Abstract
Background: The progression of cognitive decline is accelerated in older adults with sarcopenia, but the protective dietary factors have remained uncertain. Objective: This study aims to investigate the association between dietary factors and cognitive decline in older adults, and to explore the potential mediating effects of sarcopenic components. Methods: Data from the Mr. OS and Ms. OS cohort study in Hong Kong (N = 3146, aged ≥65 years) were used. Cognitive function was assessed based on the Mini-Mental State Examination (MMSE). Sarcopenic status was assessed according to the Asian Working Group for Sarcopenia 2019 updated consensus. Dietary protein intake and adherence to dietary patterns were assessed using a food frequency questionnaire. Linear regression was used to examine the associations between dietary factors and MMSE scores. Mediation analysis was conducted to identify the possible mediators in the diet–cognition associations. Results: Sarcopenia and its components were associated with baseline MMSE and MMSE changes. Positive associations were observed for plant protein intake (β = 0.79, 95% CI 0.24–1.35) and dietary patterns such as the Dietary Approaches to Stop Hypertension (DASH) diet (β = 0.14, 95% CI 0.02–0.26) and diets with lower Dietary Inflammatory Index (DII) scores (β = −0.18, 95% CI −0.26–−0.09) with better MMSE outcomes. Protective effects were more profound in participants with sarcopenia/severe sarcopenia. The effects of the DASH diet and DII were more profound in female participants, while higher adherence to the Mediterranean–DASH Intervention for Neurodegenerative Delay (MIND) diet was associated with an increment in MMSE score in male participants with sarcopenia. Handgrip strength and physical performance are significant mediators in the diet–cognition associations. Conclusions: The protective effects of healthy dietary patterns were beneficial, especially for participants with sarcopenia, while handgrip strength and walking speed potentially mediated the associations.
1. Introduction
Dementia is a significant global health issue, with millions of new cases diagnosed each year, leading to a high economic cost and a considerable impact on quality of life. The number of people living with dementia could nearly triple from approximately 57 million in 2019 to 152 million by 2050 [1]. Cognitive decline is a preclinical stage of dementia, which can be reverted to normal cognition [2]. Therefore, identifying high-risk populations and modifiable factors is important. On the one hand, biological aging is a major contributor to cognitive decline; the role of sarcopenia in accelerating cognitive decline has been recognized in recent years. Sarcopenia, as characterized by the age-related loss of skeletal muscle mass and strength [3], affects 12.9% of the world’s aging population [4]. From a physiological perspective, sarcopenia-related muscle loss might accelerate cognitive decline via changes in myokine secretion, proinflammatory cytokine production, and poor vascular homeostasis [5]. From an epidemiological perspective, a meta-analysis of 18,788 participants worldwide showed that the likelihood of developing cognitive impairment was significantly higher in individuals with sarcopenia than in those without (odds ratio: 1.75), and there are significantly lower Mini-Mental State Examination (MMSE) scores among people with sarcopenia than among their counterparts (mean difference = −2.23) [6]. Other components of sarcopenia, such as low gait speed and grip strength, also predict the risk of cognitive decline and dementia [7]. It is worth noting that low walking speed is not only an important phenotype of sarcopenia but also the core diagnostic feature of motoric cognitive risk syndrome (MCR). Longitudinal studies have shown that both sarcopenia and MCR are associated with cognitive decline within 3–4 years [8,9,10]. In terms of sarcopenia management, previous literature suggests the importance of nutrition in bone health via bone mineralization; recent evidence has suggested the benefit of nutrition being extended to sarcopenia, an interconnected condition [11,12].
In view of the linkage between sarcopenia and cognitive decline, it is important to identify lifestyle factors that may exacerbate or mitigate the progression of cognitive decline for individuals with sarcopenia. The potential of nutritional interventions has been recognized via their role in reducing oxidative stress and inflammation, as well as improving vascular health and neuronal cell signaling [13,14]. However, research evidence from nutritional interventions is limited and mainly focused on individual dietary supplement trials [15]. Moreover, dietary patterns have gained increased attention, as they account for the combination of and synergy among various food types, with stronger practical value [16]. Accumulating evidence suggests that some dietary patterns, such as the Dietary Approaches to Stop Hypertension (DASH) diet, the Mediterranean diet, the Mediterranean–Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) diet, and the anti-inflammatory diet may have a protective effect on cognitive impairment in the general population [17,18]. While details may vary, there are shared features in healthy dietary patterns, e.g., the consumption of green leafy vegetables, nuts, and fish, which showed independent positive associations with cognitive function [19]. The association between fish consumption and cognitive function also accords with previous studies that focused on assessing dietary protein intake [20,21]. While the current dietary recommendation for people with sarcopenia focuses on adequate protein intake to improve muscle health, there is potential to extend the benefit to cognitive outcomes, as well as identify dietary patterns that can improve cognitive health.
However, there has been no prospective cohort analysis on dietary factors related to the changes in cognitive function in older adults with sarcopenia. In this study, we used data from the Mr. OS and Ms. OS cohort in Hong Kong. This cohort was established between 2001 and 2003, recruiting about 4000 community-dwelling older adults aged 65 years and above. It was initially aimed to investigate osteoporosis, sarcopenia, and other aging-related health outcomes, and through multiple follow-ups has become an important resource for geriatric research. To fill the research gap, we set out three objectives. First, we assessed the relationship between sarcopenia and its components with cognitive decline in older adults. Second, we examined the association between protein intake, dietary patterns, and cognitive decline in general older adults and a sub-cohort of participants with sarcopenia, and also conducted analysis by sex. Third, we explored the sarcopenic components as potential mediators in the diet–cognition associations. We hypothesized that higher protein intake and better adherence to healthy dietary patterns are linked to slower cognitive decline as measured by the MMSE, with part of this relationship mediated by sarcopenia-related factors such as muscle strength and physical performance.
2. Materials and Methods
2.1. Study Design and Population
The Mr. OS and Ms. OS cohort study recruited 2000 men and 2000 women aged 65 or above living in the Hong Kong community between 2001 and 2003 [22]. A stratified sample was used to equally assign one-third of the participants into three age groups: 65–69, 70–74, and ≥75. A total of 3991 participants without missing data on exposure, outcome, and covariates at baseline were included in the present study. Of these, 3146 participants with completed MMSE scores at baseline and in the fourth year were included in the analysis of MMSE change.
This study was conducted following the guidelines set out in the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong (CRE: 2003.102). Written informed consent was obtained from all participants.
2.2. Cognitive Outcomes
Cognitive function was assessed based on the Mini-Mental State Examination (MMSE) [23] at baseline and reassessed in the fourth year. MMSE assessment includes several domains: orientation, immediate and short-term recall, attention and calculation, word finding, figure construction, reading and writing skills, and ability to follow a three-step command. The total scores range from 0 to 30. MMSE change was calculated as the year-4 MMSE score minus the baseline MMSE score.
2.3. Sarcopenia Assessment
Sarcopenia was assessed at baseline according to the Asian Working Group for Sarcopenia (AWGS) 2019 updated consensus [24]. Participants were classified into four groups: non-sarcopenia (healthy); possible sarcopenia (low muscle strength, defined as grip strength <28 kg in men and <18 kg in women, or low physical performance, defined as 6 m walk <1.0 m/s or 5-time chair stand ≥12 s); sarcopenia (low appendicular skeletal muscle mass (ASM) index (ASM/height2) according to dual-energy X-ray absorptiometry (DXA), <7.0 kg/m2 in men and <5.4 kg/m2 in women, with either low muscle strength or low physical performance); and severe sarcopenia (low ASM with low muscle strength and low physical performance).
2.4. Dietary Assessment
Dietary intake was assessed at baseline using a validated food frequency questionnaire (FFQ) with 280 food items, which was previously validated against 24 h dietary recalls in the older Hong Kong population [25]. The FFQ was administered through face-to-face interviews by trained staff. Participants were asked to report the frequency and portion size for each food item consumed over the past 12 months. A food photo catalog with standard portion sizes was provided to assist in portion estimation [26]. The calculation of daily protein intake used the food tables derived from McCance and Widdowson [27] and the Chinese Medical Sciences Institute [28]. Energy-adjusted intakes were computed using the residual method [29]. Total protein intake was calculated as the sum of animal and plant protein [30]. Animal protein sources included red meat, processed meat, poultry, fish and shellfish, eggs, milk products, animal giblets, and others [30]. Plant protein sources included cereals, vegetables, fruits, soybeans, nuts and seeds, and others [30].
The Diet Quality Index—International (DQI-I) assessment includes four aspects of the diet with subcomponents: variety, adequacy, moderation, and overall balance. The overall score ranges from 0 to 100. Due to insufficient information to calculate the category of empty-calorie foods under the ‘moderation’ aspect in this study, the scoring range for moderation was adjusted to 0–24 instead of the usual 0–30, and the total score of the DQI-I ranged from 0 to 94 instead of 0 to 100, with a high score indicating high quality.
The DASH score was calculated based on the method developed by Mellen et al. [31]. The DASH target intakes included nine nutrients (i.e., total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, potassium, and sodium). One score would be given when achieving each target intake. A score of 0.5 was assigned when achieving a nutrient target between the DASH target and the nutrient content of the control group’s diet in the DASH trial. The total score ranged from 0 to 9, with a higher score representing greater adherence.
The MIND score was calculated based on 15 food groups associated with the prevention of cognitive impairment: ten beneficial food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, and wine) and five unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food). Each food group was given a score of 0, 0.5, or 1 based on the frequency and proportion of consumption [32]. However, this study lacked sufficient information on the consumption of olive oil and the frequency of consuming fish (not fried), beans, poultry, red meat and products, and fast-fried foods, so the maximum MIND score in our study was 9 instead of 15.
The Mediterranean Diet Score (MDS) was calculated using the methods developed by Trichopoulou et al. [33]. One score was given when the consumption of beneficial components (vegetables, legumes, fruits and nuts, cereal, and fish) was at or above the sex-specific median, and the consumption of detrimental components was below the median cutoff. A value of 1 was assigned if daily consumption of ethanol was between 10 and 50 g for men or 5 and 25 g for women.
The dietary inflammatory index (DII) was calculated based on 30 literature-derived food parameters related to chronic inflammation [34]. The DII score was calculated as the sum of the standardized scores of 30 food parameters. A higher and positive DII score indicates a more proinflammatory diet, while a lower and negative one indicates a more anti-inflammatory diet.
2.5. Justification for Inclusion of Dietary Patterns
There is growing evidence supporting the use of holistic dietary patterns rather than individual nutrients or food groups in promoting cognitive health among older adults [35]. We included five established dietary pattern scores (MIND, DASH, MDS, DQI-I, and DII) because of their known relevance to cognitive and brain health [36]. We also aimed to examine whether these patterns may influence cognition indirectly through sarcopenic components.
The MIND diet has been linked to slower cognitive decline and a reduced risk of dementia, with a focus on plant-based foods such as green leafy vegetables, berries, whole grains, and nuts [19]. The DASH and MDS scores were selected due to their well-documented roles in supporting cardiovascular and brain health [36]. The DQI-I reflects overall dietary quality and has been validated among East Asian populations, including older Hong Kong adults [37]. The DII captures the inflammatory potential of the diet and has been associated with neuroinflammation and cognitive decline [38,39].
All five dietary pattern scores have been applied in the Mr. and Ms. OS cohort in Hong Kong [37,40].
2.6. High-Sensitivity C-Reactive Protein (hs-CRP)
To measure the levels of chronic inflammation, fasting serum samples were collected from the participants at baseline and stored at −80 °C. The serum hs-CRP level was measured using a commercially available enzyme-linked immunosorbent assay (Vitros Fusion 5,1, Vitros Chemistry Products, Raritan, NJ, USA). The intra-assay and inter-assay coefficients of variation (CVs) were 1.1–1.4% and 3.7–6.2%, respectively.
2.7. Covariates
A standardized, structured interview was performed to collect baseline information on age, sex, body mass index, systolic blood pressure, education level, smoking habit (never, former, or current smoker), alcohol use (≥12 alcoholic drinks in the past year), and self-reported medical history (diabetes, stroke, heart attack, angina, congestive heart failure, or cancer). Physical activity level was assessed using the Physical Activity Scale for the Elderly (PASE) score, with a higher score indicating greater intensity of physical activity [41].
2.8. Statistical Analysis
Baseline characteristics were stratified by sarcopenia status using means with standard deviations for continuous variables and frequencies with percentages for categorical variables. One-way ANOVA tests and chi-square tests were used for continuous variables and categorical variables, respectively. In this study, MMSE scores and their changes, protein intake (g/kg/day), dietary pattern scores (DQI-I, DASH, MIND, Mediterranean, DII), BMI, SBP, PASE score, handgrip strength, walking speed, chair stand time, and muscle mass were treated as continuous variables. Sex, education level, smoking status, alcohol drinking, and medical history (diabetes, stroke, heart attack, angina, heart failure, cancer) were treated as categorical variables. Sarcopenia status (healthy, probable, sarcopenia/severe sarcopenia) and its diagnostic components (low handgrip strength, low physical performance, low muscle mass) were also considered categorical. Dietary exposures were analyzed both as continuous variables and as quartile categories to test linear trends and distributional effects. Linear regression was used to estimate the coefficient (β) and 95% CIs for the association between sarcopenia and MMSE scores (baseline MMSE and MMSE change), in addition to the association between dietary factors and MMSE score among all participants and a sub-cohort of participants with sarcopenia/severe sarcopenia. To enhance statistical power, we combined participants with sarcopenia and severe sarcopenia into a single group for analysis. The sex-stratified analyses were also conducted for the diet–cognition associations. The models were adjusted for sex, age, dietary energy, body mass index, physical activity, systolic blood pressure, medical history, smoking habit, alcohol drinking, and education level. We additionally performed sensitivity analyses excluding BMI and PASE score, as these covariates may lie on the causal pathway between diet and cognition.
Mediation analysis was conducted to explore the potential mediation effect of sarcopenia-related mediators (handgrip strength, walking speed, time to complete 5 stands, and muscle mass) and hs-CRP in the association between dietary factors and cognitive outcomes among all participants and participants with sarcopenia/severe sarcopenia. We integrated two regression models, one to regress the cognitive outcomes on the exposure and the mediator, and a linear model to regress the mediator on the exposure, with adjustment for covariates [42,43]. The mediation analysis was performed using the “mediation” package in R. The proportion mediated was estimated on the coefficient scale, and the 95% CIs were obtained using bootstrapping. All analyses were carried out with SPSS statistical software version 27.0.1 (SPSS, Inc., Chicago, IL, USA) and R 4.3.2 software. We assumed that dietary factors at baseline influence sarcopenia-related components, which in turn affect the cognitive outcomes at year 4. A 2-sided p value of less than 0.05 is considered statistically significant.
3. Results
3.1. Characteristics of OS Study Participants
After excluding 9 participants with missing data on dietary factors or covariates and 845 participants with no year-4 MMSE score records, a total of 3146 older adults were included in the analysis. The baseline characteristics of these participants are presented by sarcopenia status in Table 1. A total of 1572 (50.0%) and 576 (18.3%) participants were diagnosed with probable sarcopenia and sarcopenia/severe sarcopenia, respectively. Compared with healthy participants, older adults with sarcopenia/severe sarcopenia tended to be males; be of older age; be less likely to have post-secondary education; have lower PASE scores, BMI, and systolic blood pressure; be more likely to be current smokers; have a stroke history; have a higher protein intake (total protein, animal protein, and plant protein); have lower scores for the DQI-I, DASH, MIND, and Mediterranean diet; and have higher DII scores. Supplementary Table S1 shows the sex-specific levels of dietary intake in all participants and participants with sarcopenia/severe sarcopenia. Compared with all women, men had higher energy and protein intakes, and lower DQI-I, DASH diet, MIND diet, and DII scores. However, in the participants with sarcopenia/severe sarcopenia, there was no significant difference in plant protein intake compared to women.

Table 1.
Baseline characteristics of participants in Mr OS and Ms OS study according to sarcopenic status (N = 3146).
3.2. Sarcopenia and Cognitive Outcomes
Table 2 shows the associations between baseline sarcopenia status, diagnosis components, and MMSE scores. Compared with the non-sarcopenia group, probable sarcopenia was significantly associated with a greater reduction in MMSE scores (β = −0.51, 95% CI = −0.76 to −0.26), and sarcopenia/severe sarcopenia was related to a greater reduction in MMSE scores (β = −0.52, 95% CI = −0.85 to −0.19).

Table 2.
Associations of sarcopenia and its diagnosis components with MMSE scores among participants in Mr OS and Ms OS study.
For three diagnosis components of sarcopenia, low handgrip strength was associated with a greater reduction in MMSE scores (β = −0.61, 95% CI = −0.97 to −0.25). Per 1-unit higher handgrip strength was associated with a higher baseline MMSE (β = 0.03, 95% CI = 0.01 to 0.05) and increment in MMSE scores (β = 0.04, 95% CI = 0.02 to 0.06). Low physical performance was associated with a greater reduction in MMSE scores (β = −0.46, 95% CI = −0.69 to −0.23). Per 1-unit higher walking speed was associated with a higher baseline MMSE (β = 0.83, 95% CI = 0.30 to 1.36) and MMSE score increment (β = 0.71, 95% CI = 0.17 to 1.24). Per 1-unit longer time to complete five stands was associated with a greater reduction in MMSE scores (β = −0.04, 95% CI = −0.07 to −0.02). Per 1-unit greater muscle mass was associated with lower baseline MMSE scores (β = −0.25, 95% CI = −0.47 to −0.02).
3.3. Protein Intake, Dietary Patterns, and Cognitive Outcomes
The associations between protein intake, dietary patterns, and MMSE scores among all participants are summarized in Figure 1 and Figure 2. Full regression results are provided in Supplementary Tables S3 and S4. Per 1-unit increment in total protein intake was associated with a lower baseline MMSE in males (β = −0.42, 95% CI = −0.75 to −0.09) and higher baseline MMSE in females (β = 0.51, 95% CI = 0.02 to 1.01). Per 1-unit increment in animal protein intake was associated with a greater reduction in MMSE scores in all participants (β = −0.36, 95% CI = −0.67 to −0.05) and in males (β = −0.48, 95% CI = −0.90 to −0.06). Per 1-unit increment in plant protein intake was associated with an increment in MMSE scores in all participants (β = 0.79, 95% CI = 0.24 to 1.35) and in females (β = 0.88, 95% CI = 0.04 to 1.73).
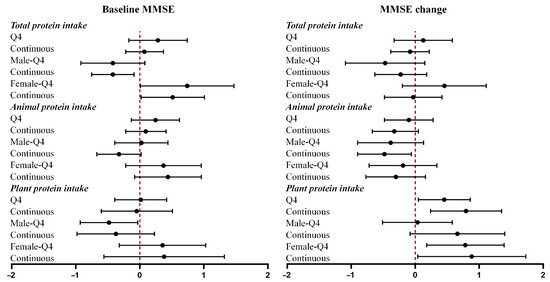
Figure 1.
Associations of protein intake with baseline MMSE and MMSE change. Forest plots showing β coefficients (95% CI) for associations of total, animal, and plant protein intake with baseline MMSE and MMSE change, stratified by sex. Q4 = highest quartile vs. Q1 (reference). Continuous = per 1-unit increment.

Figure 2.
Associations of dietary patterns with baseline MMSE and MMSE change. Forest plots showing β coefficients (95% CI) for associations of dietary pattern scores (DQI-I, DASH, MIND, MDS, and DII) with baseline MMSE and MMSE change, stratified by sex. Q4 = highest quartile vs. Q1 (reference). Continuous = per 1-unit increment.
Per 1-unit increment in DQI-I was associated with a higher baseline MMSE (β = 0.01, 95% CI = 0.00 to 0.03). Per 1-unit increment in DQI-I was associated with an increment in MMSE scores in all participants (β = 0.02, 95% CI = 0.00 to 0.03) and all females (β = 0.02, 95% CI = 0.00 to 0.04). The highest quartile of the DQI was associated with an increment in MMSE scores in all participants (β = 0.34, 95% CI = 0.03 to 0.64). The highest quartile of the DASH diet (β = 0.46, 95% CI = 0.03 to 0.89) and per 1-unit increment in the DASH diet score (β = 0.14, 95% CI = 0.02 to 0.26) were associated with an increment in MMSE scores in females. Per 1-unit increment in the MIND diet score was associated with a higher baseline MMSE in females (β = 0.21, 95% CI = 0.00 to 0.41) and increment in MMSE scores in males (β = 0.18, 95% CI = 0.02 to 0.34). The highest quartile of the MDS (β = 0.44, 95% CI = 0.01 to 0.87) and per 1-unit increment in the MDS (β = 0.11, 95% CI = 0.01 to 0.21) were associated with an increment in MMSE scores in males. †The sex × exposure interaction (tested on the continuous term) is significant at p < 0.05. The sex-stratified estimates are exploratory.
The highest quartile of the DII score and per 1-unit increment in DII score were associated with baseline MMSE in all participants (Q4: β = −0.54, 95% CI = −0.89 to −0.19; continuous: β = −0.18, 95% CI = −0.26 to −0.09) and in females (Q4: β = −0.93, 95% CI = −1.48 to −0.37; continuous: β = −0.28, 95% CI = −0.41 to −0.14). The highest quartile of the DII score and per 1-unit increment in DII score were associated with MMSE reductions in all participants (Q4: β = −0.35, 95% CI = −0.70 to −0.00; continuous: β = −0.10, 95% CI = −0.19 to −0.02). Per 1-unit increment in DII score was also associated with MMSE reduction in males (Q4: β = −0.35, 95% CI = −0.70 to −0.00; continuous: β = −0.10, 95% CI = −0.19 to −0.02). The results of the regression analysis among participants with sarcopenia/severe sarcopenia are presented in Supplementary Tables S5 and S6. Sensitivity analyses excluding BMI and PASE score yielded results largely consistent with the main findings (Supplementary Table S7).
3.4. Mediation Analysis
Potential mediators that were significantly associated with exposures, as well as with baseline MMSE/MMSE change, were further investigated in mediation analysis (Table 3). Supplementary Table S8 presents the results for the significant associations between exposures (dietary factors) and mediators (sarcopenia-related components). For all participants, handgrip strength, walking speed, and time to complete five stands are significant mediators in the association between dietary factors and MMSE scores. For the overall effect of the DQI-I and DII on baseline MMSE scores, walking speed mediated 8.04% and 7.43% of the total effects, respectively. Time to complete five stands mediated 9.31% and 37.22% of the total effect of the DQI-I and DII on MMSE change, respectively. Handgrip strength mediated 8.05% of the total effect of animal protein on MMSE change. For all males, the associations of total protein with baseline MMSE were mediated by handgrip strength (13.36%). The associations of the DII with MMSE change were mediated by time to complete five stands (15.05%). The model diagram for mediation analysis is shown in Figure 3.

Table 3.
Significant mediating effects of sarcopenia-related components on the association of dietary factors with MMSE scores among all participants in Mr OS and Ms OS study.

Figure 3.
Model diagram for significant mediating effects of sarcopenia-related components on the association of dietary factors with MMSE scores. Notes: (A) Mediating effects of sarcopenia-related components on the association of dietary factors with baseline MMSE scores in all participants. (B) Mediating effects of sarcopenia-related components on the association of dietary factors with MMSE change in all participants. (C) Mediating effects of sarcopenia-related components on the association of dietary factors with baseline MMSE scores in all male participants. Abbreviations: DQI-I, Diet Quality Index—International; MMSE, Mini-Mental State Examination; DII, dietary inflammation index. * p < 0.05.
4. Discussion
To the best of our knowledge, this is one of the first prospective cohort studies to investigate the associations between dietary factors and cognitive decline in older adults with and without sarcopenia, and to further explore the diet–muscle–cognition triad through mediation analysis. Our findings demonstrated the adverse effect of sarcopenia and two sarcopenic components (i.e., poor handgrip strength and low physical performance) on cognitive function, which was consistent with previous studies [44,45,46,47]. The association between sarcopenia and worse cognitive health also indicated the importance of identifying protective dietary factors, especially in this vulnerable population. Our mediation analyses further showed that two sarcopenic components, handgrip strength and walking speed, exhibited significant mediating effects in the diet–cognition associations.
In our study, the protective effect of plant protein intake on cognitive function was found among all participants, but the effect was debatable. Consistent with our findings on the more pronounced effect of plant protein on the increment in MMSE scores, previous studies have also highlighted the cognitive benefits of plant protein over animal protein [20,48]. A study using two US cohorts reported that substituting every 5% of energy from animal protein with plant protein was associated with 16% lower odds of subjective cognitive decline [20]. The effect of protein intake on cognitive function may be explained by the impact of dietary proteins on the specific amino acids which are essential for synthesizing neurotransmitters [49]. Research has indicated that tryptophan, which is a necessary protein for brain health and cognitive processes, may be more efficiently utilized in synthesizing neurotransmitters when derived from plant sources compared to animal sources [50]. Our findings together with the previous evidence suggested that the neuroprotective effects of plant proteins could potentially make them a superior protein source for mitigating cognitive decline. In the sex-stratified analysis, the impact of protein intake was notably greater in women, consistent with previous findings [51,52]. Some studies have suggested that women may need more protein intake than men due to higher levels of protein oxidation [53]. However, the baseline data (Supplementary Table S1) in our cohort showed significantly lower protein consumption among women than men, potentially resulting in a heightened effect. Current evidence on the associations between protein intake and cognitive function is inconclusive [20,51,54,55], which may be attributed to differences in study design, the length of follow-up, and the characteristics of the participants, and there remains a lack of evidence among people with sarcopenia.
Our findings on dietary patterns in all participants were generally in line with the results from the previous literature. Many studies have been conducted to explore the association of dietary patterns with cognitive decline. A systematic review [18] suggested an overall protective effect of the Mediterranean, MIND, DASH, and anti-inflammatory diets on cognitive function in older adults. Common features exist in the three dietary patterns (Mediterranean, MIND, and DASH diets), such as emphasizing the high consumption of grains, fresh fruit, vegetables, and nuts [18]. Together with the anti-inflammatory diet with a low DII score, abundant bioactive compounds such as carotenoids, polyphenols, and antioxidant vitamins are consumed [17]. These nutrients have been reported to be antioxidants and anti-inflammatory agents and have neuroprotective properties [56]. When compared to previous literature, our findings demonstrated that the benefits of these dietary patterns in slowing cognitive decline are more profound in older adults with sarcopenia. Our results also revealed sex differences in the effects of specific dietary factors on cognitive decline. Higher adherence to the DASH diet was associated with an increment in MMSE score in female participants with sarcopenia, while higher adherence to the MIND diet was associated with an increment in MMSE score in male participants with sarcopenia. The protective effect of the DASH diet in women was also reported to lower the prevalence of prospective subjective cognitive complaints in women [57]. A Japanese study using the MMSE for cognition assessment found a negative association between soy food, isoflavones, and cognitive decline specifically in elderly women, rather than men [58]. Additionally, calcium intake and dairy products were observed to be inversely associated with cognitive decline [59,60], while inadequate calcium intake is frequently noted among elderly women. This may offer a potential explanation for the observed effects of the DASH diet in females, as the DASH diet emphasizes the intake of soy food and low-fat dairy products. Meanwhile, the sex difference in the cognitive effect of MIND diet adherence remains inconclusive. A study using data from UK Biobank observed an inverse association between the MIND diet and dementia only in women [61], while an insignificant association in females was reported in other studies [62]. The risk effect of the DII on cognitive decline was only found in women (in both all women and women with sarcopenia) in our study, which was consistent with previous studies showing that inflammatory markers such as CRP had a statistically significant negative association with cognitive functioning for females but not males [63]. More future studies may explore the diet–cognition associations according to sex and sarcopenic status, and the underlying mechanisms.
In the mediation analysis, our results showed that the associations between several dietary patterns and cognitive outcomes were significantly mediated by handgrip strength and physical performance (walking speed and time to complete five stands) in all participants. Previous research suggested that sarcopenia was linked to cognitive decline via proinflammatory cytokine production caused by the reduction in myokine secretion under low skeletal muscle mass [5]. Interestingly, a mediating effect of low muscle mass between the associations of healthy dietary patterns and cognitive outcomes was not observed. Both the frontal lobe and hippocampus are vulnerable to neurodegenerative changes, and these changes have been linked to decreased gait speed and subjective cognitive complaints [64,65]. In other words, physical performance may serve as the early marker of cognitive impairment, echoing the concept of motoric cognitive risk syndrome [66].
Previous literature has reported the roles of healthy dietary patterns in improvements in handgrip strength [67] and gait speed [68,69]. In our study, we revealed that a higher DQI-I and DII and protein intake could influence cognitive function via muscle strength and physical performance. Additionally, it is worth noting that our results indicated that protein intake was related to lower MMSE scores, which might be mediated by reduced handgrip strength. The association between higher protein consumption and low handgrip strength could potentially be attributed to participants with sarcopenia (low handgrip strength) consciously increasing their protein intake, which is observed in our cohort (Table 1). However, participants with sarcopenia had lower adherence to several healthy dietary patterns compared with the healthy participants, which might explain the associations between dietary patterns and cognitions in this sub-cohort. Several common features of healthy dietary patterns, such as the emphasis on vegetables, berries, whole grains, fish, olives, and nuts, have been reported to be beneficial to physical function and grip strength [70,71]. The rich contents of inorganic nitrate, polyphenols, and n-3 fatty acids from these food components have been shown to support muscle strength and enhance physical function [70,71]. Our findings suggest that greater adherence to healthy dietary patterns, regardless of their specific types, may have beneficial effects on cognition for older adults in general, as well as for those with sarcopenia.
The choice of a 4-year follow-up period in this study is reasonable, because previous studies have shown that both sarcopenia and MCR can predict cognitive decline or dementia risk within about 3 years of follow-up. Therefore, evaluating the protective effects of dietary factors within this time window has direct clinical and public health significance. The present study has several strengths and implications. We conducted objective physical measurements for sarcopenia diagnosis, as well as the dietary intake measurement using a validated FFQ. With the prospective study design and large cohort with few missing data, our results provide evidence for the association between diet, sarcopenia, and cognitive outcomes. The single assessment of dietary intake at baseline may not have reflected the long-term dietary intakes of the participants, which may have been influenced by the diagnosis and medication of diseases during follow-up. However, we contend that dietary changes over four years may not be substantial, as several nutrition cohorts (e.g., Nurse’s Health Study, Health Professional Follow-up Study, Women’s Health Initiative) have conducted cumulative dietary assessments in three to four years. In addition, the temporal assumption is inherent in the mediation model and represents a limitation, as dietary intake was only measured at baseline. In addition, as the data were collected about two decades ago, dietary habits and lifestyle may differ from those of today’s older population, and the findings should be interpreted with caution when generalized to current cohorts. Moreover, some studies suggested a better validity of using the MoCA rather than the MMSE for mild cognitive impairment assessment [72], but only MMSE data are accessible for our cohort. Residual confounding factors such as stress and depressive symptoms would exist, although the models have been carefully adjusted for other potential confounders. For future research, there remains a need for longitudinal cohort studies to further track the long-term associations between dietary intake and the progression of cognitive decline in populations with sarcopenia, in addition to the effects of nutrition interventions on muscle and cognitive functions.
5. Conclusions
In conclusion, protein intake and dietary patterns were significantly associated with cognitive outcomes in community-dwelling older Hong Kong adults, and such associations were more profound in participants with sarcopenia/severe sarcopenia, potentially mediated by handgrip strength and walking speed.
These findings suggest that promoting a healthy diet is an evidence-based public health strategy, which may help delay cognitive and physical function decline in older adults, especially in those with sarcopenia, a population at high risk of cognitive decline. Future studies should combine evidence from long-term follow-up and explore integrated interventions pertaining to nutrition and exercise to further improve cognitive and physical health in older populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17193070/s1. Supplemental Methods: Detailed description of sarcopenia assessment, dietary pattern score calculation, and mediation analysis; Figure S1: Baseline MMSE scores and MMSE change by sarcopenia status; Table S1: Sex-specific levels of energy, protein intake, and adherence to dietary patterns of all participants and participants with sarcopenia or severe sarcopenia; Table S2: Baseline characteristics of participants in Mr. OS and Ms. OS study with or without year-4 MMSE scores; Table S3: Association of dietary protein with MMSE scores among all participants in Mr OS and Ms OS study (full regression results); Table S4: Association of dietary patterns with MMSE scores among all participants in Mr OS and Ms OS study (full regression results); Table S5: Association of dietary protein with MMSE scores among participants with sarcopenia/severe sarcopenia in Mr. OS and Ms. OS study; Table S6: Association of dietary patterns with MMSE scores among participants with sarcopenia/severe sarcopenia in Mr. OS and Ms. OS study; Table S7: Sensitivity analysis excluding BMI and PASE score in the significant diet–cognition models; Table S8: Significant associations of dietary factors with mediators among all participants in Mr. OS and Ms. OS study; Table S9: Quartile cutoff points for protein intake and dietary patterns in different groups.
Author Contributions
Conceptualization, Y.J.; methodology, Y.J. and K.K.-h.L.; formal analysis, Y.J. and S.L.; writing—original draft preparation, Y.J. and G.L.; writing—review and editing, G.L., S.L., J.L. (Jenny Lee), V.C., Z.L., J.L. (Jason Leung), K.L. (Kingson Lai), T.W.A., K.L. (Kuen Lam), T.K., K.T.C., J.W. and K.K.-h.L.; supervision, J.W. and K.K.-h.L.; funding acquisition, K.K.-h.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Hong Kong Polytechnic University Start-up Fund for New Recruits BE91.
Institutional Review Board Statement
The Mr. OS and Ms. OS study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong (CRE: 2003.102, renewed on 27 August 2005).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical reasons.
Acknowledgments
We thank all the OS study participants for their contributions in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Rabin, L.A.; Smart, C.M.; Amariglio, R.E. Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Annu. Rev. Clin. Psychol. 2017, 13, 369–396. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyere, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef]
- Jo, D.; Yoon, G.; Kim, O.Y.; Song, J. A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction. Biomed. Pharmacother. 2022, 147, 112636. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Liu, M.; Liu, S.; Zhao, Z.; Chen, H. Association between sarcopenia and cognitive impairment in the older people: A meta-analysis. Eur. Geriatr. Med. 2022, 13, 771–787. [Google Scholar] [CrossRef]
- Orchard, S.G.; Polekhina, G.; Ryan, J.; Shah, R.C.; Storey, E.; Chong, T.T.; Lockery, J.E.; Ward, S.A.; Wolfe, R.; Nelson, M.R.; et al. Combination of gait speed and grip strength to predict cognitive decline and dementia. Alzheimer’s Dement. 2022, 14, e12353. [Google Scholar] [CrossRef]
- Tessier, A.-J.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Association of low muscle mass with cognitive function during a 3-year follow-up among adults aged 65 to 86 years in the Canadian longitudinal study on aging. JAMA Netw. Open 2022, 5, e2219926. [Google Scholar] [CrossRef]
- Maniscalco, L.; Veronese, N.; Ragusa, F.S.; Vernuccio, L.; Dominguez, L.J.; Smith, L.; Matranga, D.; Barbagallo, M. Sarcopenia using muscle mass prediction model and cognitive impairment: A longitudinal analysis from the English longitudinal study on ageing. Arch. Gerontol. Geriatr. 2024, 117, 105160. [Google Scholar] [CrossRef]
- Chung, J.; Byun, S. Motoric cognitive risk and incident dementia in older adults. JAMA Netw. Open 2023, 6, e2338534. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and nutrition impact on osteoporosis and sarcopenia—The incidence of osteosarcopenia: A narrative review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef]
- Mocini, E.; Cardinali, L.; Di Vincenzo, O.; Moretti, A.; Baldari, C.; Iolascon, G.; Migliaccio, S. An Integrated Nutritional and Physical Activity Approach for Osteosarcopenia. Nutrients 2025, 17, 2842. [Google Scholar] [CrossRef]
- Gadgil, M.D.; Appel, L.J.; Yeung, E.; Anderson, C.A.; Sacks, F.M.; Miller, E.R., 3rd. The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: Results from the OmniHeart trial. Diabetes Care 2013, 36, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Surkan, P.J.; Esmaillzadeh, A.; Willett, W.C. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J. Nutr. 2011, 141, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Sikkes, S.A.M.; Tang, Y.; Jutten, R.J.; Wesselman, L.M.P.; Turkstra, L.S.; Brodaty, H.; Clare, L.; Cassidy-Eagle, E.; Cox, K.L.; Chetelat, G.; et al. Toward a theory-based specification of non-pharmacological treatments in aging and dementia: Focused reviews and methodological recommendations. Alzheimer’s Dement. 2021, 17, 255–270. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, F.; Du, Y.; Zheng, J.; An, Y.; Lu, Y. Higher Adherence to the AMED, DASH, and CHFP Dietary Patterns Is Associated with Better Cognition among Chinese Middle-Aged and Elderly Adults. Nutrients 2023, 15, 3974. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimer’s Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef]
- Huang, L.; Tao, Y.; Chen, H.; Chen, X.; Shen, J.; Zhao, C.; Xu, X.; He, M.; Zhu, D.; Zhang, R.; et al. Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet and Cognitive Function and its Decline: A Prospective Study and Meta-analysis of Cohort Studies. Am. J. Clin. Nutr. 2023, 118, 174–182. [Google Scholar] [CrossRef]
- Yeh, T.S.; Yuan, C.; Ascherio, A.; Rosner, B.A.; Blacker, D.; Willett, W.C. Long-term dietary protein intake and subjective cognitive decline in US men and women. Am. J. Clin. Nutr. 2022, 115, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, X.; Lutz, M.W.; Ju, S.Y.; Liu, K.; Guo, G.; Zeng, Y.; Yao, Y. Interaction between APOE epsilon4 and dietary protein intake on cognitive decline: A longitudinal cohort study. Clin. Nutr. 2021, 40, 2716–2725. [Google Scholar] [CrossRef]
- Yang, J.; Yang, A.; Yeung, S.; Woo, J.; Lo, K. Joint Associations of Food Groups with All-Cause and Cause-Specific Mortality in the Mr. OS and Ms. OS Study: A Prospective Cohort. Nutrients 2022, 14, 3915. [Google Scholar] [CrossRef]
- Chiu, H.F.; Lee, H.; Chung, W.; Kwong, P. Reliability and validity of the Cantonese version of Mini-Mental State Examination. East Asian Arch. Psychiatry 1994, 4, 25. [Google Scholar]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Woo, J.; Leung, S.S.F.; Ho, S.C.; Lam, T.H.; Janus, E.D. A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr. Res. 1997, 17, 1633–1641. [Google Scholar] [CrossRef]
- Chan, R.S.; Yu, B.; Leung, J.; Lee, J.; Auyeung, T.; Kwok, T.; Woo, J. How dietary patterns are related to inflammaging and mortality in community-dwelling older Chinese adults in Hong Kong—A prospective analysis. J. Nutr. Health Aging 2019, 23, 181–194. [Google Scholar] [CrossRef]
- Paul, A.; Southgate, D.A. McCance and Widdowson’s the Composition of Foods; HM Stationery Office: London, UK, 1978. [Google Scholar]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Beijing, China, 2009; Volume 42, pp. 795–799. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Li, S.-Y.; Lu, Z.-H.; Leung, J.C.; Kwok, T.C. Association of dietary protein intake, inflammation with muscle mass, physical performance and incident sarcopenia in Chinese community-dwelling older adults. J. Nutr. Health Aging 2024, 28, 100163. [Google Scholar] [CrossRef]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch. Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Tessier, A.-J.; Wang, F.; Korat, A.A.; Eliassen, A.H.; Chavarro, J.; Grodstein, F.; Li, J.; Liang, L.; Willett, W.C.; Sun, Q. Optimal dietary patterns for healthy aging. Nat. Med. 2025, 31, 1644–1652. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Dietary patterns and risk of frailty in Chinese community-dwelling older people in Hong Kong: A prospective cohort study. Nutrients 2015, 7, 7070–7084. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, S.; Sun, M.; Yang, Y.; Wang, L.; Wu, C.; Li, P. Association between dietary inflammatory index and cognitive impairment: A meta-analysis. Front. Aging Neurosci. 2023, 14, 1007629. [Google Scholar] [CrossRef]
- Fang, B.; Wang, Z.; Nan, G. Dietary inflammatory potential and the risk of cognitive impairment: A meta-analysis of prospective cohort studies. J. Nutr. Health Aging 2025, 29, 100428. [Google Scholar] [CrossRef]
- Li, S.-Y.; Lu, Z.-H.; Leung, J.; Su, Y.; Yu, B.; Kwok, T. Dietary patterns modify the association between body mass index and mortality in older adults. Clin. Nutr. 2025, 46, 20–29. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef]
- Gong, J.H.; Lo, K.; Liu, Q.; Li, J.; Lai, S.; Shadyab, A.H.; Arcan, C.; Snetselaar, L.; Liu, S. Dietary Manganese, Plasma Markers of Inflammation, and the Development of Type 2 Diabetes in Postmenopausal Women: Findings From the Women’s Health Initiative. Diabetes Care 2020, 43, 1344–1351. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, W.; Ren, R.; Wang, Y.; Wang, G. Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J. Cachexia Sarcopenia Muscle 2022, 13, 2944–2952. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; Palazuelos-González, R.; Rivera-Almaraz, A.; Manrique-Espinoza, B. Longitudinal association of sarcopenia and mild cognitive impairment among older Mexican adults. J. Cachexia Sarcopenia Muscle 2021, 12, 1848–1859. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Moon, J.H.; Moon, J.H.; Kim, K.M.; Choi, S.H.; Lim, S.; Park, K.S.; Kim, K.W.; Jang, H.C. Sarcopenia as a Predictor of Future Cognitive Impairment in Older Adults. J. Nutr. Health Aging 2016, 20, 496–502. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Moraca, M.; Rotundo, S.; Colica, C.; Provenzano, F.; Terracciano, R.; Greco, M.; Foti, D.; et al. Impact of legumes and plant proteins consumption on cognitive performances in the elderly. J. Transl. Med. 2017, 15, 109. [Google Scholar] [CrossRef]
- van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Feskanich, D.; Niu, C.; Dopfel, R.; Holmes, M.D.; Hankinson, S.E. Dietary correlates of urinary 6-sulfatoxymelatonin concentrations in the Nurses’ Health Study cohorts. Am. J. Clin. Nutr. 2009, 90, 975–985. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, W.; Zhang, D. Association between Dietary Protein Intake and Cognitive Function in Adults Aged 60 Years and Older. J. Nutr. Health Aging 2020, 24, 223–229. [Google Scholar] [CrossRef]
- Lee, L.; Kang, S.A.; Lee, H.O.; Lee, B.H.; Park, J.S.; Kim, J.H.; Jung, I.K.; Park, Y.J.; Lee, J.E. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health 2001, 115, 133–138. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef]
- Ding, B.; Xiao, R.; Ma, W.; Zhao, L.; Bi, Y.; Zhang, Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: A cross-sectional study. BMJ Open 2018, 8, e018573. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Song, Y.; Wu, F.; Sharma, S.; Clendenen, T.V.; India-Aldana, S.; Afanasyeva, Y.; Gu, Y.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Chen, Y. Mid-life adherence to the Dietary Approaches to Stop Hypertension (DASH) diet and late-life subjective cognitive complaints in women. Alzheimer’s Dement. 2024, 20, 1076–1088. [Google Scholar] [CrossRef]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef]
- Cherbuin, N. Higher dietary intakes of potassium, calcium and magnesium are associated with a reduced risk of developing vascular dementia. Evid. Based Ment. Health 2013, 16, 26. [Google Scholar] [CrossRef]
- Talaei, M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Dairy, soy, and calcium consumption and risk of cognitive impairment: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1541–1552. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Agarwal, P.; Holland, T.M.; van Dam, R.M. MIND Dietary Pattern and Its Association with Cognition and Incident Dementia in the UK Biobank. Nutrients 2022, 15, 32. [Google Scholar] [CrossRef]
- Berendsen, A.M.; Kang, J.H.; Feskens, E.J.M.; de Groot, C.; Grodstein, F.; van de Rest, O. Association of Long-Term Adherence to the MIND Diet with Cognitive Function and Cognitive Decline in American Women. J. Nutr. Health Aging 2018, 22, 222–229. [Google Scholar] [CrossRef]
- Canon, M.E.; Crimmins, E.M. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J. Nutr. Health Aging 2011, 15, 695–698. [Google Scholar] [CrossRef]
- Ezzati, A.; Katz, M.J.; Lipton, M.L.; Lipton, R.B.; Verghese, J. The association of brain structure with gait velocity in older adults: A quantitative volumetric analysis of brain MRI. Neuroradiology 2015, 57, 851–861. [Google Scholar] [CrossRef]
- Nadkarni, N.K.; Nunley, K.A.; Aizenstein, H.; Harris, T.B.; Yaffe, K.; Satterfield, S.; Newman, A.B.; Rosano, C.; Health, A.B.C.S. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J. Gerontol. Ser. A 2014, 69, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Tian, Q.; Carlson, M.C.; Xue, Q.L.; Ferrucci, L. Motoric cognitive risk syndrome: Integration of two early harbingers of dementia in older adults. Ageing Res. Rev. 2020, 58, 101022. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Zhang, S.; Wang, X.; Zhang, J.; et al. Anti-inflammatory dietary pattern is associated with handgrip strength decline: A prospective cohort study. Eur. J. Nutr. 2023, 62, 3207–3216. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, M.D.M.; Julibert, A.; Argelich, E.; Aparicio-Ugarriza, R.; Palacios, G.; Pons, A.; Gonzalez-Gross, M.; Tur, J.A. Western and Mediterranean Dietary Patterns and Physical Activity and Fitness among Spanish Older Adults. Nutrients 2017, 9, 704. [Google Scholar] [CrossRef]
- Shahar, D.R.; Houston, D.K.; Hue, T.F.; Lee, J.S.; Sahyoun, N.R.; Tylavsky, F.A.; Geva, D.; Vardi, H.; Harris, T.B. Adherence to mediterranean diet and decline in walking speed over 8 years in community-dwelling older adults. J. Am. Geriatr. Soc. 2012, 60, 1881–1888. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Jin, Y.; Simonsick, E.M.; Tucker, K.L.; Ferrucci, L.; Tanaka, T. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is associated with physical function and grip strength in older men and women. Am. J. Clin. Nutr. 2022, 115, 625–632. [Google Scholar] [CrossRef]
- Pasdar, Y.; Moradi, S.; Saedi, S.; Moradinazar, M.; Rahmani, N.; Hamzeh, B.; Najafi, F. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet in relation to age-associated poor muscle strength; a cross-sectional study from the Kurdish cohort study. Sci. Rep. 2022, 12, 11866. [Google Scholar] [CrossRef]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).