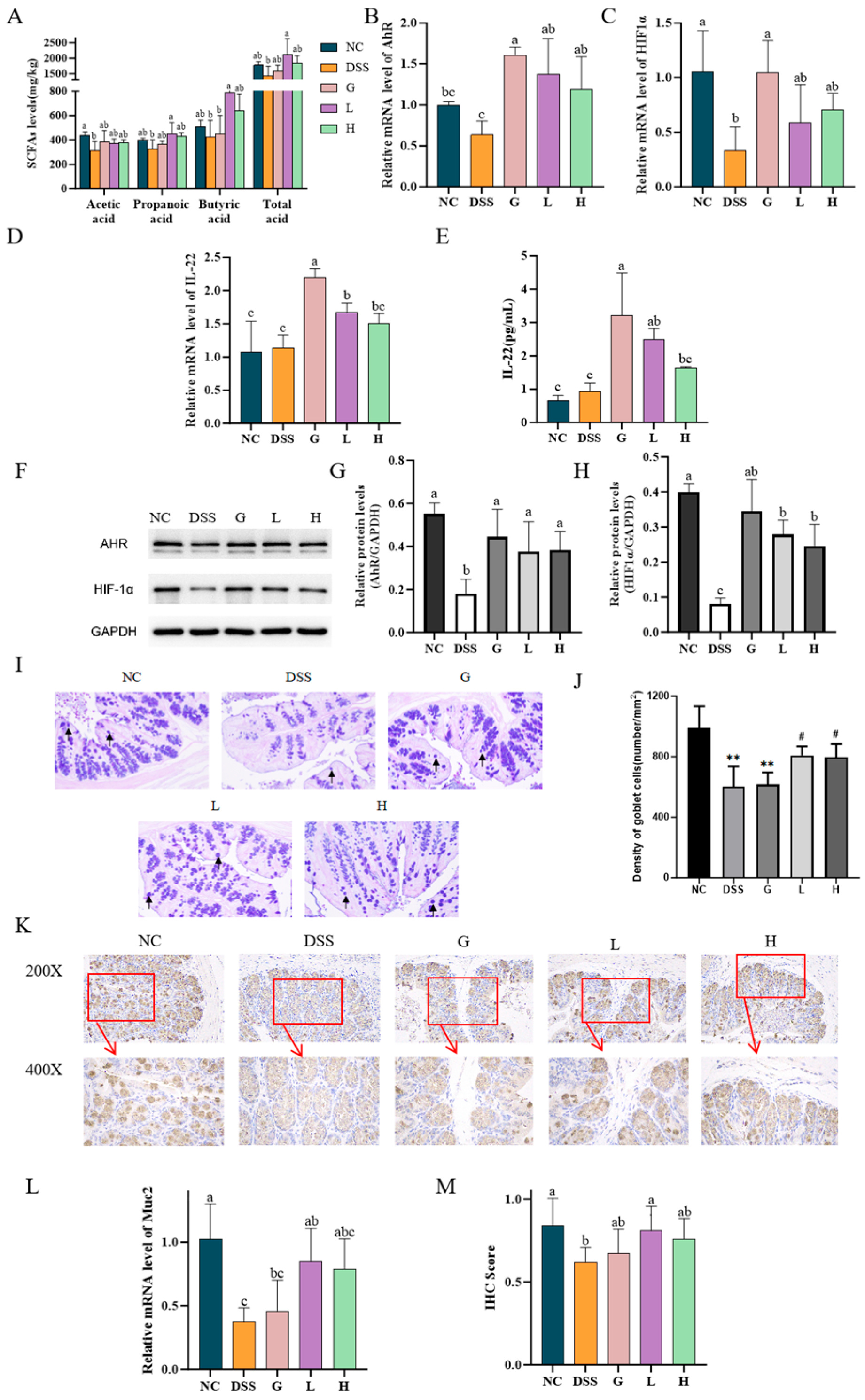
3.5. L. paracasei L21 and H-L21 Alleviate DSS-Induced Colitis Through SCFA-Mediated Activation of AhR/HIF1α, Induction of IL-22 Production
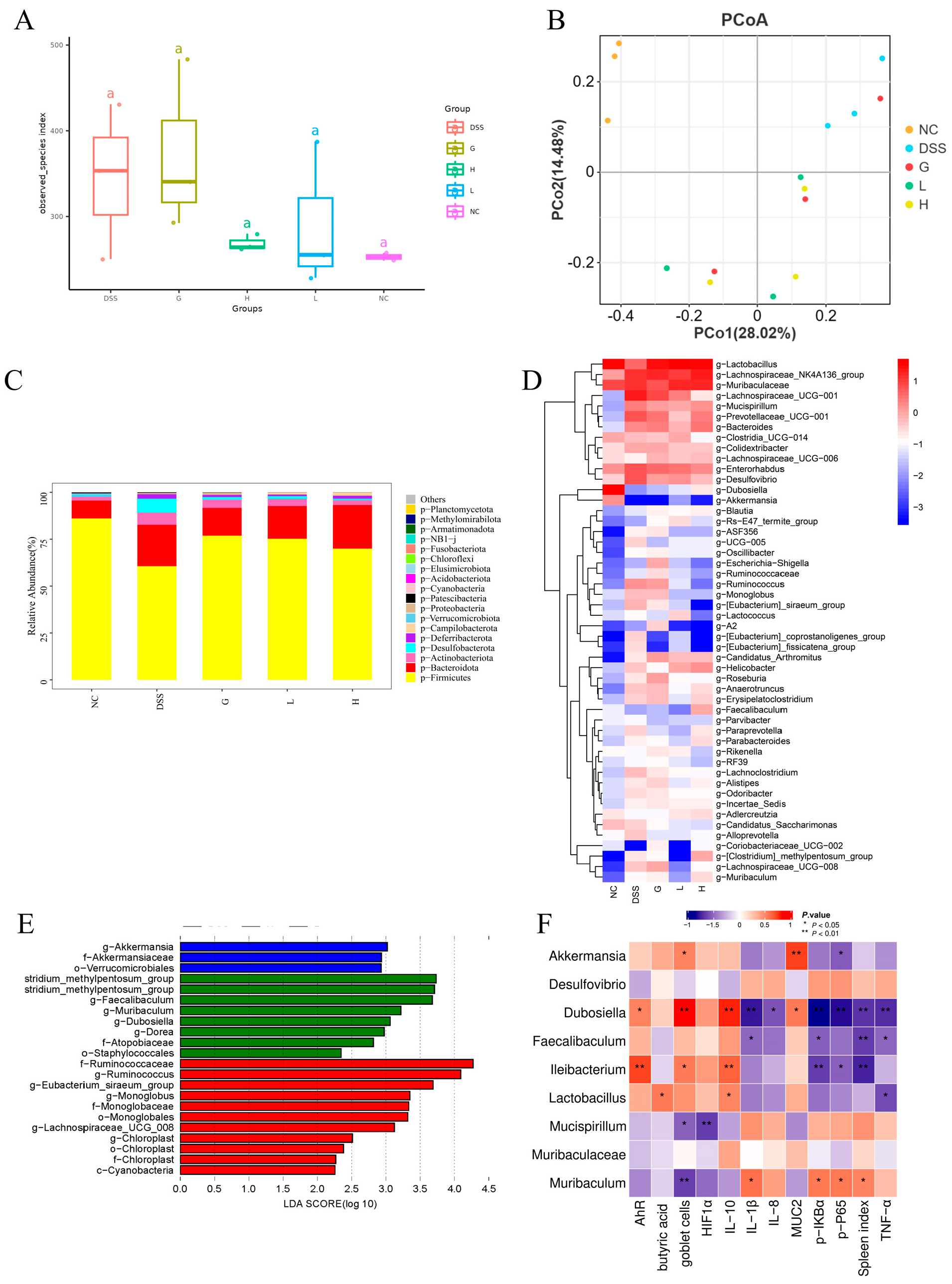
3.6. Lactobacillus paracasei L21 and H-L21 Reverse DSS-Induced Gut Microbiota Imbalance in UC Mice
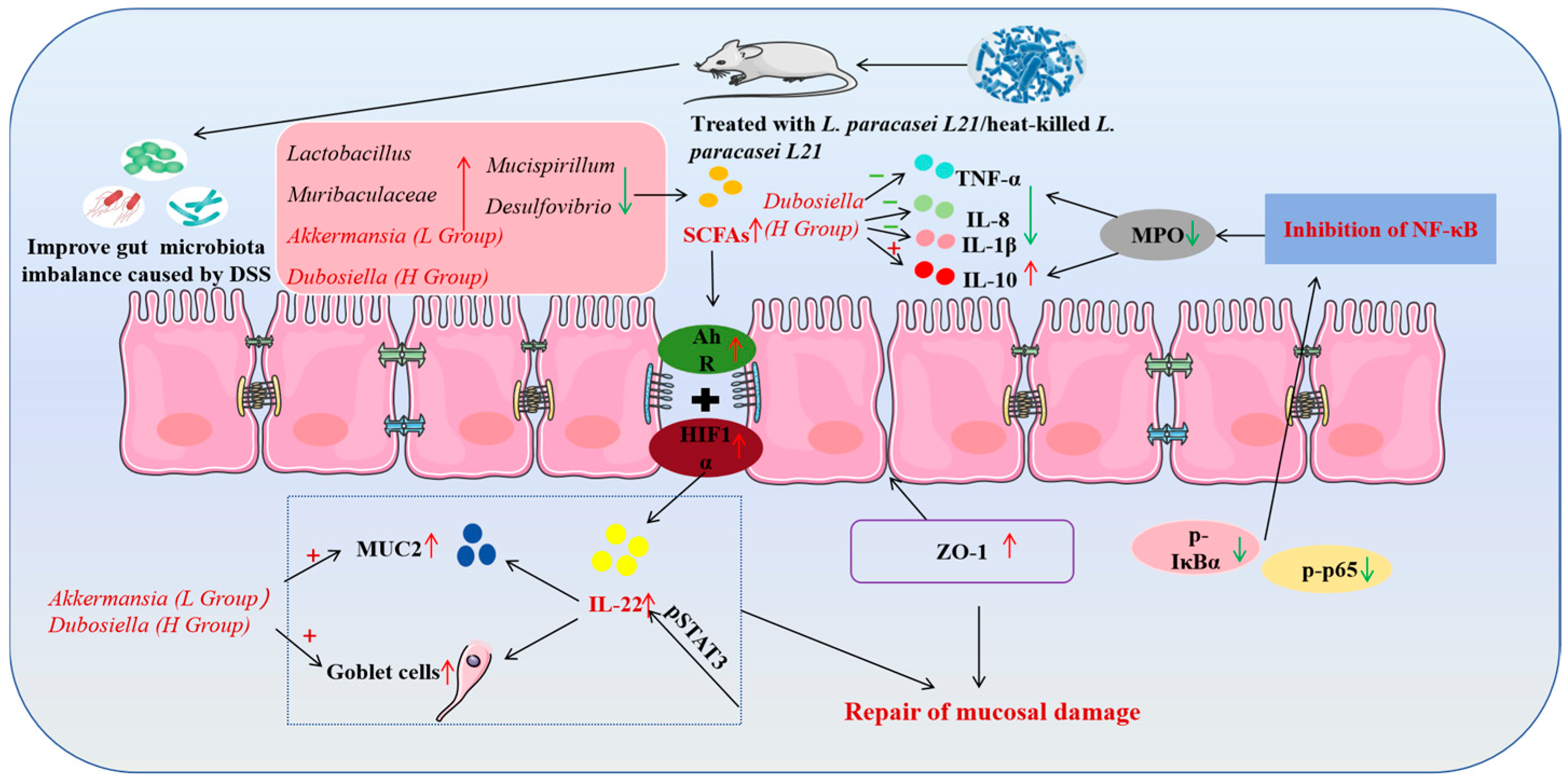
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Purpose | Gene Name | Primer Sequence (5′-3′) |
|---|---|---|
| Primer for 16SrRNA gene amplification | 16S rRNA (27F) | AGAGTTTGATCCTGGCTCAG |
| 16S rRNA (1492R) | CTACGGCTACCTTGTTACGA | |
| Primers for housekeeping gene amplification | pheS (F) | CAYCCNGCHCGYGAYATGC |
| pheS (R) | GGRTGRACCATVCCNGCHCC | |
| Sequences of RT-qPCR (intestinal barrier, cells) | ZO-1 | GCCAAGCCAGTCCATTCTCAGAG TCCATAGCATCATTTCGGGTTTCC |
| Occludin | CAACGGCAAAGTGAATGGCAAGAG TCATCCACGGACAAGGTCAGAGG | |
| Claudin-1 | CTTCTGGGTTTCATCCTGGCTTCG CCTGAGCAGTCACGATGTTGTCC | |
| Sequences of RT-qPCR (intestinal barrier, animals) | ZO-1 | CTTCTCTTGCTGGCCCTAAAC TGGCTTCACTTGAGGTTTCTG |
| Occludin | CACACTTGCTTGGGACAGAG TAGCCATAGCCTCCATAGCC | |
| Claudin-1 | TCTACGAGGGACTGTGGATG TCAGATTCAGCTAGGAGTCG | |
| MUC2 | TGGTCCAGGGTTTCTTACTCC TGATGAGGTGGCAGACAGGAGAC | |
| IL-22 and related signaling pathways | IL-22 | AACAGCAGGTCCAGTTCCCC GCTCCTGTCACA TCAGCGGT |
| AhR | TTGGTTGTGATGCCAAAGGGC CATGCGGATGTGGGATTCTGC | |
| HIF1α | AGCTTCTGTTA TGAGGCTCACC TGACTTGATGTTCATCGTCCTC | |
| Reference gene (cells) | β-actin | GTTGACATCCGTAAAGACCT CCACCAATCCACACAGAGTA |
| Reference gene (animals) | GAPDH | TCAAGAAGGTGGTGAAGCAG AAGGTGGAAGAGTGGGAGTTG |
| Ingredients | Energy Content |
|---|---|
| Protein (Casein) | 17.8% |
| Carbohydrates (dextrin, sucrose, cornstarch, cellulose) | 64.3% |
| Fats (soybean oil) | 7% |
| Water | 6.6% |
| Mineral (ash content) | 4.17% |
| Vitamin and amino acid complexes (Methionine, choline, vitamins, etc.) | 0.13% |
| Total | 100% |
| Energy (kcal/kg) | 3766 |
| Score | Weight Loss Rate (%) | Faecal Patterns | Occult Blood/Hematochezia |
|---|---|---|---|
| 0 | 0 | Normal | Normal feces |
| 1 | 1−5 | − | − |
| 2 | 5−10 | Loose but formed feces | Feces with a small amount of blood |
| 3 | 10−15 | − | − |
| 4 | >15 | Diarrhea | Feces with a large amount of blood |
| Purpose | Primary/Secondary Antibodies | Antibody Name | Dilution Ratio | Manufacturers |
|---|---|---|---|---|
| Immunofluorescence | Primary antibodies | ZO-1 | 1:1000 | Proteintech Group, Inc (Wuhan, China) |
| Primary antibodies | Occludin | 1:500 | Proteintech Group, Inc (Wuhan, China) | |
| Primary antibodies | Claudin1 | 1:200 | Affinity Biosciences (Cincinnati, OH, USA) | |
| Secondary antibodies | HRP-labelled rabbit anti-goat | 1:400 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP labelled goat anti-rabbit | 1:400 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP labelled goat anti-mouse | 1:400 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP labelled goat anti-rat | 1:200 | SeraCare (Milford, MA, USA) | |
| Immunohistochemistry | Primary antibodies | ZO-1 | 1:200 | Proteintech Group, Inc (Wuhan, China) |
| Primary antibodies | Occludin | 1:400 | Proteintech Group, Inc (Wuhan, China) | |
| Primary antibodies | Claudin1 | 1:100 | Affinity Biosciences (Cincinnati, OH, USA) | |
| Primary antibodies | MUC2 | 1:1000 | Guge Biological Technology (Wuhan, China) | |
| Secondary antibodies | HRP-labelled rabbit anti-goat | 1:100 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP-labelled goat anti-rabbit | 1:500 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP-labelled goat anti-mouse | 1:500 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP-labelled goat anti-rat | 1:500 | SeraCare (Milford, MA, USA) | |
| Secondary antibodies | HRP-labelled goat anti-rabbit-mouse universal secondary antibody | 1:1 | DAKO (Copenhagen, Denmark) | |
| Western Blot | Primary antibodies | GAPDH | 1:10000 | Proteintech (Chicago, IL, USA) |
| Primary antibodies | AHR | 1:5000 | Proteintech (Chicago, IL, USA) | |
| Primary antibodies | HIF-1α | 1:2000 | Bioss (Beijing, China) | |
| Primary antibodies | P65 | 1:1000 | ZenBioScience (Chengdu, China) | |
| Primary antibodies | p-P65 | 1:1000 | Cell Signaling Technology (MA, USA) | |
| Primary antibodies | p-IκBα | 1:2000 | Affinity Biosciences (Cincinnati, OH, USA) | |
| Secondary antibodies | HRP-goat anti rabbit | 1:50,000 | Seracare (Milford, MA, USA) | |
| Secondary antibodies | HRP-goat anti mouse | 1:50,000 | Seracare (Milford, MA, USA) |
References
- Danne, C.; Skerniskyte, J.; Marteyn, B.; Sokol, H. Neutrophils: From IBD to the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 184–197. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and its postbiotics alleviate dextran sulfate sodium-induced colitis in mice, association with modulating inflammation and intestinal microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yang, R.; Shi, J.; An, N.; Shan, S.; Li, Z.; Dong, X. Polyphenol from foxtail millet bran alleviates experimental colitis in mice by remodulating intestinal fungal community. Food Sci. Hum. Wellness 2024, 13, 3339–3350. [Google Scholar] [CrossRef]
- Guzzo, G.L.; Andrews, J.M.; Weyrich, L.S. The neglected gut microbiome: Fungi, protozoa, and bacteriophages in inflammatory bowel disease. Inflamm. Bowel Dis. 2022, 28, 1112–1122. [Google Scholar] [CrossRef]
- Zeng, W.; He, D.; Xing, Y.; Liu, J.; Su, N.; Zhang, C.; Wang, Y.; Xing, X. Internal connections between dietary intake and gut microbiota homeostasis in disease progression of ulcerative colitis: A review. Food Sci. Hum. Wellness 2021, 10, 119–130. [Google Scholar] [CrossRef]
- Vacca, M.; Sommella, E.M.; Liso, M.; Verna, G.; Scarano, A.; Sila, A.; Curlo, M.; Mastronardi, M.; Petroni, K.; Tonelli, C. Anthocyanins from purple corn affect gut microbiota and metabolome in inflammatory bowel disease patients under infliximab infusion: The SiCURA pilot study. Food Sci. Hum. Wellness 2024, 13, 3536–3543. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Gong, J.; Cao, L.; Xu, D.; Yu, Q.; Wang, X.; Chen, Y. Perceived stigma and self-efficacy of patients with inflammatory bowel disease-related stoma in China: A cross-sectional study. Front. Med. 2022, 9, 813367. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Feiqiao, B.Y.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153, e4114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Q.; Quan, K.-Y.; Feng, C.-J.; Zhang, T.; He, Q.-W.; Kwok, L.-Y.; Chen, Y.-F. The Lactobacillus gasseri G098 strain mitigates symptoms of DSS-induced inflammatory bowel disease in mice. Nutrients 2022, 14, 3745. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Pei, C.; Cai, K.; Huang, W.; Xiao, X.; Zhang, X.; Liang, R.; Chen, Y.; Xie, Z.; Li, P. Lactobacillus acidophilus and its metabolite ursodeoxycholic acid ameliorate ulcerative colitis by promoting Treg differentiation and inhibiting M1 macrophage polarization. Front. Microbiol. 2024, 15, 1302998. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, L.; Wei, S.; Chen, Z.; Ma, X. Effect of yeast Saccharomyces cerevisiae supplementation on serum antioxidant capacity, mucosal sIgA secretions and gut microbial populations in weaned piglets. J. Integr. Agric. 2017, 16, 2029–2037. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Buccigrossi, V.; Poeta, M.; Cioffi, V.; Terranova, S.; Nunziata, F.; Lo Vecchio, A.; Guarino, A. Lacticaseibacillus rhamnosus GG counteracts rotavirus-induced ion secretion and enterocyte damage by inhibiting oxidative stress and apoptosis through specific effects of living and postbiotic preparations. Front. Cell. Infect. Microbiol. 2022, 12, 854989. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Sabahi, S.; Homayouni Rad, A.; Aghebati-Maleki, L.; Sangtarash, N.; Ozma, M.A.; Karimi, A.; Hosseini, H.; Abbasi, A. Postbiotics as the new frontier in food and pharmaceutical research. Crit. Rev. Food Sci. Nutr. 2023, 63, 8375–8402. [Google Scholar] [CrossRef]
- Cuevas-González, P.; Liceaga, A.; Aguilar-Toalá, J. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Chung, I.-C.; OuYang, C.-N.; Yuan, S.-N.; Lin, H.-C.; Huang, K.-Y.; Wu, P.-S.; Liu, C.-Y.; Tsai, K.-J.; Loi, L.-K.; Chen, Y.-J. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Screening for potential novel probiotics with dipeptidyl peptidase IV-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front. Microbiol. 2020, 10, 2855. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, J.; Zhang, Y.; Li, X.; Zhang, N.; Liu, F.; Jiao, Y. In vitro hypoglycemic activities of Lactobacilli and Bifidobacterium strains from healthy children’s sources and their effect on stimulating GLP-1 secretion in STC-1 cells. Foods 2024, 13, 519. [Google Scholar] [CrossRef]
- Samba-Mondonga, M.; Constante, M.; Fragoso, G.; Calvé, A.; Santos, M.M. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLoS ONE 2019, 14, e0208677. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.H.J.; Akol, H.; Bloemena, E.; PEña, A.S.; Meuwissen, S.G.M.; Rees, E.P.V. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Mazhar, S.; Khokhlova, E.; Colom, J.; Simon, A.; Deaton, J.; Rea, K. In vitro and in silico assessment of probiotic and functional properties of Bacillus subtilis DE111®. Front. Microbiol. 2023, 13, 1101144. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Ali, S.A. Evaluation of probiotic properties of lactic acid bacteria isolated from dairy products. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; p. 112016. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Tsironi, T.; Tsantes, A.G.; Tsakali, E.; Van Impe, J.F.; Houhoula, D. Probiotic properties and antioxidant activity in vitro of lactic acid bacteria. Microorganisms 2023, 11, 1264. [Google Scholar] [CrossRef]
- Li, B.; Pan, L.-L.; Sun, J. Novel probiotic lactic acid bacteria were identified from healthy infant feces and exhibited anti-inflammatory capacities. Antioxidants 2022, 11, 1246. [Google Scholar] [CrossRef]
- Topçu, K.C.; Kaya, M.; Kaban, G. Probiotic properties of lactic acid bacteria strains isolated from pastırma. LWT 2020, 134, 110216. [Google Scholar] [CrossRef]
- Saito, K.; Tomita, S.; Nakamura, T. Aggregation of Lactobacillus brevis associated with decrease in pH by glucose fermentation. Biosci. Biotechnol. Biochem. 2019, 83, 1523–1529. [Google Scholar] [CrossRef]
- Yan, S.; Yin, L.; Dong, R. Inhibition of IEC-6 cell proliferation and the mechanism of ulcerative colitis in C57BL/6 mice by dandelion root polysaccharides. Foods 2023, 12, 3800. [Google Scholar] [CrossRef]
- Husien, H.M.; Peng, W.; Essa, M.O.A.; Adam, S.Y.; Ur Rehman, S.; Ali, R.; Saleh, A.A.; Wang, M.; Li, J. The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro. Animals 2024, 14, 3508. [Google Scholar] [CrossRef]
- Jeffrey, M.P.; Strap, J.L.; Jones Taggart, H.; Green-Johnson, J.M. Suppression of intestinal epithelial cell chemokine production by Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0389 is mediated by secreted bioactive molecules. Front. Immunol. 2018, 9, 2639. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, A.; Goettert, M.I.; de Souza, C.F.V. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, M.; McClements, D.J.; Liu, X.; Liu, F. Recent advances in the design and fabrication of probiotic delivery systems to target intestinal inflammation. Food Hydrocoll. 2022, 125, 107438. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Dai, L.; Jie, S.; Bi, S.; Qing, Q.; Chen, J.; Wang, L. Angiopoietin-2 silence alleviates lipopolysaccharide-induced inflammation, barrier dysfunction and endoplasmic reticulum stress of intestinal epithelial cells by blocking Notch signaling pathway. Bioengineered 2021, 12, 8116–8124. [Google Scholar] [CrossRef]
- Niu, M.-M.; Guo, H.-X.; Cai, J.-W.; Meng, X.-C. Bifidobacterium breve alleviates DSS-induced colitis in mice by maintaining the mucosal and epithelial barriers and modulating gut microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, J.-M.; Yang, F.-E.; Ji, X.-M.; Li, C.-Y.; Lv, S.-W.; Wang, S. A novel mediation strategy of DSS-induced colitis in mice based on an iron-enriched probiotic and in vivo bioluminescence tracing. J. Agric. Food Chem. 2020, 68, 12028–12038. [Google Scholar] [CrossRef]
- Hervé, J.-C.; Derangeon, M.; Sarrouilhe, D.; Bourmeyster, N. Influence of the scaffolding protein Zonula Occludens (ZOs) on membrane channels. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 595–604. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. CME Exam 1: The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function But Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Ahmad, W.; Din, A.U.; Khan, T.M.; Rehman, M.U.; Hassan, A.; Aziz, T.; Alharbi, M.; Wu, J. Lacticaseibacillus paracasei BNCC345679 revolutionizes DSS-induced colitis and modulates gut microbiota. Front. Microbiol. 2024, 15, 1343891. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Bhatia, R.; Sharma, S.; Bhadada, S.K.; Bishnoi, M.; Kondepudi, K.K. Lactic acid bacterial supplementation ameliorated the lipopolysaccharide-induced gut inflammation and dysbiosis in mice. Front. Microbiol. 2022, 13, 930928. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fan, Y.; Liu, K.; Lonan, P.; Liao, F.; Huo, Y.; Zhong, X.; Liang, Y.; Wang, Y.; Hou, S. Edible bird’s nest ameliorates dextran sulfate sodium-induced ulcerative colitis in C57BL/6J mice by restoring the Th17/Treg cell balance. Front. Pharmacol. 2021, 12, 632602. [Google Scholar] [CrossRef]
- Chen, M.; Yao, H.; Tan, H.; Huang, W.; Wu, Q.; Nie, S. Impact of Bifidobacterium longum NSP001 on DSS-induced colitis in conventional and humanised mice. Food Sci. Hum. Wellness 2023, 12, 1109–1118. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Zhang, R.; Si, D.; Petitte, J.N.; Ahmad, B.; Zhang, M. A novel peptide ameliorates LPS-induced intestinal inflammation and mucosal barrier damage via its antioxidant and antiendotoxin effects. Int. J. Mol. Sci. 2019, 20, 3974. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Germano, P.M.; Oh, S.; Wang, S.; Wang, J.; Lee, R.; Paige, H.; Yang, S.; Henning, S.M.; Zhong, J. Pomegranate Extract Improves Colitis in IL-10 Knockout Mice Fed a High Fat High Sucrose Diet. Mol. Nutr. Food Res. 2022, 66, 2100730. [Google Scholar] [CrossRef]
- Dai, C.; Zheng, C.-Q.; Meng, F.-j.; Zhou, Z.; Sang, L.-x.; Jiang, M. VSL# 3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell. Biochem. 2013, 374, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Spatz, M.; Da Costa, G.; Michaudel, C.; Lapiere, A.; Danne, C.; Agus, A.; Michel, M.-L.; Netea, M.G.; Langella, P. Deletion of both Dectin-1 and Dectin-2 affects the bacterial but not fungal gut microbiota and susceptibility to colitis in mice. Microbiome 2022, 10, 91. [Google Scholar] [CrossRef]
- Lu, Q.; Stappenbeck, T.S. Local barriers configure systemic communications between the host and microbiota. Science 2022, 376, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Park, J.-H.; Jung, H.K. Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J. Microbiol. Biotechnol. 2020, 30, 477. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B. Probiotic properties of Loigolactobacillus coryniformis NA-3 and in vitro comparative evaluation of live and heat-killed cells for antioxidant, anticancer and immunoregulatory activities. Foods 2023, 12, 1118. [Google Scholar] [CrossRef]
- Pyclik, M.J.; Srutkova, D.; Razim, A.; Hermanova, P.; Svabova, T.; Pacyga, K.; Schwarzer, M.; Górska, S. Viability status-dependent effect of Bifidobacterium longum ssp. longum CCM 7952 on prevention of allergic inflammation in mouse model. Front. Immunol. 2021, 12, 707728. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W. Probiotic consortia and their metabolites ameliorate the symptoms of inflammatory bowel diseases in a colitis mouse model. Microbiol. Spectr. 2022, 10, e0065722. [Google Scholar] [CrossRef]
- Kim, W.-K.; Jang, Y.J.; Seo, B.; Han, D.H.; Park, S.; Ko, G. Administration of Lactobacillus paracasei strains improves immunomodulation and changes the composition of gut microbiota leading to improvement of colitis in mice. J. Funct. Foods 2019, 52, 565–575. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis alleviates DSS-induced colitis by inflammatory cytokines and gut microbiota modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef]
- Van Zyl, W.F.; Deane, S.M.; Dicks, L.M. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, K.; Wu, Y.; Yang, Y.; Tso, P.; Wu, Z. Interactions between intestinal microbiota and host immune response in inflammatory bowel disease. Front. Immunol. 2017, 8, 942. [Google Scholar] [CrossRef]
- Xiang, X.; Jiang, Q.; Shao, W.; Li, J.; Zhou, Y.; Chen, L.; Deng, S.; Zheng, B.; Chen, Y. Protective effects of shrimp peptide on dextran sulfate sodium-induced colitis in mice. Front. Nutr. 2021, 8, 773064. [Google Scholar] [CrossRef]
- Thananimit, S.; Pahumunto, N.; Teanpaisan, R. Characterization of short chain fatty acids produced by selected potential probiotic Lactobacillus strains. Biomolecules 2022, 12, 1829. [Google Scholar] [CrossRef]
- Tralongo, P.; Tomasello, G.; Sinagra, E.; Damiani, P.; Leone, A.; Palumbo, V.D.; Giammanco, M.; Di Majo, D.; Damiani, F.; Abruzzo, A. The role of butyric acid as a protective agent against inflammatory bowel diseases. Euromediterr. Biomed. J. 2014, 9, 24–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.; Wang, Y.; Liang, L.; Zhang, Z.; Tan, X.; Tao, S.; Wu, Z.; Dong, M.; Zheng, J. Whole intestinal microbiota transplantation is more effective than fecal microbiota transplantation in reducing the susceptibility of DSS-induced germ-free mice colitis. Front. Immunol. 2023, 14, 1143526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.-Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- He, K.-Y.; Lei, X.-Y.; Wu, D.-H.; Zhang, L.; Li, J.-Q.; Li, Q.-T.; Yin, W.-T.; Zhao, Z.-L.; Liu, H.; Xiang, X.-Y. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef]
- Lécuyer, E.; Rakotobe, S.; Lengliné-Garnier, H.; Lebreton, C.; Picard, M.; Juste, C.; Fritzen, R.; Eberl, G.; McCoy, K.D.; Macpherson, A.J. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 2014, 40, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yeo, S.; Kang, S.; Huh, C.S. Longitudinal microbiome analysis in a dextran sulfate sodium-induced colitis mouse model. Microorganisms 2021, 9, 370. [Google Scholar] [CrossRef]
- Shenghua, P.; Ziqin, Z.; Shuyu, T.; Huixia, Z.; Xianglu, R.; Jiao, G. An integrated fecal microbiome and metabolome in the aged mice reveal anti-aging effects from the intestines and biochemical mechanism of FuFang zhenshu TiaoZhi (FTZ). Biomed. Pharmacother. 2020, 121, 109421. [Google Scholar] [CrossRef]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.; Gálvez, E.J.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Herp, S.; Durai Raj, A.C.; Salvado Silva, M.; Woelfel, S.; Stecher, B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Med. Microbiol. Immunol. 2021, 210, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Shi, F.; Zhang, X.; Zhang, Y.; Bi, K.; Chen, X.; Li, L.; Diao, H. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells. Gut Microbes 2022, 14, 2096994. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.; Wagenaar, G.T.; Garssen, J.; Folkerts, G.; Henricks, P.A. Pro-and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Yang, S.; Shang, J.; Liu, L.; Tang, Z.; Meng, X. Strains producing different short-chain fatty acids alleviate DSS-induced ulcerative colitis by regulating intestinal microecology. Food Funct. 2022, 13, 12156–12169. [Google Scholar] [CrossRef]
- Hou, Q.; Ye, L.; Liu, H.; Huang, L.; Yang, Q.; Turner, J.; Yu, Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Duarte-Silva, M.; de Barros Cardoso, C.R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Glal, D.; Sudhakar, J.N.; Lu, H.-H.; Liu, M.-C.; Chiang, H.-Y.; Liu, Y.-C.; Cheng, C.-F.; Shui, J.-W. ATF3 sustains IL-22-induced STAT3 phosphorylation to maintain mucosal immunity through inhibiting phosphatases. Front. Immunol. 2018, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, L.; Jiao, Y.; Lu, X.; Zhang, N.; Li, X.; Zheng, S.; Li, B.; Liu, F.; Zuo, P. Lacticaseibacillus paracasei L21 and Its Postbiotics Ameliorate Ulcerative Colitis Through Gut Microbiota Modulation, Intestinal Barrier Restoration, and HIF1α/AhR-IL-22 Axis Activation: Combined In Vitro and In Vivo Evidence. Nutrients 2025, 17, 2537. https://doi.org/10.3390/nu17152537
Chen J, Zhang L, Jiao Y, Lu X, Zhang N, Li X, Zheng S, Li B, Liu F, Zuo P. Lacticaseibacillus paracasei L21 and Its Postbiotics Ameliorate Ulcerative Colitis Through Gut Microbiota Modulation, Intestinal Barrier Restoration, and HIF1α/AhR-IL-22 Axis Activation: Combined In Vitro and In Vivo Evidence. Nutrients. 2025; 17(15):2537. https://doi.org/10.3390/nu17152537
Chicago/Turabian StyleChen, Jingru, Linfang Zhang, Yuehua Jiao, Xuan Lu, Ning Zhang, Xinyi Li, Suo Zheng, Bailiang Li, Fei Liu, and Peng Zuo. 2025. "Lacticaseibacillus paracasei L21 and Its Postbiotics Ameliorate Ulcerative Colitis Through Gut Microbiota Modulation, Intestinal Barrier Restoration, and HIF1α/AhR-IL-22 Axis Activation: Combined In Vitro and In Vivo Evidence" Nutrients 17, no. 15: 2537. https://doi.org/10.3390/nu17152537
APA StyleChen, J., Zhang, L., Jiao, Y., Lu, X., Zhang, N., Li, X., Zheng, S., Li, B., Liu, F., & Zuo, P. (2025). Lacticaseibacillus paracasei L21 and Its Postbiotics Ameliorate Ulcerative Colitis Through Gut Microbiota Modulation, Intestinal Barrier Restoration, and HIF1α/AhR-IL-22 Axis Activation: Combined In Vitro and In Vivo Evidence. Nutrients, 17(15), 2537. https://doi.org/10.3390/nu17152537






