Post-Exercise Cognition and Prefrontal Hemodynamic Responses in Athletes: An Investigation of Low vs. High Glycemic Index Breakfast
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
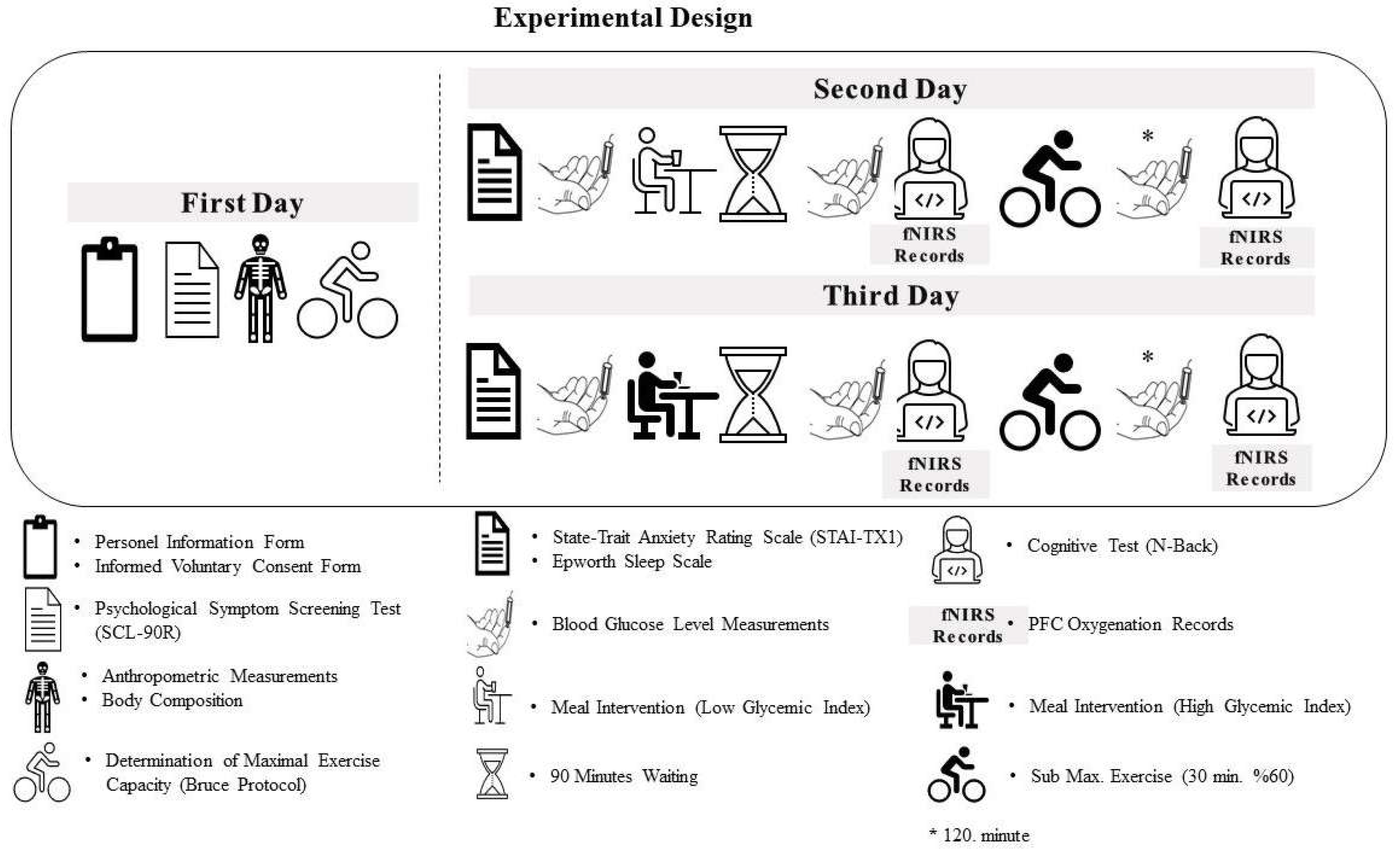
2.2. Experimental Design
2.3. Measurement of Blood Glucose Levels
2.4. Nutritional Intervention Planning: High Glycemic Index (HGI) and Low-Glycemic-Index (LGI) Breakfast Menus
2.5. Measurement of Cognitive Performance
2.6. Exercise Protocol
2.7. The Functional Near-Infrared Spectroscopy (fNIRS) Recordings
2.8. Statistical Analysis
3. Results
3.1. Demographic Information, Body Composition, and Physiologic Parameters
3.2. Blood Glucose Level Results
3.3. Results of “3-Back Test”
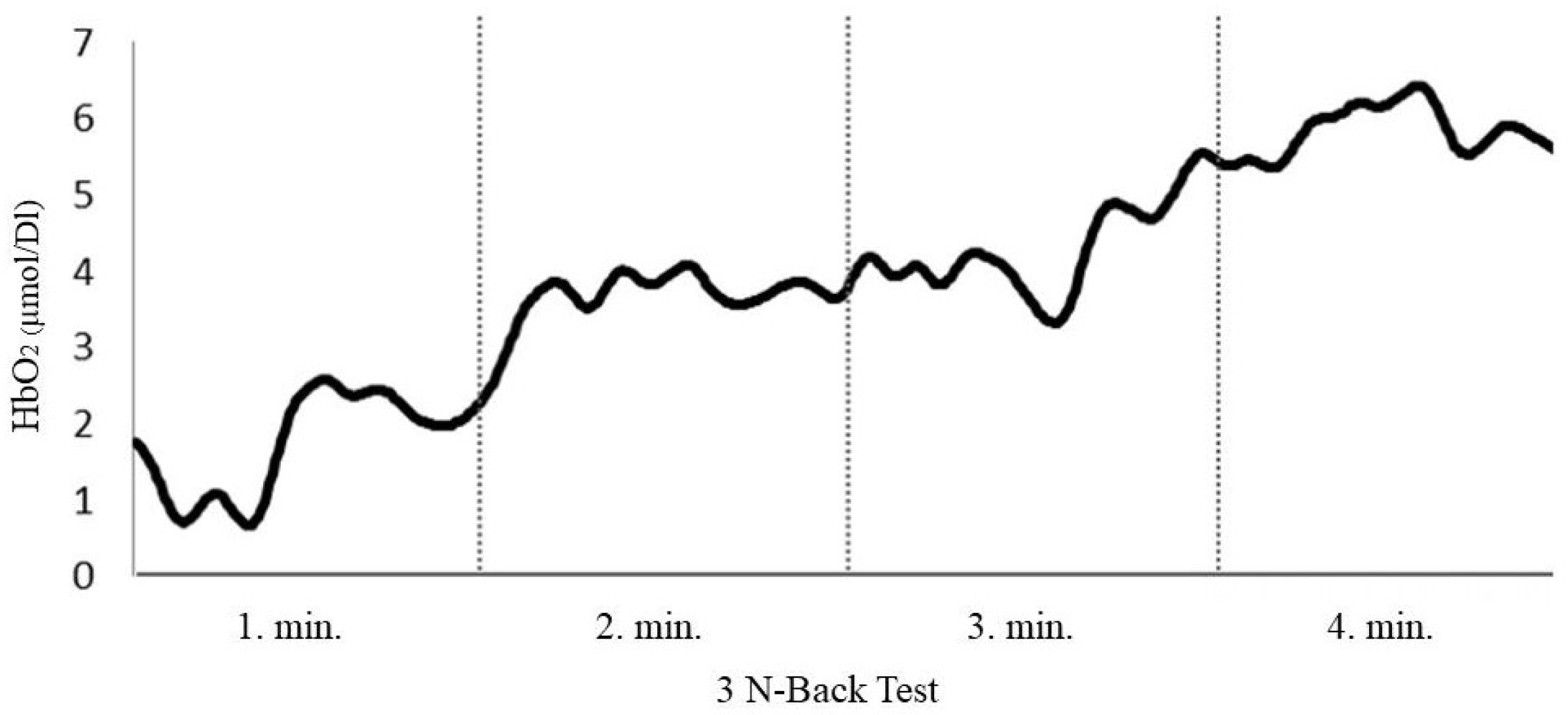
3.4. HbO2 Measurements During Cognitive Tests
3.5. The Relationship Between Blood Glucose Levels, Changes in Brain Hemodynamics, and Results of the “3-Back Test”
4. Discussion
4.1. The GI Value of Breakfast and the Combined Effect of Exercise on Blood Glucose Levels
4.2. The GI Value of Breakfast and the Combined Effect of Exercise on Working Memory
4.3. The GI Value of Breakfast and the Combined Effect of Exercise on Brain Hemodynamics
4.4. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ersoy, G.E. Spor Yapanlar Için Beslenme Sorular ve Cevapları ile Açıklamalı Sözlük; Ankara, Nobel Yayınları: Çankaya, Turkey, 2004; Volume 3. [Google Scholar]
- Jalolov, N.; Imamova, A.; Sultonov, E.Y. Proper nutrition of athletes, martial arts. Web Sci. 2023, 4. [Google Scholar]
- Thomas, F.; Pretty, C.G.; Desaive, T.; Chase, J.G. Blood glucose levels of subelite athletes during 6 days of free living. J. Diabetes Sci. Technol. 2016, 10, 1335–1343. [Google Scholar] [CrossRef]
- Campbell, S.C.; Wisniewski, P.J. Nutritional recommendations for athletes. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 255–271. [Google Scholar] [CrossRef]
- Chua, M.T.; Balasekaran, G.; Ihsan, M.; Aziz, A.R. Effects of Pre-Exercise High and Low Glycaemic Meal on Intermittent Sprint and Endurance Exercise Performance. Sports 2019, 7, 188. [Google Scholar] [CrossRef]
- Moore, L.J.; Midgley, A.W.; Thurlow, S.; Thomas, G.; Mc Naughton, L.R. Effect of the glycaemic index of a pre-exercise meal on metabolism and cycling time trial performance. J. Sci. Med. Sport 2010, 13, 182–188. [Google Scholar] [CrossRef]
- Philippou, E.; Constantinou, M. The influence of glycemic index on cognitive functioning: A systematic review of the evidence. Adv. Nutr. 2014, 5, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, C.; Benton, D.; Brennan, A.; Young, H. The impact of glycaemic load on cognitive performance: A meta-analysis and guiding principles for future research. Neurosci. Biobehav. Rev. 2022, 141, 104824. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.K.; Sünram-Lea, S.I.; Wesnes, K.A. Acute ingestion of different macronutrients differentially enhances aspects of memory and attention in healthy young adults. Biol. Psychol. 2012, 89, 477–486. [Google Scholar] [CrossRef]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef]
- Trecroci, A.; Duca, M.; Cavaggioni, L.; Rossi, A.; Scurati, R.; Longo, S.; Merati, G.; Alberti, G.; Formenti, D. Relationship between cognitive functions and sport-specific physical performance in youth volleyball players. Brain Sci. 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Mancı, E.; Gümüş, H.; Kayatekin, B.M. Validity and reliability of the wearable bioelectrical impedance measuring Device. Spor Performans Araştırmaları Derg. 2018, 10, 44–55. [Google Scholar] [CrossRef]
- Hsia, D.; Casaburi, R.; Pradhan, A.; Torres, E.; Porszasz, J. Physiological responses to linear treadmill and cycle ergometer exercise in COPD. Eur. Respir. J. 2009, 34, 605–615. [Google Scholar] [CrossRef]
- Jagim, A.R.; Camic, C.L.; Kisiolek, J.; Luedke, J.; Erickson, J.; Jones, M.T.; Oliver, J.M. Accuracy of resting metabolic rate prediction equations in athletes. J. Strength Cond. Res. 2018, 32, 1875–1881. [Google Scholar] [CrossRef]
- Laforgia, J.; Withers, R.; Van der Ploeg, G.; Gunn, S. The accuracy of resting metabolic rate prediction equations in male athletes. In Proceedings of the Nutrition Society of Australia; Nutrition Society of Australia: Crows Nest, NSW, Australia, 2000; Volume 24, p. 270. [Google Scholar]
- McInnis, K.; Balady, G.J. Comparison of submaximal exercise responses using the Bruce vs modified Bruce protocols. Med. Sci. Sports Exerc. 1994, 26, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Piper, B.J.; Li, V.; Eiwaz, M.A.; Kobel, Y.V.; Benice, T.S.; Chu, A.M.; Olsen, R.H.; Rice, D.Z.; Gray, H.M.; Mueller, S.T. Executive function on the psychology experiment building language tests. Behav. Res. Methods 2012, 44, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.T.; Piper, B.J. The psychology experiment building language (PEBL) and the PEBL test battery. J. Neurosci. Methods 2014, 222, 250–259. [Google Scholar] [CrossRef]
- Mancı, E.; Ozdalyan, F.; Kosova, S.; Gumus, H.; Gencoglu, C. Comparison of the Cognitive Skills of Adolescent Basketball Players and Sedentary Adolescents. Turk. J. Sport Exerc. 2021, 23, 320–327. [Google Scholar]
- Ferrier, D. Lippincott Görsel Anlatımlı Çalışma Kitapları: Biyokimya; Ulukaya, E., Ed.; Nobel Tıp Kitapevleri: İstanbul, Turkey, 2019; p. 272. [Google Scholar]
- Aksoy, M. Beslenme Biyokimyası; Hatiboğlu Yayınları: Ankara, Turkey, 2008. [Google Scholar]
- Harvey, R.; Ferrier, D. Lippincott Görsel Anlatımlı Çalışma Kitapları: Biyokimya, 5th ed.; Nobel Tıp Kitabevleri: İstanbul, Turkey, 2014. [Google Scholar]
- Kasnakoğlu, H. Türkiye Tarım İstatistikleri: Bütünleşik Bir Veri Tabanı Önerisi. Ekonomi-Tek 2022, 11, 63–105. [Google Scholar]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Argüero-Fonseca, A.; Martínez-Soto, J.; Barrios, F.A.; Villaseñor, T.D.J.; Cabrera, H.E.; González-Santos, L.; Aguirre-Ojeda, D.P.; Pimienta, D.P.; González, O.U.R.; Marchioro10, D.M. Effects of an n-back task on indicators of perceived cognitive fatigue and fatigability in healthy adults. Acta Biomed 2023, 94, e2022294. [Google Scholar]
- Piper, B. Evaluation of the Test–Retest Reliability of the PEBL Continuous Performance Test in a Normative Sample; PEBL Technical Report Series; 2012; Available online: https://pebl.sourceforge.net/ (accessed on 17 September 2025).
- Brush, C.J.; Olson, R.L.; Ehmann, P.J.; Osovsky, S.; Alderman, B.L. Dose–response and time course effects of acute resistance exercise on executive function. J. Sport Exerc. Psychol. 2016, 38, 396–408. [Google Scholar] [CrossRef]
- Ayaz, H.; Onaral, B. Analytical Software and Stimulus-Presentation Platform to Utilize, Visualize, and Analyze Near-Infrared Spectroscopy Measures; Drexel University: Philadelphia, PA, USA, 2005. [Google Scholar]
- Manci, E.; Herold, F.; Özdalyan, F.; Benli, M.D.; Bozkurt, Ç.; Gençtürk, U.; Gebel, A.; Güdücü, Ç.; Günay, E.; Bediz, C.Ş. Determining Cognitive Performance in Athletes: A Systematic Review Focused on Methodology of Applying Cognitive Tests. Scand. J. Psychol. 2025, 66, 523–552. [Google Scholar] [CrossRef]
- Yücel, M.A.; Lühmann, A.v.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E. Best practices for fNIRS publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Bauernfeind, G.; Wriessnegger, S.C.; Neuper, C. Focal frontal (de) oxyhemoglobin responses during simple arithmetic. Int. J. Psychophysiol. 2010, 76, 186–192. [Google Scholar] [CrossRef]
- Von Lühmann, A.; Ortega-Martinez, A.; Boas, D.A.; Yücel, M.A. Using the general linear model to improve performance in fNIRS single trial analysis and classification: A perspective. Front. Hum. Neurosci. 2020, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Mancı, E.; Deniz, O.C.; Guducu, C.; Gunay, E.; Bediz, C.S. Hemodynamic changes in athletes’ brains: Is there any adaptation? Gen. Physiol. Biophys. 2021, 40, 387–396. [Google Scholar] [CrossRef]
- Mancı, E.; Günay, E.; Güdücü, C.; Özgören, M.; Bediz, C. Brain hemodynamic changes during sprint interval cycling exercise and recovery periods. Sci. Sports 2023, 38, 75–83. [Google Scholar] [CrossRef]
- Wu, C.-L.; Williams, C. A low glycemic index meal before exercise improves endurance running capacity in men. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Aydın, C.; Gökdemir, K.; Cicioğlu, İ. Aerobik ve anaerobik egzersiz sonrası insülin ve kan glikoz değerlerinin incelenmesi. Spor Bilim. Derg. 2000, 11, 47–55. [Google Scholar]
- Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; López, E.J.; Visier-Alfonso, M.E.; Redondo-Tébar, A.; Cavero-Redondo, I. Comparative effect of low-glycemic index versus high-glycemic index breakfasts on cognitive function: A systematic review and meta-analysis. Nutrients 2019, 11, 1706. [Google Scholar] [CrossRef]
- Adolphus, K.; Lawton, C.L.; Champ, C.L.; Dye, L. The effects of breakfast and breakfast composition on cognition in children and adolescents: A systematic review. Adv. Nutr. 2016, 7, 590S–612S. [Google Scholar] [CrossRef]
- Fischer, K.; Colombani, P.C.; Langhans, W.; Wenk, C. Cognitive performance and its relationship with postprandial metabolic changes after ingestion of different macronutrients in the morning. Br. J. Nutr. 2001, 85, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bediz, C.S.; Oniz, A.; Guducu, C.; Ural Demirci, E.; Ogut, H.; Gunay, E.; Cetinkaya, C.; Ozgoren, M. Acute Supramaximal Exercise Increases the Brain Oxygenation in Relation to Cognitive Workload. Front. Hum. Neurosci. 2016, 10, 174. [Google Scholar] [CrossRef]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Chen, F.-C.; Pan, C.-Y.; Wang, C.-H.; Huang, T.-H.; Chen, T.-C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology 2014, 41, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Gaylor, C.; Young, H.; Benton, D. The Impact of Glycemic Load on Cognitive Performance in Adults: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2021, 5, 905. [Google Scholar] [CrossRef]
- Guerriero, M.A.; Dipace, A.; Monda, A.; De Maria, A.; Polito, R.; Messina, G.; Monda, M.; di Padova, M.; Basta, A.; Ruberto, M.; et al. Relationship between sedentary lifestyle, physical activity, and stress in university students and their life habits: A scoping review with PRISMA checklist (PRISMA-ScR). Brain Sci. 2025, 15, 78. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, T.; Mei, L.; Li, A.; Zhang, C. Influence of acute combined physical and cognitive exercise on cognitive function: An NIRS study. PeerJ 2019, 7, e7418. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.-H.; Hsu, W.-C.; Tseng, H.-Y.; Wang, R.-Y. Effect of exergaming versus combined exercise on cognitive function and brain activation in frail older adults: A randomised controlled trial. Ann. Phys. Rehabil. Med. 2021, 64, 101492. [Google Scholar] [CrossRef]
- Sezen, I.H. Investigating the Validity and Reliability of Hemodynamic Measurements Obtained Via Functional Near-Infrared Spectroscopy (FNIRS) by Using N-Back Task. Master’s Thesis, İzmir Ekonomi Üniversitesi, İzmir, Turkey, 2017. [Google Scholar]

| Breakfast with High Glycemic Index (GI Value) | Breakfast with Low Glycemic Index (GI Value) | ||
|---|---|---|---|
| White bread (75) | 125 g | Whole wheat bread (49) | 130 g |
| Raisin (65) | 40 g | Apple (36) | 300 g |
| White cheese (30) | 90 g | White cheese (30) | 90 g |
| Milk, full-fat (39) | 260 mL | Milk, full-fat (39) | 240 mL |
| Tomato (30) | 200 g | Tomato (30) | 200 g |
| Energy = 845 kcal | Energy = 845 kcal | ||
| 58% carbohydrate | 58% carbohydrate | ||
| 17% protein | 17% protein | ||
| 25% fat | 25% fat |
| Characteristics | ± SD |
|---|---|
| Age (year) | 19 ± 1.5 |
| Height (cm) | 180.0 ± 0.10 |
| Weight (kg) | 76.2 ± 10.4 |
| BMI (kg/m2) | 23.9 ± 2.9 |
| Fat mass (kg) | 10.1 ± 6.3 |
| Muscle mass (kg) | 37.8 ± 3.8 |
| Lean tissue mass (kg) | 66.1 ± 6.5 |
| VO2max (mL/kg/min) | 49.0 ± 6.7 |
| Resting heart rate (bpm) | 59.7 ± 10.2 |
| Target heart rate (bpm) | 137.6–166.7 ± 17.3–15.5 |
| Blood Glucose Level (mg/dL) | ||||
|---|---|---|---|---|
| Breakfast with HGI | Breakfast with LGI | Z | p | |
| ± SD | ± SD | |||
| Fasting | 84.3 ± 9.6 | 86.5 ± 5.6 | −0.26 | 0.798 |
| Pre-exercise | 96.4 ± 9.3 | 78.0 ± 13.6 | −2.30 | 0.022 * |
| Post-exercise | 101.6 ± 10.7 | 84.4 ± 6.6 | −2.81 | 0.005 ** |
| Blood Glucose Level (mg/dL) | ||||
| Pre-exercise | Post-exercise | Z | p | |
| ± SD | ± SD | |||
| Breakfast with HGI | 96.4 ± 9.3 | 101.6 ± 10.7 | −0.67 | 0.506 |
| Breakfast with LGI | 78.0 ± 13.6 | 84.4 ± 6.6 | −1.39 | 0.166 |
| 3-Back Test | Breakfast with HGI | Breakfast with HGI | Z | p |
|---|---|---|---|---|
| ± SD | ± SD | |||
| Percentage of Correct Answer (%) | ||||
| Pre-exercise | 35.6 ± 18.2 | 37.0 ± 15.3 | −0.26 | 0.798 |
| Post-exercise | 48.5 ± 18.0 | 42.3 ± 15.6 | −1.31 | 0.191 |
| Percentage of Wrong Answers (%) | ||||
| Pre-exercise | 19.4 ± 9.6 | 18.4 ± 8.54 | −0.77 | 0.441 |
| Post-exercise | 18.5 ± 11.5 | 18.0 ± 9.0 | −0.42 | 0.677 |
| RT_correct (ms) | ||||
| Pre-exercise | 572.6 ± 109.2 | 572.5 ± 183.7 | −0.66 | 0.508 |
| Post-exercise | 515.0 ± 144.3 | 525.7 ± 159.5 | −0.61 | 0.541 |
| RT_correct (ms) | ||||
| Pre-exercise | 549.6 ± 129.7 | 528.9 ± 162.9 | −0.36 | 0.721 |
| Post-exercise | 516.1 ± 136.5 | 549.8 ± 120.7 | −0.76 | 0.445 |
| Pre-exercise | Post-exercise | Z | p | |
| ± SD | ± SD | |||
| Percentage of Correct Answer (%) | ||||
| Breakfast with HGI | 35.6 ± 18.2 | 48.5 ± 18.0 | −1.68 | 0.098 |
| Breakfast with LGI | 37.0 ± 15.3 | 42.3 ± 15.6 | −0.97 | 0.331 |
| Percentage of Wrong Answers (%) | ||||
| Breakfast with HGI | 19.4 ± 9.6 | 18.5 ± 11.5 | −0.97 | 0.333 |
| Breakfast with LGI | 18.4 ± 8.5 | 18.0 ± 9.0 | −0.77 | 0.444 |
| RT_correct (ms) | ||||
| Breakfast with HGI | 572.6 ± 109.2 | 515.0 ± 144.3 | −1.72 | 0.086 |
| Breakfast with LGI | 572.5 ± 183.7 | 525.7 ± 159.5 | −1.17 | 0.241 |
| RT_correct (ms) | ||||
| Breakfast with HGI | 549.6 ± 129.7 | 516.1 ± 136.5 | −1.00 | 0.314 |
| Breakfast with LGI | 528.9 ± 162.9 | 549.8 ± 120.7 | −0.05 | 0.959 |
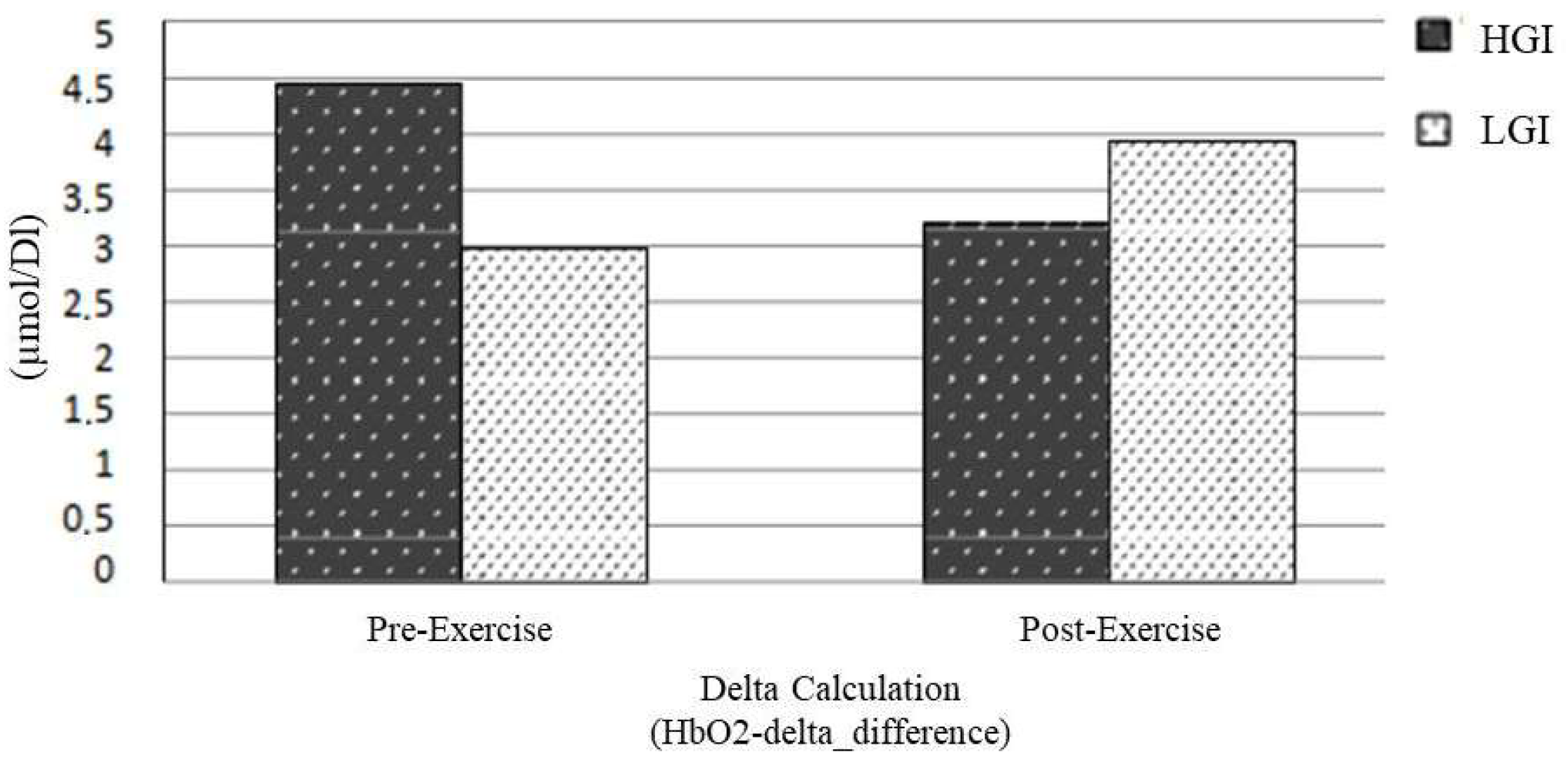
| HbO2-Delta_Difference (μmol/L) | Breakfast with HGI | Breakfast with LGI | Z | p |
|---|---|---|---|---|
| ± SD | ± SD | |||
| Pre-exercise | 4.5 ± 2.3 | 3.0 ± 2.0 | −1.84 | 0.066 |
| Post-exercise | 3.2 ± 2.3 | 3.9 ± 2.4 | −0.178 | 0.859 |
| HbO2-delta_difference (μmol/L) | Pre-exercise | Post-exercise | Z | p |
| ± SD | ± SD | |||
| Breakfast with HGI | 4.45 ± 2.32 | 3.20 ± 2.27 | −1.68 | 0.093 |
| Breakfast with LGI | 2.98 ± 1.96 | 3.94 ± 2.41 | −1.36 | 0.173 |
| Time Point (min) | ± SD) | ± SD) | Between-Condition Difference |
|---|---|---|---|
| Fasting (0) | 84.3 ± 9.6 | 86.5 ± 5.6 | – |
| Pre-exercise (90) | 96.4 ± 9.3 | 78.0 ± 13.6 | ↑ HGI (p < 0.05) * |
| Post-exercise (120) | 101.6 ± 10.7 | 84.4 ± 6.6 | ↑ HGI (p < 0.05) * |
| Incremental AUC (0–120 min) | 622.5 mg·min·dL−1 | 0 mg·min·dL−1 | ↑ HGI (p < 0.01) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bediz, Ç.; Bertan, F.; Günay, E.; Mancı, E.; Bediz, C.Ş. Post-Exercise Cognition and Prefrontal Hemodynamic Responses in Athletes: An Investigation of Low vs. High Glycemic Index Breakfast. Nutrients 2025, 17, 3296. https://doi.org/10.3390/nu17203296
Bediz Ç, Bertan F, Günay E, Mancı E, Bediz CŞ. Post-Exercise Cognition and Prefrontal Hemodynamic Responses in Athletes: An Investigation of Low vs. High Glycemic Index Breakfast. Nutrients. 2025; 17(20):3296. https://doi.org/10.3390/nu17203296
Chicago/Turabian StyleBediz, Çiğdem, Ferya Bertan, Erkan Günay, Egemen Mancı, and Cem Şeref Bediz. 2025. "Post-Exercise Cognition and Prefrontal Hemodynamic Responses in Athletes: An Investigation of Low vs. High Glycemic Index Breakfast" Nutrients 17, no. 20: 3296. https://doi.org/10.3390/nu17203296
APA StyleBediz, Ç., Bertan, F., Günay, E., Mancı, E., & Bediz, C. Ş. (2025). Post-Exercise Cognition and Prefrontal Hemodynamic Responses in Athletes: An Investigation of Low vs. High Glycemic Index Breakfast. Nutrients, 17(20), 3296. https://doi.org/10.3390/nu17203296






