New Anthropometry-Based Formulae to Predict 24 h Sodium Excretion from Spot Urine
Abstract
1. Introduction
2. Materials and Methods
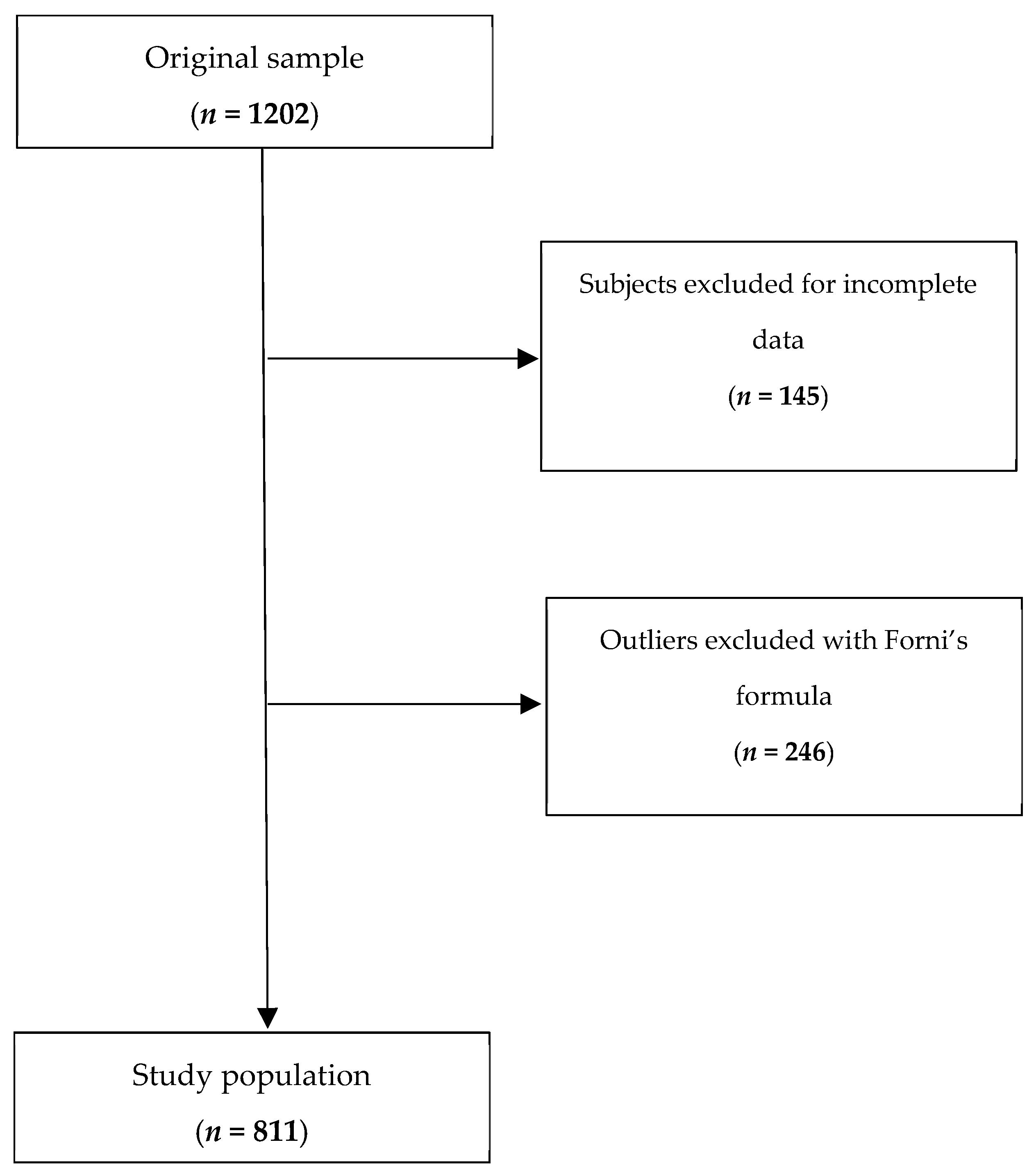
2.1. Study Design and Population
2.2. Data Collection Procedures
2.3. Data Preprocessing and Cleaning
2.4. Model Development
2.5. Model Evaluation
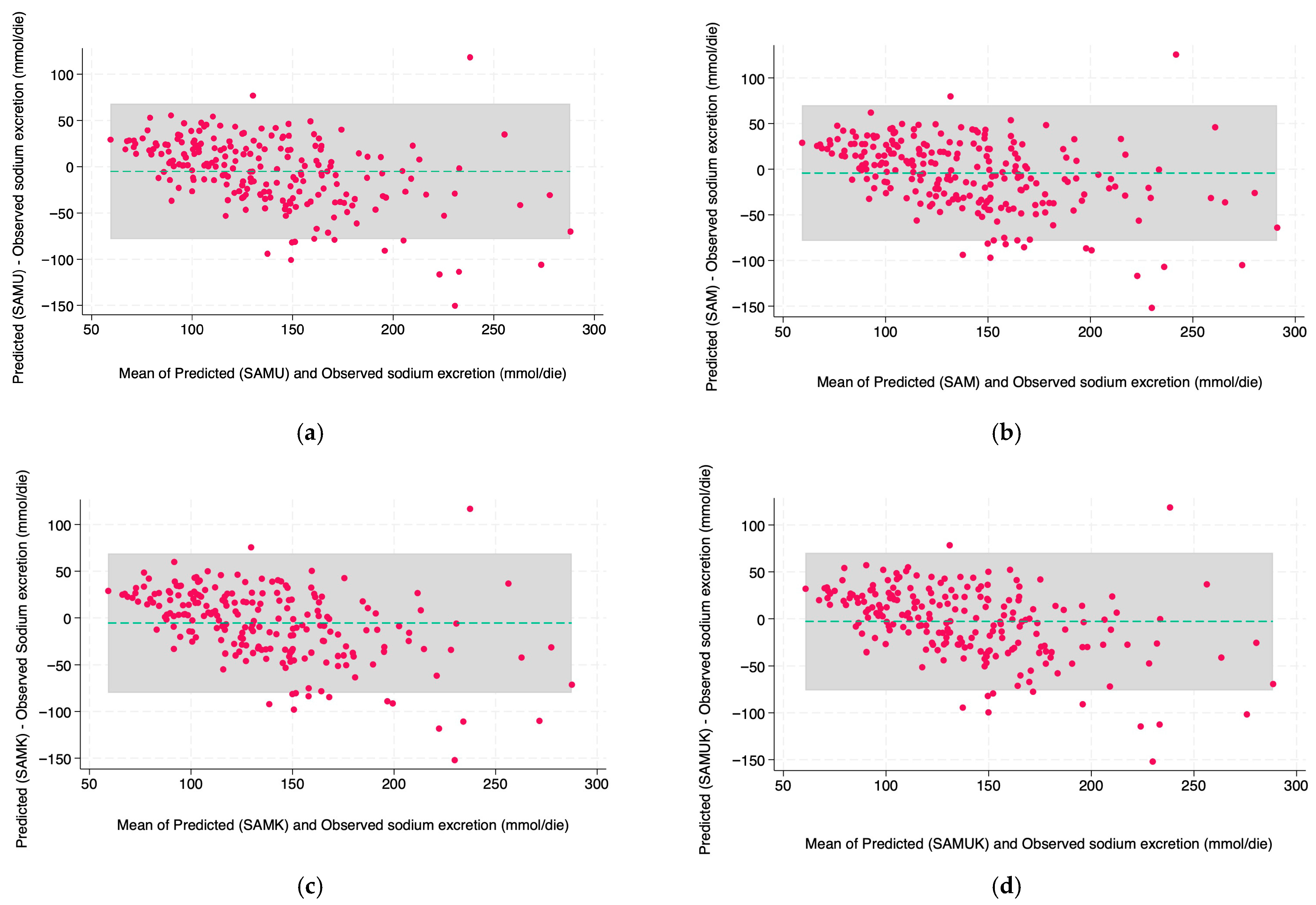
3. Results
Timed Nocturnal Urine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| CVD | Cardiovascular disease |
| SAM | Swiss anthropometric model |
| SAMK | Swiss anthropometric model with potassium |
| SAMUK | Swiss anthropometric model with urea and potassium |
| SAMU | Swiss anthropometric model with urea |
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Horowitz, B.; Miskulin, D.; Zager, P. Epidemiology of hypertension in CKD. Adv. Chronic Kidney Dis. 2015, 22, 88–95. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Nichols, R.; Dyer, A.R.; Stamler, R.; Kesteloot, H.; Marmot, M. Intersalt revisited: Further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ 1996, 312, 1249–1253. [Google Scholar] [CrossRef]
- Stamler, J.; Chan, Q.; Daviglus, M.L.; Dyer, A.R.; Van Horn, L.; Garside, D.B.; Miura, K.; Wu, Y.; Ueshima, H.; Zhao, L.; et al. Relation of Dietary Sodium (Salt) to Blood Pressure and Its Possible Modulation by Other Dietary Factors: The INTERMAP Study. Hypertension 2018, 71, 631–637. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children. 2012. Available online: https://www.who.int/publications/i/item/9789241504836 (accessed on 1 March 2025).
- Thijssen, S.; Kitzler, T.M.; Levin, N.W. Salt: Its role in chronic kidney disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2008, 18, 18–26. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- WHO. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk. 2007. Available online: https://www.who.int/publications/i/item/9789241547178 (accessed on 1 March 2025).
- Thout, S.R.; Santos, J.A.; McKenzie, B.; Trieu, K.; Johnson, C.; McLean, R.; Arcand, J.; Campbell, N.R.C.; Webster, J. The Science of Salt: Updating the evidence on global estimates of salt intake. J. Clin. Hypertens. 2019, 21, 710–721. [Google Scholar] [CrossRef]
- Chelbi, S.T.; Gianini, J.; Gagliano, V.; Theiler, K.; Alonzo, G.L.; Marot, P.; Ackermann, D.; Durrer, I.; Beuschlein, F.; Suter, P.; et al. Swiss Salt Study 2, second survey on salt consumption in Switzerland: Main results. Food Risk Assess Eur. 2024, 2, 0031E. [Google Scholar] [CrossRef]
- WHO. Who Global Report On Sodium Intake Reduction. 2023. Available online: https://www.who.int/publications/i/item/9789240069985 (accessed on 1 March 2025).
- Leiba, A.; Vald, A.; Peleg, E.; Shamiss, A.; Grossman, E. Does dietary recall adequately assess sodium, potassium, and calcium intake in hypertensive patients? Nutrition 2005, 21, 462–466. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Maalouf, J.; Elliott, P.; Loria, C.M.; Patel, S.; Bowman, B.A. Use of Urine Biomarkers to Assess Sodium Intake: Challenges and Opportunities. Annu. Rev. Nutr. 2015, 35, 349–387. [Google Scholar] [CrossRef]
- Saran, R.; Padilla, R.L.; Gillespie, B.W.; Heung, M.; Hummel, S.L.; Derebail, V.K.; Pitt, B.; Levin, N.W.; Zhu, F.; Abbas, S.R.; et al. A Randomized Crossover Trial of Dietary Sodium Restriction in Stage 3–4 CKD. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 399–407. [Google Scholar] [CrossRef]
- McLean, R.M. Measuring population sodium intake: A review of methods. Nutrients 2014, 6, 4651–4662. [Google Scholar] [CrossRef]
- Kamińska, J.; Dymicka-Piekarska, V.; Tomaszewska, J.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit. Rev. Clin. Lab. Sci. 2020, 57, 345–364. [Google Scholar] [CrossRef]
- WHO. Sodium Intakes Around the World. 2006. Available online: https://iris.who.int/server/api/core/bitstreams/20fb3e48-ef9a-410b-b21f-c63a547ab8c7/content (accessed on 1 March 2025).
- Forni Ogna, V.; Ogna, A.; Vuistiner, P.; Pruijm, M.; Ponte, B.; Ackermann, D.; Gabutti, L.; Vakilzadeh, N.; Mohaupt, M.; Martin, P.Y.; et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015, 13, 40. [Google Scholar] [CrossRef]
- Huang, L.; Crino, M.; Wu, J.H.; Woodward, M.; Barzi, F.; Land, M.A.; McLean, R.; Webster, J.; Enkhtungalag, B.; Neal, B. Mean population salt intake estimated from 24-h urine samples and spot urine samples: A systematic review and meta-analysis. Int. J. Epidemiol. 2016, 45, 239–250. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef]
- Brown, I.J.; Dyer, A.R.; Chan, Q.; Cogswell, M.E.; Ueshima, H.; Stamler, J.; Elliott, P. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: The INTERSALT study. Am. J. Epidemiol. 2013, 177, 1180–1192. [Google Scholar] [CrossRef]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef]
- Xu, J.; Du, X.; Bai, Y.; Fang, L.; Liu, M.; Ji, N.; Zhong, J.; Yu, M.; Wu, J. Assessment and validation of spot urine in estimating the 24-h urinary sodium, potassium, and sodium/potassium ratio in Chinese adults. J. Hum. Hypertens. 2020, 34, 184–192. [Google Scholar] [CrossRef]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar]
- Fujisawa, C.; Umegaki, H.; Sugimoto, T.; Huang, C.H.; Fujisawa, H.; Sugimura, Y.; Kuzuya, M.; Toba, K.; Sakurai, T. Older adults with a higher frailty index tend to have electrolyte imbalances. Exp. Gerontol. 2022, 163, 111778. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.R.; Bailey, M.A. Nondipping Blood Pressure: Predictive or Reactive Failure of Renal Sodium Handling? Physiology 2021, 36, 21–34. [Google Scholar] [CrossRef]
- O’Brien, E.; Sheridan, J.; O’Malley, K. Dippers and non-dippers. Lancet 1988, 2, 397. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.S.; McFetridge-Durdle, J.A.; Dean, C.R. Night-time blood pressure patterns and target organ damage: A review. Can. J. Cardiol. 2007, 23, 132–138. [Google Scholar] [CrossRef]
- Viggiano, J.; Coutinho, D.; Clark-Cutaia, M.N.; Martinez, D. Effects of a high salt diet on blood pressure dipping and the implications on hypertension. Front. Neurosci. 2023, 17, 1212208. [Google Scholar] [CrossRef]
- Uzu, T.; Kazembe, F.S.; Ishikawa, K.; Nakamura, S.; Inenaga, T.; Kimura, G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension 1996, 28, 139–142. [Google Scholar] [CrossRef]
- Sachdeva, A.; Weder, A.B. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension 2006, 48, 527–533. [Google Scholar] [CrossRef]
- Weinberger, M.H. Salt sensitivity of blood pressure in humans. Hypertension 1996, 27 3 Pt 2, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, A.S.; Refaat, S.A.; Bentley, B.L.; Rahmani, J. Association between intake of sodium, potassium, sodium-to-potassium ratio, and blood pressure among US adults. Int. J. Vitam. Nutr. Res. 2023, 93, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Bontić, A.; Kezić, A.; Pavlović, J.; Baralić, M.; Gajić, S.; Petrovic, K.; Ristanović, V.K.; Petrović, O.; Stjepanović, V.; Stanković, S.; et al. Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients. Metabolites 2024, 14, 460. [Google Scholar] [CrossRef]
- Alwis, U.S.; Delanghe, J.; Dossche, L.; Walle, J.V.; Van Camp, J.; Monaghan, T.F.; Roggeman, S.; Everaert, K. Could Evening Dietary Protein Intake Play a Role in Nocturnal Polyuria? J. Clin. Med. 2020, 9, 2532. [Google Scholar] [CrossRef]
- Del Giorno, R.; Gabutti, S.; Troiani, C.; Stefanelli, K.; Falciano, R.; Graziano, E.; Rochat Negro, T.; Gabutti, L. Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study. J. Clin. Med. 2019, 8, 1527. [Google Scholar] [CrossRef]
- Mann, S.J.; Gerber, L.M. Estimation of 24-hour sodium excretion from spot urine samples. J. Clin. Hypertens. 2010, 12, 174–180. [Google Scholar] [CrossRef]
- Peng, Y.; Li, W.; Wang, Y.; Chen, H.; Bo, J.; Wang, X.; Liu, L. Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in Chinese Adults. PLoS ONE 2016, 11, e0149655. [Google Scholar] [CrossRef]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Gabutti, L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: A population-based study. Ann. Med. 2021, 53, 1–16. [Google Scholar] [CrossRef]
- Gagliano, V.; Gehrig, D.; Del Giorno, R.; Gianini, J.; Gabutti, L. A Population-Based Scoring System to Assess the Impact of Individual Risk Factors on Vascular Health. Aging Dis. 2024, 15, 1373–1383. [Google Scholar]
- WHO. Sodium Reduction. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/sodium-reduction (accessed on 1 March 2025).
- Kesteloot, H.E.; Joossens, J.V. Relationship between dietary protein intake and serum urea, uric acid and creatinine, and 24-hour urinary creatinine excretion: The BIRNH Study. J. Am. Coll. Nutr. 1993, 12, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, K.; Imai, K.; Abe, S.; Iwamoto, M.; Kawate, H.; Moriguchi, R.; Ono, M.; Ueno, H.; Miya, M.; Tsuda, H.; et al. Food intake and dietary patterns that affect urinary sodium excretion in young women. J. Clin. Hypertens. 2020, 22, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 811) | Training (n = 574, 70.8%) | Test (n = 237, 29.2%) | p-Value |
|---|---|---|---|---|
| BMI (kg/m2) | 25.21 ± 4.4 [24.5 (22.2–27.5)] | 25.25 ± 4.44 [24.5 (22.2–27.7)] | 25.14 ± 4.31 [24.6 (22.1–27.3)] | 0.813 |
| Sex, n (%) | ||||
| Male | 352 (43.4) | 325 (56.6) | 135 (57.0) | 0.983 |
| Female | 459 (56.6) | 249 (43.4) | 102 (43.0) | |
| Weight (kg) | 71.97 ± 15.8 [71.0 (60.0–81.0)] | 72.26 ± 15.94 [71.0 (60.0–81.0)] | 71.25 ± 15.46 [70.0 (60.0–80.0)] | 0.431 |
| Height (cm) | 168.51 ± 9.95 [167.94 (161.09–175.12)] | 168.78 ± 10.33 [167.94 (161.11–175.96)] | 167.86 ± 8.95 [167.94 (160.96–173.95)] | 0.349 |
| Age (years) | 51.19 ± 13.7 [51.0 (42.0–60.0)] | 50.87 ± 13.9 [51.0 (42.0–60.0)] | 51.96 ± 13.21 [51.0 (43.0–61.0)] | 0.310 |
| Expected creatinine excretion, 24 h Forni (mmol) [20] | 12.04 ± 3.57 [11.05 (9.22–14.92)] | 12.1 ± 3.66 [11.08 (9.2–15.17)] | 11.87 ± 3.36 [10.98 (9.27–14.53)] | 0.592 |
| Observed creatinine excretion, 24 h (mmol) | 12.46 ± 3.81 [11.83 (9.41–15.15)] | 12.45 ± 3.91 [11.76 (9.25–15.36)] | 12.47 ± 3.55 [11.84 (9.85–14.92)] | 0.677 |
| Expected creatinine excretion, 24 h Kawasaki (mmol) | 14.21 ± 2.94 [14.15 (12.05–16.01)] | 14.3 ± 3.03 [14.37 (12.03–16.04)] | 13.98 ± 2.72 [13.79 (12.09–15.76)] | 0.185 |
| Expected creatinine excretion, 24 h Tanaka (mmol) | 12.75 ± 3.16 [12.5 (10.37–14.76)] | 12.84 ± 3.22 [12.53 (10.39–14.88)] | 12.55 ± 3.0 [12.29 (10.3–14.43)] | 0.322 |
| Urine volume, 24 h (L) | 1.81 ± 0.78 [1.7 (1.2–2.25)] | 1.82 ± 0.8 [1.7 (1.2–2.25)] | 1.77 ± 0.74 [1.6 (1.2–2.1)] | 0.494 |
| Urine volume, day (L) | 1.27 ± 0.66 [1.1 (0.8–1.6)] | 1.27 ± 0.67 [1.15 (0.8–1.64)] | 1.26 ± 0.64 [1.1 (0.8–1.6)] | 0.980 |
| Urine volume, night (L) | 0.54 ± 0.29 [0.5 (0.35–0.7)] | 0.55 ± 0.29 [0.5 (0.35–0.7)] | 0.52 ± 0.27 [0.45 (0.35–0.6)] | 0.137 |
| Na concentration, day (mmol/L) | 89.94 ± 50.19 [79.0 (52.0–121.0)] | 89.08 ± 51.16 [77.0 (50.0–120.0)] | 92.0 ± 47.82 [82.0 (55.0–123.0)] | 0.207 |
| Na concentration, night (mmol/L) | 90.49 ± 45.4 [82.0 (54.0–121.0)] | 89.56 ± 45.19 [81.0 (54.0–118.0)] | 92.72 ± 45.94 [82.0 (55.0–125.0)] | 0.394 |
| Creatinine concentration, day (mmol/L) | 8.24 ± 4.85 [7.0 (4.6–10.8)] | 8.21 ± 4.88 [7.0 (4.53–10.8)] | 8.3 ± 4.78 [7.1 (4.8–11.0)] | 0.676 |
| Creatinine concentration, night (mmol/L) | 9.75 ± 5.43 [8.4 (5.4–13.1)] | 9.74 ± 5.62 [8.35 (5.3–13.08)] | 9.78 ± 4.95 [8.4 (5.7–13.4)] | 0.406 |
| Creatinine excretion, day (mmol) | 8.16 ± 2.78 [7.76 (6.0–9.98)] | 8.14 ± 2.83 [7.71 (5.96–9.96)] | 8.23 ± 2.67 [7.9 (6.24–9.98)] | 0.516 |
| Creatinine excretion, night (mmol) | 4.29 ± 1.73 [4.0 (3.08–5.12)] | 4.32 ± 1.77 [4.05 (3.08–5.12)] | 4.24 ± 1.62 [3.87 (3.08–5.11)] | 0.603 |
| Night hours (h) | 8.29 ± 2.09 [8.07 (7.07–9.24)] | 8.33 ± 2.07 [8.11 (7.13–9.29)] | 8.21 ± 2.14 [7.96 (6.9–9.18)] | 0.305 |
| Hourly creatinine excretion (mmol/h) | 0.52 ± 0.16 [0.49 (0.39–0.63)] | 0.52 ± 0.16 [0.49 (0.39–0.64)] | 0.52 ± 0.15 [0.49 (0.41–0.62)] | 0.677 |
| Hourly Na excretion (mmol/h) | 5.34 ± 2.75 [4.75 (3.43–6.79)] | 5.33 ± 2.79 [4.79 (3.36–6.66)] | 5.34 ± 2.66 [4.71 (3.56–6.93)] | 0.809 |
| Na concentration, night/creatinine concentration, night | 10.57 ± 5.0 [9.55 (7.06–12.92)] | 10.64 ± 5.2 [9.61 (7.07–13.01)] | 10.39 ± 4.49 [9.46 (7.03–12.86)] | 0.895 |
| Na excretion, 24 h (mmol) | 139.14 ± 56.05 [133.7 (96.0–172.9)] | 138.26 ± 56.47 [132.32 (96.05–170.69)] | 141.29 ± 55.08 [135.2 (96.0–175.5)] | 0.468 |
| Na excretion, day (mmol) | 95.31 ± 43.97 [90.25 (63.2–120.0)] | 94.11 ± 43.57 [88.8 (63.2–118.61)] | 98.22 ± 44.9 [92.0 (63.2–126.35)] | 0.294 |
| Na excretion, night (mmol) | 43.83 ± 24.51 [38.5 (26.8–55.58)] | 44.15 ± 25.36 [38.5 (26.6–55.5)] | 43.08 ± 22.35 [38.4 (27.2–56.4)] | 0.991 |
| K excretion, night (mmol/L) | 28.9 ± 15.95 [26.0 (17.0–37.0)] | 28.51 ± 16.38 [25.0 (16.0–37.0)] | 29.81 ± 14.89 [27.0 (19.0–38.0)] | 0.083 |
| Urea excretion, night (mmol/L) | 281.18 ± 142.63 [250.55 (166.78–375.38)] | 280.13 ± 145.02 [250.2 (162.1–381.8)] | 283.64 ± 137.14 [251.05 (173.38–371.18)] | 0.563 |
| K, night/creatinine, night | 3.35 ± 1.46 [3.1 (2.25–4.09)] | 3.34 ± 1.48 [3.08 (2.31–4.07)] | 3.35 ± 1.43 [3.19 (2.17–4.14)] | 0.745 |
| Urea, night/creatinine night | 0.53 ± 0.52 [0.37 (0.2–0.69)] | 0.55 ± 0.55 [0.37 (0.2–0.71)] | 0.49 ± 0.46 [0.37 (0.2–0.64)] | 0.539 |
| Predicted Na excretion, Kawasaki 24 h (mmol) | 64.9 ± 27.87 [58.94 (44.61–81.59)] | 65.83 ± 29.0 [59.31 (44.71–82.75)] | 62.67 ± 24.83 [57.36 (43.54–76.38)] | 0.314 |
| Predicted Na excretion, Tanaka 24 h (mmol) | 65.62 ± 22.8 [61.41 (49.06–79.29)] | 66.33 ± 23.69 [61.59 (49.06–80.29)] | 63.91 ± 20.42 [61.1 (49.06–75.12)] | 0.337 |
| Predicted Na excretion, INTERSALT 24 h (mmol) | 97.06 ± 24.95 [93.0 (79.26–114.68)] | 96.95 ± 24.96 [92.73 (79.27–114.74)] | 97.33 ± 24.98 [94.05 (79.23–114.52)] | 0.971 |
| Model | R2 | RMSE | Mean Difference | Relative Error (Mean ± SD) | Error > 40% * |
|---|---|---|---|---|---|
| Swiss anthropometric model with potassium (SAMK) | 0.52 | 38.06 | −5.5 (37.99) | 3.65 ± 28.22 | 52 (21.94%) |
| Swiss anthropometric model (SAM) | 0.53 | 37.99 | −4.23 (37.91) | 4.49 ± 28.52 | 38 (16.03%) |
| Swiss anthropometric model with urea and potassium (SAMUK) | 0.54 | 37.38 | −2.86 (37.31) | 5.54 ± 28.46 | 51 (21.54%) |
| Swiss anthropometric model with urea (SAMU) | 0.53 | 37.48 | −5.01 (37.42) | 3.80 ± 27.98 | 49 (20.68%) |
| Model | R2 | RMSE | Mean Difference | Relative Error (Mean ± SD) | Error > 40% * |
|---|---|---|---|---|---|
| Swiss anthropometric model with potassium (SAMK) | 0.57 | 36.12 | 10.37 (36.04) | 15.9 ± 30.22 | 68 (28.69%) |
| Swiss anthropometric model (SAM) | 0.58 | 35.92 | −3.88 (35.84) | 4.03 ± 26.65 | 29 (12.24%) |
| Swiss anthropometric model with urea and potassium (SAMUK) | 0.58 | 35.52 | −2.76 (35.45) | 4.80 ± 26.58 | 50 (21.10%) |
| Swiss anthropometric model with urea (SAMU) | 0.58 | 35.54 | −2.60 (35.48) | 4.95 ± 26.55 | 51 (21.52%) |
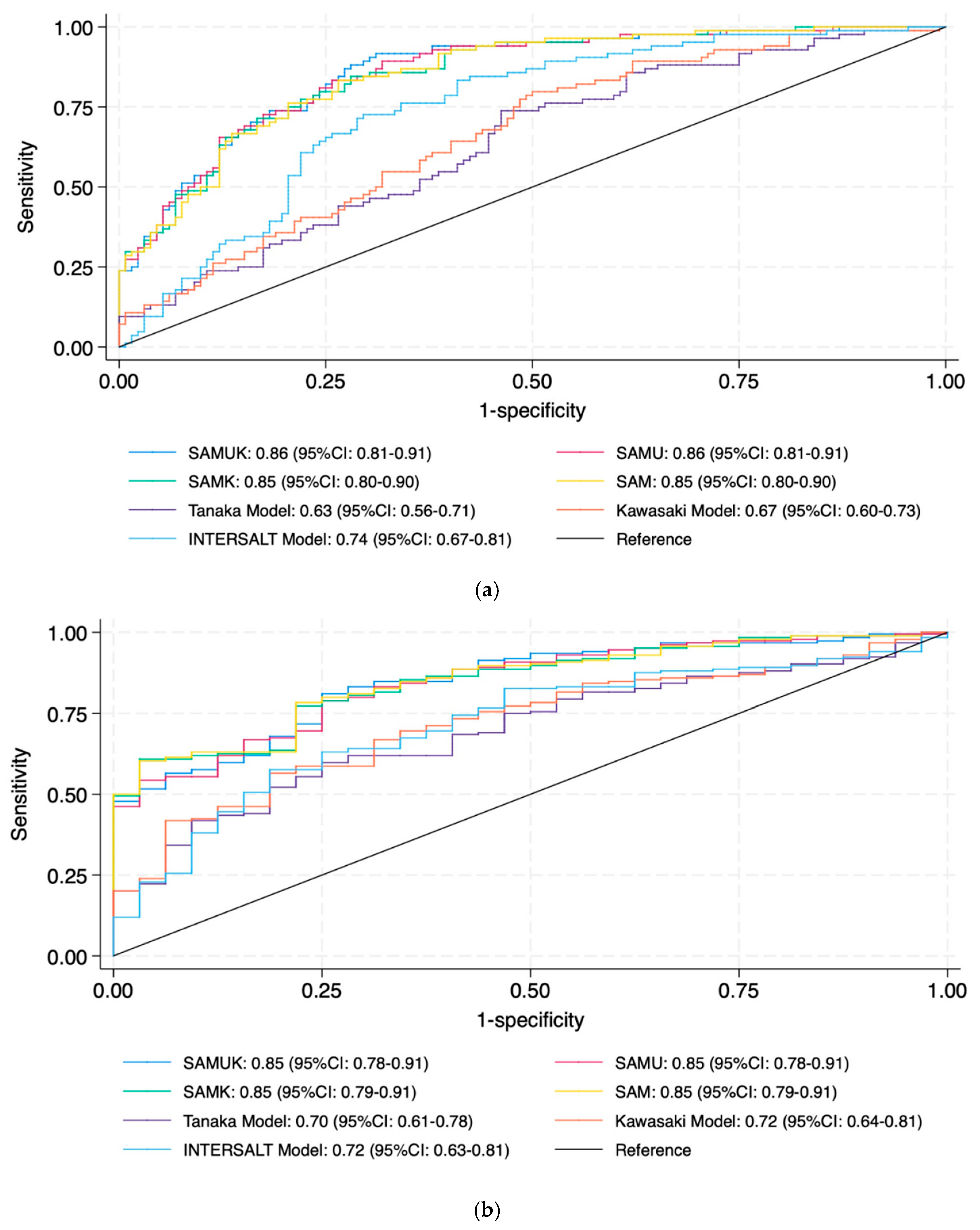
| Model | AUC (95% CI) |
|---|---|
| Cut-off: 150 mmol | |
| SAMUK | 0.88 (0.84–0.93) |
| SAMU | 0.89 (0.84–0.93) |
| SAMK | 0.88 (0.84–0.93) |
| SAM | 0.88 (0.83–0.93) |
| Tanaka | 0.64 (0.57–0.72) |
| Kawasaki | 0.67 (0.60–0.74) |
| INTERSALT | 0.75 (0.68–0.81) |
| Cut-off: 85 mmol | |
| SAMUK | 0.87 (0.81–0.92) |
| SAMU | 0.87 (0.81–0.92) |
| SAMK | 0.87 (0.82–0.92) |
| SAM | 0.87 (0.82–0.92) |
| Tanaka | 0.70 (0.61–0.78) |
| Kawasaki | 0.72 (0.64–0.81) |
| INTERSALT | 0.72 (0.63–0.81) |
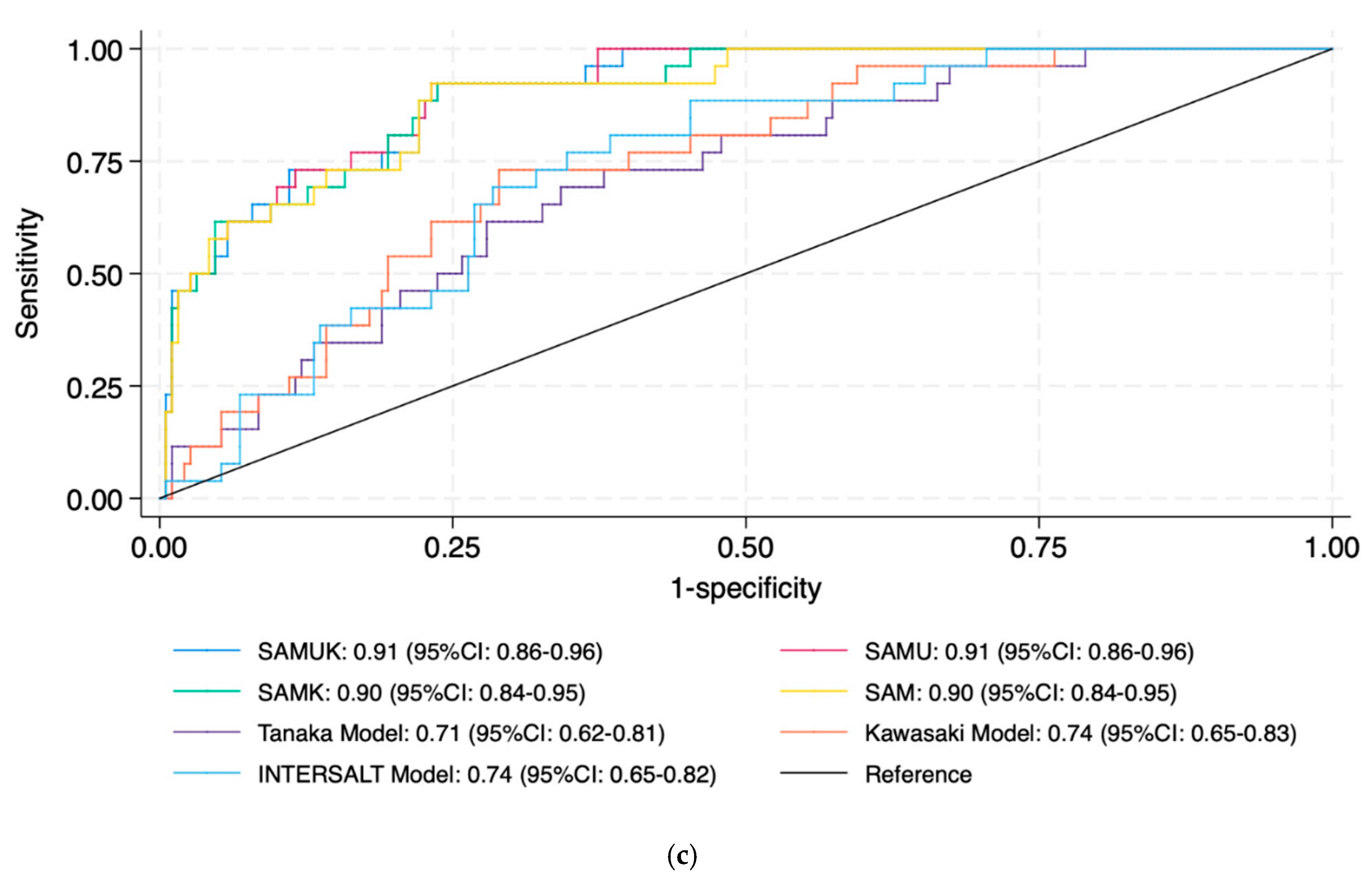
| Cut-off: 200 mmol | |
| SAMUK | 0.92 (0.87–0.96) |
| SAMU | 0.92 (0.88–0.97) |
| SAMK | 0.92 (0.87–0.96) |
| SAM | 0.92 (0.87–0.96) |
| Tanaka | 0.71 (0.62–0.81) |
| Kawasaki | 0.74 (0.65–0.83) |
| INTERSALT | 0.74 (0.65–0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandonà, M.; Holzner, K.; Garo, M.L.; Del Giorno, R.; Gabutti, L. New Anthropometry-Based Formulae to Predict 24 h Sodium Excretion from Spot Urine. Nutrients 2025, 17, 3284. https://doi.org/10.3390/nu17203284
Zandonà M, Holzner K, Garo ML, Del Giorno R, Gabutti L. New Anthropometry-Based Formulae to Predict 24 h Sodium Excretion from Spot Urine. Nutrients. 2025; 17(20):3284. https://doi.org/10.3390/nu17203284
Chicago/Turabian StyleZandonà, Martina, Karin Holzner, Maria Luisa Garo, Rosaria Del Giorno, and Luca Gabutti. 2025. "New Anthropometry-Based Formulae to Predict 24 h Sodium Excretion from Spot Urine" Nutrients 17, no. 20: 3284. https://doi.org/10.3390/nu17203284
APA StyleZandonà, M., Holzner, K., Garo, M. L., Del Giorno, R., & Gabutti, L. (2025). New Anthropometry-Based Formulae to Predict 24 h Sodium Excretion from Spot Urine. Nutrients, 17(20), 3284. https://doi.org/10.3390/nu17203284






