Dairy-Gut Microbiome Interactions: Implications for Immunity, Adverse Reactions to Food, Physical Performance and Cardiometabolic Health—A Narrative Review
Abstract
1. Introduction
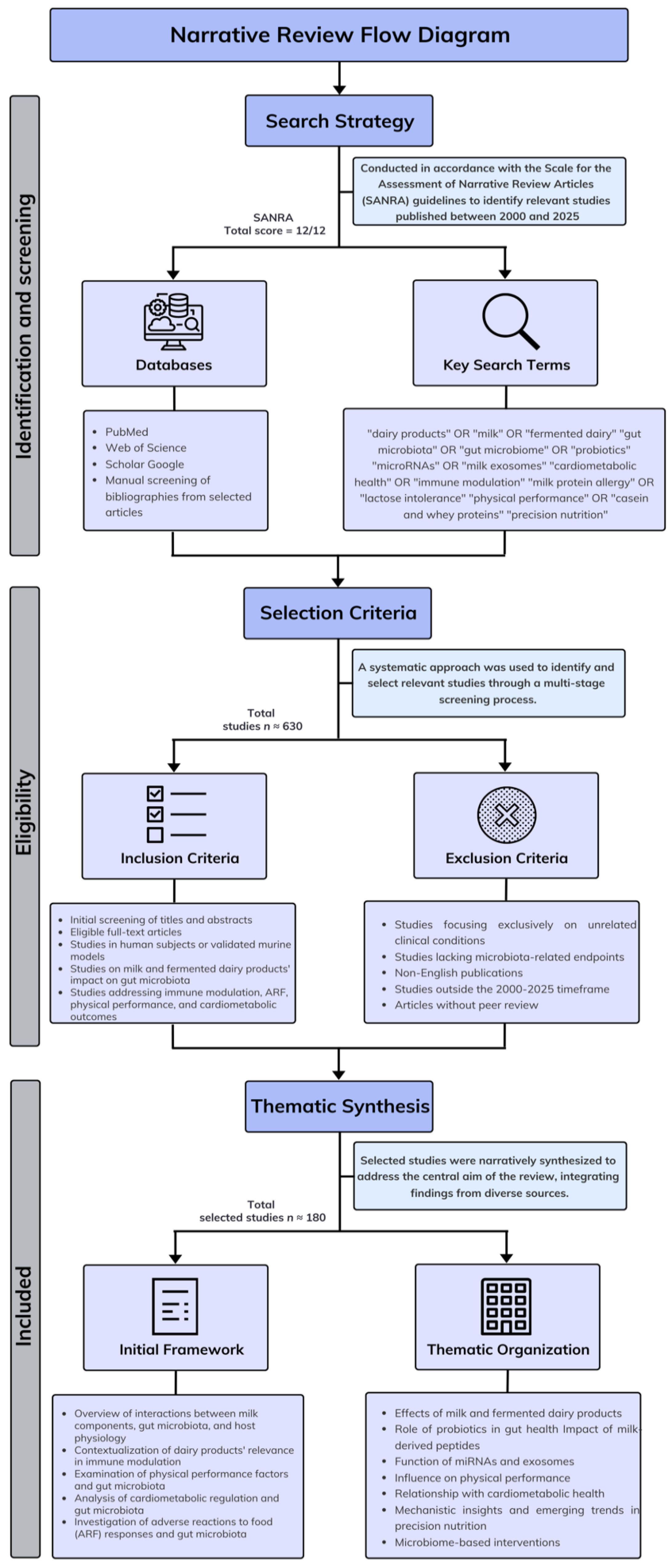
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Process
2.3. Data Synthesis
2.4. Exclusion Criteria
2.5. Study Quality
3. Results
3.1. Nutritional and Functional Composition of Milk and Fermented Dairy Products
3.1.1. Macronutrients and Micronutrients
Carbohydrates
Proteins
| Proteins | Concentration (g/L) | Biological Function | References |
|---|---|---|---|
| Caseins | |||
| α-casein | 13 | Antioxidant, mineral transport | [32] |
| β-casein | 9.3 | Antioxidant, antihypertensive, mineral transport | [32] |
| κ-casein | 3.5 | Mineral transport, inhibition of platelet aggregation | [32] |
| Total whey proteins | |||
| β-lactoglobulin | 7.5 | Affinity for retinol and fatty acids, antioxidant | [33] |
| α-lactoalbumin | 1.2 | Calcium transport, immunomodulatory function, lactose production | [34] |
| Inmunoglobulin G1 | 9 | Immune protection | [35] |
| Albumin | 30 | Transport | [33] |
| Lactoferrin | 0.2 | Antimicrobial, iron absorption, antihypertensive | [36] |
| Lactoperoxidase | 0.03 | Antimicrobial | [37] |
| Lisozime | 0.0004 | Antimicrobial, synergy with immunoglobulins and lactoferrin | [38] |
Lipids
Vitamins and Minerals
3.1.2. Other Bioactive Compounds
Milk Fat Globule Membrane
Milk-Derived Exosomes
Milk-Derived microRNAs
3.1.3. Fermented and Processed Dairy Products: Microbial and Nutritional Profiles
| Product | Shelf Life (4 °C) | Fermentation Microorganism | Description | References |
|---|---|---|---|---|
| Cheese | months | Lactococcus, Lactobacillus, Streptococcus, Propionibacter | Fermented solid food product obtained from curdled milk of ruminant animals | [85,86] |
| Sour cream | 28 days | Lactococcus lactis subsp. lactis | Fermented cream to which salt is added. It has a smooth texture and a tangy flavor | [87] |
| Crème Fraîche | 10 days | Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, Streptococcus | Fermented cream originating from France, with a higher fat content than sour cream | [88] |
| Filmjölk | 14 days | Lactococcus lactis, Leuconostoc | Fermented dairy product of Scandinavian origin, produced from bovine milk | [89] |
| Yogurt | 40 days | Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricum | Fermented milk produced using thermophilic microorganisms | [82] |
| Kéfir | 14 days | Lactobacillus acidophilus, Saccharomyces cerevisae, Lactobacillus kefiranofaciens, Lactococcus lactis, Leuconostoc spp., Kluyveromyces marxianus, Kazachstania spp., Bifidobacterium bifidum | Fermented dairy beverage, similar in consistency to liquid yogurt, traditionally originating from the Caucasian region | [90,91,92] |
| Koumiss | 14 days | Lactobacillus acidophilus, Kluyveromyces marxianus, Saccharomyces cerevisae | Fermented dairy product of Asian origin, traditionally produced from mare’s milk | [93,94] |
| Viili | 14 days | Lactococcus lactis, Leuconostoc mesenteroides, Geotrichum candidum | Fermented milk product of Scandinavian origin, produced through surface mold fermentation | [89] |
| Buttermilk | 10 days | Lactococcus lactis, Leuconostoc mesenteroides | Fermented dairy product obtained from pasteurized milk using mesophilic lactic acid bacteria, characterized by a lower viscosity than cream and a slightly acidic flavor | [95] |
| Acidophilus milk | 14 days | Lactobacillus acidophilus | Fermented milk product inoculated with Lactobacillus acidophilus, a probiotic bacterium commonly used for its health-promoting properties | [96] |
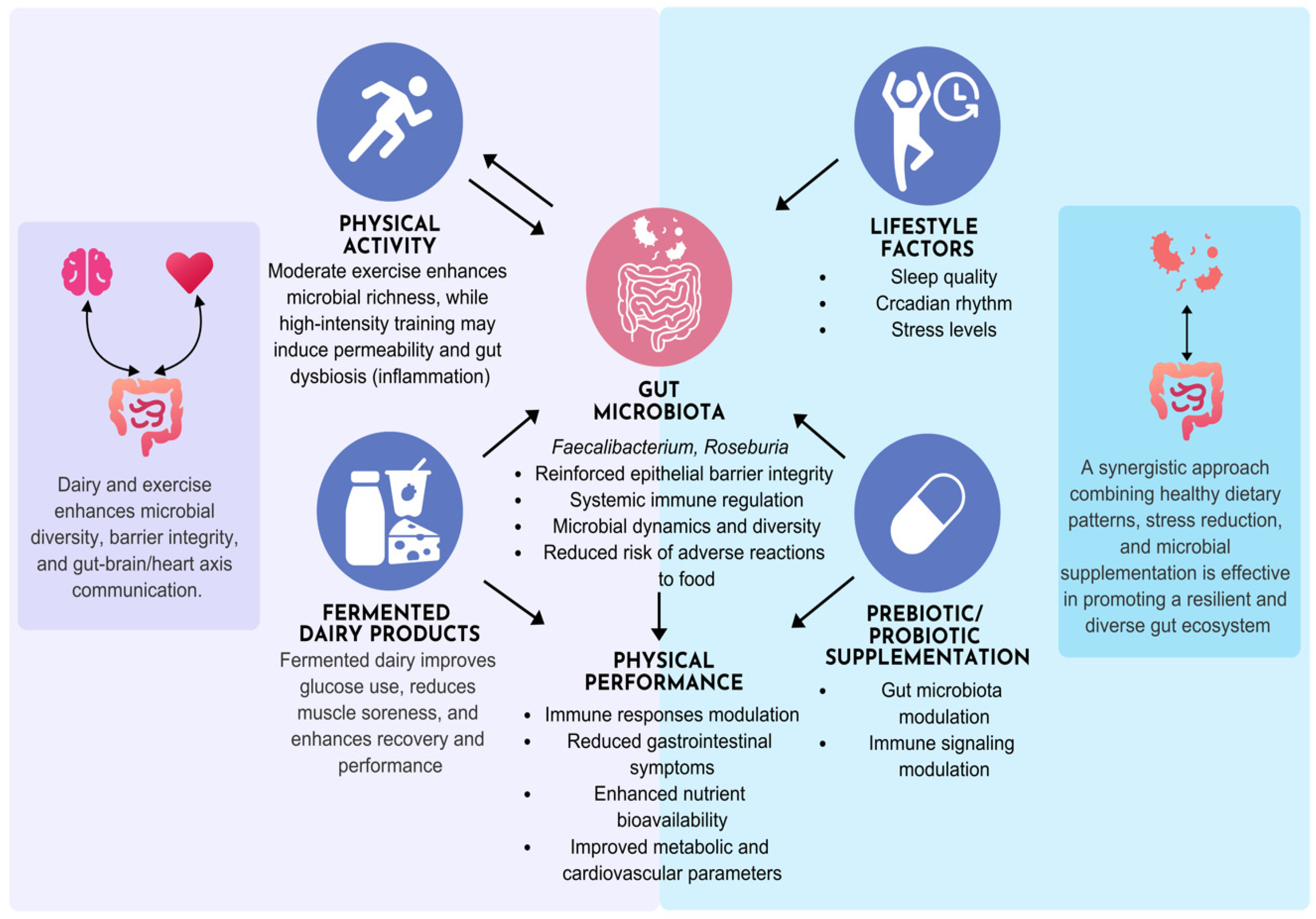
3.2. Dairy-Gut Microbiota Interactions: Consequences for Immune Regulation and Dietary Sensitivities
3.2.1. Gut Microbiota-Mediated Effects of Dairy Consumption
3.2.2. Dairy-Gut Microbiota, and Immunonutrition: A Tripartite Interaction
3.3. Dairy Intake and Cardiometabolic Health
3.3.1. Dairy Fat and Lipid Metabolism
3.3.2. Dairy Intake and Hypertension
3.3.3. Effects of Dairy Consumption on Systemic Inflammation
3.4. Gut Microbiome-Heart Axis and Its Interaction with Cardiometabolic Health
3.5. Physical Activity and Lifestyle Modulation in Dairy Gut-Microbiome Interactions
3.6. Metagenomics and Personalized Nutrition Approaches
4. Discussion
4.1. Research Gaps
4.2. Clinical Implications
4.3. Limitations of This Review
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ACE | Angiotensin-converting enzyme |
| ARF | Adverse reactions to food |
| BMOs | Bovine milk oligosaccharides |
| CLA | Conjugated linoleic acid |
| CMPA | Cow’s milk protein allergy |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DASH | Dietary approaches to stop hypertension |
| FMT | Fecal Microbiota Transplantation |
| GABA | γ-aminobutyric acid |
| GALT | Gut-associated lymphoid tissue |
| HELENA | Healthy lifestyle in Europe by nutrition in adolescence |
| ImP | Imidazole Propionate |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MFGM | Milk fat globule membrane |
| miRNAs | microRNAs |
| NGS | Next-generation sequencing |
| NF-κB | Nuclear factor kappa B |
| PAGln | Phenylacetyl glutamine |
| SANRA | Assessment of narrative review articles |
| SCFAs | Short-chain fatty acids |
| TLR | Toll-like receptor |
| TMAO | Trimethylamine N-oxide |
| UHT | Ultra-high temperature |
References
- Evershed, R.P.; Payne, S.; Sherratt, A.G.; Copley, M.S.; Coolidge, J.; Urem-Kotsu, D.; Kotsakis, K.; Ozdoğan, M.; Ozdoğan, A.E.; Nieuwenhuyse, O.; et al. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 2008, 455, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.J.; Olson, D.W. A 100-year review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Gut microbes and host physiology: What happens when you host billions of guests? Front. Endocrinol. 2014, 5, 91. [Google Scholar] [CrossRef]
- Gray, W.R.; Jacobs, J.P. Modulation of host physiology and pathophysiology by the gut microbiome. Nutrients 2024, 16, 361. [Google Scholar] [CrossRef]
- Nestel, P.J.; Mori, T.A. Dairy foods: Beneficial effects of fermented products on cardiometabolic health. Curr. Nutr. Rep. 2023, 12, 478–485. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Role of fermented dairy products in the health benefits of a mediterranean diet. Aging Clin. Exp. Res. 2024, 36, 75. [Google Scholar] [CrossRef]
- Ejima, R.; Mishima, R.; Sen, A.; Yamaguchi, K.; Mitsuyama, E.; Kaneko, H.; Kimura, M.; Arai, S.; Muto, N.; Hiraku, A.; et al. The impact of fermented milk products containing Bifidobacterium longum BB536 on the gut environment: A randomized double-blind placebo-controlled trial. Nutrients 2024, 16, 3580. [Google Scholar] [CrossRef]
- de Jesus, L.C.L.; Freitas, A.D.S.; Dutra, J.D.C.F.; Campos, G.M.; Américo, M.F.; Laguna, J.G.; Dornelas, E.G.; Carvalho, R.D.O.; Vital, K.D.; Fernandes, S.O.A.; et al. Lactobacillus delbrueckii CIDCA 133 fermented milk modulates inflammation and gut microbiota to alleviate acute colitis. Food Res. Int. 2024, 186, 114322. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Jie, Z.; Liang, S.; Ding, Q.; Li, F.; Sun, X.; Lin, Y.; Chen, P.; Cai, K.; Wang, X.; Zhang, T.; et al. Dairy consumption and physical fitness tests associated with fecal microbiome in a Chinese cohort. Med. Microecol. 2021, 9, 10038. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124, Erratum in: PLoS Med. 2022, 19, e1004085. https://doi.org/10.1371/journal.pmed.1004085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.; Wallace, D.; O’Donnell, C.; Greene, D.; Hennessy, D.; O’Shea, N.; Tobin, J.T.; Fenelon, M.A. Trend analysis and prediction of seasonal changes in milk composition from a pasture-based dairy research herd. J. Dairy Sci. 2023, 106, 2326–2337. [Google Scholar] [CrossRef]
- Thum, C.; Roy, N.C.; Everett, D.W.; McNabb, W.C. Variation in milk fat globule size and composition: A source of bioactives for human health. Crit. Rev. Food Sci. Nutr. 2023, 63, 87–113. [Google Scholar] [CrossRef]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition-a review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Alm, L. Effect of fermentation on lactose, glucose, and galactose content in milk and suitability of fermented milk products for lactose intolerant individuals. J. Dairy Sci. 1982, 65, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W. Fermentation, fermented foods and lactose intolerance. Eur. J. Clin. Nutr. 2002, 56, 50–55. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern.-Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Wultańska, D.; Pituch, H. The prebiotic effect of human milk oligosaccharides 3′- and 6′-sialyllactose on adhesion and biofilm formation by Clostridioides difficile—Pilot study. Microbes. Infect. 2022, 24, 104929. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Szajewska, H.; et al. Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Larke, J.A.; Heiss, B.E.; Ehrlich, A.; Taft, D.H.; Raybould, H.E.; Mills, D.A.; Slupsky, C.M. Milk oligosaccharide-driven persistence of Bifidobacterium pseudocatenulatum modulates local and systemic microbial metabolites upon synbiotic treatment in conventionally colonized mice. Microbiome 2023, 11, 194. [Google Scholar] [CrossRef]
- Chauhan, D.D.A.P.; Deepak, D.; Chauhan, S. Cow milk oligosaccharides and theirr to infant nutrition. Biol. Life Sci. Forum 2023, 29, 19. [Google Scholar]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-related aspects of milk proteins. Iran. J. Pharm. Res. 2016, 15, 573–591. [Google Scholar]
- Horstman, A.M.H.; Huppertz, T. Milk proteins: Processing, gastric coagulation, amino acid availability and muscle protein synthesis. Crit. Rev. Food Sci. Nutr. 2023, 63, 10267–10282. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive peptides of whey: Obtaining, activity, mechanism of action, and further applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10351–10381. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The functional role of lactoferrin in intestine mucosal immune system and inflammatory bowel disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of bovine immunoglobulins on immune function, allergy, and infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Petrova, S.Y.; Khlgatian, S.V.; Emel’yanova, O.Y.; Pishulina, L.A.; Berzhets, V.M. Current data about milk caseins. Russ. J. Bioorg. Chem. 2022, 48, 273–280. [Google Scholar] [CrossRef]
- Alberghina, D.; Giannetto, C.; Vazzana, I.; Ferrantelli, V.; Piccione, G. Reference intervals for total protein concentration, serum protein fractions, and albumin/globulin ratios in clinically healthy dairy cows. J. Vet. Diagn. Investig. 2011, 23, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, H.A.; Collins, J.; Lukman, R.; Saxton, R.; Andersen, T.; McDougal, O.M. SVR Chemometrics to quantify β-lactoglobulin and α-Lactalbumin in milk using MIR. Foods 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Caffin, J.P.; Poutrel, B.; Rainard, P. Physiological and pathological factors influencing bovine immunoglobulin G1 concentration in milk. J. Dairy Sci. 1983, 66, 2161–2166. [Google Scholar] [CrossRef]
- Harmon, R.J.; Schanbacher, F.L.; Ferguson, L.C.; Smith, K.L. Concentration of lactoferrin in milk of normal lactating cows and changes occurring during mastitis. Am. J. Vet. Res. 1975, 36, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Kussendrager, K.D.; van Hooijdonk, A.C. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br. J. Nutr. 2000, 84, S19–S25. [Google Scholar] [CrossRef]
- Goudswaard, J.; Bakker-de Koff, E.C.; van Ravenswaaij-Kraan, H.P. Lysozyme and its presence in bovine milk and serum. Tijdschr. Diergeneeskd. 1978, 103, 445–450. [Google Scholar]
- Wang, S.; Liu, Y.; Cai, H.; Li, Y.; Zhang, X.; Liu, J.; Sun, R.; Fang, S.; Yu, B. Decreased risk of all-cause and heart-specific mortality is associated with low-fat or skimmed milk consumption compared with whole milk intake: A cohort study. Clin. Nutr. 2021, 40, 5568–5575. [Google Scholar] [CrossRef]
- Mansbridge, R.J.; Blake, J.S. Nutritional factors affecting the fatty acid composition of bovine milk. Br. J. Nutr. 1997, 78, 37–47. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The role of α-linolenic acid and its oxylipins in human cardiovascular diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Weiss, W.P. A 100-year review: From ascorbic acid to zinc-mineral and vitamin nutrition of dairy cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef]
- Lieben, L.; Carmeliet, G. Vitamin D signaling in osteocytes: Effects on bone and mineral homeostasis. Bone 2013, 54, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Finglas, P.M.; Roe, M.A.; Pinchen, H.M.; Church, S. McCance and Widdowson’s the Composition of Foods, 7th Summary ed.; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Cerbulis, J.; Farrell, H.M., Jr. Composition of the milks of dairy cattle. II. Ash, calcium, magnesium, and phosphorus. J. Dairy Sci. 1976, 59, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Sachdeva, B.; Arora, S.; Kapila, S.; Wadhwa, B.K. Bioavailability of vitamin D2 and calcium from fortified milk. Food Chem. 2014, 147, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Castillo, L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Jia, M.; Luo, J.; Gao, B.; Huangfu, Y.; Bao, Y.; Li, D.; Jiang, S. Preparation of synbiotic milk powder and its effect on calcium absorption and the bone microstructure in calcium deficient mice. Food Funct. 2023, 14, 3092–3106. [Google Scholar] [CrossRef]
- Hidayat, K.; Zhang, L.L.; Rizzoli, R.; Guo, Y.X.; Zhou, Y.; Shi, Y.J.; Su, H.W.; Liu, B.; Qin, L.Q. The effects of dairy product supplementation on bone health indices in children aged 3 to 18 years: A meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 1187–1196. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Lappe, J.; O’Brien, K.O.; Wang, D.D.; Sahni, S.; Weaver, C.M. Dairy intake and bone health across the lifespan: A systematic review and expert narrative. Crit. Rev. Food Sci. Nutr. 2021, 61, 3661–3707. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Gallier, S.; Tolenaars, L.; Prosser, C. Whole goat milk as a source of fat and milk fat globule membrane in infant formula. Nutrients 2020, 12, 3486. [Google Scholar] [CrossRef]
- Yang, M.T.; Lan, Q.Y.; Liang, X.; Mao, Y.Y.; Cai, X.K.; Tian, F.; Liu, Z.Y.; Li, X.; Zhao, Y.R.; Zhu, H.L. Lactational changes of phospholipids content and composition in Chinese breast milk. Nutrients 2022, 14, 1539. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Melo, T.; Mauricio, T.; Ferreira, H.; Domingues, P.; Domingues, R. The non-enzymatic oxidation of phosphatidylethanolamine and phosphatidylserine and their intriguing roles in inflammation dynamics and diseases. FEBS Lett. 2024, 598, 2174–2189. [Google Scholar] [CrossRef] [PubMed]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wuren, T.; Zhai, B.T.; Liu, Y.; Er, D. Milk-derived exosomes in the regulation of nutritional and immune functions. Food Sci. Nutr. 2024, 12, 7048–7059. [Google Scholar] [CrossRef]
- Danev, N.; Harman, R.M.; Oliveira, L.; Huntimer, L.; Van de Walle, G.R. Bovine milk-derived cells express transcriptome markers of pluripotency and secrete bioactive factors with regenerative and antimicrobial activity. Sci. Rep. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef]
- He, Y.; He, Z.; Leone, S.; Liu, S. Milk exosomes transfer oligosaccharides into macrophages to modulate immunity and attenuate adherent-invasive E. coli (AIEC) infection. Nutrients 2021, 13, 3198. [Google Scholar] [CrossRef]
- Kim, K.U.; Kim, J.; Jang, H.; Dan, K.B.; Kim, B.K.; Ji, Y.W.; Yi, D.Y.; Min, H. Protective effects of human breast milk-derived exosomes on inflammatory bowel disease through modulation of immune cells. npj Sci. Food 2025, 9, 34. [Google Scholar] [CrossRef]
- Cui, Z.; Amevor, F.K.; Zhao, X.; Mou, C.; Pang, J.; Peng, X.; Liu, A.; Lan, X.; Liu, L. Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J. Nanobiotechnol. 2023, 21, 496. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, 5041. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Cho, Y.E.; Kim, H.W.; Min, K.Y.; Hwang, J.H.; Kim, D.H.; Yin, G.N.; Lim, J.H.; Kwun, I.S.; Baek, M.C.; Kim, D.K. Regulation of mast cell activation by extracellular vesicles in cow’s milk casein-induced allergic responses. Mol. Cell. Toxicol. 2022, 18, 177–184. [Google Scholar] [CrossRef]
- Ma, X.; Xia, J.; Yuan, J.; Meng, X.; Chen, H.; Li, X. Blockade of exosome release alleviates the hypersensitive reaction by influencing the T helper cell population in cow’s milk allergic mice. Food Funct. 2024, 15, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, B.; Ross, S.A.; Zempleni, J. Nutrition, microRNAs, and human health. Adv. Nutr. 2017, 8, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef]
- van Herwijnen, M.J.C.; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Hoen, E.N.M.N.; Wauben, M.H.M. Abundantly present miRNAs in milk-derived extracellular vesicles are conserved between mammals. Front. Nutr. 2018, 5, 81. [Google Scholar] [CrossRef]
- Fu, X.; Dong, B.; Tian, Y.; Lefebvre, P.; Meng, Z.; Wang, X.; Pattou, F.; Han, W.; Wang, X.; Lou, F.; et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J. Clin. Investig. 2015, 125, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, Y.M.; Su, Y.D.; Zuo, F.; Wu, B.; Nian, X. MiR-26a regulated adipogenic differentiation of ADSCs induced by insulin through CDK5/FOXC2 pathway. Mol. Cell. Biochem. 2021, 476, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [PubMed]
- Mar-Aguilar, F.; Arreola-Triana, A.; Mata-Cardona, D.; Gonzalez-Villasana, V.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Evidence of transfer of miRNAs from the diet to the blood still inconclusive. PeerJ 2020, 8, e9567. [Google Scholar] [CrossRef]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-enriched milk and dairy products increase gut microbiota diversity: A comparative study. Nutr. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef]
- Zhao, J.; Gong, J.; Liang, W.; Zhang, S. Microbial diversity analysis and isolation of thermoresistant lactic acid bacteria in pasteurized milk. Sci. Rep. 2024, 14, 29705. [Google Scholar] [CrossRef]
- Quigley, L.; McCarthy, R.; O’Sullivan, O.; Beresford, T.P.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C.; Cotter, P.D. The microbial content of raw and pasteurized cow milk as determined by molecular approaches. J. Dairy Sci. 2013, 96, 4928–4937. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Hou, J.; Huang, G.; Zhao, S.; Zheng, N.; Wang, J. Loss of bioactive microRNAs in cow’s milk by ultra-high-temperature treatment but not by pasteurization treatment. J. Sci. Food Agric. 2022, 102, 2676–2685. [Google Scholar] [CrossRef]
- Dan, T.; Hu, H.; Tian, J.; He, B.; Tai, J.; He, Y. Influence of different ratios of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus on fermentation characteristics of yogurt. Molecules 2023, 28, 2123. [Google Scholar] [CrossRef]
- Gutiérrez, N.; Garrido, D. Species deletions from microbiome consortia reveal key metabolic interactions between gut microbes. mSystems 2019, 4, e00185-19. [Google Scholar] [CrossRef]
- Tidona, F.; Zago, M.; Carminati, D.; Giraffa, G. The Reduction of salt in different cheese categories: Recent advances and future challenges. Front. Nutr. 2022, 9, 859694. [Google Scholar] [CrossRef]
- Elcheninov, A.G.; Zayulina, K.S.; Klyukina, A.A.; Kremneva, M.K.; Kublanov, I.V.; Kochetkova, T.V. Metagenomic insights into the taxonomic and functional features of traditional fermented milk products from Russia. Microorganisms 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Breton, J.; Richoux, R.; Nurdin, M.; Deutsch, S.M.; Falentin, H.; Hervé, C.; Chuat, V.; Lemée, R.; Maguin, E.; et al. Combining selected immunomodulatory Propionibacterium freudenreichii and Lactobacillus delbrueckii strains: Reverse engineering development of an anti-inflammatory cheese. Mol. Nutr. Food Res. 2016, 60, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mo, L.; Pan, L.; Yao, C.; Ren, D.; An, X.; Tsogtgerel, T.; Zhang, H.; Liu, W. Bacterial microbiota and metabolic character of traditional sour cream and butter in buryatia, Russia. Front. Microbiol. 2018, 9, 2496. [Google Scholar] [CrossRef]
- Lhomme, E.; Lattanzi, A.; Dousset, X.; Minervini, F.; De Angelis, M.; Lacaze, G.; Onno, B.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of sixteen French traditional sourdoughs. Int. J. Food Microbiol. 2015, 215, 161–170. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Abrahamsen, R.K. Traditional and modern Nordic fermented milk products: A review. Int. Dairy J. 2023, 142, 105641. [Google Scholar] [CrossRef]
- Çıtar Dazıroğlu, M.E.; Acar Tek, N.; Cevher Akdulum, M.F.; Yılmaz, C.; Yalınay, A.M. Effects of kefir consumption on gut microbiota and health outcomes in women with polycystic ovary syndrome. Food Sci. Nutr. 2024, 12, 5632–5646. [Google Scholar] [CrossRef]
- Kazou, M.; Grafakou, A.; Tsakalidou, E.; Georgalaki, M. Zooming into the microbiota of home-made and industrial kefir produced in Greece using classical microbiological and amplicon-based metagenomics analyses. Front. Microbiol. 2021, 12, 621069. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef]
- Guo, L.; Ya, M.; Guo, Y.S.; Xu, W.L.; Li, C.D.; Sun, J.P.; Zhu, J.J.; Qian, J.P. Study of bacterial and fungal community structures in traditional koumiss from Inner Mongolia. J. Dairy Sci. 2019, 102, 1972–1984. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, X.; Yuan, H. Detection and identification of wild yeast in Koumiss. Food Microbiol. 2012, 31, 301–308. [Google Scholar] [CrossRef]
- Bellengier, P.; Richard, J.; Foucaud, C. Associative growth of Lactococcus lactis and Leuconostoc mesenteroides strains in milk. J. Dairy Sci. 1997, 80, 1520–1527. [Google Scholar] [CrossRef]
- Kumar, A.; Rawat, M.; Kunde, Y.A.; Davenport, K.W.; Al-Sadi, R.; Chain, P.S.G.; Ma, T.Y. The complete genome sequence of probiotic Lactobacillus acidophilus ATCC 9224 isolated from sour milk. Microbiol. Resour. Announc. 2024, 13, e0067723. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Smidt, H.; de Vos, W.M. Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 2007, 9, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Shapira, M. Gut microbiotas and host evolution: Scaling up symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Calatayud Arroyo, M.; García-Mantrana, I.; Parra-Llorca, A.; Escuriet, R.; Martínez-Costa, C.; Collado, M.C. Perinatal environment shapes microbiota colonization and infant growth: Impact on host response and intestinal function. Microbiome 2020, 8, 167. [Google Scholar] [CrossRef]
- Suárez-Martínez, C.; Santaella-Pascual, M.; Yagüe-Guirao, G.; Martínez-Graciá, C. Infant gut microbiota colonization: Influence of prenatal and postnatal factors, focusing on diet. Front. Microbiol. 2023, 14, 1236254. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Li, P.; Chen, G.; Zhang, J.; Pei, C.; Chen, Y.; Gong, J.; Deng, S.; Cai, K.; Li, H.; Wang, D.; et al. Live Lactobacillus acidophilus alleviates ulcerative colitis via the SCFAs/mitophagy/NLRP3 inflammasome axis. Food Funct. 2022, 13, 2985–2997. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Karp, G. Cell Biology, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Angima, G.; Qu, Y.; Park, S.H.; Dallas, D.C. Prebiotic strategies to manage lactose intolerance symptoms. Nutrients 2024, 16, 1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Yang, Y.; Shoaie, S.; Zhang, C.; Ji, B.; Wei, Y. Advances in the relationships between cow’s milk protein allergy and gut microbiota in infants. Front. Microbiol. 2021, 12, 716667. [Google Scholar] [CrossRef] [PubMed]
- Moriki, D.; León, E.D.; García-Gamero, G.; Jiménez-Hernández, N.; Artacho, A.; Pons, X.; Koumpagioti, D.; Dinopoulos, A.; Papaevangelou, V.; Priftis, K.N.; et al. Specific gut microbiome signatures in children with cow’s milk allergy. Nutrients 2024, 16, 2752. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Torres-Fuentes, C.; Heeney, D.D.; Marco, M.L. Synergy between probiotic Lactobacillus casei and milk to maintain barrier integrity of intestinal epithelial cells. J. Agric. Food Chem. 2019, 67, 1955–1962. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Peng, C.; Yu, Z.; Li, B.; Zhang, W.; Sun, Z.; Zhang, H. Fermented milk containing Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis V9 alleviated constipation symptoms through regulation of intestinal microbiota, inflammation, and metabolic pathways. J. Dairy Sci. 2020, 103, 11025–11038. [Google Scholar] [CrossRef]
- Salminen, S.; Salminen, E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand. J. Gastroenterol. 1997, 222, 45–48. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-dose lactulose as a prebiotic for improved gut health and enhanced mineral absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef] [PubMed]
- JanssenDuijghuijsen, L.; Looijesteijn, E.; van den Belt, M.; Gerhard, B.; Ziegler, M.; Ariens, R.; Tjoelker, R.; Geurts, J. Changes in gut microbiota and lactose intolerance symptoms before and after daily lactose supplementation in individuals with the lactase nonpersistent genotype. Am. J. Clin. Nutr. 2024, 119, 702–710. [Google Scholar] [CrossRef]
- Forsgård, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Campbell, E.; Hesser, L.A.; Berni Canani, R.; Carucci, L.; Paparo, L.; Patry, R.T.; Nagler, C.R. A lipopolysaccharide-enriched cow’s milk allergy microbiome promotes a TLR4-dependent proinflammatory intestinal immune iesponse. J. Immunol. 2024, 212, 702–714. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Yang, X.; Huazeng, B.; Cheng, Q. Influences of non-IgE-mediated cow’s milk protein allergy-associated gut microbial dysbiosis on regulatory T cell-mediated intestinal immune tolerance and homeostasis. Microb. Pathog. 2021, 158, 105020. [Google Scholar] [CrossRef]
- Dong, P.; Feng, J.J.; Yan, D.Y.; Lyu, Y.J.; Xu, X. Early-life gut microbiome and cow’s milk allergy- a prospective case—Control 6-month follow-up study. Saudi J. Biol. Sci. 2018, 25, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Moriki, D.; Francino, M.P.; Koumpagioti, D.; Boutopoulou, B.; Rufián-Henares, J.Á.; Priftis, K.N.; Douros, K. The role of the gut microbiome in cow’s milk allergy: A clinical approach. Nutrients 2022, 14, 4537. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Ioannis, P.; Chiara, P.; Eleonora, F.; Roubini, C.; Vittorio, D. Biological effects of milk proteins and their peptides with emphasis on those related to the gastrointestinal ecosystem. J. Dairy Res. 2005, 72, 66–72. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Stephen-Victor, E.; Chatila, T.A. Regulation of oral immune tolerance by the microbiome in food allergy. Curr. Opin. Immunol. 2019, 60, 141–147. [Google Scholar] [CrossRef]
- Iweala, O.I.; Nagler, C.R. The microbiome and food allergy. Annu. Rev. Immunol. 2019, 37, 377–403. [Google Scholar] [CrossRef] [PubMed]
- Mehaudy, R.; Jáuregui, M.B.; Vinderola, G.; Guzmán, L.; Martínez, J.; Orsi, M.; Parisi, C. Cow’s milk protein allergy; new knowledge from a multidisciplinary perspective. Arch. Argent. Pediatr. 2022, 120, 200–206. [Google Scholar]
- Ağagündüz, D.; Yılmaz, B.; Şahin, T.Ö.; Güneşliol, B.E.; Ayten, Ş.; Russo, P.; Spano, G.; Rocha, J.M.; Bartkiene, E.; Özogul, F. Dairy lactic acid bacteria and their potential function in dietetics: The food-gut-health axis. Foods 2021, 10, 3099. [Google Scholar] [CrossRef]
- Xu, J.; Sheikh, T.M.M.; Shafiq, M.; Khan, M.N.; Wang, M.; Guo, X.; Yao, F.; Xie, Q.; Yang, Z.; Khalid, A.; et al. Exploring the gut microbiota landscape in cow milk protein allergy: Clinical insights and diagnostic implications in pediatric patients. J. Dairy Sci. 2025, 108, 73–89. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2024, 64, 5984–6000. [Google Scholar] [CrossRef]
- Di Costanzo, M.; Vella, A.; Infantino, C.; Morini, R.; Bruni, S.; Esposito, S.; Biasucci, G. Probiotics in infancy and childhood for food allergy prevention and treatment. Nutrients 2024, 16, 297. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.È.; Desroches, S.; Couture, P.; Lamarche, B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y.; et al. Consumption of dairy products and the risk of overweight or obesity, hypertension, and type 2 diabetes mellitus: A dose-response meta-analysis and systematic review of cohort studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef]
- O’Sullivan, T.A.; Schmidt, K.A.; Kratz, M. Whole-fat or reduced-fat dairy product intake, adiposity, and cardiometabolic health in children: A systematic review. Adv. Nutr. 2020, 11, 928–950. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Mouratidou, T.; Jiménez-Pavón, D.; Huybrechts, I.; Cuenca-García, M.; Mistura, L.; Gottrand, F.; González-Gross, M.; Dallongeville, J.; Kafatos, A.; et al. Is dairy consumption associated with low cardiovascular disease risk in European adolescents? Results from the HELENA Study. Pediatr. Obes. 2014, 9, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Rudkowska, I. Dairy nutrients and their effect on inflammatory profile in molecular studies. Mol. Nutr. Food Res. 2015, 59, 1249–1263. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Berg, E.; de Campos Zani, S.C.; Chan, C.B. Cow’s milk bioactive molecules in the regulation of glucose homeostasis in human and animal studies. Foods 2024, 13, 2837. [Google Scholar] [CrossRef]
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: A systematic review and meta-analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef]
- Iuliano, S.; Hare, D.L.; Vogrin, S.; Poon, S.; Robbins, J.; French, C.; Seeman, E. Consumption of dairy foods to achieve recommended levels for older adults has no deleterious effects on serum lipids. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.A.; Cromer, G.; Burhans, M.S.; Kuzma, J.N.; Hagman, D.K.; Fernando, I.; Murray, M.; Utzschneider, K.M.; Holte, S.; Kraft, J.; et al. Impact of low fat and full-fat dairy foods on fasting lipid profile and blood pressure: Exploratory endpoints of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 882–892. [Google Scholar] [CrossRef]
- Hirahatake, K.M.; Bruno, R.S.; Bolling, B.W.; Blesso, C.; Alexander, L.M.; Adams, S.H. Dairy foods and dairy fats: New perspectives on pathways implicated in cardiometabolic health. Adv. Nutr. 2020, 11, 266–279. [Google Scholar] [CrossRef]
- Soerensen, K.V.; Thorning, T.K.; Astrup, A.; Kristensen, M.; Lorenzen, J.K. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am. J. Clin. Nutr. 2014, 95, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of dietary approaches to stop hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Verberne, L.D.; Ding, E.L.; Engberink, M.F.; Geleijnse, J.M. Dairy consumption and incidence of hypertension: A dose-response meta-analysis of prospective cohort studies. Hypertension 2012, 60, 1131–1137. [Google Scholar] [CrossRef]
- Contreras, M.; Sanchez, D.; Sevilla, M.A.; Recio, I.; Amigo, L. Resistance of casein-derived bioactive peptides to simulated gastrointestinal digestion. Int. Dairy J. 2013, 32, 71–78. [Google Scholar] [CrossRef]
- Manzanares, P.; Salom, J.B.; García-Tejedor, A.; Fernández-Musoles, R.; Ruiz-Giménez, P.; Gimeno-Alcañíz, J.V. Unraveling the mechanisms of action of lactoferrin-derived antihypertensive peptides: ACE inhibition and beyond. Food Funct. 2015, 6, 2440–2452. [Google Scholar] [CrossRef]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I-converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- Domínguez-González, K.N.; Cruz Guerrero, A.E.; Márquez, H.G.; Gómez Ruiz, L.C.; García-Garibay, M.; Rodríguez Serrano, G.M. The antihypertensive effect of fermented milks. Rev. Argent. Microbiol. 2014, 46, 58–65. [Google Scholar] [PubMed]
- Engberink, M.F.; Hendriksen, M.A.H.; Schouten, E.G.; van Rooij, F.J.A.; Hofman, A.; Witteman, J.C.M.; Geleijnse, J.M. Inverse association between dairy intake and hypertension: The Rotterdam Study. Am. J. Clin. Nutr. 2009, 89, 1877–1883. [Google Scholar] [CrossRef]
- Boelsma, E.; Kloek, J. IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr. J. 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kawaguchi, K.; Yamamoto, N. Study of the mechanism of antihypertensive peptides VPP and IPP in spontaneously hypertensive rats by DNA microarray analysis. Eur. J. Pharmacol. 2009, 620, 71–77. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells. PLoS ONE 2015, 10, e0117492. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, P.W.; van der Zander, K.; Kroon, A.A.; Rennenberg, R.M.; Koning, M.M. Dose-dependent lowering of blood pressure by dairy peptides in mildly hypertensive subjects. Blood Press. 2009, 18, 44–50. [Google Scholar] [CrossRef]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, Q.; Guan, X.; Tang, Y.; Chen, X.; Deng, J.; Fan, J. Effects of fermented dairy products on inflammatory biomarkers: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 471–482. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ashtary-Larky, D.; Mehrbod, M.; Kouhi Sough, N.; Salehi Omran, H.; Dolatshahi, S.; Amirani, N.; Asbaghi, O. Impacts of supplementation with milk proteins on inflammation: A systematic review and meta-analysis. Inflammopharmacology 2025, 33, 1061–1083. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.H.; Zampelas, A.D.; Chrysohoou, C.A.; Stefanadis, C.I. Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: The ATTICA study. J. Am. Coll. Nutr. 2010, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mellett, N.; Pally, S.; Wong, G.; Barlow, C.K.; Croft, K.; Mori, T.A.; Meikle, P.J. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br. J. Nutr. 2013, 110, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, Y.; Kawano, S.; Takenokuchi, M.; Nishimura, K.; Sakai, Y.; Chin, T.; Saura, R.; Kurosaka, M.; Kumagai, S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis. Rheum. 2009, 60, 1294–1304. [Google Scholar] [CrossRef]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs. Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Cheng, W.; Dai, Q.; Wei, Z.; Guo, M.; Chen, F.; Qiao, S.; Hu, J.; Wang, J.; et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc. Res. 2023, 119, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Li, J.; Wang, X.; Zhang, M.; Du, M.; Jiang, W.; Li, C. Butyrate and propionate are negatively correlated with obesity and glucose levels in patients with type 2 diabetes and obesity. Diabetes. Metab. Syndr. Obes. 2024, 17, 1533–1541. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Six, I.; Flissi, N.; Lenglet, G.; Louvet, L.; Kamel, S.; Gallet, M.; Massy, Z.A.; Liabeuf, S. Uremic toxins and vascular dysfunction. Toxins 2020, 12, 404. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 2020, 180, 862–877. [Google Scholar] [CrossRef]
- Covasa, M.; Stephens, R.W.; Toderean, R.; Cobuz, C. Intestinal sensing by gut microbiota: Targeting gut peptides. Front. Endocrinol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Burton, K.J.; Krüger, R.; Scherz, V.; Münger, L.H.; Picone, G.; Vionnet, N.; Bertelli, C.; Greub, G.; Capozzi, F.; Vergères, G. Trimethylamine-N-Oxide postprandial response in plasma and urine is lower after fermented compared to non-fermented dairy consumption in healthy adults. Nutrients 2020, 12, 234. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut microbiota and derived short-chain fatty acids are linked to evolution of heart failure patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Robles-Vera, I.; Mañanes, D.; Galán, M.; Femenía-Muiña, M.; Redondo-Urzainqui, A.; Barrero-Rodríguez, R.; Papaioannou, E.; Amores-Iniesta, J.; Devesa, A. Imidazole propionate is a driver and therapeutic target in atherosclerosis. Nature 2025, 645, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical exercise and the gut microbiome: A bidirectional relationship influencing health and performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC. Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef]
- Pantoja-Arévalo, L.; Gesteiro, E.; Pérez-Ruiz, M.; López-Seoane, J.; Wusterhausen, P.; Matthias, T.; Urrialde, R.; González-Gross, M. The multifactorial approach and the food allergen-specific substitutive diet as a tool to manage and ameliorate adverse reactions to foodstuffs in adulthood: Study protocol for a randomized controlled trial-the ALASKA study. Trials 2024, 25, 494. [Google Scholar] [CrossRef]
- Iwasa, M.; Aoi, W.; Mune, K.; Yamauchi, H.; Furuta, K.; Sasaki, S.; Takeda, K.; Harada, K.; Wada, S.; Nakamura, Y.; et al. Fermented milk improves glucose metabolism in exercise-induced muscle damage in young healthy men. Nutr. J. 2013, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Aykut, M.N.; Erdoğan, E.N.; Çelik, M.N.; Gürbüz, M. An updated view of the effect of probiotic supplement on sports performance: A detailed review. Curr. Nutr. Rep. 2024, 13, 251–263. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Ma, T.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Nie, H.; Huang, J.; Mu, G. Fermented dairy products as precision modulators of gut microbiota and host health: Mechanistic insights, clinical evidence, and future directions. Foods 2025, 14, 1946. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S.; Assem, F.M.; El-Sayyad, G.S.; Hasanien, Y.A.; Elfadil, D.; Soliman, T.N. Impact of fermented milk on gut microbiota and human health: A comprehensive review. Curr. Microbiol. 2025, 82, 107. [Google Scholar] [CrossRef]
- Maccaferri, S.; Biagi, E.; Brigidi, P. Metagenomics: Key to human gut microbiota. Dig. Dis. 2011, 29, 525–530. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-microbiota interactions: An emerging priority for precision medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Rao, J.; Qiu, P.; Zhang, Y.; Wang, X. Gut microbiota trigger host liver immune responses that affect drug-metabolising enzymes. Front. Immunol. 2024, 15, 1511229. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zheng, Q.; Li, L. The efficacy of probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in irritable bowel syndrome: A systematic review and network meta-analysis. Nutrients 2024, 16, 2114. [Google Scholar] [CrossRef]
- Guo, W.; Liu, S.; Khan, M.Z.; Wang, J.; Chen, T.; Alugongo, G.M.; Li, S.; Cao, Z. Bovine milk microbiota: Key players, origins, and potential contributions to early-life gut development. J. Adv. Res. 2024, 59, 49–64. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017, 25, 1243–1253.e5. [Google Scholar] [CrossRef]
- Shuai, M.; Zuo, L.S.; Miao, Z.; Gou, W.; Xu, F.; Jiang, Z.; Ling, C.W.; Fu, Y.; Xiong, F.; Chen, Y.M.; et al. Multi-omics analyses reveal relationships among dairy consumption, gut microbiota and cardiometabolic health. eBioMedicine 2021, 66, 103284. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Z.; Zhang, X.; Chen, L.; Gu, Q.; Li, P. Lactobacillus paracasei ZFM54 alters the metabolomic profiles of yogurt and the co-fermented yogurt improves the gut microecology of human adults. J. Dairy Sci. 2024, 107, 5280–5300. [Google Scholar] [CrossRef]
- Chang, Y.H.; Jeong, C.H.; Cheng, W.N.; Choi, Y.; Shin, D.M.; Lee, S.; Han, S.G. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 2021, 104, 7415–7425. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M.; Kostrzynska, M. Lactic acid bacteria and bifidobacteria attenuate the proinflammatory response in intestinal epithelial cells induced by Salmonella enterica serovar Typhimurium. Can. J. Microbiol. 2013, 59, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-derived microRNAs of human milk and their effects on infant health and development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef]
- Yuan, M.; Singer, M.R.; Pickering, R.T.; Moore, L.L. Saturated fat from dairy sources is associated with lower cardiometabolic risk in the Framingham Offspring Study. Am. J. Clin. Nutr. 2022, 116, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Cella, V.; Bimonte, V.M.; Sabato, C.; Paoli, A.; Baldari, C.; Campanella, M.; Lenzi, A.; Ferretti, E.; Migliaccio, S. Nutrition and physical activity-induced changes in gut microbiota: Possible implications for human health and athletic performance. Foods 2021, 10, 3075. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, J.M.A.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Labayen, I.; Ruiz, J.R. Impact of cow’s milk intake on exercise performance and recovery of muscle function: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Modrego, J.; Ortega-Hernández, A.; Sánchez-González, S.; Corbatón-Anchuelo, A.; Gómez-Garre, D. Analysis of the gut microbiota profile targeted to multiple hypervariable regions of 16S rRNA in a hypertensive heart failure rat model. Methods Cell Biol. 2024, 188, 183–203. [Google Scholar]

| Bacteria | Role & Relevance | References |
|---|---|---|
| Bifidobacterium longum | Promotes gut barrier integrity, reduces inflammation, and is commonly enriched by BMOs | [22] |
| Bifidobacterium breve | Supports immune modulation and is frequently found in infant gut microbiota | [23,24] |
| Bifidobacterium bifidum | Known for adhesion to intestinal mucosa and competitive exclusion of pathogens | [23] |
| Bifidobacterium pseudocatenulatum | Demonstrates persistence and metabolic activity when paired with BMOs like 2′-fucosyllactose | [24] |
| Bifidobacterium animalis subsp. lactis (CNCM I-3446) | Used in symbiotic formulas; shows strong bifidogenic effects when combined with BMOs | [23] |
| Lactobacillus spp. (e.g., L. rhamnosus, L. casei) | Though less dominant, some strains benefit from BMOs and contribute to gut health | [22] |
| Parabacteroides distasonis | Emerging evidence suggests BMOs may promote its growth, with anti-inflammatory potential | [25] |
| Vitamin | Raw Milk | UHT-Processed Milk |
|---|---|---|
| A (µg) | 37 | 21 |
| D (IU) | 38 | 20 |
| E (mg) | 0.06 | 0.31 |
| B1 (mg) | 0.03 | 0.04 |
| B2 (mg) | 0.20 | 0.21 |
| B3 (mg) | 0.20 | 0.10 |
| B6 (mg) | 0.06 | 0.05 |
| B12 (µg) | 0.40 | 0.30 |
| C (mg) | 2.00 | 1.00 |
| Folate (µg) | 8.00 | 2.00 |
| Pantothenic Acid (mg) | 0.60 | 0.34 |
| Casein | Peptide Fraction | Amino Acid Sequence | Inhibitory Concentration 50 (μM) |
|---|---|---|---|
| αs1 | 146–147 | YP | 720 |
| 194–199 | TTMPLW | 51 | |
| 142–147 | LAYFYP | 65 | |
| 157–164 | DAYPSGAW | 98 | |
| β | 114–115 | YP | 720 |
| 74–76 | IPP | 5 | |
| 84–86 | VPP | 9 | |
| 193–198 | YQEPVL | 280 | |
| 108–113 | EMPFPK | 423 | |
| 177–183 | AVPYPQR | 274 | |
| 11–20 | LVYPFPGPIH | 89 | |
| 11–26 | LVYPFPGPIPNSLPQN | 71 | |
| κ | 58–59 | YP | 720 |
| 108–110 | IPP | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrego, J.; Pantoja-Arévalo, L.; Gómez-Garre, D.; Gesteiro, E.; González-Gross, M. Dairy-Gut Microbiome Interactions: Implications for Immunity, Adverse Reactions to Food, Physical Performance and Cardiometabolic Health—A Narrative Review. Nutrients 2025, 17, 3312. https://doi.org/10.3390/nu17203312
Modrego J, Pantoja-Arévalo L, Gómez-Garre D, Gesteiro E, González-Gross M. Dairy-Gut Microbiome Interactions: Implications for Immunity, Adverse Reactions to Food, Physical Performance and Cardiometabolic Health—A Narrative Review. Nutrients. 2025; 17(20):3312. https://doi.org/10.3390/nu17203312
Chicago/Turabian StyleModrego, Javier, Lisset Pantoja-Arévalo, Dulcenombre Gómez-Garre, Eva Gesteiro, and Marcela González-Gross. 2025. "Dairy-Gut Microbiome Interactions: Implications for Immunity, Adverse Reactions to Food, Physical Performance and Cardiometabolic Health—A Narrative Review" Nutrients 17, no. 20: 3312. https://doi.org/10.3390/nu17203312
APA StyleModrego, J., Pantoja-Arévalo, L., Gómez-Garre, D., Gesteiro, E., & González-Gross, M. (2025). Dairy-Gut Microbiome Interactions: Implications for Immunity, Adverse Reactions to Food, Physical Performance and Cardiometabolic Health—A Narrative Review. Nutrients, 17(20), 3312. https://doi.org/10.3390/nu17203312








