Potential of Traditional Chinese Medicine Brucea javanica in Cancer Treatment: A Review of Chemical Constituents, Pharmacology, and Clinical Applications
Abstract
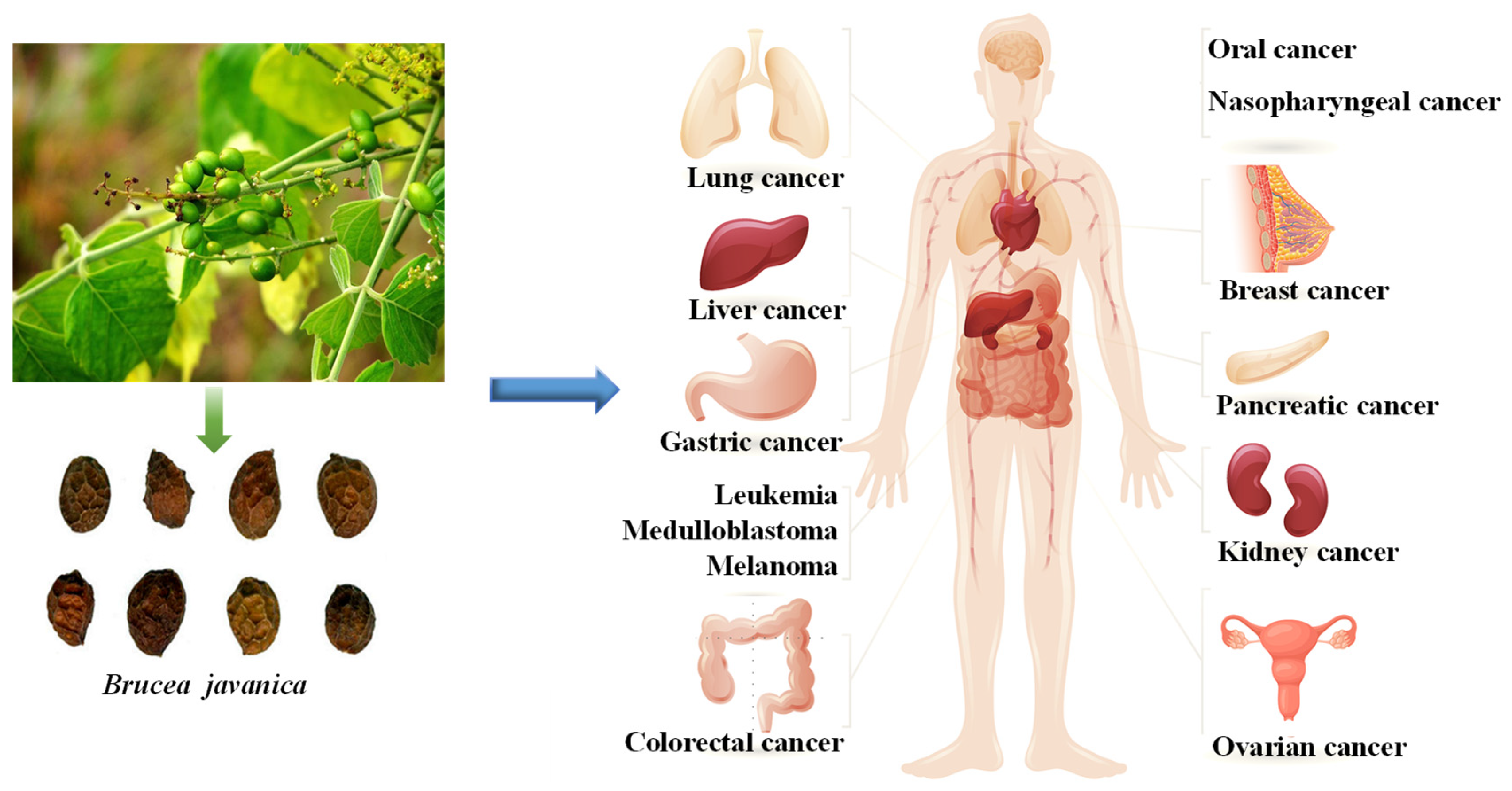
1. Introduction
2. Anticancer Chemical Ingredients
3. Anticancer Effects
3.1. Anti-Lung Cancer
3.2. Anti-Digestive System Cancer
3.3. Anti-Reproductive System Cancer
3.4. Anti-Leukemia
3.5. Other Cancers
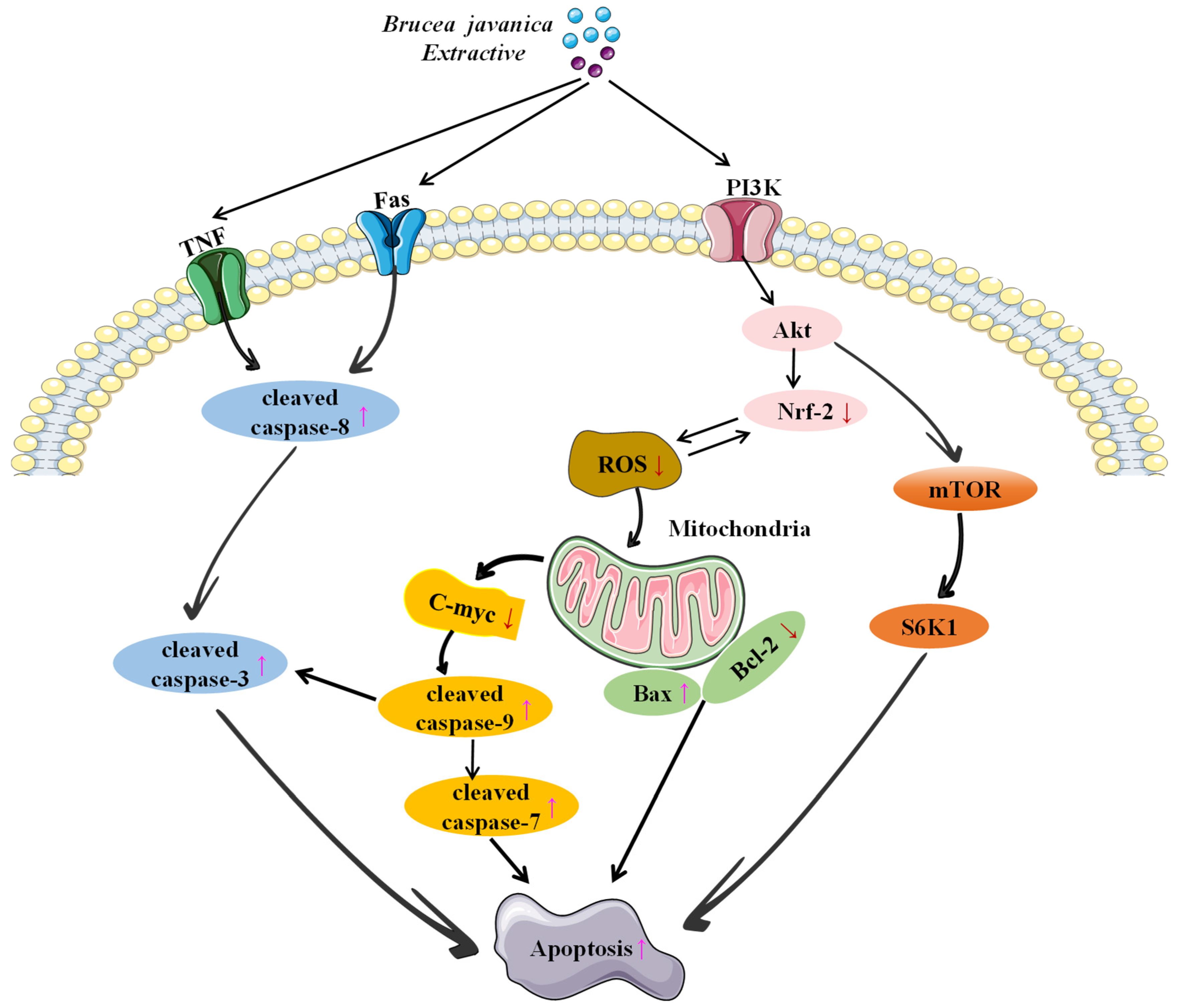
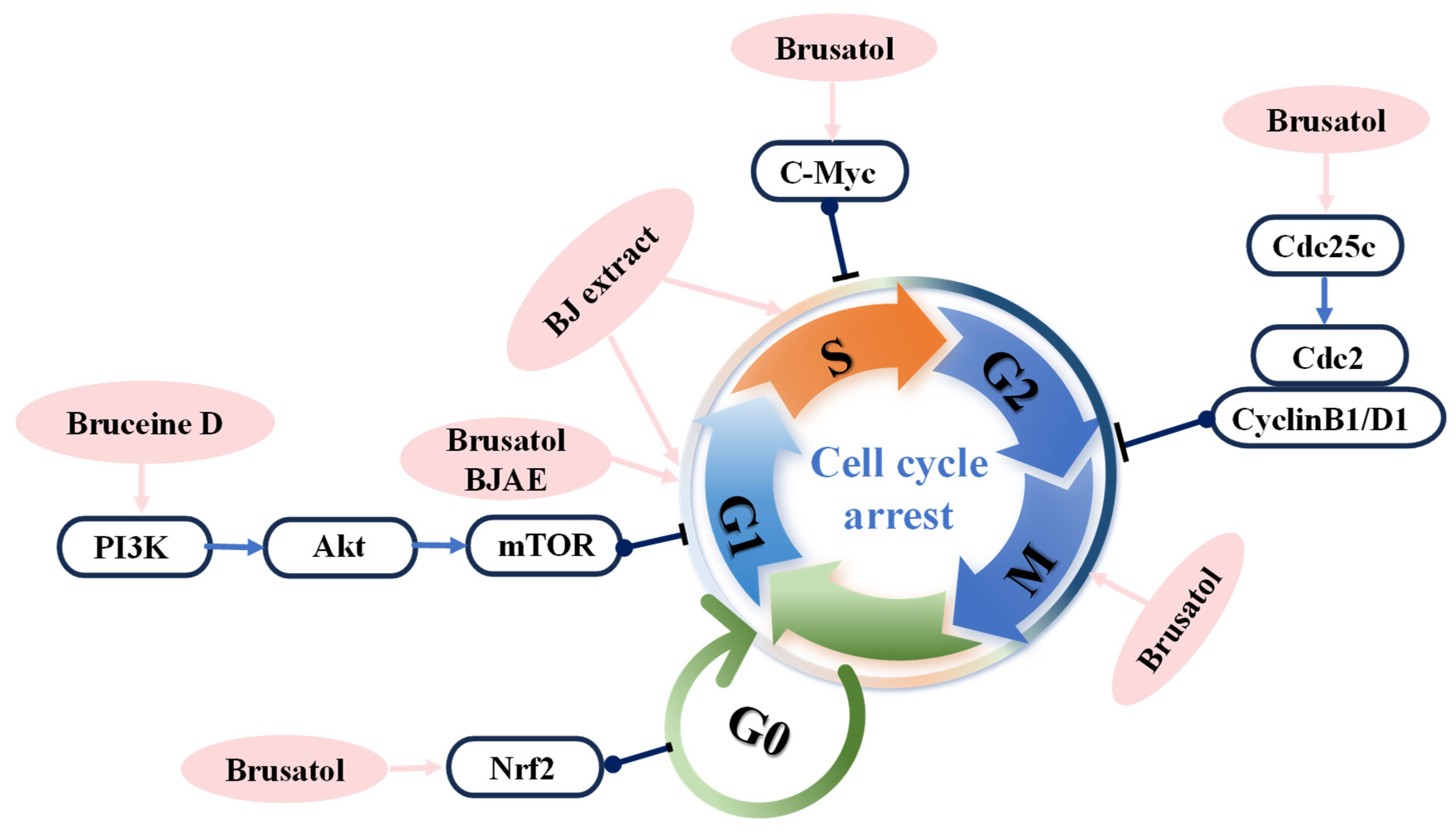
4. Anticancer Mechanisms
4.1. Inducing Apoptosis
4.2. Inhibition of Cell Proliferation and Inducing Cell Cycle Arrest
4.3. Inhibition of Migration/Invasion
5. BJ Antitumor Clinical Preparations Research
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwartz, S.M. Epidemiology of cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef]
- Kalachaveedu, M.; Senthil, R.; Azhagiyamanavalan, S.; Ravi, R.; Meenakshisundaram, H.; Dharmarajan, A. Traditional medicine herbs as natural product matrices in cancer chemoprevention: A trans pharmacological perspective (scoping review). Phytother. Res. 2023, 37, 1539–1573. [Google Scholar] [CrossRef]
- Fu, B.; Wang, N.; Tan, H.Y.; Li, S.; Cheung, F.; Feng, Y. Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: A review on experimental and clinical evidences. Front. Pharmacol. 2018, 9, 1394. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35 (Suppl.), S276–S304. [Google Scholar] [CrossRef]
- Hu, X.; Huang, W.; Fan, M. Emerging therapies for breast cancer. J. Hematol. Oncol. 2017, 10, 98. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Development of potential antitumor agents from the scaffolds of plant-derived terpenoid lactones. J. Med. Chem. 2020, 63, 15410–15448. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Liu, K.; Chen, Y.; Zhang, Y.; Zhang, Z.; Li, H. Investigating the immune mechanism of natural products in the treatment of lung cancer. Front. Pharmacol. 2024, 15, 1289957. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Chen, H.; Li, R.; Zhang, F.; Yao, Q.; Guo, Y. A scientometric visualization analysis for natural products on cancer research from 2008 to 2020. Front. Pharmacol. 2021, 12, 650141. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Y.; Wang, R.; Najafi, M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis 2022, 27, 647–667. [Google Scholar] [CrossRef]
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf. B Biointerfaces 2023, 228, 113419. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Deng, H.; Niu, T.; Qu, Y.; Qian, Z. Stimulus-responsive nano-prodrug strategies for cancer therapy: A focus on camptothecin delivery. Small Methods 2024, 8, e2301271. [Google Scholar] [CrossRef]
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, X.; Ho, P.C.; Chan, S.Y.; Heng, P.W.; Chan, E.; Duan, W.; Koh, H.L.; Zhou, S. Herb-drug interactions: A literature review. Drugs 2005, 65, 1239–1282. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Shang, X.Y.; Huang, X.X.; Yao, G.D.; Song, S.J. Brusatol: A potential anti-tumor quassinoid from Brucea javanica. Chin. Herb. Med. 2020, 12, 359–366. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Li, X.; Deng, Q.; Yang, H.; Chen, L.; Bai, L. A review of Brucea javanica: Metabolites, pharmacology and clinical application. Front. Pharmacol. 2023, 14, 1317620. [Google Scholar] [CrossRef]
- Tao, S.; Rojo de la Vega, M.; Chapman, E.; Ooi, A.; Zhang, D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef]
- Bagheri, E.; Hajiaghaalipour, F.; Nyamathulla, S.; Salehen, N. The apoptotic effects of Brucea javanica fruit extract against HT29 cells associated with p53 upregulation and inhibition of NF-κB translocation. Drug Des. Dev. Ther. 2018, 12, 657–671. [Google Scholar] [CrossRef]
- Zhao, M.; Lau, S.T.; Leung, P.S.; Che, C.T.; Lin, Z.X. Seven quassinoids from Fructus Bruceae with cytotoxic effects on pancreatic adenocarcinoma cell lines. Phytother. Res. 2011, 25, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; He, D.; Dang, J.; Al, E. Treatment of prostatic carcinoma (stage C to D) with fructus bruceae emulsion. J. Mod. Urol. 1998, 68–71. [Google Scholar]
- Shan, G.Y.; Zhang, S.; Li, G.W.; Chen, Y.S.; Liu, X.A.; Wang, J.K. Clinical evaluation of oral Fructus bruceae oil combined with radiotherapy for the treatment of esophageal cancer. Chin. J. Integr. Med. 2011, 17, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Q.; Huang, X.E.; Wu, X.Y.; Liu, J.; Wang, L.; Tang, J.H. Safety of Brucea javanica and cantharidin combined with chemotherapy for treatment of NSCLC patients. Asian Pac. J. Cancer Prev. 2014, 15, 8603–8605. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, X.; Xu, Z.; Ye, T.; Shi, Q. The Efficacy of Brucea javanica oil emulsion injection as adjunctive therapy for advanced non-small-cell lung cancer: A meta-analysis. Evid. Based Complement. Altern. Med. 2016, 2016, 5928562. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Cao, L.; Wu, J.; Lu, T.; Li, S.; Li, J. Efficacy and safety of Brucea javanica oil emulsion injection in the treatment of gastric cancer: A systematic review and meta-analysis. Front. Nutr. 2021, 8, 784164. [Google Scholar] [CrossRef]
- Rohilla, S.; Dureja, H.; Chawla, V. Cytoprotective agents to avoid chemotherapy induced sideeffects on normal cells: A review. Curr. Cancer Drug Targets 2019, 19, 765–781. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhou, H.Y.; Wang, Y.T.; Chen, W.; Qi, S.Y.; Cao, J.L.; Li, G.H. Assessment on the efficacy and safety of Aidi injection combined with vinorelbine and cisplatin for treatment of advanced nonsmall cell lung cancer. Chin. Med. J. 2016, 129, 723–730. [Google Scholar] [CrossRef]
- Chen, H.; Bai, J.; Fang, Z.-F.; Yu, S.-S.; Ma, S.-G.; Xu, S.; Li, Y.; Qu, J.; Ren, J.-H.; Li, L.; et al. Indole alkaloids and quassinoids from the stems of Brucea mollis. J. Nat. Prod. 2011, 74, 2438–2445. [Google Scholar] [CrossRef]
- Chen, Q.J.; Ouyang, M.A.; Tan, Q.W.; Zhang, Z.K.; Wu, Z.J.; Lin, Q.Y. Constituents from the seeds of Brucea javanica with inhibitory activity of Tobacco mosaic virus. J. Asian Nat. Prod. Res. 2009, 11, 539–547. [Google Scholar] [CrossRef]
- Hong-Wu, W.; Yan-Qing, L.; Zi-Jun, Y.; Shou-Lian, W.; Jin-Qing, Y.E. A gas chromatography-mass spectrometry analysis of the essential oils from Brucea javanica extracted with different methods. Fine Chem. 2011, 28, 668–670. [Google Scholar]
- Liu, J.H.; Jin, H.Z.; Zhang, W.D.; Yan, S.K.; Shen, Y.H. Chemical constituents of plants from the genus Brucea. Chem. Biodivers. 2009, 6, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, R.; Wang, S.; Tan, W.; Hu, Y.; Peng, X.; Wang, Y. Chemical components, pharmacological properties, and nanoparticulate delivery systems of Brucea javanica. Int. J. Nanomed. 2013, 8, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Cuendet, M.; Gills, J.J.; Pezzuto, J.M. Brusatol-induced HL-60 cell differentiation involves NF-kappaB activation. Cancer Lett. 2004, 206, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Hao, J.; Xu, Z.; Huang, R.; Zhang, N.; Qiu, S. A new quassinoid from fruits of Brucea javanica. Nat. Prod. Res. 2013, 27, 2016–2021. [Google Scholar] [CrossRef]
- Kim, I.H.; Takashima, S.; Hitotsuyanagi, Y.; Hasuda, T.; Takeya, K. New quassinoids, javanicolides C and D and javanicosides B--F, from seeds of Brucea javanica. J. Nat. Prod. 2004, 67, 863–868. [Google Scholar] [CrossRef]
- Ye, Q.M.; Bai, L.L.; Hu, S.Z.; Tian, H.Y.; Ruan, L.J.; Tan, Y.F.; Hu, L.P.; Ye, W.C.; Zhang, D.M.; Jiang, R.W. Isolation, chemotaxonomic significance and cytotoxic effects of quassinoids from Brucea javanica. Fitoterapia 2015, 105, 66–72. [Google Scholar] [CrossRef]
- Duan, Z.K.; Zhang, Z.J.; Dong, S.H.; Wang, Y.X.; Song, S.J.; Huang, X.X. Quassinoids: Phytochemistry and antitumor prospect. Phytochemistry 2021, 187, 112769. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, J.Y.; Sun, F.; Yan, J.J.; Wang, J.L.; Zhang, Z.X.; Zhang, Y.; Wang, T. Relationship between structural characteristics and plant sources along with pharmacology research of quassinoids. Chem. Pharm. Bull. 2019, 67, 654–665. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, R.; Liu, B.; Zou, J.; Wang, L.; Xia, J. Quantitative determination of contents of three components in Brucea javanica by HPLC. Zhongguo Zhong Yao Za Zhi 2011, 36, 1979–1981. [Google Scholar]
- Hu, S.Z.; Jin, L.; Yu, T.; Tian, H.Y.; Jiang, R.W. Brusatol. Acta Crystallogr. Sect. E Crystallogr. Commun. 2012, 68, o1592–o1593. [Google Scholar] [CrossRef] [PubMed]
- Fukamiya, N.; Lee, K.H.; Muhammad, I.; Murakami, C.; Okano, M.; Harvey, I.; Pelletier, J. Structure-activity relationships of quassinoids for eukaryotic protein synthesis. Cancer Lett. 2005, 220, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hitotsuyanagi, Y.; Kim, I.H.; Hasuda, T.; Yamauchi, Y.; Takeya, K. A structure–activity relationship study of brusatol, an antitumor quassinoid. Tetrahedron 2006, 62, 4262–4271. [Google Scholar] [CrossRef]
- Luyengi, L.; Suh, N.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. A lignan and four terpenoids from Brucea javanica that induce differentiation with cultured HL-60 promyelocytic leukemia cells. Phytochemistry 1996, 43, 409–412. [Google Scholar] [CrossRef]
- Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer-epidemiology, pathogenesis, treatment and molecular aspect (review of literature). Int. J. Mol. Sci. 2025, 26, 2049. [Google Scholar] [CrossRef]
- Shalata, W.; Naamneh, R.; Najjar, W.; Asla, M.; Abu Gameh, A.; Abu Amna, M.; Saiegh, L.; Agbarya, A. Current and emerging therapeutic strategies for limited- and extensive-stage small-cell lung cancer. Med. Sci. 2025, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Fukamiya, N.; Okano, M.; Tagahara, K.; Lee, K.H. Bruceanols D, E, and F three new cytotoxic quassinoids from Brucea antidysenterica. J. Nat. Prod. 1993, 56, 2091–2097. [Google Scholar] [CrossRef]
- Fukamiya, N.; Okano, M.; Tagahara, K.; Aratani, T.; Lee, K.H. Antitumor agents, 93. Bruceanol C, a new cytotoxic quassinoid from Brucea antidysenterica. J. Nat. Prod. 1988, 51, 349–352. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, C.F.; Li, X.Y.; Chen, J.C.; Li, Y.; Qiu, M.H. One new pregnane glycoside from the seeds of cultivated Brucea javanica. Arch. Pharm. Res. 2011, 34, 1297–1300. [Google Scholar] [CrossRef]
- Fukamiya, N.; Okano, M.; Miyamoto, M.; Tagahara, K.; Lee, K.H. Antitumor agents, 127. Bruceoside C, a new cytotoxic quassinoid glucoside, and related compounds from Brucea javanica. J. Nat. Prod. 1992, 55, 468–475. [Google Scholar] [CrossRef]
- Ohnishi, S.; Fukamiya, N.; Okano, M.; Tagahara, K.; Lee, K.H. Bruceosides D, E, and F, three new cytotoxic quassinoid glucosides from Brucea javanica. J. Nat. Prod. 1995, 58, 1032–1038. [Google Scholar] [CrossRef]
- Chen, H.; Ma, S.G.; Fang, Z.F.; Bai, J.; Yu, S.S.; Chen, X.G.; Hou, Q.; Yuan, S.P.; Chen, X. Tirucallane triterpenoids from the stems of Brucea mollis. Chem. Biodivers. 2013, 10, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Chang, L.C.; Park, E.J.; Cuendet, M.; Santarsiero, B.D.; Mesecar, A.D.; Mehta, R.G.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Bioactive constituents of the seeds of Brucea javanica. Planta Med. 2002, 68, 730–733. [Google Scholar] [CrossRef]
- Tan, B.; Huang, Y.; Lan, L.; Zhang, B.; Ye, L.; Yan, W.; Wang, F.; Lin, N. Bruceine D induces apoptosis in human non-small cell lung cancer cells through regulating JNK pathway. Biomed. Pharmacother. 2019, 117, 109089. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Huang, H.; Li, J.; Zhu, Y.; Huang, R.; Qiu, S.X. Chemical composition and cytotoxic activities of petroleum ether fruit extract of fruits of Brucea javanica (Simarubaceae). Trop. J. Pharm. Res. 2013, 12, 735–742. [Google Scholar] [CrossRef]
- Lau, F.Y.; Chui, C.H.; Gambari, R.; Kok, S.H.; Kan, K.L.; Cheng, G.Y.; Wong, R.S.; Teo, I.T.; Cheng, C.H.; Wan, T.S.; et al. Antiproliferative and apoptosis-inducing activity of Brucea javanica extract on human carcinoma cells. Int. J. Mol. Med. 2005, 16, 1157–1162. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, C.Y.; Fan, P.W.; Hsieh, C.H.; Lin, H.Y.; Lee, M.C.; Fang, K. The aqueous extract of Brucea javanica suppresses cell growth and alleviates tumorigenesis of human lung cancer cells by targeting mutated epidermal growth factor receptor. Drug Des. Dev. Ther. 2016, 10, 3599–3609. [Google Scholar] [CrossRef]
- Makong, Y.S.; Mouthé Happi, G.; Djouaka Bavoua, J.L.; Wansi, J.D.; Nahar, L.; Kamdem Waffo, A.F.; Martin, C.; Sewald, N.; Sarker, S.D. Cytotoxic stilbenes and canthinone alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules 2019, 24, 4412. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Stoop, T.F.; Javed, A.A.; Oba, A.; Koerkamp, B.G.; Seufferlein, T.; Wilmink, J.W.; Besselink, M.G. Pancreatic cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef]
- Connor, A.A.; Gallinger, S. Pancreatic cancer evolution and heterogeneity: Integrating omics and clinical data. Nat. Rev. Cancer 2022, 22, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ye, W.; Huang, C.; Lou, B.; Zhang, J.; Yu, D.; Huang, X.; Chen, B.; Zhou, M. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.T.; Lin, Z.X.; Liao, Y.; Zhao, M.; Cheng, C.H.; Leung, P.S. Bruceine D induces apoptosis in pancreatic adenocarcinoma cell line PANC-1 through the activation of p38-mitogen activated protein kinase. Cancer Lett. 2009, 281, 42–52. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Z.X.; Leung, P.S.; Chen, L.H.; Zhao, M.; Liang, J. Involvement of the mitochondrial pathway in bruceine D-induced apoptosis in Capan-2 human pancreatic adenocarcinoma cells. Int. J. Mol. Med. 2012, 30, 93–99. [Google Scholar] [CrossRef]
- Zhang, P.; Tao, W.; Lu, C.; Fan, L.; Jiang, Q.; Yang, C.; Shang, E.; Cheng, H.; Che, C.; Duan, J.; et al. Bruceine A induces cell growth inhibition and apoptosis through PFKFB4/GSK3β signaling in pancreatic cancer. Pharmacol. Res. 2021, 169, 105658. [Google Scholar] [CrossRef] [PubMed]
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef]
- Osumi, H.; Shinozaki, E.; Yamaguchi, K.; Zembutsu, H. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci. 2019, 110, 1148–1155. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhao, N.; Zhang, G.J.; Yu, S.S.; Wu, L.J.; Qu, J.; Ma, S.G.; Chen, X.G.; Zhang, T.Q.; Bai, J.; et al. Bioactive quassinoids from the seeds of Brucea javanica. J. Nat. Prod. 2012, 75, 683–688. [Google Scholar] [CrossRef]
- Oh, E.T.; Kim, C.W.; Kim, H.G.; Lee, J.S.; Park, H.J. Brusatol-mediated inhibition of c-Myc increases HIF-1α degradation and causes cell death in colorectal cancer under hypoxia. Theranostics 2017, 7, 3415–3431. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, B.; Shi, Q.; Wang, X.; Wang, D.; Zhu, L. Brusatol inhibits HIF-1 signaling pathway and suppresses glucose uptake under hypoxic conditions in HCT116 cells. Sci. Rep. 2016, 6, 39123. [Google Scholar] [CrossRef]
- Evans, J.P.; Winiarski, B.K.; Sutton, P.A.; Jones, R.P.; Ressel, L.; Duckworth, C.A.; Pritchard, D.M.; Lin, Z.X.; Fretwell, V.L.; Tweedle, E.M.; et al. The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget 2018, 9, 27104–27116. [Google Scholar] [CrossRef]
- Bagheri, E.; Hajiaghaalipour, F.; Nyamathulla, S.; Salehen, N.A. Ethanolic extract of Brucea javanica inhibit proliferation of HCT-116 colon cancer cells via caspase activation. RSC Adv. 2018, 8, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Lai, Z.Q.; Liao, H.J.; Xie, J.H.; Xian, Y.F.; Chen, Y.L.; Ip, S.P.; Lin, Z.X.; Su, Z.R. Synergistic antitumor effect of brusatol combined with cisplatin on colorectal cancer cells. Int. J. Mol. Med. 2018, 41, 1447–1454. [Google Scholar] [CrossRef]
- Donne, R.; Lujambio, A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 2023, 77, 1773–1796. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, X.; Wang, L.; Jiang, S.; Luo, H.; Chen, Y.; Peng, C. The pathogenesis of liver cancer and the therapeutic potential of bioactive substances. Front. Pharmacol. 2022, 13, 1029601. [Google Scholar] [CrossRef]
- Ye, R.; Dai, N.; He, Q.; Guo, P.; Xiang, Y.; Zhang, Q.; Hong, Z.; Zhang, Q. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 105, 962–973. [Google Scholar] [CrossRef]
- Chen, J.H.; Kim, S.H.; Fan, P.W.; Liu, C.Y.; Hsieh, C.H.; Fang, K. The aqueous extract of Chinese medicinal herb Brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Des. Dev. Ther. 2016, 10, 2003–2013. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Matulonis, U.A. Clinical and translational advances in ovarian cancer therapy. Nat. Cancer 2023, 4, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Zhang, X.; Peng, J.; Zhang, S. What predicts the clinical benefits of PARP inhibitors in platinum-sensitive recurrent ovarian cancer: A real-world single-center retrospective cohort study from China. Front. Oncol. 2022, 12, 955124. [Google Scholar] [CrossRef]
- Qi, Y.J.; Su, G.H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.Z.; Shao, Z.M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef]
- Pan, L.; Chin, Y.W.; Chai, H.B.; Ninh, T.N.; Soejarto, D.D.; Kinghorn, A.D. Bioactivity-guided isolation of cytotoxic constituents of Brucea javanica collected in Vietnam. Bioorganic Med. Chem. 2009, 17, 2219–2224. [Google Scholar] [CrossRef]
- Chumkaew, P.; Srisawat, T. Antimalarial and cytotoxic quassinoids from the roots of Brucea javanica. J. Asian Nat. Prod. Res. 2017, 19, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Haryanti, S.; Sholikhah, I.Y.M.; Widiyastuti, Y. Cytotoxic and anti-migratory effects of Brucea javanica fruits extract on 4T1 breast cancer cells. AIP Conf. Proc. 2019, 2202, 020097. [Google Scholar] [CrossRef]
- Su, Z.; Ma, Z.; Liu, K.; Li, T.; Zhou, B. Quassilactones A and B, structural characterization of a new class of norquassinoids from Brucea javanica. J. Nat. Med. 2020, 74, 599–605. [Google Scholar] [CrossRef]
- Ciantra, Z.; Paraskevopoulou, V.; Aifantis, I. The rewired immune microenvironment in leukemia. Nat. Immunol. 2025, 26, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Britton, R.W.; Lacadie, J.A.; Ziegler, M.F.; Sigel, C.W. Tumor inhibitors. 100. Isolation and structural elucidation of bruceantin and bruceantinol, new potent antileukemic quassinoids from Brucea antidysenterica. J. Org. Chem. 1975, 40, 648–654. [Google Scholar] [CrossRef]
- Okano, M.; Lee, K.H.; Hall, I.H.; Boettner, F.E. Antitumor agents. 39. Bruceantinoside-A and -B, novel antileukemic quassinoid glucosides from Brucea antidysenterica. J. Nat. Prod. 1981, 44, 470–474. [Google Scholar] [CrossRef]
- Lee, K.H.; Hayashi, N.; Okano, M.; Nozaki, H.; Ju-Ichi, M. Antitumor agents, 65. Brusatol and cleomiscosin-A, antileukemic principles from Brucea javanica. J. Nat. Prod. 1984, 47, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Fukamiya, N.; Aratani, T.; Juichi, M.; Lee, K.H. Antitumor agents, 74. Bruceanol-A and -B, two new antileukemic quassinoids from Brucea antidysenterica. J. Nat. Prod. 1985, 48, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, T.; Yoshimura, S.; Tsuyuki, T.; Takahashi, T.; Honda, T. Yadanzioside P, a new antileukemic quassinoid glycoside from Brucea javanica (L.) Merr with the 3-O-(beta-D-glucopyranosyl) bruceantin structure. Chem. Pharm. Bull. 1986, 34, 4447–4450. [Google Scholar] [CrossRef]
- Fukamiya, N.; Okano, M.; Tagahara, K.; Aratani, T.; Muramoto, Y.; Lee, K.H. Antitumor agents, 90. Bruceantinoside C, a new cytotoxic quassinoid glycoside from Brucea antidysenterica. J. Nat. Prod. 1987, 50, 1075–1079. [Google Scholar] [CrossRef]
- Toyota, T.; Fukamiya, N.; Okano, M.; Tagahara, K.; Chang, J.J.; Lee, K.H. Antitumor agents, 118. The isolation and characterization of bruceanic acid A, its methyl ester, and the new bruceanic acids B, C, and D, from Brucea antidysenterica. J. Nat. Prod. 1990, 53, 1526–1532. [Google Scholar] [CrossRef]
- Kim, I.H.; Hitotsuyanagi, Y.; Takeya, K. Quassinoid glucosides from seeds of Brucea amarissima. Phytochemistry 2004, 65, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Mata-Greenwood, E.; Cuendet, M.; Sher, D.; Gustin, D.; Stock, W.; Pezzuto, J.M. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc. Leukemia 2002, 16, 2275–2284. [Google Scholar] [CrossRef]
- Sakaki, T.; Yoshimura, S.; Ishibashi, M.; Tsuyuki, T.; Takahashi, T.; Honda, T.; Nakanishi, T. Structures of new quassinoid glycosides, yadanziosides A, B, C, D, E, G, H, and new quassinoids, dehydrobrusatol and dehydrobruceantinol from Brucea javanica (L.) Merr. Bull. Chem. Soc. Jpn. 1985, 58, 2680–2686, Erratum in Bull. Chem. Soc. Jpn. 1986, 59, 3541–3546. [Google Scholar] [CrossRef]
- Anderson, M.M.; O’Neill, M.J.; Phillipson, J.D.; Warhurst, D.C. In vitro cytotoxicity of a series of quassinoids from Brucea javanica fruits against KB cells. Planta Med. 1991, 57, 62–64. [Google Scholar] [CrossRef]
- Chumkaew, P.; Srisawat, T. New neolignans from the seeds of Myristica fragrans and their cytotoxic activities. J. Nat. Med. 2019, 73, 273–277. [Google Scholar] [CrossRef]
- Majid, M.Z.; Zaini, Z.M.; Razak, F.A. Apoptosis-inducing effect of three medicinal plants on oral cancer cells KB and ORL-48. Sci. World J. 2014, 2014, 125353. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, Y.; Wang, P.; Gao, W. Brusatol inhibits amyloid-β-induced neurotoxicity in U-251 cells via regulating the Nrf2/HO-1 pathway. J. Cell. Biochem. 2019, 120, 10556–10563. [Google Scholar] [CrossRef]
- Tang, X.; Fu, X.; Liu, Y.; Yu, D.; Cai, S.J.; Yang, C. Blockade of glutathione metabolism in IDH1-mutated glioma. Mol. Cancer Ther. 2020, 19, 221–230. [Google Scholar] [CrossRef]
- Lee, J.; Mawla, I.; Kim, J.; Loggia, M.L.; Ortiz, A.; Jung, C.; Chan, S.T.; Gerber, J.; Schmithorst, V.J.; Edwards, R.R.; et al. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain 2019, 160, 550–560. [Google Scholar] [CrossRef]
- Xie, J.; Lai, Z.; Zheng, X.; Liao, H.; Xian, Y.; Li, Q.; Wu, J.; Ip, S.; Xie, Y.; Chen, J.; et al. Apoptotic activities of brusatol in human non-small cell lung cancer cells: Involvement of ROS-mediated mitochondrial-dependent pathway and inhibition of Nrf2-mediated antioxidant response. Toxicology 2021, 451, 152680. [Google Scholar] [CrossRef]
- Moyer, A.; Tanaka, K.; Cheng, E.H. Apoptosis in cancer biology and therapy. Annu. Rev. Pathol. Mech. Dis. 2025, 20, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lai, Z.Q.; Leung, A.W.N.; Leung, P.S.; Li, Z.S.; Lin, Z.X. Exploring brusatol as a new anti-pancreatic cancer adjuvant: Biological evaluation and mechanistic studies. Oncotarget 2017, 8, 84974–84985. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Xu, J.; Lu, J.; Cai, L.; Li, Q.; Wang, C.; Su, Z. Brusatol inhibits tumor growth and increases the efficacy of cabergoline against pituitary adenomas. Oxidative Med. Cell. Longev. 2021, 2021, 6696015. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Wei, C.; Lu, Z.; Cai, R.; Pan, D.; Zhang, H.; Liang, B.; Zhang, Z. Anticancer effects of brusatol in nasopharyngeal carcinoma through suppression of the Akt/mTOR signaling pathway. Cancer Chemother. Pharmacol. 2020, 85, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Xie, C.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea javanica. J. Ethnopharmacol. 2013, 147, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, T.; Chen, H.; Su, J.; Wang, X.; Cao, Y.; Li, Q. Brusatol reverses lipopolysaccharide-induced epithelial-mesenchymal transformation and induces apoptosis through PI3K/Akt/NF-κB pathway in human gastric cancer SGC-7901 cells. Anticancer. Drugs 2021, 32, 394–404. [Google Scholar] [CrossRef]
- Ya-Fang, T.; Juan, L.I.; Shu-Zhi, H.U.; Ren-Wang, J. Inhibitory effects of brusatol on human prostate cancer cells DU145 and its molecular mechanism. Guihaia 2015, 35, 431–436. [Google Scholar]
- Xiao, Z.; Chow, S.C.; Li, C.H.; Tang, S.C.; Tsui, S.K.W.; Lin, Z.; Chen, Y. Role of microRNA-95 in the anticancer activity of Brucein D in hepatocellular carcinoma. Eur. J. Pharmacol. 2014, 728, 141–150. [Google Scholar] [CrossRef]
- Glaviano, A.; Singh, S.K.; Lee, E.H.C.; Okina, E.; Lam, H.Y.; Carbone, D.; Reddy, E.P.; O’Connor, M.J.; Koff, A.; Singh, G.; et al. Cell cycle dysregulation in cancer. Pharmacol. Rev. 2025, 77, 100030. [Google Scholar] [CrossRef]
- Min, W.; Qili, L.; Shuai, X.U.; Pinyu, L.I.; Juntao, L.I. Study on the mechanism of the influence of Brusatol for the Nrf2-Notch1 axis of non-small cell lung cancer. China Med. Her. 2018, 15, 16–19. [Google Scholar]
- Cheng, Z.; Yuan, X.; Qu, Y.; Li, X.; Wu, G.; Li, C.; Zu, X.; Yang, N.; Ke, X.; Zhou, J.; et al. Bruceine D inhibits hepatocellular carcinoma growth by targeting β-catenin/jagged1 pathways. Cancer Lett. 2017, 403, 195–205. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Y.; Wei, C.; Chen, Y.; Ji, Z. The anti-migration and anti-invasion effects of Bruceine D in human triple-negative breast cancer MDA-MB-231 cells. Exp. Ther. Med. 2020, 19, 273–279. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Li, D.; Li, M.; Su, Z.; Lai, X.; Zhou, C.; Chen, S.; Li, S.; Yang, X.; et al. Ethanol extract of Brucea javanica seed inhibit triple-negative breast cancer by restraining autophagy via PI3K/Akt/mTOR pathway. Front. Pharmacol. 2020, 11, 606. [Google Scholar] [CrossRef]
- Wang, M.; Shi, G.; Bian, C.; Nisar, M.F.; Guo, Y.; Wu, Y.; Li, W.; Huang, X.; Jiang, X.; Bartsch, J.W.; et al. UVA irradiation enhances Brusatol-mediated inhibition of melanoma growth by downregulation of the Nrf2-mediated antioxidant response. Oxidative Med. Cell. Longev. 2018, 2018, 9742154. [Google Scholar] [CrossRef] [PubMed]
- Bovilla, V.R.; Kuruburu, M.G.; Bettada, V.G.; Krishnamurthy, J.; Sukocheva, O.A.; Thimmulappa, R.K.; Shivananju, N.S.; Balakrishna, J.P.; Madhunapantula, S.V. Targeted inhibition of anti-inflammatory regulator Nrf2 results in breast cancer retardation in vitro and in vivo. Biomedicines 2021, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, B.; Li, Y.; Yuan, Q. Potential mechanisms of cancer prevention and treatment by sulforaphane, a natural small molecule compound of plant-derived. Mol. Med. 2024, 30, 94. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, M.R.; Zhang, J.; Reinhart-King, C.A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021, 33, 1307–1321. [Google Scholar] [CrossRef]
- Abdelwahed, K.S. Pseurotin a as a Novel PCSK9 Axis Lead Modulator for the Control of Breast and Prostate Malignancy Recurrences. Ph.D. Thesis, University of Louisiana at Monroe, Monroe, LA, USA, 2021. [Google Scholar]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial-mesenchymal transition: A new target in anticancer drug discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 26, 83–94. [Google Scholar] [CrossRef]
- He, T.; Zhou, F.; Su, A.; Zhang, Y.; Xing, Z.; Mi, L.; Li, Z.; Wu, W. Brusatol: A potential sensitizing agent for cancer therapy from Brucea javanica. Biomed. Pharmacother. 2023, 158, 114134. [Google Scholar] [CrossRef]
- Déry, M.A.; Michaud, M.D.; Richard, D.E. Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Xu, Y.; Ma, Q.; Guo, F.; Zhao, Y.; Tao, Y.; Li, M.; Guo, J. Nrf2/HO-1 axis regulates the angiogenesis of gastric cancer via targeting VEGF. Cancer Manag. Res. 2021, 13, 3155–3169. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, Q.; Li, Q.; Pan, R. Silencing of BRF2 inhibits the growth and metastasis of lung cancer cells. Mol. Med. Rep. 2020, 22, 1767–1774. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Chou, H.C.; Liou, M.H.; Liao, E.C.; Cheng, C.T.; Chang, S.J.; Chan, H.L. Role of IGFBP-2 in oral cancer metastasis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, Z.; Jia, R.; Wang, N.A.; Shi, M.; Wang, Y.; Xiang, S.; Zhang, Q.; Xu, L. CD151-enriched migrasomes mediate hepatocellular carcinoma invasion by conditioning cancer cells and promoting angiogenesis. J. Exp. Clin. Cancer Res. 2024, 43, 160. [Google Scholar] [CrossRef]
- Guo, C.; Wei, L.I.; Xiao-Hui, L.I. The inhibitory effects of brusatol on the migration of human non-small cell lung carcinoma A549 cells and its molecular mechanism. Chin. J. Gerontol. 2016, 36, 3897–3899. [Google Scholar]
- So, T.H.; Chan, S.K.; Lee, V.H.; Chen, B.Z.; Kong, F.M.; Lao, L.X. Chinese Medicine in cancer treatment—How is it practised in the east and the West? Clin. Oncol. 2019, 31, 578–588. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese Medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Shou-Xiang, Y.; Peng, J.; Pharmacy, D.O. Literature analysis on adverse drug reactions induced by Brucea Jananica Oil Injection. Drug Eval. Res. 2017, 40, 266–269. [Google Scholar]
- Li, K.W.; Liang, Y.Y.; Wang, Q.; Li, Y.; Zhou, S.J.; Wei, H.C.; Zhou, C.Z.; Wan, X.H. Brucea javanica: A review on anticancer of its pharmacological properties and clinical researches. Phytomedicine 2021, 86, 153560. [Google Scholar] [CrossRef]
- Rong, S.U.N.; Qian, Y. Research development on toxicity of fructus Bruceae based on efficacy and material basis. Chin. J. Pharmacovigil. 2010, 7, 159. [Google Scholar]
- Hang, Y.; Feng, P.P.; Jiang, H.H.; Liu, K.; Zhang, D.; Li, S.Y. High-throughput and rapid characteristics analysis of Brucea javanica oil and its oral drugs by ultra high resolution mass spectrometry. J. Instrum. Anal. 2023, 42, 531–540. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.Y.; Zhou, F.; Wang, L.H.; Zhang, W.; Sha, S.; Wu, C.F. Seed oil of Brucea javanica induces apoptotic death of acute myeloid leukemia cells via coth the death receptors and the mitochondrial-related pathways. Evid. Based Complement. Altern. Med. 2011, 2011, 965016. [Google Scholar] [CrossRef]
- Shi, W.R.; Liu, Y.; Wang, X.T.; Huang, Q.Y.; Cai, X.R.; Wu, S.R. Antitumor efficacy and mechanism in hepatoma H22-bearing mice of Brucea javanica oil. Evid. Based Complement. Altern. Med. 2015, 2015, 217494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, Z.; Zhang, B.; Huang, Y.; Qiu, H.; Chen, P.; Guo, G.F. Involvement of autophagy inhibition in Brucea javanica oil emulsion-induced colon cancer cell death. Oncol. Lett. 2015, 9, 1425–1431. [Google Scholar] [CrossRef]
- Wang, D.; Qu, X.; Zhuang, X.; Geng, G.; Hou, J.; Xu, N.; Li, W.; Hu, T.; Chen, Y.S. Seed oil of Brucea javanica induces cell cycle arrest and apoptosis via reactive oxygen species-mediated mitochondrial dysfunction in human lung cancer cells. Nutr. Cancer 2016, 68, 1394–1403. [Google Scholar] [CrossRef]
- Nan, Z.; Feng-Juan, H.; Gui-Yuan, W.; Yu-Hua, L.I. Effect of Brucea javanica fruit oil emulsion combination with cisplatin on the growth of human ovarian cancer SKOV3 cells nude mouse orthotopic transplantation tumor. Lett. Biotechnol. 2015, 26, 519–523. [Google Scholar]
- Zhao, N.; Li, Y.H.; Wu, X.K.; Wang, G.Y.; Cai, D.Y.; Han, F.J. Effect of Brucea javanica fruit oil emulsion combined cisplatin on the growth inhibition of transplanted tumor in human ovarian cancer SKOV3 nude mice: An experimental study. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015, 35, 57–62. [Google Scholar]
- Zhonghui, S. Experiment study of oleum fructus bruceae vein emulsion on ovarian cancer cell strain. Mod. J. Integr. Tradit. Chin. West. Med. 2009, 18, 1591–1592. [Google Scholar]
- Wang, Y.; Chen, B.; Xiao, M.; Wang, X.; Peng, Y. Brucea javanica oil emulsion promotes autophagy in ovarian cancer cells through the miR-8485/LAMTOR3/mTOR/ATG13 signaling axis. Front. Pharmacol. 2022, 13, 935155. [Google Scholar] [CrossRef]
- Pan, P.; Yang, B.X.; Ge, X.L. Brucea javanica seed oil enhances the radiosensitivity of esophageal cancer by inhibiting hypoxia-inducible factor 1α, in vitro and in vivo. Oncol. Lett. 2018, 15, 3870–3875. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.H.; Zhang, W.W.; Zhang, H.H.; Jiao, G.H. Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis. World J. Gastroenterol. 2019, 25, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, J.F.; Wang, Y.M.; Chen, W.H.; Qian, S.; Zhou, Z.G.; Xu, M. Brucea javanica oil emulsion suppresses tumor growth in human cervical cancer cells through inhibition of the E6 oncogene and induction of apoptosis. Transl. Cancer Res. 2020, 9, 918–929. [Google Scholar] [CrossRef]
- Wang, T.; Dou, Y.; Lin, G.; Li, Q.; Nie, J.; Chen, B.; Xie, J.; Su, Z.; Zeng, H.; Chen, J.; et al. The anti-hepatocellular carcinoma effect of Brucea javanica oil in ascitic tumor-bearing mice: The detection of brusatol and its role. Biomed. Pharmacother. 2021, 134, 111122. [Google Scholar] [CrossRef]
- Su, J.; Chen, X.; Xiao, Y.; Li, D.; Li, M.; Li, H.; Huang, J.; Lai, Z.; Su, Z.; Xie, Y.; et al. Bruceae fructus oil inhibits triple-negative breast cancer by restraining autophagy: Dependence on the gut microbiota-mediated amino acid regulation. Front. Pharmacol. 2021, 12, 727082. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.G.; Yao, H.P.; Xie, L.P. Brucea javanica oil induces apoptosis in T24 bladder cancer cells via upregulation of caspase-3, caspase-9, and inhibition of NF-kappaB and COX-2 expressions. Am. J. Chin. Med. 2010, 38, 613–624. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, S.L.; Wang, L.H.; Jia, L.N.; Su, G.Y.; Liu, X.Q.; Zhou, F.; Breslin, P.; Meng, R.; Li, Q.Y.; et al. Seed oil of Brucea javanica induces apoptosis through the PI3K/Akt signaling pathway in acute lymphocytic leukemia Jurkat cells. Chin. J. Nat. Med. 2021, 19, 608–620. [Google Scholar] [CrossRef]
- Jin, W.; Han, H.; Zhou, S.; Wang, Y.; Dong, T.; Zhao, C. Therapeutic efficacy of Brucea javanica oil emulsion (BJOE) combined with transcatheter hepatic arterial chemoembolization (TACE) in patients with primary liver cancer. Int. J. Clin. Exp. Med. 2015, 8, 18954–18962. [Google Scholar] [PubMed]
- Chen, J.; Chen, S.; Yang, X.; Wang, S.; Wu, W. Efficacy and safety of Brucea javanica oil emulsion injection as adjuvant therapy for cancer: An overview of systematic reviews and meta-analyses. Phytomedicine 2022, 102, 154141. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, K.; Hu, X. Comparative efficacy and safety of Chinese medicine injections combined with capecitabine and oxaliplatin chemotherapies in treatment of colorectal cancer: A bayesian network meta-analysis. Front. Pharmacol. 2022, 13, 1004259. [Google Scholar] [CrossRef]
- Wu, J.R.; Liu, S.Y.; Zhu, J.L.; Zhang, D.; Wang, K.H. Efficacy of Brucea javanica oil emulsion injection combined with the chemotherapy for treating gastric cancer: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2018, 2018, 6350782. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhang, H.F.; Li, J.Y.; Diao, Y.R.; Huang, M.J.; Gao, D.Y.; Liang, C.H.; Luo, Z.Q. Effectiveness and safety of Brucea javanica oil assisted TACE versus TACE in the treatment of liver cancer: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2024, 15, 1337179. [Google Scholar] [CrossRef] [PubMed]
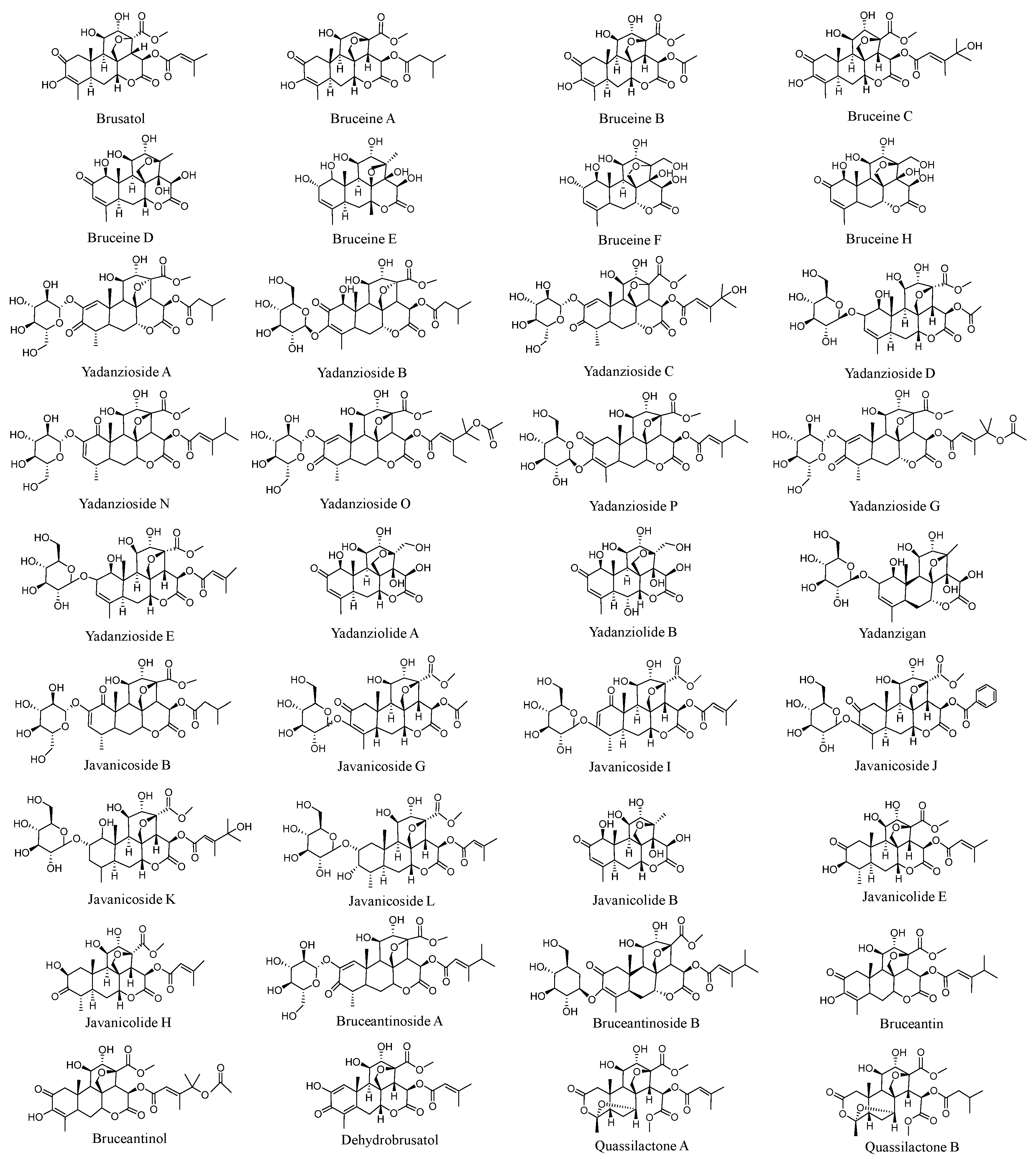

| Cancer Cell Line | Compound/Extract | IC50 | Reference | Cancer Cell Line | Compound/Extract | IC50 | Reference |
|---|---|---|---|---|---|---|---|
| HL-60 | Brusatol | 0.06 μmol/L | [49] | MCF-7 | Brusatol | 0.08 μmol/L | [49] |
| Bruceine B | 0.27 μmol/L | Bruceine B | 0.54 μmol/L | ||||
| Bruceine D | 1.14 μmol/L | Bruceine D | 6.75 μmol/L | ||||
| Bruceine E | 4.48 μmol/L | Bruceine E | 17.77 μmol/L | ||||
| Yadanziolide A | 26.32 μmol/L | Yadanziolide A | 14.61 μmol/L | ||||
| SMMC-7721 | Brusatol | <0.064 μmol/L | [49] | BJ dichloromethane extract | 55.1 μg/mL | [58] | |
| Bruceine B | 0.15 μmol/L | BJ methanol extract | 80.5 μg/mL | ||||
| Bruceine D | 0.88 μmol/L | BJ ethanol extract | 15.12 μg/mL | [55] | |||
| Bruceine E | 4.27 μmol/L | BJ ethyl acetatel extract | 3.28 μg/mL | ||||
| Yadanziolide A | 12.35 μmol/L | BJ petroleum ether extract | 15.15 μg/mL | ||||
| A-549 | Bruceine B | 0.24 μmol/L | [49] | BJ n-butyl alcohol extract | 30.92 μg/mL | ||
| Bruceine D | 3.30 μmol/L | SW480 | Brusatol | 0.10 μmol/L | [49] | ||
| Bruceine E | 7.62 μmol/L | Bruceine B | 0.30 μmol/L | ||||
| Yadanziolide A | 17.05 μmol/L | Bruceine D | 7.78 μmol/L | ||||
| BJ dichloromethane extract | 50.0 μg/mL | [58] | Bruceine E | 28.48 μmol/L | |||
| BJ methanol extract | 75.2 μg/mL | PANC-1 | Brusatol | 0.36 mmol/L | [21] | ||
| BJ water extract | 50 μg/mL | [56] | SW1990 | Brusatol | 0.10 mmol/L | [21] | |
| BJ ethanol extract | 8.79 μg/mL | [55] | Hs68 | Bruceine D | >30 μmol/L | [63] | |
| BJ ethyl acetatel extract | 0.02 μg/mL | HCT-8 | Bruceine B | 2 μmol/L | [68] | ||
| BJ petroleum ether extract | 9.14 μg/mL | Bruceine D | 2 μmol/L | ||||
| BJ n-butyl alcohol extract | 17.47 μg/mL | Bruceine E | 6.70 μmol/L | ||||
| Hep3B | Brusatol | 0.69 μmol/L | [76] | Bruceine H | 1.30 μmol/L | ||
| BJ water extract | 50 μg/mL | [56] | HCT116 | Brusatol | 15 nmol/L | [69] | |
| BJ water extract | 4 mg/mL | [77] | BJ ethanol extract | 8.90 μg/mL | [72] | ||
| Huh7 | Brusatol | 0.34 μmol/L | [76] | CT26 | Brusatol | 373 nmol/L | [70] |
| LM3 | Brusatol | 12.49 μmol/L | [76] | HT29 | BJ ethanol extract | 48 μg/mL | [20] |
| Bel-7404 | Brusatol | 18.04 nmol/L | [76] | BGC-823 | javanicolide H | 0.52 μmol/L | [68] |
| HepG2 | Bruceine B | 0.81 μmol/L | [68] | SKOV3 | Bruceine B | 0.12 μmol/L | [68] |
| Bruceine D | 1.2 μmol/L | Bruceine D | 0.76 μmol/L | ||||
| Bruceine E | 2.9 μmol/L | Bruceine E | 2.2 μmol/L | ||||
| Bruceine H | 2.8 μmol/L | Bruceine H | 0.33 μmol/L | ||||
| javanicolide H | >10 μmol/L | javanicolide H | 0.23 μmol/L | ||||
| javanicolide E | >10 μmol/L | javanicolide E | 1.49 μmol/L | ||||
| Bel-7402 | Yadanziolide B | 4.24 μmol/L | [29] | MDA-MB231 | BJ water extract | 50 μg/mL | [56] |
| Bel-7404 | Bruceantinol | 10 μmol/L | [35] | Brusatol | 0.081 μmol/L | [37] | |
| P-388 | javanicoside B | 5.6 μg/mL | [96] | Bruceantinol | 0.088 μmol/L | ||
| javanicolides C | >100 μg/mL | Bruceine A | 0.228 μmol/L | ||||
| javanicolides D | 18 μg/mL | Bruceantarin | 0.238 μmol/L | ||||
| javanicosides C | 18 μg/mL | HeLa | quassilactones A | 78.95 μmol/L | [86] | ||
| javanicosides D | 89 μg/mL | quassilactones B | 92.57 μmol/L | ||||
| javanicosides E | 16 μg/mL | KB | BJ extract | 24.37 μg/mL | [101] | ||
| javanicosides F | 50 μg/mL | ORL-48 | BJ extract | 6.67 μg/mL | [101] | ||
| H1650 | Brusatol | 24.27 ng/mL | [105] | U251 | Bruceanol | 20 nmol/L | [102] |
| PC9 | Brusatol | 18.40 ng/mL | [105] | HCC827 | Brusatol | 73.3 ng/mL | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yang, H.; Zhou, Y.; Guo, R.; Liu, J.; Wei, F.; Lin, Y. Potential of Traditional Chinese Medicine Brucea javanica in Cancer Treatment: A Review of Chemical Constituents, Pharmacology, and Clinical Applications. Nutrients 2025, 17, 3285. https://doi.org/10.3390/nu17203285
Xu W, Yang H, Zhou Y, Guo R, Liu J, Wei F, Lin Y. Potential of Traditional Chinese Medicine Brucea javanica in Cancer Treatment: A Review of Chemical Constituents, Pharmacology, and Clinical Applications. Nutrients. 2025; 17(20):3285. https://doi.org/10.3390/nu17203285
Chicago/Turabian StyleXu, Weiyin, Hongmei Yang, Yanan Zhou, Rixin Guo, Jing Liu, Feng Wei, and Yongqiang Lin. 2025. "Potential of Traditional Chinese Medicine Brucea javanica in Cancer Treatment: A Review of Chemical Constituents, Pharmacology, and Clinical Applications" Nutrients 17, no. 20: 3285. https://doi.org/10.3390/nu17203285
APA StyleXu, W., Yang, H., Zhou, Y., Guo, R., Liu, J., Wei, F., & Lin, Y. (2025). Potential of Traditional Chinese Medicine Brucea javanica in Cancer Treatment: A Review of Chemical Constituents, Pharmacology, and Clinical Applications. Nutrients, 17(20), 3285. https://doi.org/10.3390/nu17203285






