Activation of Sirtuin3 by 6,4′-Dihydroxy-7-methoxyflavanone Against Myoblasts Senescence by Attenuating D-Galactose-Induced Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DMF and D-Gal
2.2. Cell Culture
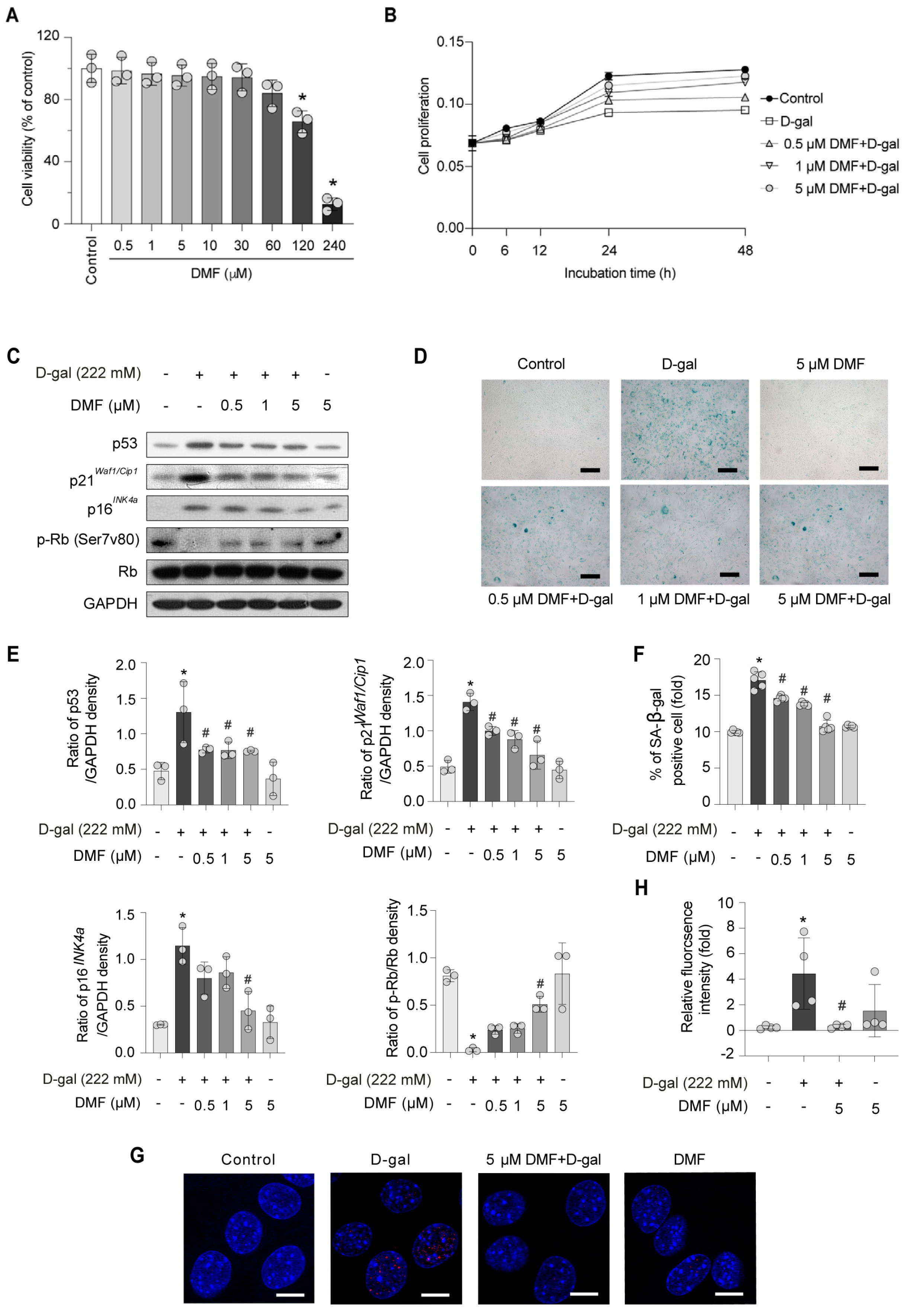
2.3. Cell Viability Assay
2.4. Senescence-Associated β-Galactosidase Staining Assay
2.5. Western Blotting Analysis
2.6. Immunofluorescence
2.7. Detection of ROS
2.8. RNA Interference
2.9. SIRT3 Deacetylase Activity Assay
2.10. Mitochondrial Membrane Potential (MMP) Measurement
2.11. ATP Production
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Statistical Analysis
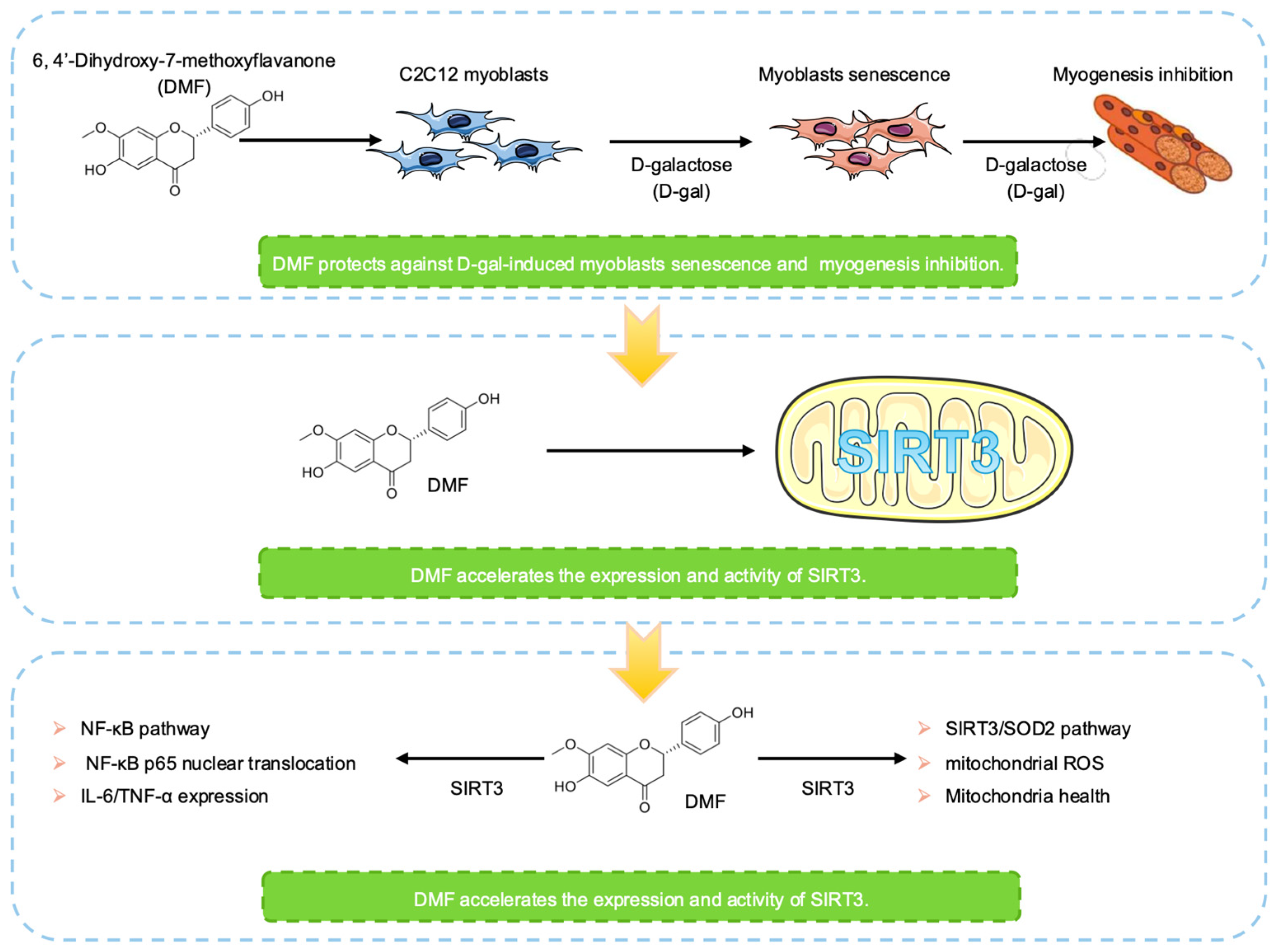
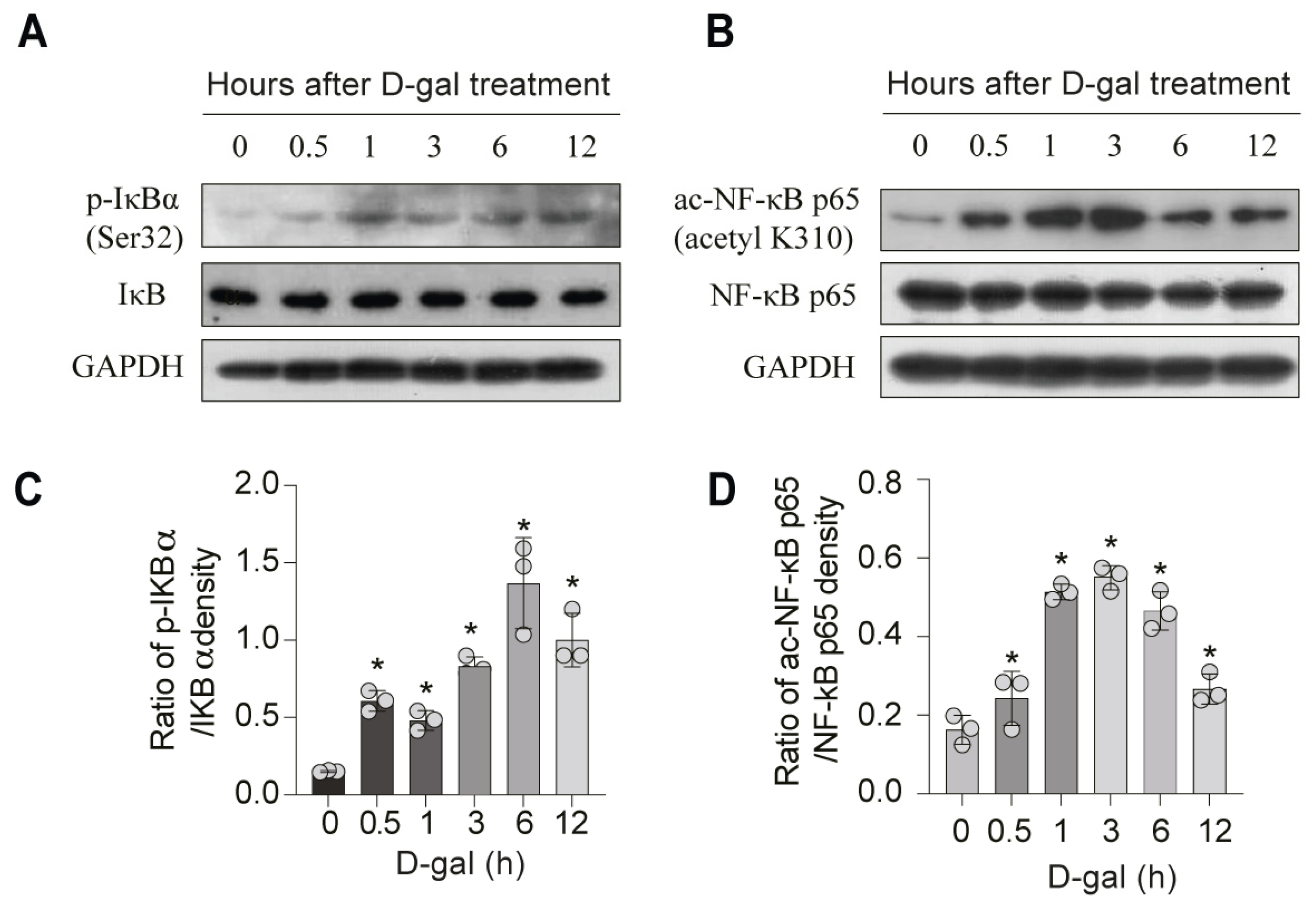
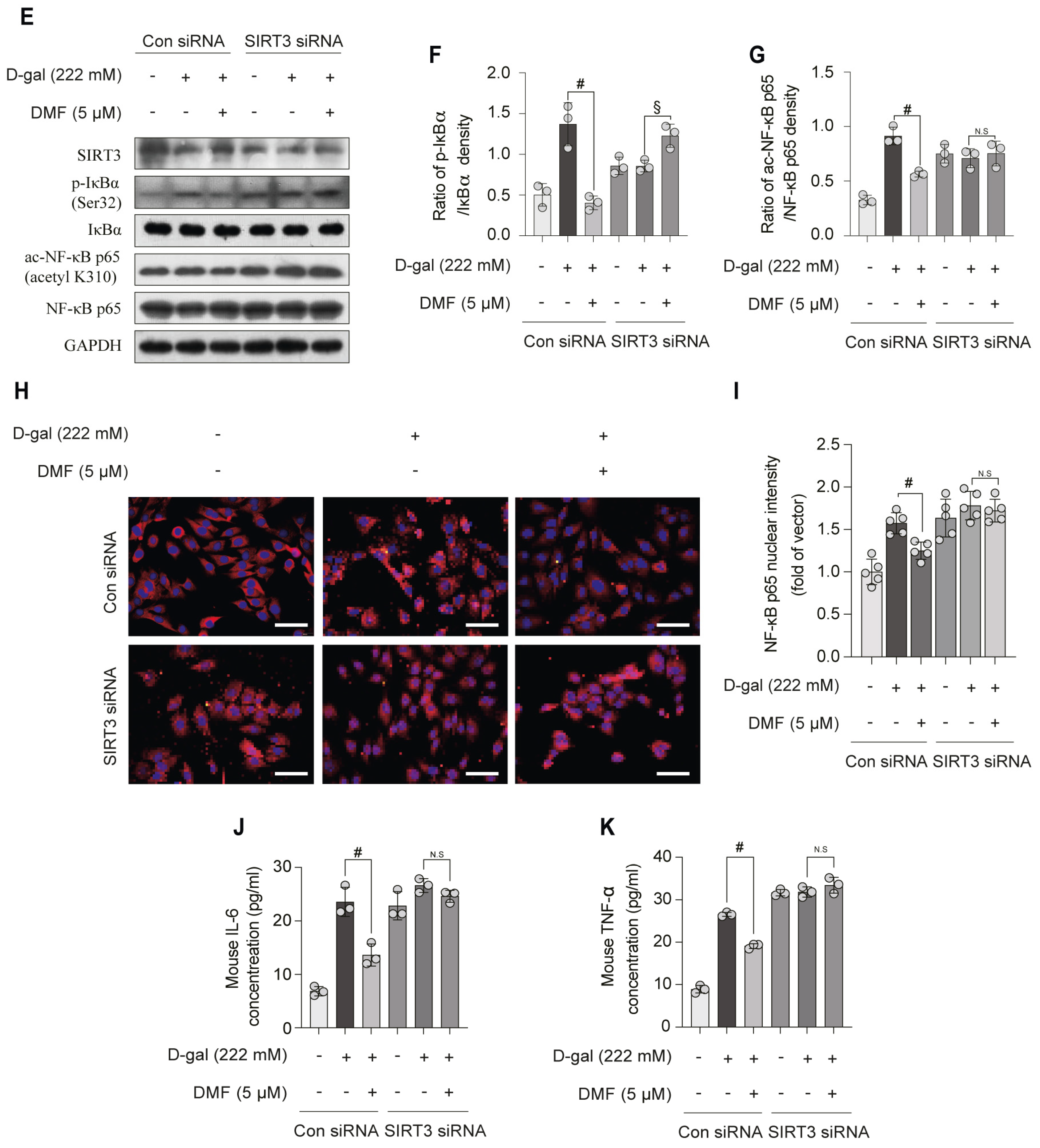
3. Results
3.1. DMF Attenuates Myoblasts Senescence in C2C12
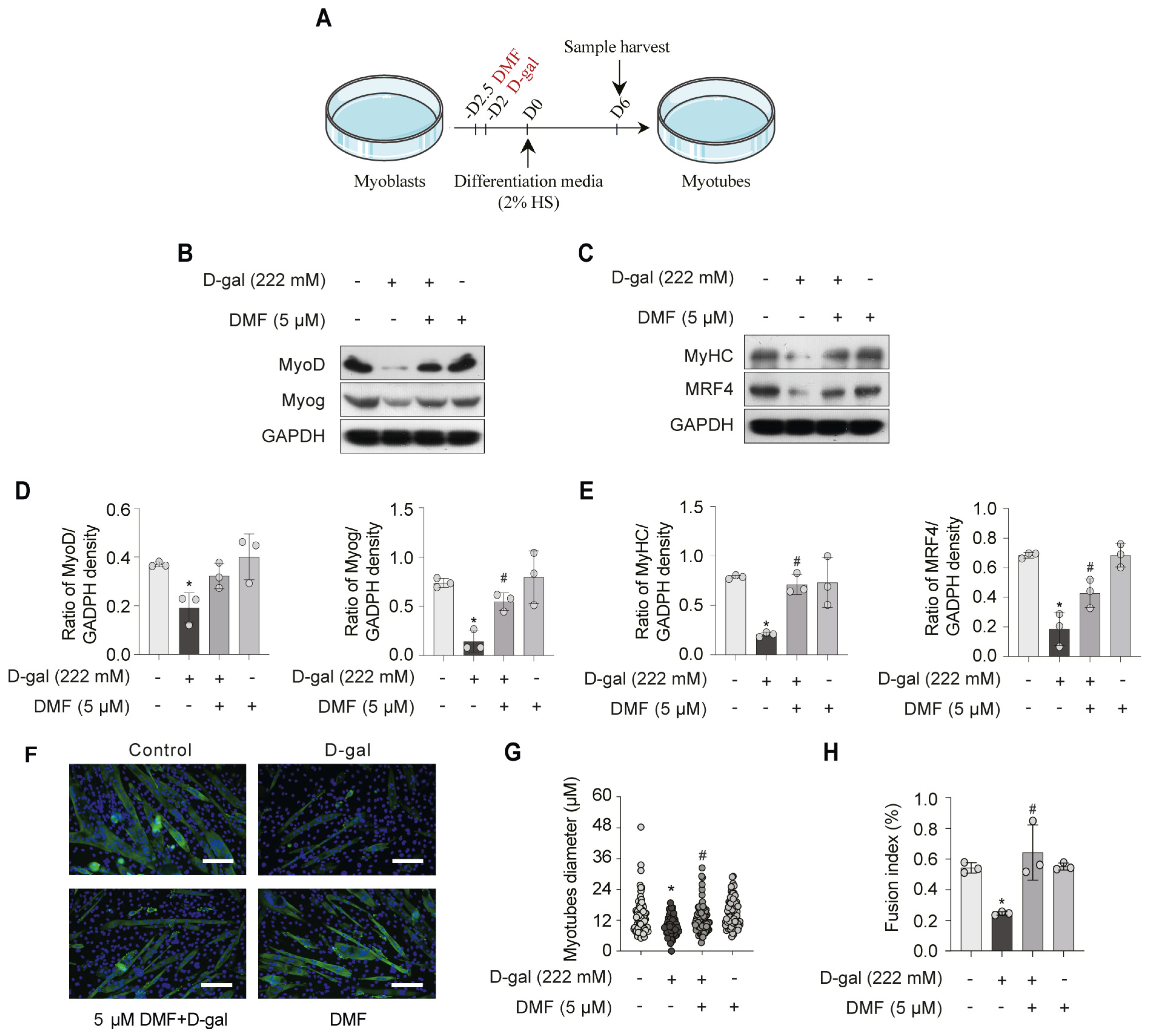
3.2. DMF Promotes Myogenesis by Alleviating D-Gal-Induced Senescence in Myoblasts
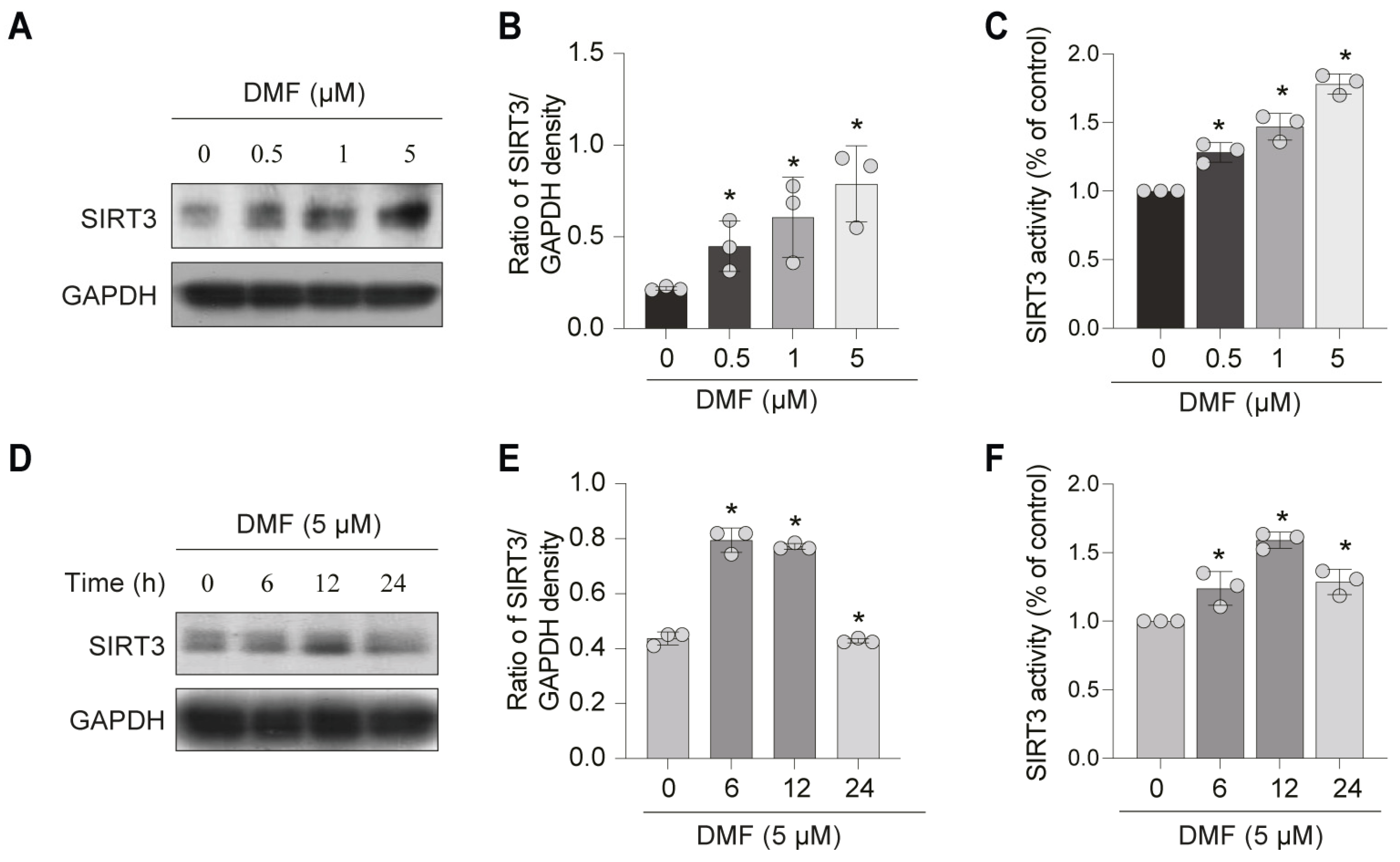
3.3. DMF, a SIRT3 Activator, Enhances SOD2 Activity and Inhibits Mitochondrial ROS (mtROS) in C2C12 Myoblasts
3.4. DMF Attenuates D-Gal-Induced NF-κB Activation in a SIRT3-Dependent Manner
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ac-NF-κB p65 | acetylated nuclear Factor kappa B p65 |
| ANOVA | analysis of variance |
| Con | Control |
| D6 | 6 days |
| DMF | 6,4′-Dihydroxy-7-methoxyflavanone |
| D-gal | D-galactose |
| DMSO | Dimethyl sulfoxide |
| DW | double-distilled water |
| DAPI | 4′6-diamidino-2-phenylindole |
| d/D | Day |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DCFH-DA | 2′,7′-Dichlorofluorescin diacetate |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| GSA | Goat Serum Albumin |
| HS | horse serum |
| IL-6 | ILterleukin-6 |
| MTS | [3-(4,5-dimethylthiazol- 2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] test so-lution |
| MyoD | Myoblast determination protein 1 |
| MyoG | Myogenin |
| MyHC | Myosin heavy chain |
| MRF4 | Muscle-specific regulatory factor 4 |
| MMP | Mitochondrial membrane potential |
| NAD | Nicotinamide adenine dinucleotide |
| N.S. | not significant |
| P/S | penicillin/streptomycin |
| PMSF | Phenylmethanesulfonyl fluoride |
| PVDF | Polyvinylidene fluoride |
| p-Rb | Phospho-Rb |
| p-H2A.X | Phospho-H2A histone family member X |
| p-IκBα | Phosphorylated IκBα |
| PBS | phosphate buffer saline |
| ROS | Reactive oxygen species |
| mtROS | mitochondrial ROS |
| RT | Room temperature |
| SA-β-gal | Senescence-associated β-galactosidase |
| SEM | standard error of the mean |
| siRNAs | Small interfering RNAs |
| SOD2 | Superoxide Dismutase 2 |
| SIRT3 | Sirtuin3 |
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kubben, N.; Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 2017, 18, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Cellular senescence: Molecular mechanisms, in vivo significance, and redox considerations. Antioxid. Redox. Signal. 2009, 11, 59–98. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Passos, J.F.; Khosla, S.; Tchkonia, T.; Kirkland, J.L. Reducing Senescent Cell Burden in Aging and Disease. Trends. Mol. Med. 2020, 26, 630–638. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 Modulates Redox Homeostasis during Ischemia/Reperfusion in the Aging Heart. Antioxidants 2020, 9, 858. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.J.; et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 2018, 17, e12679. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- He, J.; Shangguan, X.; Zhou, W.; Cao, Y.; Zheng, Q.; Tu, J.; Hu, G.; Liang, Z.; Jiang, C.; Deng, L.; et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat. Commun. 2021, 12, 4371. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef]
- Motti, M.L.; Tafuri, D.; Donini, L.; Masucci, M.T.; De Falco, V.; Mazzeo, F. The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19). Nutrients 2022, 14, 1000. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeong, G.S. Arylbenzofuran isolated from Dalbergia odorifera suppresses lipopolysaccharide-induced mouse BV2 microglial cell activation, which protects mouse hippocampal HT22 cells death from neuroinflammation-mediated toxicity. Eur. J. Pharmacol. 2014, 728, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2, 4, 5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/β-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, W.; Yang, M. Antioxidant activities of compounds isolated from Dalbergia odorifera T. Chen and their inhibition effects on the decrease of glutathione level of rat lens induced by UV irradiation. Food Chem. 2007, 104, 715–720. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.S.; Jeong, G.S.; Jeong, G.S.; Kim, Y.C. Involvement of heme oxygenase-1 induction in the cytoprotective and immunomodulatory activities of 6,4′-dihydroxy-7-methoxyflavanone in murine hippocampal and microglia cells. Eur. J. Pharmacol. 2012, 674, 153–162. [Google Scholar] [CrossRef]
- Im, N.K.; Choi, J.Y.; Oh, H.; Kim, Y.C.; Jeong, G.S. 6, 4′-Dihydroxy-7-methoxyflavanone inhibits osteoclast differentiation and function. Biol. Pharm. Bull. 2013, 36, 796–801. [Google Scholar] [CrossRef]
- Li, B.S.; Zhu, R.Z.; Choi, B.-M. 6,4′-dihydroxy-7-methoxyflavanone protects against H2O2-induced cellular senescence by inducing SIRT1 and inhibiting phosphatidylinositol 3-kinase/Akt pathway activation. Mol. Cell. Biochem. 2021, 476, 863–872. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; Von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef]
- Guo, A.; Huang, K.; Lu, Q.; Tao, B.; Li, K.; Jiang, D. TRIM16 facilitates SIRT-1-dependent regulation of antioxidant response to alleviate age-related sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 2056–2070. [Google Scholar] [CrossRef]
- Rygiel, K.A.; Picard, M.; Turnbull, D.M. The ageing neuromuscular system and sarcopenia: A mitochondrial perspective. J. Physiol. 2016, 594, 4499–4512. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Sorianello, E.; Segalés, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Muñoz, J.P.; Sánchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, Z.; Chang, L.; Chen, H.; Ren, L.; Huang, Z.; Jiao, Y.; Xia, H.; Yang, C.; Luo, K.; et al. Therapeutic silicate biomaterials for sarcopenia treatment by inhibiting inflammation and enhancing muscle regeneration through regulation of Sarcolipin/SIRT signaling pathway. Bioact. Mater. 2025, 51, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.L.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Choi, P.G.; Park, S.H.; Jeong, H.Y.; Kim, H.S.; Hahm, J.H.; Seo, H.D.; Ahn, J.; Jung, C.H. Geniposide attenuates muscle atrophy via the inhibition of FoxO1 in senescence-accelerated mouse prone-8. Phytomedicine 2024, 123, 155281. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat. Metab. 2024, 6, 433–447. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, B.H.; Wang, X.; Huang, C.P.; Xu, S.M.; Wang, J.L.; Huang, T.E.; Xiao, W.L.; Tian, X.L.; Lan, X.Q.; et al. Handelin alleviates cachexia- and aging-induced skeletal muscle atrophy by improving protein homeostasis and inhibiting inflammation. J. Cachexia Sarcopenia Muscle 2024, 15, 173–188. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Gu, Y.; Zhou, L.; Chen, R.; Liu, Y.; Wan, Z.; Liang, Z.; Wang, Y.; Ji, R.; Liu, Z. Activation of Sirtuin3 by 6,4′-Dihydroxy-7-methoxyflavanone Against Myoblasts Senescence by Attenuating D-Galactose-Induced Oxidative Stress and Inflammation. Nutrients 2025, 17, 3298. https://doi.org/10.3390/nu17203298
Li B, Gu Y, Zhou L, Chen R, Liu Y, Wan Z, Liang Z, Wang Y, Ji R, Liu Z. Activation of Sirtuin3 by 6,4′-Dihydroxy-7-methoxyflavanone Against Myoblasts Senescence by Attenuating D-Galactose-Induced Oxidative Stress and Inflammation. Nutrients. 2025; 17(20):3298. https://doi.org/10.3390/nu17203298
Chicago/Turabian StyleLi, Bingsi, Yuxuan Gu, Libing Zhou, Rui Chen, Yiwei Liu, Zexuan Wan, Ziyi Liang, Yukang Wang, Renlei Ji, and Zhian Liu. 2025. "Activation of Sirtuin3 by 6,4′-Dihydroxy-7-methoxyflavanone Against Myoblasts Senescence by Attenuating D-Galactose-Induced Oxidative Stress and Inflammation" Nutrients 17, no. 20: 3298. https://doi.org/10.3390/nu17203298
APA StyleLi, B., Gu, Y., Zhou, L., Chen, R., Liu, Y., Wan, Z., Liang, Z., Wang, Y., Ji, R., & Liu, Z. (2025). Activation of Sirtuin3 by 6,4′-Dihydroxy-7-methoxyflavanone Against Myoblasts Senescence by Attenuating D-Galactose-Induced Oxidative Stress and Inflammation. Nutrients, 17(20), 3298. https://doi.org/10.3390/nu17203298





