Digital Microinterventions in Nutrition: Virtual Culinary Medicine Programs and Their Effectiveness in Promoting Plant-Based Diets—A Narrative Review
Abstract
1. Introduction
2. Methods
3. The “Food Is Medicine” Movement: Nutrition Prescribed as Treatment
4. Digital Nutrition Interventions
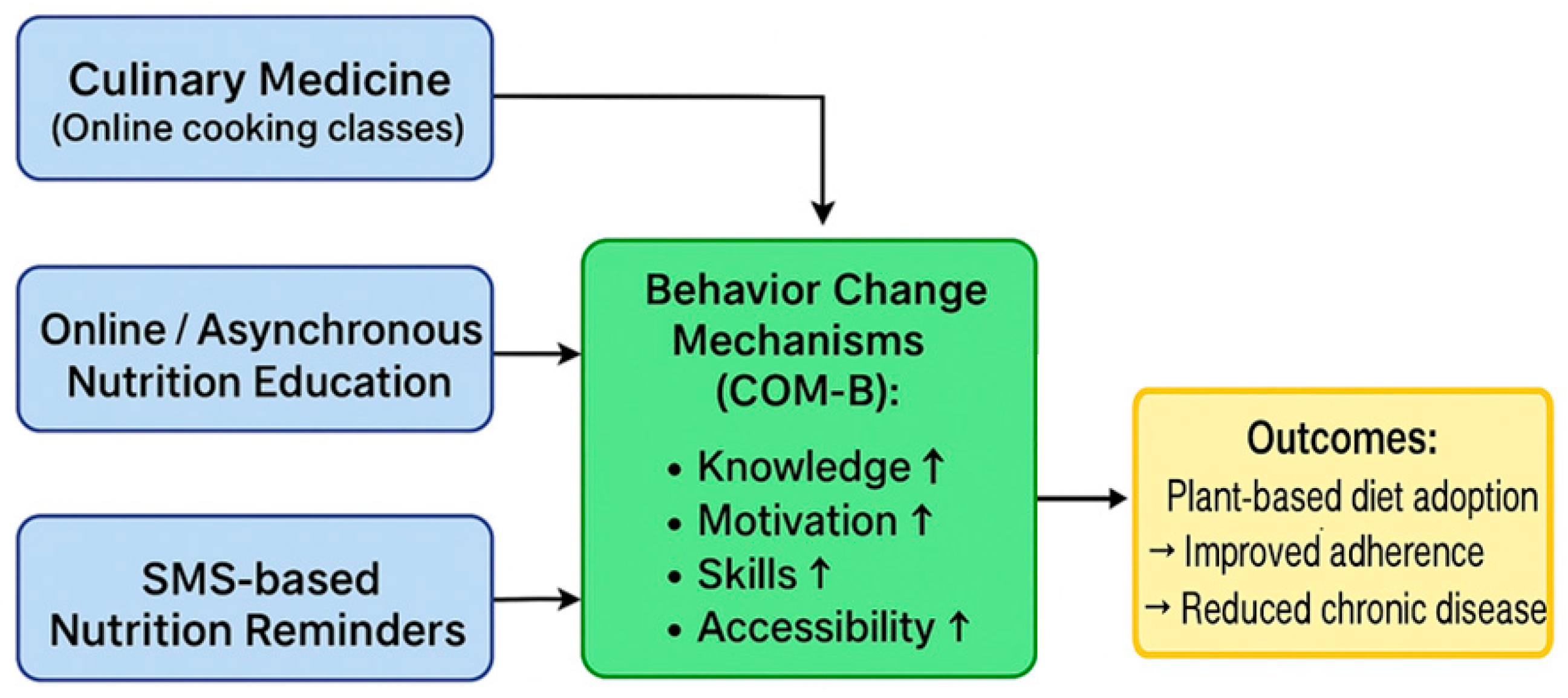
4.1. Online Cooking Courses/Culinary Medicine
4.2. Digital Nutrition Education
4.3. Conceptual Framework of Digital Nutrition Interventions
- Knowledge and literacy enhancement: Interactive content, educational videos and expert materials improve nutritional literacy.
- Motivation reinforcement: Goal setting, feedback, and social support sustain engagement.
- Skill development: Culinary medicine programs provide practical cooking skills, while short digital content (e.g., brief videos, app-based tips) facilitates home adaptation.
- Accessibility increase: Asynchronous online platforms, mobile technologies (apps, SMS), and web-based resources lower barriers to entry, especially for individuals facing geographic or temporal constraints.
5. Summary of Previous Research and Findings
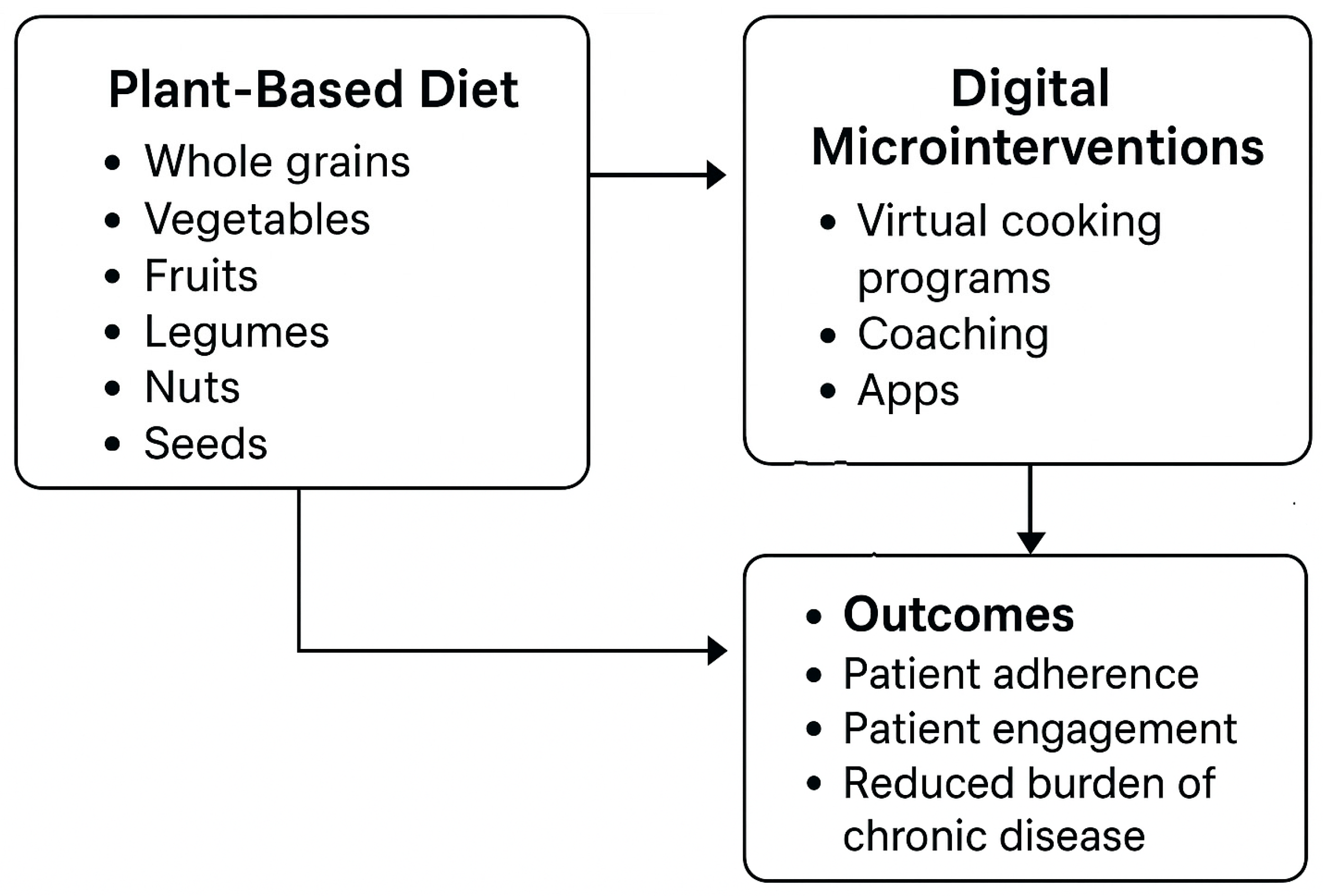
5.1. Promoting Plant-Based Diets in Healthy Populations
5.2. Digital Nutrition Interventions in Patients with Chronic Diseases
5.2.1. SMS- and Email-Based Digital Interventions and Dietary Adherence
5.2.2. Digital Nutrition Interventions Through Mobile Applications and Smartphone-Based Systems
5.2.3. Web-Based Platforms and e-Coaching
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Goni et al., 2020, Spain [85] | 720 adults post-catheter ablation for atrial fibrillation (365 intervention, 355 control); recruited from 4 hospitals | Baseline, 12, 24 months | 14-item MEDAS (phone-administered), semi-quantitative FFQ (dietitian-administered) | Multi-component remote program (website, app, printed materials, quarterly phone calls) with menus, tips, education, self-assessment tools | ↑ Mediterranean diet adherence; ↑ fruits, olive oil, whole grains, legumes, nuts, fish, white meat; ↓ refined cereals, red/processed meat, sweets; ↑ fiber and omega-3, ↓ carbohydrates and saturated fats | High retention (95.6% at 12 mo, 94.4% at 24 mo); improved lifestyle, PA, QoL, biomarkers; atrial tachyarrhythmia monitored |
| Hansel et al., 2017, France [81] | 120 adults (18–75 y) with T2DM and abdominal obesity; mean BMI 33, mean HbA1c 7.2%; 67% women | Baseline, 16 weeks | 24 h dietary recall via web-based module | Fully automated web-based e-coaching program (ANODE) with diet/PA self-monitoring, nutritional assessment, menu generator, PA prescription | ↑ DQI-I (+4.55 vs. −1.68; p < 0.001) | ↓ weight (−2.3 vs. −0.4 kg), ↓ waist circumference (−3.1 vs. −0.9 cm), ↓ HbA1c (−0.4% vs. −0.1%); ↑ VO2 max (NS) |
| Abu-Saad et al., 2019, Israel [82] | 50 overweight/obese Arab adults (40–62 y) with poorly controlled T2DM; 25 per arm (I-ACE vs. SLA) | Baseline, 3, 6, 12 months | FFQ, PA questionnaire, anthropometry | I-ACE software: interactive lifestyle assessment and education tool delivered during 4 dietitian-led in-person sessions | ↑ DM-related dietary knowledge; ↓ added sugar intake (−2.6% TEI); ↑ fiber intake (+2.7 g/1000 kcal) | Trends toward ↑ PA; ↓ HbA1c in both groups |
| Green et al., 2014, USA [91] | 101 adults (35–69 y); BMI> 26; elevated BP; Framingham 10-y CVD risk 10–25% | 6 months | 3-day food diary, self-reported fruit/vegetable intake, PA questionnaire | Dietitian-led web-based intervention: initial in-person visit, personalized plan, BP monitor, scale, pedometer, secure messaging | ↑ fruit and vegetable intake; adoption of DASH diet | ↓ weight (net −3.2 kg), improved BP control (NS), reduced CVD risk (trend), high satisfaction |
| Humalda et al., 2020, The Netherlands [86] | 99 adults with CKD (stages 1–4) or kidney transplant; mean eGFR 55 ± 22; sodium intake > 130 mmol/d | 3 months (end of intervention), 9 months (post-maintenance) | 24 h urinary sodium excretion | Web-based self-management program (SUBLIME): interactive diary, self-monitoring, goal setting, motivational e-coaching, group sessions | ↓ sodium excretion (−24.8 mmol/d vs. control; p = 0.049) at 3 months (effect attenuated by 9 months) | ↓ SBP (140→132 mmHg at 3 months); QoL, proteinuria, costs, self-management assessed (no long-term differences) |
| Kelly et al., 2020, Australia [72] | 80 adults with stage 3–4 CKD (mean age 62 ± 12; 64% male) | 3 months, 6 months | AHEI and exploratory dietary measures | Telehealth coaching: dietitian phone calls (biweekly, 3 mo) + tailored text messages, followed by 3 mo text-only support | No change in overall AHEI; improvements in vegetable, fiber, and core food group intake | ↓ weight; no effect on BP; intervention safe, no adverse events |
| Lewis et al., 2019, Australia [87] | 61 adults with class III obesity (BMI > 40), enrolled in public obesity management service | Baseline, 4, 8 months | Self-monitored dietary goals and behavior tracking | Telephone and SMS adjunct to standard care: monthly calls (10–30 min) + 3 texts/week | Improved dietary goal adherence | ↓ weight (−4.87 kg vs. +0.38 kg); ↑ self-efficacy, treatment regulation, adherence |
| Ramadas et al., 2018, Malaysia [84] | 128 adults with T2DM (HbA1c ≥ 7.0%); literate in English/Malay; internet access | Baseline, 6 months, follow-up | DKAB and DSOC questionnaires | Web-based program (myDIDeA): 6-month intervention with 12 modules, tailored dietary advice, fortnightly web access | ↑ DKAB scores (post: 11.1 vs. 6.5; follow-up: 19.8 vs. 7.6); ↑ DSOC | ↓ fasting glucose (7.9 vs. 8.9 mmol/L), ↓ HbA1c (8.5% vs. 9.1%) |
| Jahangiry et al., 2017, Iran [83] | 160 adults ≥20 y with metabolic syndrome (80 intervention, 80 control) | Baseline, 6 months | Self-reported food records, dietary questionnaires; PA (MET-min/week) | Web-based program “My Healthy Heart Profile”: tailored calorie restriction, CVD risk assessment, feedback, messaging | ↓ cholesterol (−88.4 vs. −8.3 mg/day), ↓ calories (−430 vs. −393 kcal/day), ↓ sodium (1337 vs. 1342 mmol/day); ↑ PA (moderate PA +260 vs. +102 MET-min/week) | ↑ HRQoL (general health, vitality); improvements in anthropometry, CVD risk factors |
| Lee et al., 2014, South Korea [88] | 59 breast cancer patients (stage 0–III) post-curative surgery; completed primary treatment within 12 months | Baseline, 12 weeks | 3-day dietary recall; DQI | Web-based self-management program (WSEDI) using TTM strategies (assessment, education, action planning, SMS feedback) | ↑ fruit/vegetable intake (≥5 servings/day); ↑ diet quality (DQI) | ↑ aerobic exercise (≥150 min/week); ↑ HRQoL; ↓ fatigue; ↑ self-efficacy |
| Russell et al., 2024, Australia [89] | Adults with MS (≥18 y); recruited via MSWA channels; English-speaking | Baseline, 9 weeks | Online surveys: DHQ, CNLT, FLBC | Online program “Eating Well with MS”: 7 asynchronous modules + mailed resources (recipes, workbook) | ↑ DHQ (dietary habits), ↑ CNLT (nutrition literacy), ↑ FLBC (food literacy behaviors) | Feasibility: high recruitment (n = 70), 54% completion, high acceptability |
| Russell et al., 2024, Australia [90] | 16 adults with MS (10 completed full program, 6 partial) | ~1 month post-program | Qualitative analysis (interviews, COM-B framework) | “Eating Well with MS” (asynchronous modules + resources) | Reported acquisition of nutrition knowledge, improved food literacy | Identified facilitators/barriers (social support, time, motivation); COM-B mapping confirmed impact on capability, opportunity, motivation |
5.2.4. Digital Education and the Benefits of Plant-Based Diets
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Ai et al., 2024, USA [33] | Low-income adults with type 2 diabetes (n not specified) | During sessions; follow-up not explicitly reported | Interactive videos, handouts; diabetes education metrics (knowledge, skills, self-efficacy) | Virtual Culinary Medicine Toolkit (VCMT): animated videos, infographics, recipes, interactive handouts; provider toolkit for standardized messaging | ↑ Food literacy, cooking skills, knowledge of MyPlate, carbohydrate management, and diabetes-related behaviors | ↑ Engagement, retention, self-efficacy for preparing healthy foods; ↑ perceived social support and normative beliefs |
| Kitaoka et al., 2013, Japan [94] | 71 hypertensive men (40–75 y); 39 intervention, 32 control | Baseline and post-intervention (duration not specified) | Dietary self-monitoring; urinary sodium and potassium excretion | Self-monitoring logbook with dietary education and cooking instructions | ↓ Sodium intake, ↑ potassium intake; improved sodium-to-potassium ratio | ↓ DBP (93→87 mmHg, significant); ↓ SBP (149→143 mmHg, NS); no changes in control |
| Krenek et al., 2025, USA [93] | 40 adults at risk for CVD (75% female, mean age 64.4 ± 8.6 y); ≥5% ASCVD risk; mostly college educated | Pre- and post-4-week diet interventions (crossover design) | Adherence to vegan diet; intake monitored via teaching kitchen sessions and self-report | Virtual culinary medicine teaching kitchen (8 weekly 90 min Zoom classes, group format) | Adherence to vegan diet (high vs. low EVOO); experiential cooking-based learning | ↓ Perceived stress (−19%), ↓ negative affect (−13%), ↑ positive affect (+6–8%); improved energy/fatigue and HRQoL |
| Macias-Navarro et al., 2024, USA [92] | Adults (18–70 y), ethnically diverse, T2DM with HbA1c > 7.0; recruited from community clinics; English/Spanish speakers | Baseline and post-intervention (5 sessions) | Questionnaires (dietary intake, cooking, shopping, self-management, barriers, knowledge); EMR (HbA1c, BMI, BP) | Virtual culinary medicine program: 5 × 90 min WebEx classes; bilingual delivery; asynchronous videos, handouts, culturally adapted recipes; grocery cards provided | ↑ Fruit/vegetable intake, ↑ healthy food consumption, ↑ cooking confidence, ↑ diabetes-related knowledge | Feasibility confirmed (recruitment, retention, satisfaction); trends in HbA1c, BMI, BP; ↑ diabetes self-management and self-efficacy |
5.2.5. Hybrid Programs Combining Community and Digital Elements
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Soto, A.; Guillen-Grima, F.; Morales, G.; Munoz, S.; Aguinaga-Ontoso, I. Trends in mortality from stroke in the European Union, 1996–2015. Eur. J. Neurol. 2021, 28, 182–191. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- World Health Organization. Advancing the Global Agenda on Prevention and Control of Noncommunicable Diseases 2000 to 2020: Looking Forwards to 2030; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Fekete, M.; Lehoczki, A.; Kryczyk-Poprawa, A.; Zábó, V.; Varga, J.T.; Bálint, M.; Fazekas-Pongor, V.; Csípő, T.; Rząsa-Duran, E.; Varga, P. Functional Foods in Modern Nutrition Science: Mechanisms, Evidence, and Public Health Implications. Nutrients 2025, 17, 2153. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Lehoczki, A.; Munkácsy, G.; Fekete, J.T.; Bianchini, G.; Ocana, A.; Buda, A.; Ungvari, A.; et al. Association between red and processed meat consumption and colorectal cancer risk: A comprehensive meta-analysis of prospective studies. Geroscience 2025, 47, 5123–5140. [Google Scholar] [CrossRef]
- Zábó, V.; Lehoczki, A.; Fekete, M.; Szappanos, Á.; Varga, P.; Moizs, M.; Giovannetti, G.; Loscalzo, Y.; Giannini, M.; Polidori, M.C.; et al. The role of purpose in life in healthy aging: Implications for the Semmelweis Study and the Semmelweis-EUniWell Workplace Health Promotion Model Program. Geroscience 2025, 47, 2817–2833. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Fekete, J.T.; Grosso, G.; Ungvari, A.; Gyorffy, B. Adherence to the Mediterranean diet and its protective effects against colorectal cancer: A meta-analysis of 26 studies with 2,217,404 participants. Geroscience 2025, 47, 1105–1121. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Menyhart, O.; et al. Impact of adherence to the Mediterranean diet on stroke risk. Geroscience 2025, 47, 3565–3581. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Jassal, D.S.; Ravandi, A.; Lehoczki, A. Dietary flaxseed: Cardiometabolic benefits and its role in promoting healthy aging. Geroscience 2025, 47, 2895–2923. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Alzahrani, A.A.; Maabreh, H.G.; Prasad, K.D.V.; Bokov, D.O.; Kareem, A.H.; Alawadi, A.; Ihsan, A.; Shakir, M.N.; Alasheqi, M.Q. Effect of walnut consumption on markers of endothelial function in adults: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2024, 38, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero-Peroni, B.; Diaz-Goni, V.; Beneit, N.; Oliveira, A.; Jimenez-Lopez, E.; Martinez-Vizcaino, V.; Mesas, A.E. Nut consumption is associated with a lower risk of all-cause dementia in adults: A community-based cohort study from the UK Biobank. Geroscience 2024, 47, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Sala-Vila, A.; Julvez, J.; Sabate, J.; Ros, E. Impact of Nut Consumption on Cognition across the Lifespan. Nutrients 2023, 15, 1000. [Google Scholar] [CrossRef]
- Ni, J.; Nishi, S.K.; Babio, N.; Ros, E.; Basterra-Gortari, F.J.; Corella, D.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J. Higher versus lower nut consumption and changes in cognitive performance over two years in a population at risk of cognitive decline: A cohort study. Am. J. Clin. Nutr. 2023, 118, 360–368. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Plant-Based Diets Reduce Blood Pressure: A Systematic Review of Recent Evidence. Curr. Hypertens. Rep. 2023, 25, 127–150. [Google Scholar] [CrossRef]
- Fekete, M.; Major, D.; Feher, A.; Fazekas-Pongor, V.; Lehoczki, A. Geroscience and pathology: A new frontier in understanding age-related diseases. Pathol. Oncol. Res. 2024, 30, 1611623. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. Geroscience 2025, 47, 3111–3130. [Google Scholar] [CrossRef]
- Talavera-Rodríguez, I.; Banegas, J.R.; de la Cruz, J.J.; Martínez-Gómez, D.; Ruiz-Canela, M.; Ortolá, R.; Hershey, M.S.; Artalejo, F.R.; Sotos-Prieto, M. Mediterranean lifestyle index and 24-h systolic blood pressure and heart rate in community-dwelling older adults. Geroscience 2024, 46, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortola, R.; Carballo-Casla, A.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. Plant-based diets and risk of frailty in community-dwelling older adults: The Seniors-ENRICA-1 cohort. Geroscience 2023, 45, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Lamberg-Allardt, C.; Bärebring, L.; Arnesen, E.K.; Nwaru, B.I.; Thorisdottir, B.; Ramel, A.; Söderlund, F.; Dierkes, J.; Åkesson, A. Animal versus plant-based protein and risk of cardiovascular disease and type 2 diabetes: A systematic review of randomized controlled trials and prospective cohort studies. Food Nutr. Res. 2023, 67, 9003. [Google Scholar] [CrossRef]
- Gardner, C.D.; Coulston, A.; Chatterjee, L.; Rigby, A.; Spiller, G.; Farquhar, J.W. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: A randomized trial. Ann. Intern. Med. 2005, 142, 725–733. [Google Scholar] [CrossRef]
- Thaiudom, S.; Posridee, K.; Liangchawengwong, S.; Chiaranai, C.; Chularee, S.; Samanros, A.; Oonsivilai, A.; Singha-Dong, N.; Oonsivilai, R. Plant-Based Diet for Glycemic Control, Insulin Sensitivity, and Lipid Profile in Type 2 Diabetes: A Systematic Review. Foods 2025, 14, 1919. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.P.; Vijayan, M. A review on gut microbiota and miRNA crosstalk: Implications for Alzheimer’s disease. Geroscience 2025, 47, 339–385. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O.W.; Reilly, J.M.; Wood, N.I.; Albin, J. Culinary medicine: Needs and strategies for incorporating nutrition into medical education in the United States. J. Med. Educ. Curric. Dev. 2024, 11, 23821205241249379. [Google Scholar] [CrossRef]
- Salas-Groves, E.; Galyean, S.; Alcorn, M.; Childress, A. Behavior change effectiveness using nutrition apps in people with chronic diseases: Scoping review. JMIR mHealth uHealth 2023, 11, e41235. [Google Scholar] [CrossRef]
- Chatterjee, A.; Prinz, A.; Gerdes, M.; Martinez, S. Digital interventions on healthy lifestyle management: Systematic review. J. Med. Internet Res. 2021, 23, e26931. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Dupuis, R.; Seligman, H.K. Food Is Medicine Movement-Key Actions Inside and Outside the Government. JAMA Health Forum 2023, 4, e233149. [Google Scholar] [CrossRef]
- Chang, A.R.; Bailey-Davis, L. Food Is Medicine, but Are Produce Prescriptions? Diabetes Care 2023, 46, 1140–1142. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aspry, K.E.; Garfield, K.; Kris-Etherton, P.; Seligman, H.; Velarde, G.P.; Williams, K.; Yang, E. “Food Is Medicine” Strategies for Nutrition Security and Cardiometabolic Health Equity: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 843–864. [Google Scholar] [CrossRef]
- Sommer, S.; Pelletier, A.; Roche, A.; Klein, L.; Dawes, K.; Hellerstein, S. Evaluation of dietary habits and cooking confidence using virtual teaching kitchens for perimenopausal women. BMC Public Health 2023, 23, 622. [Google Scholar] [CrossRef]
- Charles, J.A.; Wood, N.I.; Neary, S.; Moreno, J.O.; Scierka, L.; Brink, B.; Zhao, X.; Gielissen, K.A. “Zoom”ing to the Kitchen: A Novel Approach to Virtual Nutrition Education for Medical Trainees. Nutrients 2023, 15, 4166. [Google Scholar] [CrossRef]
- Krenek, A.M.; Aggarwal, M.; Chung, S.T.; Courville, A.B.; Guo, J.; Mathews, A. Plant-Based Culinary Medicine Intervention Improves Cooking Behaviors, Diet Quality, and Skin Carotenoid Status in Adults at Risk of Heart Disease Participating in a Randomized Crossover Trial. Nutrients 2025, 17, 1132. [Google Scholar] [CrossRef]
- Ai, D.; Heredia, N.I.; Cruz, V.; Guevara, D.C.; Sharma, S.V.; Woods, D.; Danho, M.; McWhorter, J.W. Development of a Culinary Medicine Toolkit to Improve Implementation of Virtual Cooking Classes for Low-Income Adults with Type 2 Diabetes. Healthcare 2024, 12, 343. [Google Scholar] [CrossRef]
- Domper, J.; Gayoso, L.; Goni, L.; Perezábad, L.; Razquin, C.; de la O, V.; Etxeberria, U.; Ruiz-Canela, M. An Intensive Culinary Intervention Programme to Promote Healthy Ageing: The SUKALMENA-InAge Feasibility Pilot Study. Nutrients 2024, 16, 1735. [Google Scholar] [CrossRef]
- Duan, Y.; Shang, B.; Liang, W.; Du, G.; Yang, M.; Rhodes, R.E. Effects of eHealth-based multiple health behavior change interventions on physical activity, healthy diet, and weight in people with noncommunicable diseases: Systematic review and meta-analysis. J. Med. Internet Res. 2021, 23, e23786. [Google Scholar] [CrossRef]
- Barnett, A.; Wright, C.; Stone, C.; Ho, N.Y.; Adhyaru, P.; Kostjasyn, S.; Hickman, I.J.; Campbell, K.L.; Mayr, H.L.; Kelly, J.T. Effectiveness of dietary interventions delivered by digital health to adults with chronic conditions: Systematic review and meta-analysis. J. Hum. Nutr. Diet. 2023, 36, 632–656. [Google Scholar] [CrossRef]
- El Khoury, C.F.; Karavetian, M.; Halfens, R.J.; Crutzen, R.; Khoja, L.; Schols, J.M. The effects of dietary mobile apps on nutritional outcomes in adults with chronic diseases: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2019, 119, 626–651. [Google Scholar] [CrossRef]
- Paramastri, R.; Pratama, S.A.; Ho, D.K.N.; Purnamasari, S.D.; Mohammed, A.Z.; Galvin, C.J.; Hsu, Y.-H.E.; Tanweer, A.; Humayun, A.; Househ, M.; et al. Use of mobile applications to improve nutrition behaviour: A systematic review. Comput. Methods Programs Biomed. 2020, 192, 105459. [Google Scholar] [CrossRef] [PubMed]
- Scarry, A.; Rice, J.; O’Connor, E.M.; Tierney, A.C. Usage of mobile applications or mobile health technology to improve diet quality in adults. Nutrients 2022, 14, 2437. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, H.; Reintjens, R.; Slipszenko, E.; Blok, S.; Somsen, G.; Tulevski, I.; Hofstra, L. Effectiveness of web-based personalised e-Coaching lifestyle interventions. Neth. Heart J. 2019, 27, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.L.; Salamanca-Sanabria, A.; Augsburger, M.; Frese, B.F.; Abend, S.; Jakob, R.; Kowatsch, T.; Haug, S. Effective behavior change techniques in digital health interventions for the prevention or management of noncommunicable diseases: An umbrella review. Ann. Behav. Med. 2023, 57, 817–835. [Google Scholar] [CrossRef]
- Young, C.; Campolonghi, S.; Ponsonby, S.; Dawson, S.L.; O’Neil, A.; Kay-Lambkin, F.; McNaughton, S.A.; Berk, M.; Jacka, F.N. Supporting Engagement, Adherence, and Behavior Change in Online Dietary Interventions. J. Nutr. Educ. Behav. 2019, 51, 719–739. [Google Scholar] [CrossRef]
- Alexander, G.L.; McClure, J.B.; Calvi, J.H.; Divine, G.W.; Stopponi, M.A.; Rolnick, S.J.; Heimendinger, J.; Tolsma, D.D.; Resnicow, K.; Campbell, M.K.; et al. A randomized clinical trial evaluating online interventions to improve fruit and vegetable consumption. Am. J. Public Health 2010, 100, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Buller, D.B.; Woodall, W.G.; Zimmerman, D.E.; Slater, M.D.; Heimendinger, J.; Waters, E.; Hines, J.M.; Starling, R.; Hau, B.; Burris-Woodall, P.; et al. Randomized trial on the 5 a day, the Rio Grande Way Website, a web-based program to improve fruit and vegetable consumption in rural communities. J. Health Commun. 2008, 13, 230–249. [Google Scholar] [CrossRef]
- Tapper, K.; Jiga-Boy, G.; Maio, G.R.; Haddock, G.; Lewis, M. Development and preliminary evaluation of an internet-based healthy eating program: Randomized controlled trial. J. Med. Internet Res. 2014, 16, e231. [Google Scholar] [CrossRef]
- Springvloet, L.; Lechner, L.; de Vries, H.; Candel, M.J.; Oenema, A. Short- and medium-term efficacy of a Web-based computer-tailored nutrition education intervention for adults including cognitive and environmental feedback: Randomized controlled trial. J. Med. Internet Res. 2015, 17, e23. [Google Scholar] [CrossRef]
- Springvloet, L.; Lechner, L.; de Vries, H.; Oenema, A. Long-term efficacy of a Web-based computer-tailored nutrition education intervention for adults including cognitive and environmental feedback: A randomized controlled trial. BMC Public Health 2015, 15, 372. [Google Scholar] [CrossRef] [PubMed]
- Franko, D.L.; Cousineau, T.M.; Trant, M.; Green, T.C.; Rancourt, D.; Thompson, D.; Ainscough, J.; Mintz, L.B.; Ciccazzo, M. Motivation, self-efficacy, physical activity and nutrition in college students: Randomized controlled trial of an internet-based education program. Prev. Med. 2008, 47, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lippke, S.; Corbet, J.M.; Lange, D.; Parschau, L.; Schwarzer, R. Intervention Engagement Moderates the Dose-Response Relationships in a Dietary Intervention. Dose Response 2016, 14, 1559325816637515. [Google Scholar] [CrossRef]
- Lange, D.; Richert, J.; Koring, M.; Knoll, N.; Schwarzer, R.; Lippke, S. Self-regulation prompts can increase fruit consumption: A one-hour randomised controlled online trial. Psychol. Health 2013, 28, 533–545. [Google Scholar] [CrossRef]
- Rodgers, R.F.; Pernal, W.; Matsumoto, A.; Shiyko, M.; Intille, S.; Franko, D.L. Capitalizing on mobile technology to support healthy eating in ethnic minority college students. J. Am. Coll. Health 2016, 64, 125–132. [Google Scholar] [CrossRef]
- Nour, M.; Chen, J.; Allman-Farinelli, M. Young adults’ engagement with a self-monitoring app for vegetable intake and the impact of social media and gamification: Feasibility study. JMIR Form. Res. 2019, 3, e13324. [Google Scholar] [CrossRef]
- Staffileno, B.A.; Tangney, C.C.; Fogg, L. Favorable Outcomes Using an eHealth Approach to Promote Physical Activity and Nutrition Among Young African American Women. J. Cardiovasc. Nurs. 2018, 33, 62–71. [Google Scholar] [CrossRef]
- Kothe, E.J.; Mullan, B.A. Factors affecting acceptability of an email-based intervention to increase fruit and vegetable consumption. BMC Public Health 2014, 14, 1020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livingstone, K.M.; Celis-Morales, C.; Navas-Carretero, S.; San-Cristobal, R.; Macready, A.L.; Fallaize, R.; Forster, H.; Woolhead, C.; O’donovan, C.B.; Cyril, M.; et al. Effect of an Internet-based, personalized nutrition randomized trial on dietary changes associated with the Mediterranean diet: The Food4Me Study. Am. J. Clin. Nutr. 2016, 104, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Alsabeeh, N.; Apovian, C.M.; Murphy, M.C.; Coffman, G.A.; Cullum-Dugan, D.; Jenkins, M.; Cabral, H. Weight, blood pressure, and dietary benefits after 12 months of a Web-based Nutrition Education Program (DASH for health): Longitudinal observational study. J. Med. Internet Res. 2008, 10, e52. [Google Scholar] [CrossRef] [PubMed]
- Glickman, O.; Kakaty-Monzo, J.; Roberts, M.; Daghigh, F. Exploring the effectiveness of virtual and in-person instruction in culinary medicine: A survey-based study. BMC Med. Educ. 2024, 24, 276. [Google Scholar] [CrossRef]
- Razavi, A.C.; Latoff, A.; Dyer, A.; Albin, J.L.; Artz, K.; Babcock, A.; Cimino, F.; Daghigh, F.; Dollinger, B.; Fiellin, M.; et al. Virtual teaching kitchen classes and cardiovascular disease prevention counselling among medical trainees. BMJ Nutr. Prev. Health 2023, 6, 6–13. [Google Scholar] [CrossRef]
- Shavit, Y.; Tepper, S.; Teodorescu, K. Exploring culinary diversity to enhance Mediterranean diet adherence: A randomized controlled trial. Appetite 2024, 201, 107597. [Google Scholar] [CrossRef]
- Ghammachi, N.; Mihrshahi, S.; Ronto, R. Web-based experiential nutrition education intervention “the green hub” to promote sustainable and healthy diets among young adults in Australia. Sustainability 2022, 14, 15207. [Google Scholar] [CrossRef]
- Peters, N.C.; Contento, I.R.; Kronenberg, F.; Coleton, M. Adherence in a 1-year whole foods eating pattern intervention with healthy postmenopausal women. Public Health Nutr. 2014, 17, 2806–2815. [Google Scholar] [CrossRef]
- Moreau, M.; Plourde, H.; Hendrickson-Nelson, M.; Martin, J. Efficacy of Nutrition Education-Based Cooking Workshops in Community-Dwelling Adults Aged 50 Years and Older. J. Nutr. Gerontol. Geriatr. 2015, 34, 369–387. [Google Scholar] [CrossRef]
- Diallo, A.F.; Falls, K.; Hicks, K.; McQueen Gibson, E.; Obaid, R.; Slattum, P.; Zanjani, F.; Price, E.; Parsons, P. The Healthy Meal Program: A food insecurity screening and referral program for urban dwelling older adults. Public Health Nurs. 2020, 37, 671–676. [Google Scholar] [CrossRef]
- Kwon, J.; Yoshida, Y.; Yoshida, H.; Kim, H.; Suzuki, T.; Lee, Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 263.e1–263.e8. [Google Scholar] [CrossRef]
- Lindsay, S.; Bellaby, P.; Smith, S.; Baker, R. Enabling healthy choices: Is ICT the highway to health improvement? Health 2008, 12, 313–331. [Google Scholar] [CrossRef]
- Delichatsios, H.K.; Hauser, M.E.; Burgess, J.D.; Eisenberg, D.M. Shared Medical Appointments: A Portal for Nutrition and Culinary Education in Primary Care-A Pilot Feasibility Project. Glob. Adv. Health Med. 2015, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Janko, R.K.; Haussmann, I.; Patel, A. The Effect of a Lecture-Based Educational Intervention to Improve the Nutrition Knowledge and Behavior of Plant-Based Seventh-Day Adventists Living in the United Kingdom. Health Sci. Rep. 2025, 8, e70440. [Google Scholar] [CrossRef] [PubMed]
- Akhu-Zaheya, L.M.; Shiyab, W.Y. The effect of short message system (SMS) reminder on adherence to a healthy diet, medication, and cessation of smoking among adult patients with cardiovascular diseases. Int. J. Med. Inform. 2017, 98, 65–75. [Google Scholar] [CrossRef]
- Cicolini, G.; Simonetti, V.; Comparcini, D.; Celiberti, I.; Di Nicola, M.; Capasso, L.M.; Flacco, M.; Bucci, M.; Mezzetti, A.; Manzoli, L. Efficacy of a nurse-led email reminder program for cardiovascular prevention risk reduction in hypertensive patients: A randomized controlled trial. Int. J. Nurs. Stud. 2014, 51, 833–843. [Google Scholar] [CrossRef]
- Donaldson, E.L.; Fallows, S.; Morris, M. A text message based weight management intervention for overweight adults. J. Hum. Nutr. Diet. 2014, 27 (Suppl. S2), 90–97. [Google Scholar] [CrossRef]
- Vinitha, R.; Nanditha, A.; Snehalatha, C.; Satheesh, K.; Susairaj, P.; Raghavan, A.; Ramachandran, A. Effectiveness of mobile phone text messaging in improving glycaemic control among persons with newly detected type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 158, 107919. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Conley, M.; Hoffmann, T.; Craig, J.C.; Tong, A.; Reidlinger, D.P.; Reeves, M.M.; Howard, K.; Krishnasamy, R.; Kurtkoti, J.; et al. A Coaching Program to Improve Dietary Intake of Patients with CKD: ENTICE-CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 330–340. [Google Scholar] [CrossRef]
- Dawson, J.; Campbell, K.L.; Craig, J.C.; Tong, A.; Teixeira-Pinto, A.; Brown, M.A.; Howard, K.; Howell, M.; Khalid, R.; Sud, K.; et al. A Text Messaging Intervention for Dietary Behaviors for People Receiving Maintenance Hemodialysis: A Feasibility Study of KIDNEYTEXT. Am. J. Kidney Dis. 2021, 78, 85–95.e1. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Nahar, N.; Banu, B.; Ali, L.; Sauerborn, R.; Souares, A. The influence of mobile phone-based health reminders on patient adherence to medications and healthy lifestyle recommendations for effective management of diabetes type 2: A randomized control trial in Dhaka, Bangladesh. BMC Health Serv. Res. 2020, 20, 520. [Google Scholar] [CrossRef]
- Islam, S.M.S.; George, E.S.; Maddison, R. Effectiveness of a mobile phone text messaging intervention on dietary behaviour in patients with type 2 diabetes: A post-hoc analysis of a randomised controlled trial. Mhealth 2021, 7, 10. [Google Scholar] [CrossRef]
- Choi, B.G.; Dhawan, T.; Metzger, K.; Marshall, L.; Akbar, A.; Jain, T.; Young, H.A.; Katz, R.J. Image-Based Mobile System for Dietary Management in an American Cardiology Population: Pilot Randomized Controlled Trial to Assess the Efficacy of Dietary Coaching Delivered via a Smartphone App Versus Traditional Counseling. JMIR Mhealth Uhealth 2019, 7, e10755. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.K.; Stephens, J.; Himmelfarb, C.R.D.; Stewart, K.J.; Hauck, S. Research Article Randomized Controlled Pilot Study Testing Use of Smartphone Technology for Obesity Treatment. J. Obes. 2013, 2013, 151597. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.J.; Park, J.I.; Jeon, H.J.; Oh, T.; Choi, H.J. Clinical efficacy and plausibility of a smartphone-based integrated online real-time diabetes care system via glucose and diet data management: A pilot study. Intern. Med. J. 2020, 50, 1524–1532. [Google Scholar] [CrossRef]
- Boels, A.M.; Vos, R.C.; Dijkhorst-Oei, L.-T.; Rutten, G.E. Effectiveness of diabetes self-management education and support via a smartphone application in insulin-treated patients with type 2 diabetes: Results of a randomized controlled trial (TRIGGER study). BMJ Open Diabetes Res. Care 2019, 7, e000981. [Google Scholar] [CrossRef]
- Dorsch, M.P.; Cornellier, M.L.; Poggi, A.D.; Bilgen, F.; Chen, P.; Wu, C.; An, L.C.; Hummel, S.L. Effects of a Novel Contextual Just-In-Time Mobile App Intervention (LowSalt4Life) on Sodium Intake in Adults with Hypertension: Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e16696. [Google Scholar] [CrossRef]
- Hansel, B.; Giral, P.; Gambotti, L.; Lafourcade, A.; Peres, G.; Filipecki, C.; Kadouch, D.; Hartemann, A.; Oppert, J.-M.; Bruckert, E.; et al. A Fully Automated Web-Based Program Improves Lifestyle Habits and HbA1c in Patients with Type 2 Diabetes and Abdominal Obesity: Randomized Trial of Patient E-Coaching Nutritional Support (The ANODE Study). J. Med. Internet Res. 2017, 19, e360. [Google Scholar] [CrossRef]
- Abu-Saad, K.; Murad, H.; Barid, R.; Olmer, L.; Ziv, A.; Younis-Zeidan, N.; Kaufman-Shriqui, V.; Gillon-Keren, M.; Rigler, S.; Berchenko, Y.; et al. Development and Efficacy of an Electronic, Culturally Adapted Lifestyle Counseling Tool for Improving Diabetes-Related Dietary Knowledge: Randomized Controlled Trial Among Ethnic Minority Adults With Type 2 Diabetes Mellitus. J. Med. Internet Res. 2019, 21, e13674. [Google Scholar] [CrossRef]
- Jahangiry, L.; Montazeri, A.; Najafi, M.; Yaseri, M.; Farhangi, M.A. An interactive web-based intervention on nutritional status, physical activity and health-related quality of life in patient with metabolic syndrome: A randomized-controlled trial (The Red Ruby Study). Nutr. Diabetes 2017, 7, e240. [Google Scholar] [CrossRef]
- Ramadas, A.; Chan, C.K.Y.; Oldenburg, B.; Hussein, Z.; Quek, K.F. Randomised-controlled trial of a web-based dietary intervention for patients with type 2 diabetes: Changes in health cognitions and glycemic control. BMC Public Health 2018, 18, 716. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.; de la O, V.; Barrio-López, M.T.; Ramos, P.; Tercedor, L.; Ibañez-Criado, J.L.; Castellanos, E.; Criao, A.I.; Ruiz, R.M.; Garcia-Bolao, I.; et al. A Remote Nutritional Intervention to Change the Dietary Habits of Patients Undergoing Ablation of Atrial Fibrillation: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e21436. [Google Scholar] [CrossRef] [PubMed]
- Humalda, J.K.; Klaassen, G.; de Vries, H.; Meuleman, Y.; Verschuur, L.C.; Straathof, E.J.M.; Laverman, G.D.; Bos, W.J.W.; van der Boog, P.J.; Vermeulen, K.M.; et al. A Self-management Approach for Dietary Sodium Restriction in Patients With CKD: A Randomized Controlled Trial. Am. J. Kidney Dis. 2020, 75, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Huang, H.C.; Hassmén, P.; Welvaert, M.; Pumpa, K.L. Adding Telephone and Text Support to an Obesity Management Program Improves Behavioral Adherence and Clinical Outcomes. A Randomized Controlled Crossover Trial. Int. J. Behav. Med. 2019, 26, 580–590. [Google Scholar] [CrossRef]
- Lee, M.K.; Yun, Y.H.; Park, H.A.; Lee, E.S.; Jung, K.H.; Noh, D.Y. A Web-based self-management exercise and diet intervention for breast cancer survivors: Pilot randomized controlled trial. Int. J. Nurs. Stud. 2014, 51, 1557–1567. [Google Scholar] [CrossRef]
- Russell, R.D.; Begley, A.; Daly, A.; Dunlop, E.; Mazahery, H.; Pham, M.N.; Grech, L.; Black, L.J. Feasibility of a co-designed online nutrition education program for people with multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 90, 105816. [Google Scholar] [CrossRef]
- Russell, R.D.; He, J.; Black, L.J.; Begley, A. Evaluating Experiences in a Digital Nutrition Education Program for People With Multiple Sclerosis: A Qualitative Study. Health Expect 2024, 27, e70012. [Google Scholar] [CrossRef]
- Green, B.B.; Anderson, M.L.; Cook, A.J.; Catz, S.; Fishman, P.A.; McClure, J.B.; Reid, R.J. e-Care for heart wellness: A feasibility trial to decrease blood pressure and cardiovascular risk. Am. J. Prev. Med. 2014, 46, 368–377. [Google Scholar] [CrossRef]
- Macias-Navarro, L.; McWhorter, J.W.; Guevara, D.C.; Bentley, S.S.; Sharma, S.V.; Torres, J.H.; Ai, D.; Heredia, N.I. A virtual culinary medicine intervention for ethnically diverse individuals with type 2 diabetes: Development of the Nourishing the Community through Culinary Medicine. Front. Nutr. 2024, 11, 1383621. [Google Scholar] [CrossRef] [PubMed]
- Krenek, A.M.; Aggarwal, M.; Chung, S.T.; Courville, A.B.; Farmer, N.; Guo, J.; Mathews, A. Influence of a Virtual Plant-Based Culinary Medicine Intervention on Mood, Stress, and Quality of Life Among Patients at Risk for Cardiovascular Disease. Nutrients 2025, 17, 1357. [Google Scholar] [CrossRef]
- Kitaoka, K.; Nagaoka, J.; Matsuoka, T.; Shigemura, C.; Harada, K.; Aoi, W.; Wada, S.; Asano, H.; Sakane, N.; Higashi, A. Dietary intervention with cooking instructions and self-monitoring of the diet in free-living hypertensive men. Clin. Exp. Hypertens. 2013, 35, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Domínguez, R.; García-Ortiz, L.; Patino-Alonso, M.C.; Sánchez-Aguadero, N.; Gómez-Marcos, M.A.; Recio-Rodríguez, J.I. Effectiveness of A Multifactorial Intervention in Increasing Adherence to the Mediterranean Diet among Patients with Diabetes Mellitus Type 2: A Controlled and Randomized Study (EMID Study). Nutrients 2019, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Lanari, C.; Barchiesi, L.; Casciari, E.; Tabascio, A.; Castellini, M.; Levorato, S.; Vannini, S.; Fornaciari, G.; Moretti, M.; et al. Effects of the “PreveDi” lifestyle modification trial on metabolic syndrome. Ann. Ig. 2015, 27, 595–606. [Google Scholar]
- Penn, L.; Ryan, V.; White, M. Feasibility, acceptability and outcomes at a 12-month follow-up of a novel community-based intervention to prevent type 2 diabetes in adults at high risk: Mixed methods pilot study. BMJ Open 2013, 3, e003585. [Google Scholar] [CrossRef]
- Shahar, S.; Adznam, S.N.; Lee, L.K.; Yusof, N.A.; Salleh, M.; Mohamed Sakian, N.I. A nutrition education intervention for anthropometric and biochemical profiles of rural older Malays with metabolic syndrome. Public Health Nurs. 2013, 30, 140–149. [Google Scholar] [CrossRef]
- Polom, J.; Boccardi, V. Employing Nutrition to Delay Aging: A Plant-Based Telomere-Friendly Dietary Revolution. Nutrients 2025, 17, 2004. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Lehoczki, A.; Fekete, J.T.; Ungvari, A.; Győrffy, B. Overweight and obesity significantly increase colorectal cancer risk: A meta-analysis of 66 studies revealing a 25–57% elevation in risk. Geroscience 2025, 47, 3343–3364. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Fehér, Á.; Szarvas, Z.; Kaposvári, C.; Horváth, K.; Lehoczki, A.; Tarantini, S.; Varga, J.T. The effectiveness of supplementation with key vitamins, minerals, antioxidants and specific nutritional supplements in COPD—A review. Nutrients 2023, 15, 2741. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Bálint, M.; Csizmadia, Z.; Tarantini, S.; Varga, J.T. The possible role of food and diet in the quality of life in patients with COPD—A state-of-the-art review. Nutrients 2023, 15, 3902. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Tarantini, S.; Fazekas-Pongor, V.; Csípő, T.; Csizmadia, Z.; Varga, J.T. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients 2023, 15, 5116. [Google Scholar] [CrossRef]
- Lehoczki, A.; Csípő, T.; Lipécz, Á.; Major, D.; Fazekas-Pongor, V.; Csík, B.; Mózes, N.; Fehér, Á.; Dósa, N.; Árva, D.; et al. Western Diet and Cognitive Decline: A Hungarian Perspective-Implications for the Design of the Semmelweis Study. Nutrients 2025, 17, 2446. [Google Scholar] [CrossRef]
- Madarász, B.; Fazekas-Pongor, V.; Szarvas, Z.; Fekete, M.; Varga, J.T.; Tarantini, S.; Csiszar, A.; Lionetti, V.; Tabák, A.G.; Ungvari, Z.; et al. Survival and longevity of European rulers: Geographical influences and exploring potential factors, including the Mediterranean diet—A historical analysis from 1354 to the twentieth century. GeroScience 2024, 46, 3801–3818. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition strategies promoting healthy aging: From improvement of cardiovascular and brain health to prevention of age-associated diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fazekas-Pongor, V.; Csiszar, A.; Kunutsor, S.K. The multifaceted benefits of walking for healthy aging: From Blue Zones to molecular mechanisms. Geroscience 2023, 45, 3211–3239. [Google Scholar] [CrossRef]
- Ungvari, Z.; Sonntag, W.E.; de Cabo, R.; Baur, J.A.; Csiszar, A. Mitochondrial protection by resveratrol. Exerc. Sport. Sci. Rev. 2011, 39, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tabak, A.G.; Adany, R.; Purebl, G.; Kaposvari, C.; Fazekas-Pongor, V.; Csípő, T.; Szarvas, Z.; Horváth, K.; Mukli, P.; et al. The Semmelweis Study: A longitudinal occupational cohort study within the framework of the Semmelweis Caring University Model Program for supporting healthy aging. Geroscience 2024, 46, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Zábó, V.; Lehoczki, A.; Buda, A.; Varga, P.; Fekete, M.; Fazekas-Pongor, V.; Moizs, M.; Giovannetti, G.; Loscalzo, Y.; Giannini, M.; et al. The role of burnout prevention in promoting healthy aging: Frameworks for the Semmelweis Study and Semmelweis-EUniWell Workplace Health Promotion Program. Geroscience 2025. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Alexander et al., 2010, USA [43] | n = 2540 adults (21–65 y), 5 health insurers, oversampled ethnic minorities | Baseline, 3, 6, 12 months | 16-item NCI FFQ, 2-item short questionnaire | Web-based MENU program: (1) control, (2) tailored web, (3) tailored web + email MI | FV intake + 2 servings/day in all arms; largest increase in arm 3 (+2.8 servings, p = 0.05) | High satisfaction, easy scalability, good acceptability |
| Buller et al., 2008, USA [44] | n = 755 adults, 65% Hispanic, 9% Native American, 88% female, rural | Baseline, 4 months | FFQ (All-Day Screener), single-item FV question | Web-based “5 a Day, the Rio Grande Way” site (recipes, tips, community info) | FV intake ↑ (FFQ: ns; single-item: significant ↑, OR = 1.84, p < 0.05) | Website usage low/variable; activity associated with FV increase |
| Lippke et al., 2016, Germany [49] | n = 701 adults, mean age 38 y, 81% female, high education | Baseline (T1), 1 week (T2), 1 month (T3) | Self-report, FV servings/day, planning scales | Internet-based action and coping planning modules (vs. active and waitlist control) | FV intake ↑ (T3); planning mediated change | Engagement moderated effect (inverse U); intervention clarified by moderated mediation |
| Moore et al., 2008, USA [56] | n = 2834 adults (EMC employees + family), 26% active at 12 months | Baseline, 12 months | Self-report (weight, BP, dietary logs); DASH 24 h recall validated vs. FFQ | Web-based DASH for Health program with weekly articles, emails, self-monitoring | FV intake ↑, soda ↓, grains ↓ | Overweight: −4.2 lbs; hypertensive: SBP −6.8 mmHg; dose–response with log-ins |
| Livingstone et al., 2016, 7 EU countries [55] | n = 1607 adults ≥18 y, 7 countries | Baseline, 3, 6 months | Online FFQ (157 items), MedDiet score (PREDIMED 14-point) | 6-month, 4-arm RCT (general advice vs. personalized nutrition [diet/phenotype/genotype]) | MedDiet score higher in PN group, highest in genotype-based PN | Baseline MedDiet score linked to BMI, physical activity; PN group showed moderate but significant improvement |
| Springvloet et al., 2015, The Netherlands [47] | n = 1349 adults (20–65 y), randomized (basic n = 456, plus n = 459, control n = 434) | Baseline, 9 months | Online questionnaire: FV, snacks, saturated fat, BMI, self-regulation | Web-based tailored nutrition education (basic: cognitive; plus: cognitive + environmental) | Basic: vegetable intake ↑ in low/medium educated (ES = 0.32); no effect in high-educated | Long-term effect limited; self-regulation change smaller in intervention than control |
| Lange et al., 2013, Germany [50] | n = 791; age M = 37.7 (14–79), 79% women, BMI M = 25.6, 70% college degree | Baseline & 1 week follow-up | Self-reported fruit intake (portions/day) | 1 h online self-regulation intervention with volitional prompts | ↑ Fruit consumption in intervention vs. control | Improved dietary planning and action control; brief intervention effective despite short duration |
| Tapper et al., 2014, UK [45] | n = 100; age M = 39, 82% female, BMI M = 27.7, 93% white | Baseline, 3, 6 months | Block Fat/Sugar/FV FFQ + BMI, WHR, HRV, IPAQ, alcohol & smoking questionnaires | Internet-based healthy eating program with 24 weekly sessions, reminders, gamified incentives | ↓ Saturated fat & added sugar intake, ↑ F&V intake | Improvements in BMI, WHR, HRV; adherence monitored; incentives improved participation |
| Franko et al., 2008, USA [48] | n = 476 undergraduates, 18–24 y, 6 US universities | Baseline, post-test, 3 & 6 months | FFQ, single-item F&V servings, Nutrition Knowledge Test | MyStudentBody.com-Nutrition, interactive web program + booster session | ↑ F&V intake (0.33 & 0.24 servings), ↑ nutrition knowledge | ↑ Motivation, self-efficacy, social support; attitude toward exercise improved |
| Springvloet et al., 2015, The Netherlands [46] | n = 1349 adults (20–65 y), general population | Baseline, 1 month, 4 months postintervention | Self-reported FV, high-energy snacks, saturated fat | Web-based computer-tailored nutrition education (basic vs. plus with environmental feedback) | FV intake ↑ (plus version), high-energy snacks ↓ (both), saturated fat ↓ (basic) | More effective than generic info, especially in high-educated; email reminders improved engagement |
| Lindsay et al., 2008, UK [65] | n = 108 adults (50–74 y) with CHD from deprived area | Baseline, 6 months | Self-reported diet (bad foods), alcohol, exercise, smoking, mental health, social support | Password-protected health portal with weekly sessions, phone support, forums | ↓ Frequency of “bad foods” | ↑ Health visits; slight improvements in diet, alcohol, smoke exposure; peer support engagement |
| Ghammachi et al., 2022, Australia [60] | n = 17 young adults (18–25 y) | Pre- and post-program (4 weeks) | Online surveys: knowledge, attitudes, practices, FV intake; Facebook engagement | 4-week web-based experiential program via private Facebook group (quizzes, videos, challenges) | Improved knowledge, attitudes, motivation; FV intake improved (pilot, no sig. testing) | Engagement data via Facebook; certificate of completion; prize draws |
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Rodgers et al., 2016, USA [51] | n = 43 minority female university students, BMI ≥ 25 and <21 subgroups | Baseline, 3 weeks, 10-week follow-up | Self-report (FV, SSB intake), baseline BMI | Mobile food photography + 3 daily motivational SMS | BMI ≥ 25: FV ↑; BMI < 21: fruit ↓, vegetables ↔; SSB ↔ | Short-term support for healthy eating; effect differed by BMI |
| Nour et al., 2019, Australia [52] | n = 97 young adults, mean 24.8 y, 49% adhered for 4 weeks | Baseline, 4 weeks | App logging (vegetable intake) + engagement data | Smartphone self-monitoring and goal-setting app ± gamification and/or Facebook support | App usage duration associated with ↑ vegetable intake (p < 0.001) | Gamification/social support had no direct effect; engagement higher in persistent users |
| Staffileno et al., 2018, USA [53] | n = 26 young African American women, 18–45 y, prehypertension | Baseline, 12 weeks | 6-item DASH screener, pedometer, BP, BMI | Web-based eHealth platform (12 modules, DASH vs. PA arm), mobile access + coaching | DASH group: DASH score ↑ (p = 0.001); large effect on FV and dairy intake | PA group: +39% steps, weight loss; engagement differed (71% vs. 48%) |
| Sommer et al., 2023, USA [30] | n = 609 peri- & postmenopausal women, mean age 58.8, 88% White | Pre- & post-intervention surveys | Self-reported weight, BMI, FFQ (FV, fish, beans, red meat, sugary drinks, grains), cooking confidence survey | NuCook virtual teaching kitchen, synchronous online classes with live cooking & nutrition discussion | ↑ FV, fish, beans; ↓ red meat, sugary drinks, white grains; small weight loss | ↑ Cooking self-efficacy & confidence; ↓ BMI in obese subgroup |
| Glickman et al., 2024, USA [57] | n = 360 osteopathic medical students (249 in-person, 111 virtual) | Post-course survey after 4-module culinary medicine course | Self-generated survey: knowledge (5 items), enjoyment (2 items), Likert scale | Virtual (Blackboard Collaborate) or in-person culinary medicine course | Knowledge ↑ in in-person group | Enjoyment ↑ in in-person group (Cohen’s d = 0.807); high satisfaction; reliability acceptable (Cronbach’s α: knowledge = 0.74, enjoyment = 0.77) |
| Charles et al., 2023, USA [31] | n = 80 physician assistant students, medical trainees | Pre-intervention, immediately post-intervention, 4 weeks post-intervention | Self-reported knowledge, attitudes, confidence, personal dietary behaviors | Interactive, single-session virtual curriculum (didactic + assessment/counseling + virtual culinary medicine via Zoom) | Knowledge ↑ (48.9%→78.9%, retained 75.8% at 4 weeks); FV counseling confidence ↑ | Attitudes improved on diet–disease reversal; scalable teaching kitchen; engagement via Zoom breakout rooms |
| Krenek et al., 2025, USA [32] | n = 40 adults, 75% female, age 64 ± 9 y, BMI 32 ± 7, ≥5% ASCVD risk | Baseline and post each 4-week diet period | ASA-24 Automated 24 h Dietary Recall, VeggieMeter® skin carotenoids | Weekly virtual vegan culinary medicine sessions via Zoom | ↑ Whole Plant Food Density, diet quality, vegan adherence | ↑ Cooking confidence, diet knowledge, perceived heart health control, CAFPAS; high satisfaction |
| Razavi et al., 2023, USA [58] | n = 1433 medical trainees (519 virtual culinary medicine, 914 standard nutrition), mean age 27, >50% women | Cross-sectional survey post-course | CHOP-MT survey: diet, MedDiet adherence, nutritional attitudes, competencies | Virtual culinary medicine (Zoom/WebEx), team-based cooking & discussions | MedDiet adherence ↑ (fruit intake OR 1.37) | ↑ Lifestyle medicine competencies (fiber OR 4.03, T2DM prevention OR 4.69, omega fatty acids OR 5.21, MedDiet counseling OR 5.73) |
| Kothe & Mullan, 2014, Australia [54] | n = 275 university students (≥18 y) | Baseline, 30 days | Self-reported FV intake (servings/day) | 30-day email intervention (daily vs. every 3-day messages) | FV intake ↑ in both groups | High-frequency messages perceived as excessive; acceptability moderated effect |
| Author, Year, Country | Population & Setting | Timeline | Measures | Intervention | Main Outcomes | Additional Findings |
|---|---|---|---|---|---|---|
| Diallo et al., 2020, USA [63] | 566 older adults (60% female, 81% African American, age 45–95, low-income) | Ongoing during program | USDA Six-Item Food Security, Lubben Social Network Scale | Healthy Meal Program: weekly congregate meals, home delivery, mobile market, 8-week Kitchen Clinic | ↑ Access to vegetables, ↑ fresh produce intake | ↓ Food insecurity, ↓ social isolation; strong engagement in education |
| Delichatsios et al., 2015, USA [66] | 70 adults with ≥1 cardiovascular risk factor, primary care | 17 SMA sessions over 4 years | Patient surveys (knowledge & satisfaction) | Shared Medical Appointments with cooking demos + nutrition education | ↑ Nutrition knowledge, ↑ cooking skills, improved dietary strategies | High satisfaction; cost-effective; labs and meds adjusted during sessions |
| Kwon et al., 2015, Japan [64] | 89 prefrail women ≥70 y | Baseline, 12 weeks, 6 months | Dietary variety score, cooking classes, HRQOL | Group-based exercise + nutrition program | ↑ Dietary variety, ↑ HRQOL domains | ↑ Handgrip, balance, walking speed |
| Peters et al., 2014, USA [61] | Healthy postmenopausal women, 50–72 y, BMI 18–30 | Baseline, 6 m, 12 m | Monthly 24 h food recalls, adherence score | Hands-on cooking + behavioral (social cognitive theory) | Significant adoption & maintenance of diet patterns | ↓ Non-adherence; psychosocial predictors important |
| Moreau et al., 2015, Canada [62] | 144 community-dwelling adults ≥50 y | Pre- & post-8 workshops | Elderly Nutrition Screening Q., session surveys | Cooking + nutrition workshops | ↑ Knowledge, confidence, ↑ intake of whole grains, F&V, milk alternatives | Confidence linked to healthier diet; autonomy unchanged |
| Shavit et al., 2024, Israel [59] | 211 adults, plant-based MedDiet (Fixed n = 95, Changing n = 116) | Baseline, 6 w, 3 m FU, 6 m FU | Food diversity, I-MEDAS, % plant foods | Weekly Zoom sessions + digital menus | Fixed menu: sustained MedDiet adherence, ↑ plant foods | High completion (97%); variety explored; taste ratings collected |
| Janko et al., 2025, UK [67] | 37 Seventh-day Adventists (vegan/veg/pesc.) | Pre, post, 4 w FU | 25-item nutrition knowledge test, follow-up survey | Single 30 min Zoom lecture by nutrition expert | ↑ Knowledge (8.5→20.0/25), ↑ supplement use | Behavior changes at 4 w; framed by Health Belief Model |
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Akhu-Zaheya & Shiyab, 2016, Jordan [68] | 160 adult outpatients with CVDs (≥18 y; excluded DM, renal, neurological disease) | Baseline, 3 months | MEDAS | SMS reminders (diet-focused) vs. placebo SMS vs. control | ↑ Mediterranean diet adherence (p < 0.001) | ↑ Medication adherence (p = 0.001); no effect on smoking |
| Dawson, 2021, Australia [73] | 130 adults on hemodialysis; ≥18 y; English-speaking | Baseline, 3, 6 months | 24 h recall, portion models, Foodworks | SMS (3/week; personalized 0–3 m, general 4–6 m) | No significant adherence to protein, K, P, Na guidelines; exploratory ↓ intake of these nutrients | Recruitment 48%, retention 88%; themes of acceptability; IDWG, electrolytes, binder use, QoL, healthcare use |
| Vinitha et al., 2019, India [71] | 248 adults with newly diagnosed T2DM, mean age 43.3 ± 8.7 y | Baseline, 3, 6, 12, 18, 24 months | 24 h recall, nutrient intake per NIN guidelines | 2–3 SMS/week on lifestyle + medication adherence | Promoted healthier dietary patterns (details not specified) | ↓ HbA1c, ↓ LDL, ↓ weight, ↓ waist, ↓ BP, ↑ QoL, ↑ PA; high acceptability |
| Yasmin et al., 2020, Bangladesh [74] | 320 adults with T2DM (160 I, 160 C; 273 completed) | Baseline, 12–16 months | Structured interviews; anthropometry & labs | Mobile phone interactive voice calls every 10 d + 24/7 call center | ↑ Adherence to dietary advice | ↑ Medication & exercise adherence, ↓ tobacco use, ↑ glycemic control |
| Cicolini et al., 2014, Italy [69] | 198 hypertensive adults, mean age 59 ± 14.5 y | Baseline, 1, 3, 6 months | Validated diet questionnaires, daily self-assessment, food group tables | Nurse-led weekly emails + phone follow-up | ↑ Fruit intake, ↓ obesity prevalence, ↑ adherence to low-salt, low-fat diet | ↓ BP, ↓ LDL, ↓ total cholesterol, ↓ TG, ↓ glucose; ↑ PA, ↑ med adherence, ↑ smoking cessation |
| Donaldson et al., 2014, UK [70] | 34 obese/overweight adults (BMI ≥ 30 or ≥28 + comorbidity); post-LEAP program | Pre- and post-12 weeks | Self-reported F&V & breakfast intake; step count; QoL questionnaires | SMS twice weekly; feedback loop with practitioner | ↑ F&V, ↑ breakfast intake, ↑ adherence to step goals | ↓ Weight (−1.6 kg), ↓ BMI (−0.6), ↓ waist (−2.2 cm), ↑ QoL, better follow-up |
| Islam et al., 2021, Bangladesh [75] | 236 adults with T2DM, ≤5 years since diagnosis, on oral meds | Baseline, 6 months | WHO STEPS survey + FFQ; weekly servings | Daily SMS × 6 months (diet, PA, meds, diabetes education) | No significant change in fruit/vegetable intake; ↓ sugared beverage intake (NS); ↑ sugar in tea (+0.94 tsp/w, p < 0.05) | HbA1c, BP, anthropometry measured; no major clinical effect; feasible |
| Kelly et al., 2020, Australia [72] | 80 adults with stage 3–4 CKD, mean age 62 ± 12 y | 3 months (end Phase 1), 6 months (end Phase 2) | AHEI; exploratory diet measures (veg, fiber, food groups) | Telehealth coaching: 3 m dietitian calls + SMS, then 3 m SMS only | No significant effect on AHEI; improved veg intake, fiber, core food groups | ↓ Weight; no BP change; safe, no adverse events |
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Choi, B.G. et al., 2019, USA [76] | 100 cardiology patients (mean age ~57, 60% male, 20–35% CAD) | Baseline, 1, 3, 6 months | Mediterranean Diet Score (MDS) | Smartphone app with asynchronous dietary counseling (custom app by Vibrent Health), 60 min RD interaction; SOC: 2 extra face-to-face sessions at 1 and 3 months | ↑ Adherence to Mediterranean diet over time in both EXP and SOC groups; no significant difference between groups | ↑ Weight loss (EXP 3.3 lbs vs. SOC 3.1 lbs, p = 0.04); ↑ diet satisfaction over time; BP, lipids, HbA1c, CRP showed no significant differences |
| Allen, J.K. et al., 2013, USA [77] | n = 68; 78% female; 49% African American; mean age 45 ± 11 years; BMI 34.3 ± 3.9 kg/m2 | Baseline and 6 months | 3-day food records analyzed via NDSR | Smartphone app (“Lose It!”) for self-monitoring diet, exercise, weight; delivered alone or with intensive/less intensive behavioral counseling | Trends toward improved dietary intake in counseling + smartphone groups (specifics not detailed) | BMI, waist circumference, physical activity, feasibility, acceptability, adherence to intervention |
| Ku, E.J. et al., 2020, South Korea [78] | 40 adults with T2DM (20 SC, 20 CC), aged 20–80; exclusion: cognitive impairment, inability to use smartphone, medications affecting glucose control | Baseline and 12 weeks | Dietary logging via Noom Coach app (SC group), SDSCA questionnaire (all participants) | Smartphone-based integrated online real-time diabetes care system: Noom Coach app for dietary logging, CareSens N NFC glucose meter, individualized text feedback, social network support (SC group) | ↑ Self-reported dietary management (general diet, specific diet) in SC vs. CC; SDSCA scores increased in both groups | Glycaemic control: higher proportion achieving A1C < 6.5% in SC vs. CC (47.1% vs. 11.1%, p = 0.019); improvements in blood glucose testing and foot care; no major adverse events reported |
| Boels, A.M. et al., 2019, The Netherlands [79] | 228 adults with T2DM, aged 40–70, on insulin ≥3 months, HbA1c > 7%; intervention n = 114, control n = 114 | Baseline, 6 months follow-up, additional 3-month sustainability follow-up (intervention group) | FFQ | Smartphone app delivering unidirectional evidence-based messages on diet, physical activity, hypoglycaemia prevention, and glucose variability; frequency, topics, and duration tailored by patient (6–9 months) | Not explicitly reported yet; app targeted dietary habits and self-management behaviors | Primary: HbA1c and % achieving HbA1c ≤ 7% without hypoglycaemia; Secondary: BMI, waist circumference, insulin dose, lipid profile, BP, hypoglycaemic events, glycaemic variability, self-management (SDSCA), physical activity, health status, diabetes-dependent QoL, treatment satisfaction, cost-effectiveness, sustainability |
| Dorsch, M.P. et al., 2020, USA [80] | 50 adults ≥ 18 y, hypertensive, iPhone users; excluded CKD, heart failure, severe HTN, insulin-treated diabetes, loop diuretics, corticosteroids, NSAIDs | Baseline and week 8 | 24 h urinary sodium (spot & collection), FFQ, ASA24 24-h dietary recall, sodium screener | Just-in-time adaptive mobile app (LowSalt4Life) with push notifications, geolocation-based suggestions, low-sodium food alternatives, restaurant/grocery search | ↓ Sodium intake (spot urine: App −462 mg vs. No App +381 mg, p = 0.03; FFQ: App −1553 mg vs. No App −515 mg, p = 0.01) | BP: App −7.5 mmHg vs. No App −0.7 mmHg (p = 0.12); Self-confidence in following low-sodium diet: no significant difference |
| Author, Year, Country | Sample Demographics | Timing of Outcome Assessment | Method of Nutrition Assessment | Type of Digital Intervention | Nutritional Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Alonso-Domínguez et al., 2019, Spain [95] | 204 adults with T2DM (25–70 yrs, mean 60.6; excluded CVD, musculoskeletal, neuropsychological disease) | Baseline, 3 months, 12 months | MEDAS questionnaire, Diet Quality Index (DQI) | Multifactorial: smartphone app (EVIDENT II) for 3 months; 90 min food workshop; weekly heart-healthy walks (5 weeks) | ↑ Adherence to Mediterranean diet (ΔMEDAS +2.2 at 3 months, sustained at 12 months); ↑ Diet quality (ΔDQI +2.5 at 3 months) | ↑ Physical activity (weekly walks attendance 80–90%); ↑ app engagement (days of use recorded); biomedical parameters monitored (glucose, HbA1c) |
| Villarini et al., 2015, Italy [96] | 186 adults aged ≥45, community pharmacy volunteers | Baseline, 6 months | Self-reported adherence to healthy diet; measured anthropometrics and clinical parameters in pharmacies | Lifestyle intervention with digital support: SMS reminders for conferences, cooking classes, physical activity sessions | Slight increase in adherence to healthy diet in males; no significant changes in diet-related biomarkers | Significant reductions in weight, BMI, total cholesterol; no significant change in waist circumference, BP, fasting glucose, triglycerides; metabolic syndrome prevalence decreased non-significantly in women; session attendance low |
| Penn et al., 2013, UK [97] | 218 adults aged 45–65 at high risk of T2D (FINDRISC ≥ 11), socioeconomically deprived area; 134 completed follow-up (61%) | Baseline, 6 months, 12 months | Self-report dietary questionnaire on fruit, vegetable, bread, milk, fat consumption; aligned with dietary advice | Ongoing support via mobile text messages, emails; newsletters with information and recipes | ↑ Adherence to healthy eating; use of healthy cooking demonstrated in sessions | Weight decreased by 5.7 kg (men) and 2.8 kg (women); waist circumference decreased 7.2 cm (men) and 6.0 cm (women); PA level increased 7.9 MET h/day (men) and 6.7 MET h/day (women); high intervention acceptability and retention |
| Shahar et al., 2013, Malaysia [98] | 47 older Malays with metabolic syndrome (60–75 yrs; 24 intervention, 23 control; 50.6% men, 49.4% women) | Baseline, 6 months | Anthropometric measurements (weight, waist); dietary counselling using culturally tailored materials | Group-based nutrition education sessions with flipcharts, booklets, placemats, cooking & exercise demonstrations (interactive visual aids, print materials) | Women: ↓ waist circumference; Men: maintained total cholesterol | Fasting blood glucose, lipid profile, BP, CRP; adherence/compliance assessed weekly then monthly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zábó, V.; Lehoczki, A.; Varga, J.T.; Szappanos, Á.; Lipécz, Á.; Csípő, T.; Fazekas-Pongor, V.; Major, D.; Fekete, M. Digital Microinterventions in Nutrition: Virtual Culinary Medicine Programs and Their Effectiveness in Promoting Plant-Based Diets—A Narrative Review. Nutrients 2025, 17, 3310. https://doi.org/10.3390/nu17203310
Zábó V, Lehoczki A, Varga JT, Szappanos Á, Lipécz Á, Csípő T, Fazekas-Pongor V, Major D, Fekete M. Digital Microinterventions in Nutrition: Virtual Culinary Medicine Programs and Their Effectiveness in Promoting Plant-Based Diets—A Narrative Review. Nutrients. 2025; 17(20):3310. https://doi.org/10.3390/nu17203310
Chicago/Turabian StyleZábó, Virág, Andrea Lehoczki, János Tamás Varga, Ágnes Szappanos, Ágnes Lipécz, Tamás Csípő, Vince Fazekas-Pongor, Dávid Major, and Mónika Fekete. 2025. "Digital Microinterventions in Nutrition: Virtual Culinary Medicine Programs and Their Effectiveness in Promoting Plant-Based Diets—A Narrative Review" Nutrients 17, no. 20: 3310. https://doi.org/10.3390/nu17203310
APA StyleZábó, V., Lehoczki, A., Varga, J. T., Szappanos, Á., Lipécz, Á., Csípő, T., Fazekas-Pongor, V., Major, D., & Fekete, M. (2025). Digital Microinterventions in Nutrition: Virtual Culinary Medicine Programs and Their Effectiveness in Promoting Plant-Based Diets—A Narrative Review. Nutrients, 17(20), 3310. https://doi.org/10.3390/nu17203310






